

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (1): 20210625.doi: 10.7503/cjcu20210625
收稿日期:2021-08-31
出版日期:2022-01-10
发布日期:2021-10-27
通讯作者:
李新雄,郑寿添
E-mail:lxx@fzu.edu.cn;stzheng@fzu.edu.cn
基金资助:
CHEN Huina, LI Xinxiong( ), ZHENG Shoutian(
), ZHENG Shoutian( )
)
Received:2021-08-31
Online:2022-01-10
Published:2021-10-27
Contact:
LI Xinxiong,ZHENG Shoutian
E-mail:lxx@fzu.edu.cn;stzheng@fzu.edu.cn
Supported by:摘要:
铌多酸三维框架材料是近年来无机合成化学与材料化学领域的研究热点. 该类材料不但可以将铌多酸的优异特性与框架结构的优点复合起来, 而且在光催化、 主客体化学、 能源转化等领域具有重要的应用前景. 本文总结了近10年来铌多酸三维框架材料的研究进展, 包括了该类材料的合成策略、 结构调控、 性质及应用探索. 此外, 还对当前该类材料的发展所面临的挑战进行了总结, 并对其发展前景进行了展望.
中图分类号:
TrendMD:
陈慧娜, 李新雄, 郑寿添. 铌多酸三维框架材料的研究进展. 高等学校化学学报, 2022, 43(1): 20210625.
CHEN Huina, LI Xinxiong, ZHENG Shoutian. Research Advance of Polyoxoniobate-based 3-Dimensional Framework Materials. Chem. J. Chinese Universities, 2022, 43(1): 20210625.
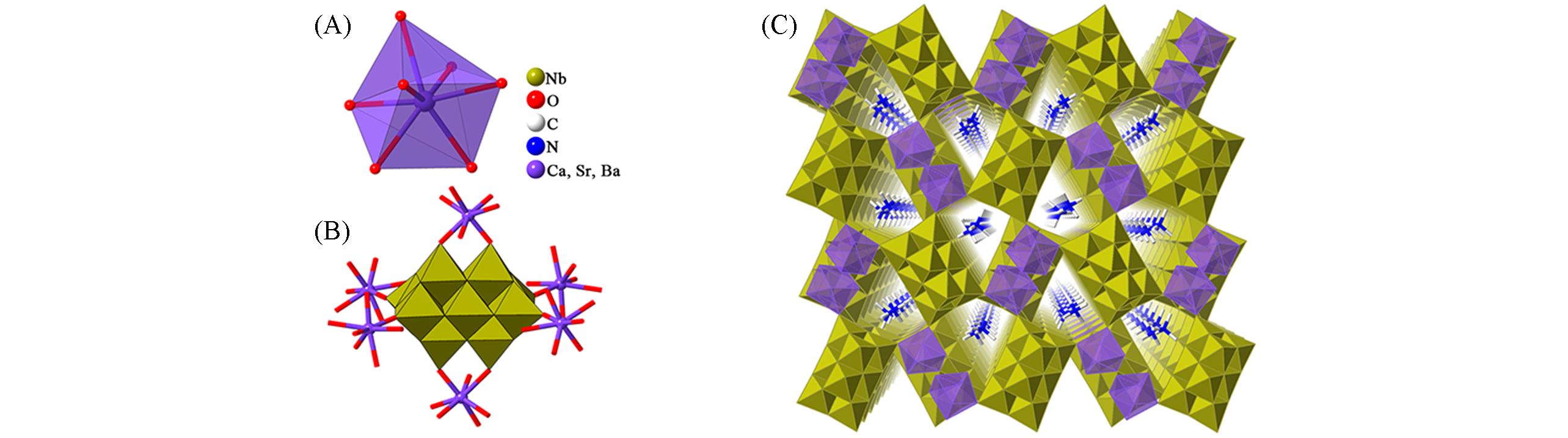
Fig.1 Framework structures based on {Nb10O28} and rare earth ions(A) Coordination patterns of calcium, strontium and barium; (B) representation of the Ca/Sr/Ba-Nb10 compounds;(C) view of the 3D framework of Ca/Sr/Ba-Nb10 compounds.
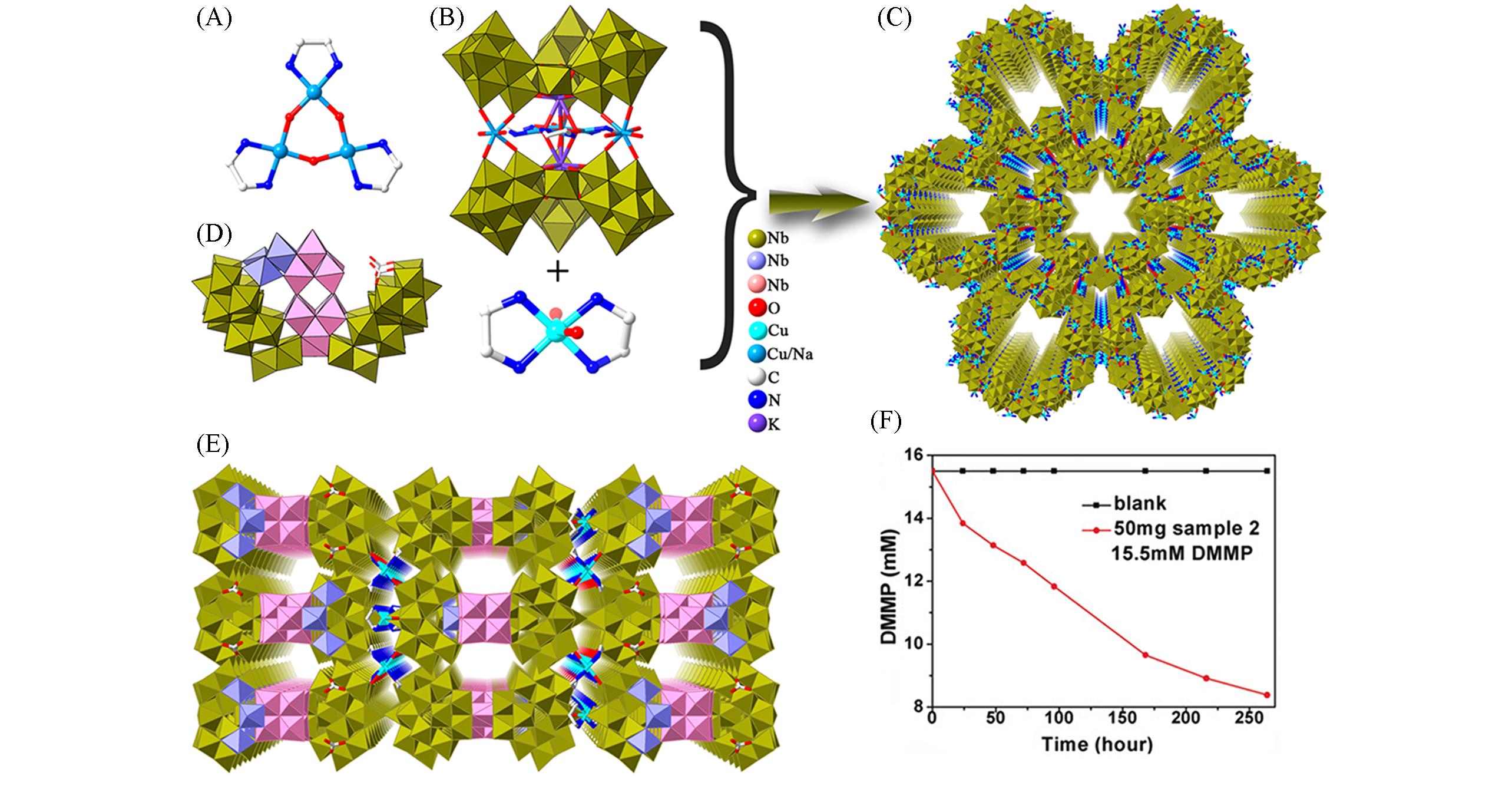
Fig.2 Framework structures based on {KNb24}2, {Nb47} polyanions and the catalytic study of {Nb47} in DMMP decomposition(A) Ball-and-stick representation of {Cu3(en)3(H2O)3}6+; (B) combine polyhedral/stick representations of the {KNb24}2 compound; (C) view of 3D framework of the {KNb24}2 compound; (D) combine polyhedral/stick representations of {Nb47}; (E) view of the 3D framework of the {Nb47} compound; (F) DMMP decomposition using {Nb47} compound[24], Copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
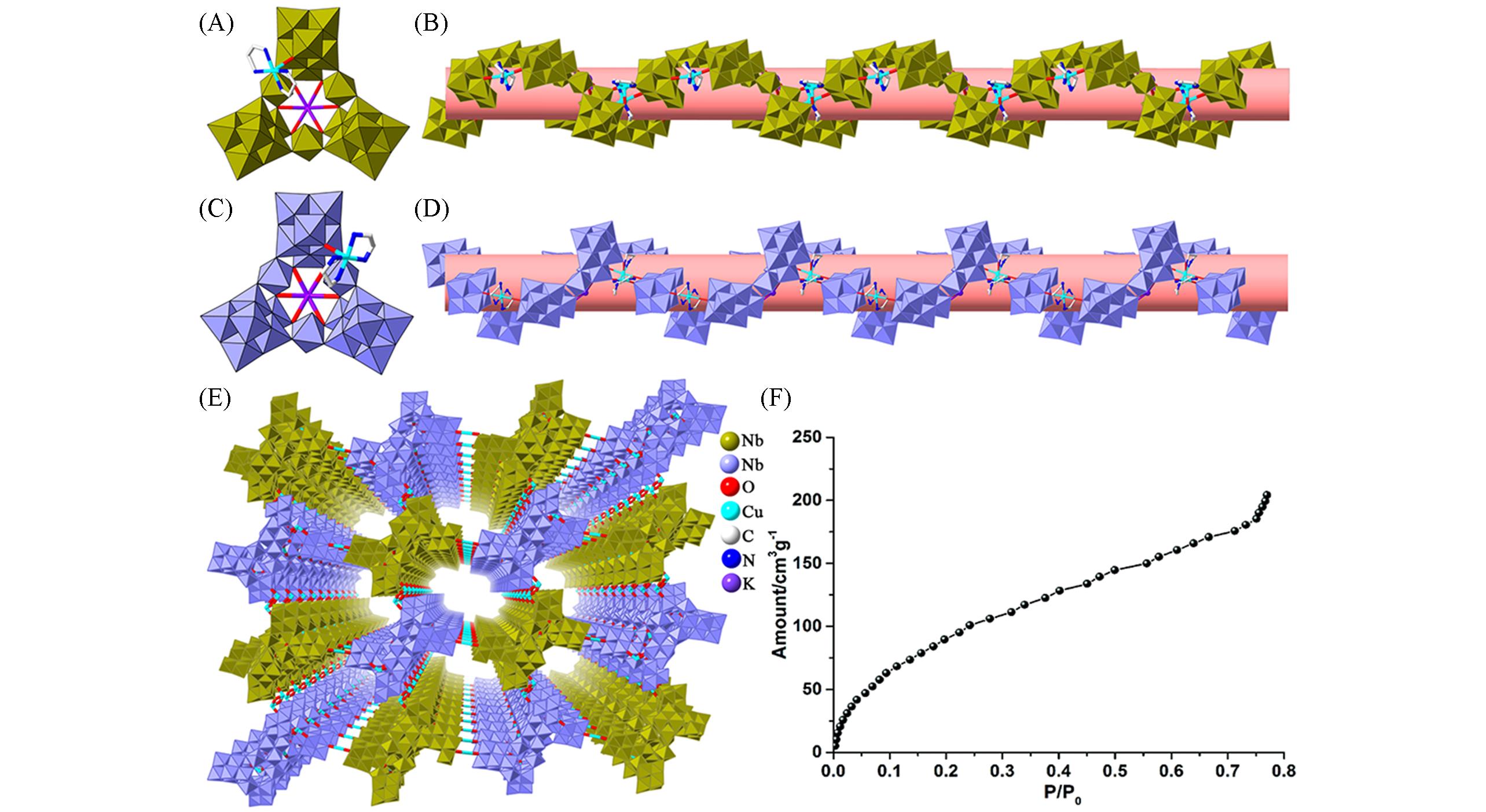
Fig.3 Framework structure of {Cu(en)2KNb24O72H10} and related water adsorption characterization(A) Polyhedral and stick representations of the {α?CuKNb24} unit; (B) polyhedral and ball?and?stick representations of the {L?α?CuKNb24}; (C) polyhedral and stick representations of the {β?CuKNb24} unit; (D) polyhedral and ball?and?stick representations of the {R?β?CuKNb24}; (E) view of the 3D framework of this compound; (F) water vapor adsorption curve[26], Copyright 2020, the Chinese Chemical Society and the Royal Society of Chemistry.
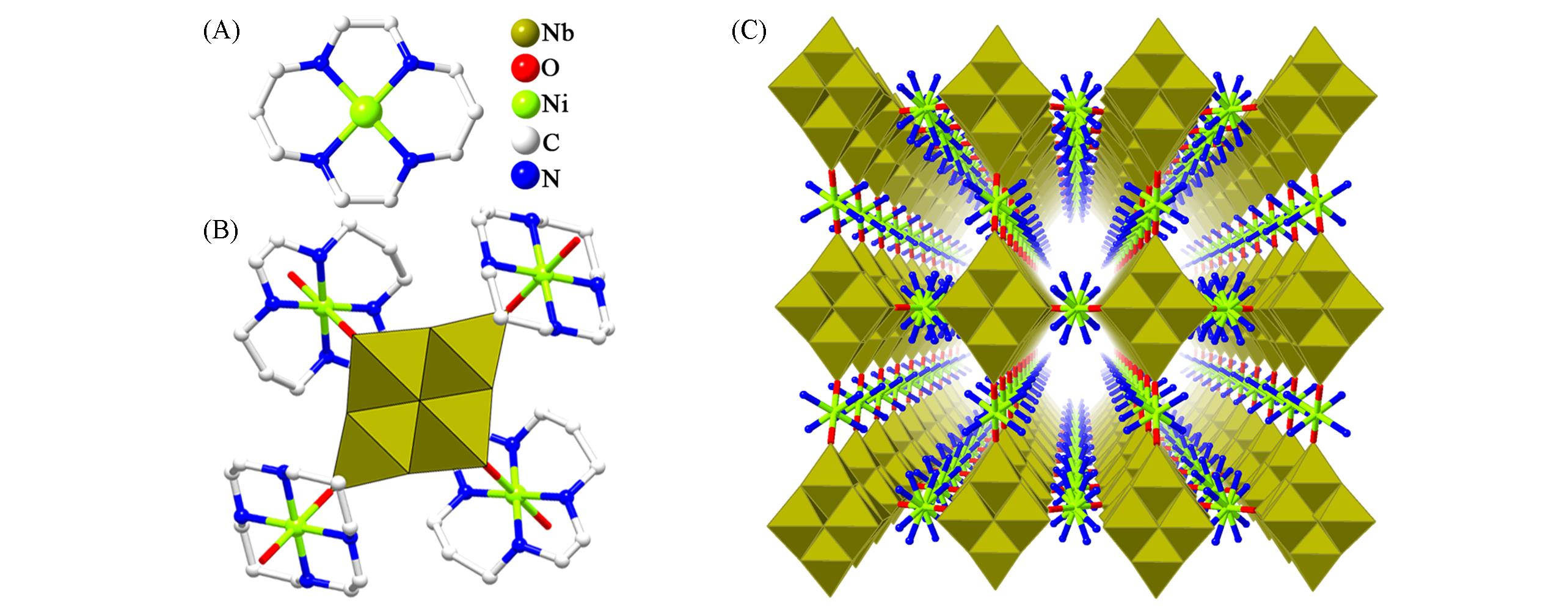
Fig.4 Framework structure of {[Ni(cyclam)]2H4Nb6O19}·12H2O(A) Ball-and-stick representation of [Ni(cyclam)]2+; (B) polyhedral and ball-and-stick representations of {[Ni(cyclam)]2H4Nb6O19}·12H2O; (C) view of the 3D framework of {[Ni(cyclam)]2H4Nb6O19}·12H2O.
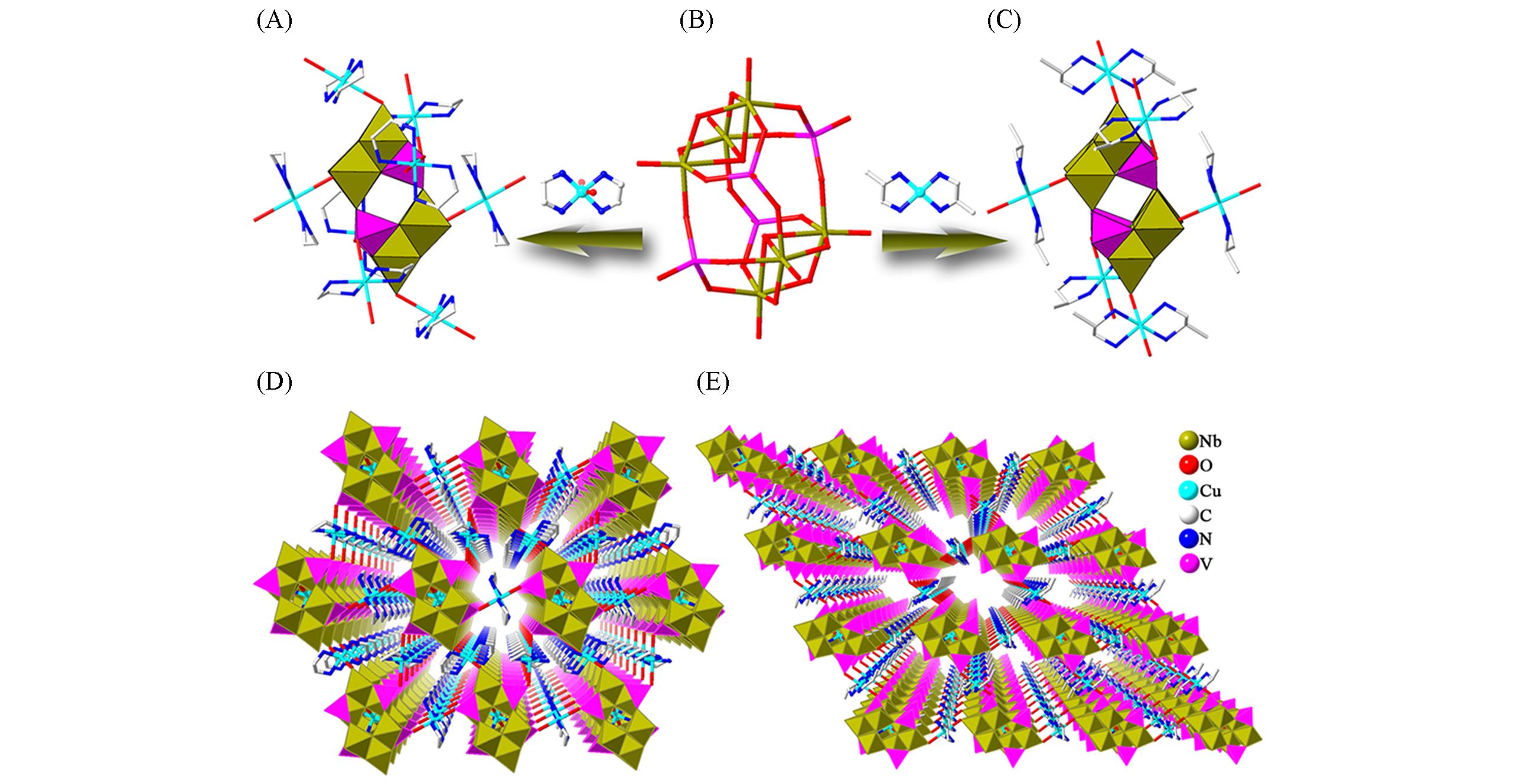
Fig.5 Framework structures based on {V4Nb6O30}(A) The linking mode of {V4Nb6O30} and the copper complexes in [Cu(en)2]3{[Cu(en)2][H2V4Nb6O30]}·12H2O; (B) stick representation of the vanadoniobate cluster {V4Nb6O30}; (C) the linking mode of {V4Nb6O30} and the copper complexes in [Cu(1,2dap)2]4·[H2V4Nb6O30]·16H2O; (D) view of the 3D framework of [Cu(en)2]3{[Cu(en)2][H2V4Nb6O30]}·12H2O; (E) view of the 3D framework of [Cu(1, 2dap)2]4[H2V4Nb6O30]·16H2O.
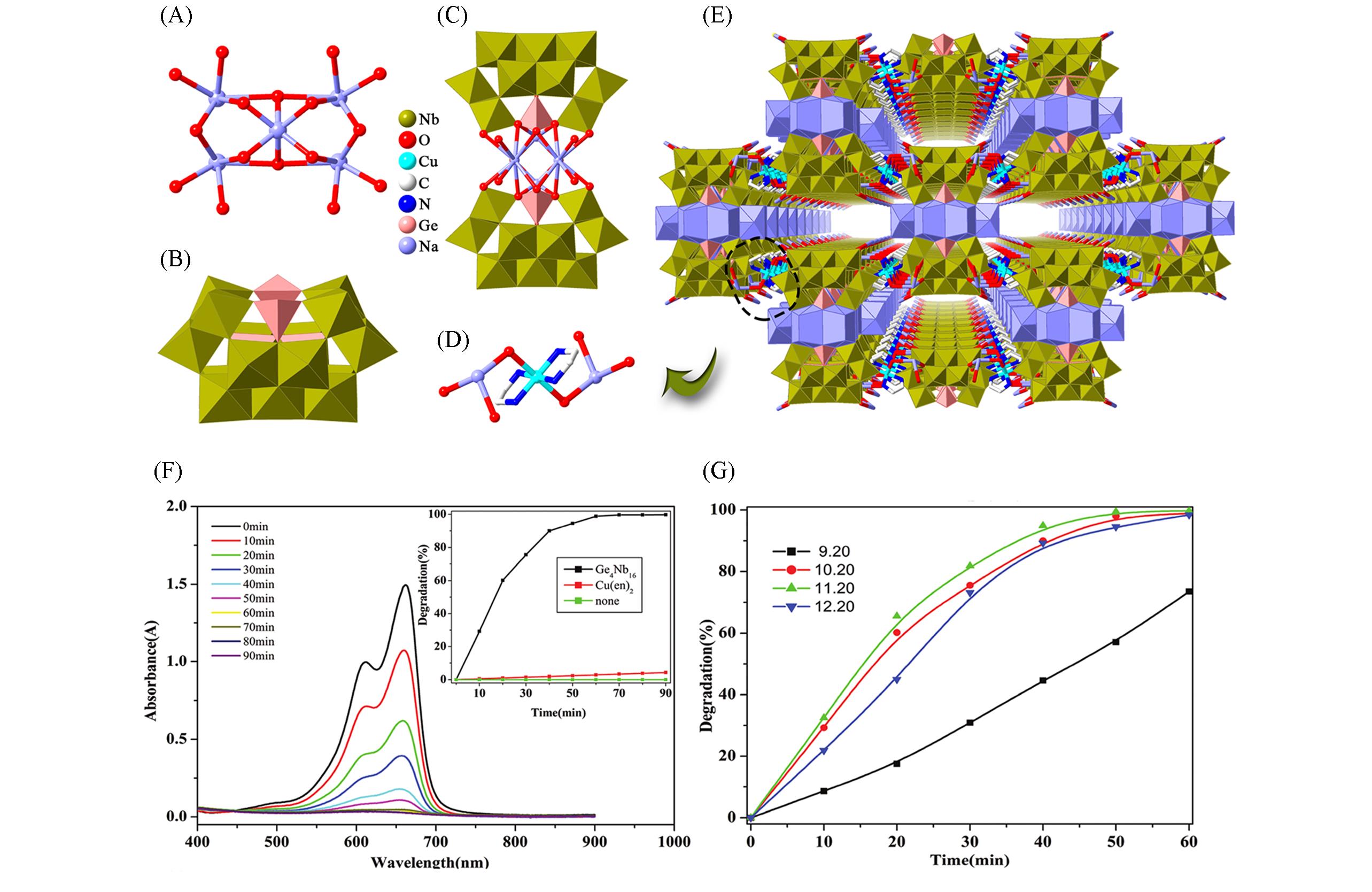
Fig.6 Framework structure based on {Ge4Nb16} and related photocatalytic characterization(A) Representations of the {Na6} clusters; (B) representations of the {Ge4Nb16} clusters; (C) representations of the {Na6Ge8Nb32} clusters; (D) the linkers between {Na6Ge8Nb32} clusters; (E) view of the 3D framework of this compound; (F) photocatalytic degradation of MB solution[34]; (G) photocatalytic degradation rate of MB solution at different pH values[34]. Copyright 2013, The Royal Society of Chemistry.
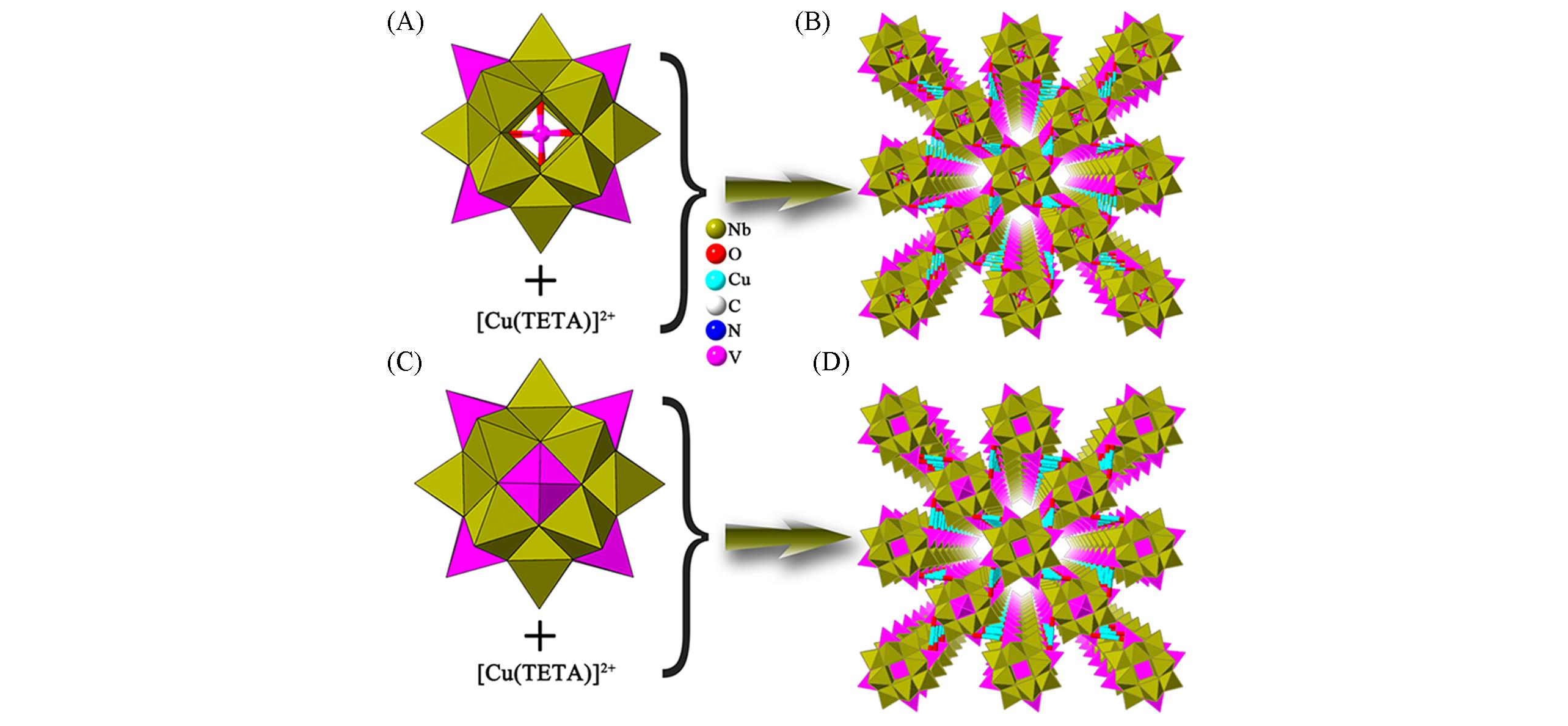
Fig.7 Framework structures based on {VNb12}(A) Polyhedral representation of {VNb12(VO)4}; (B) view of the 3D framework of [Cu(TETA)]4[VNb12(VO)4O40][OH]·10H2O;(C) polyhedral representation of {VNb12(VO)6}; (D) view of the 3D framework of [Cu(TETA)]4[VNb12(VO)6O40][OH]5·5H2O.
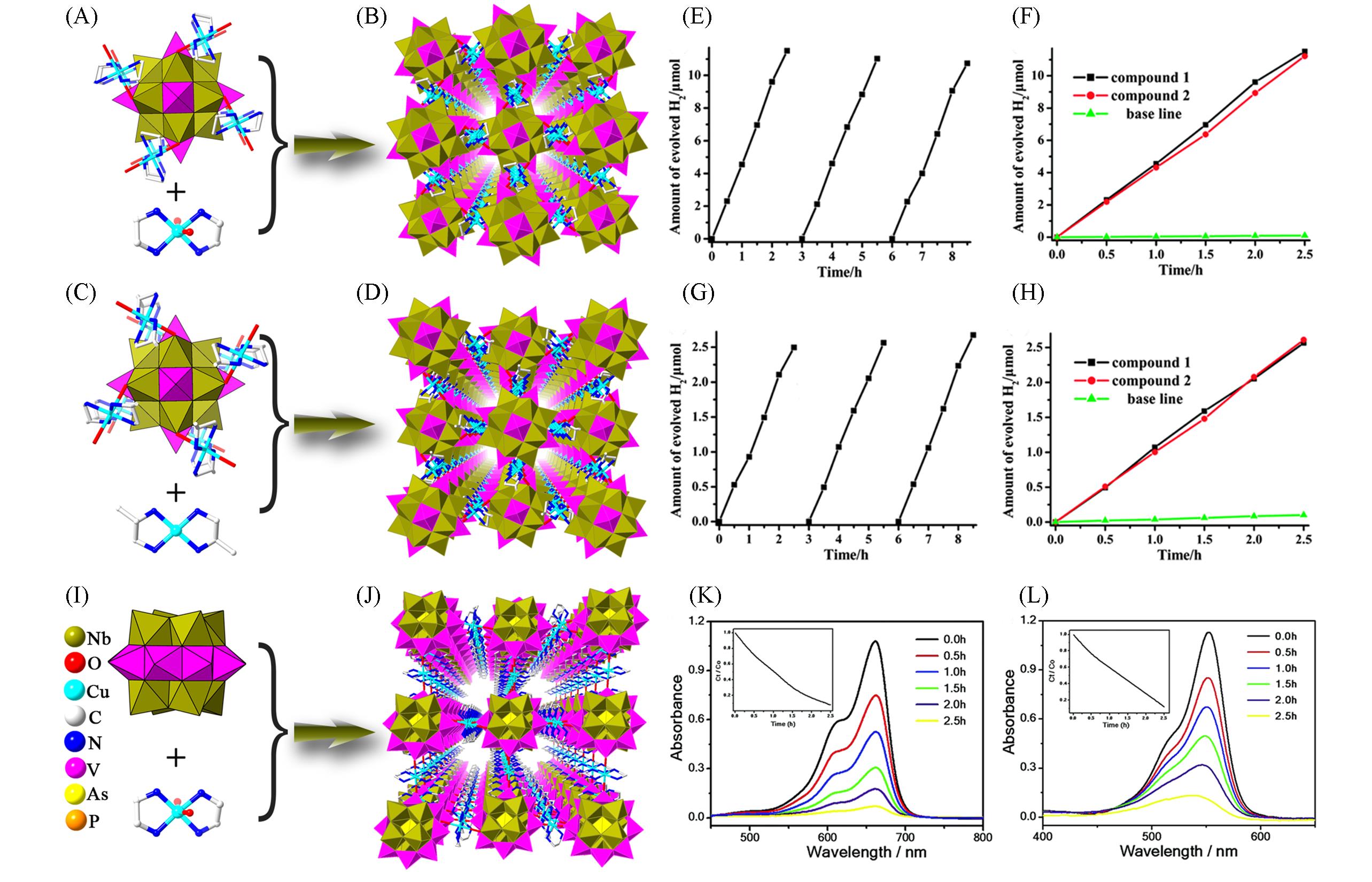
Fig.8 3D Frameworks based on {PNb12V6} and related photocatalytic characterization(A) Polyhedral and stick representations of [Cu(en)2]4[PNb12O40(VO)6]·(OH)5·8H2O; (B) view of the 3D framework of [Cu(en)2]4·[PNb12O40(VO)6]·(OH)5·8H2O; (C) polyhedral and stick representations of [Cu(dap)2]4[PNb12O40(VO)6]·(OH)5·6H2O; (D) view of the 3D framework of [Cu(dap)2]4[PNb12O40(VO)6]·(OH)5·6H2O; (E) time course of H2 evolution under 125 W Hg lamp in 1—8.5 h[39]; (F) time course of H2 evolution under 125 W Hg lamp in 1—2.5 h[39]; (G) time course of H2 evolution under 300 W Xe lamp in 1—8.5[39]; (H) time course of H2 evolution under 300 W Xe lamp in 1—2.5[39]; (I) polyhedral representation of {AsNb8V8}; (J) view of the 3D framework of {AsNb8V8}; (K) photocatalytic degradation of MB by {AsNb8V8}[40] ; (L) photocatalytic degradation of RhB by {AsNb8V8}[40]. (E—H) Copyright 2014, the Royal Society of Chemistry; (K, L) Copyright 2015, the Royal Society of Chemistry.
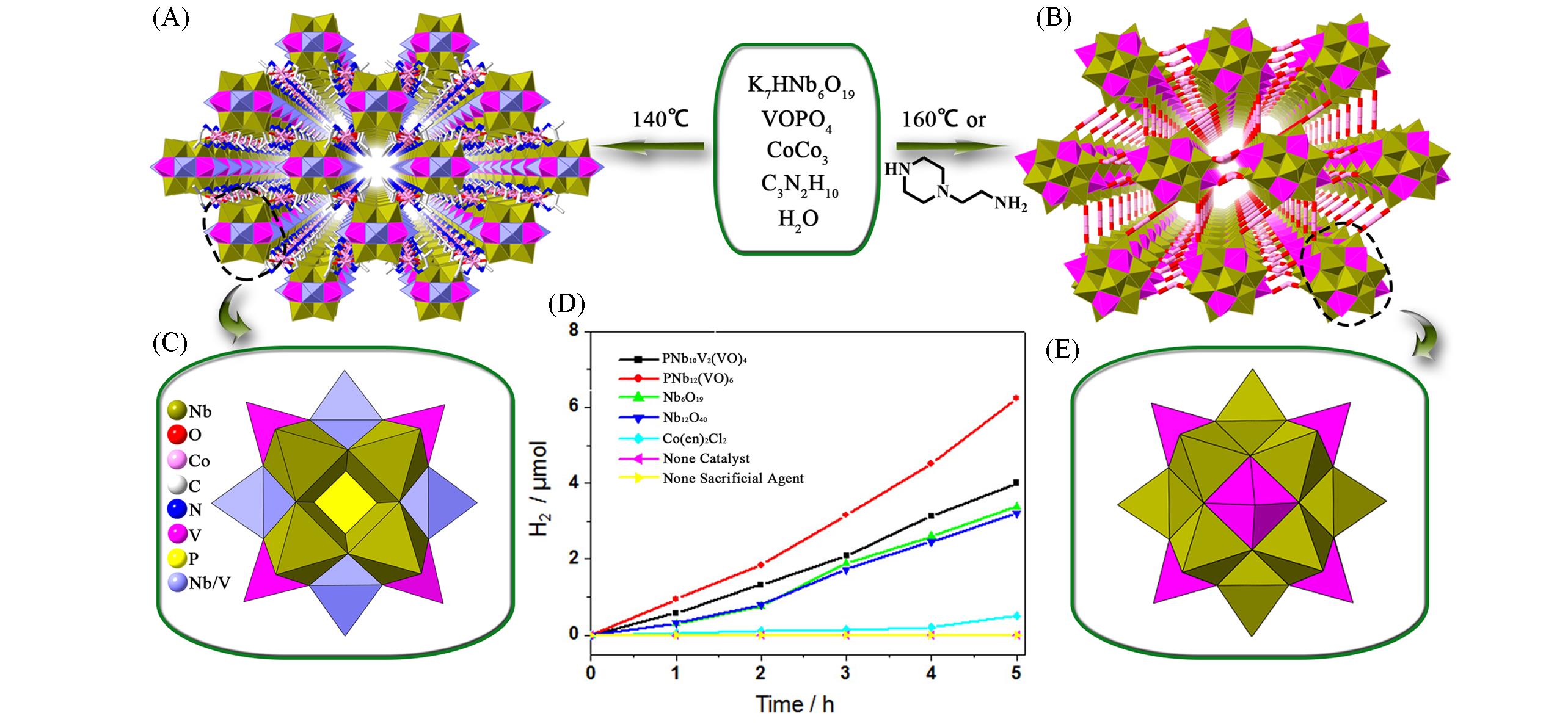
Fig.9 Framework structures based on {PNb10V2O40(VO)4}, {PNb12O40(VO)6}and related photocatalytic characterization(A) View of the 3D framework of {PNb10V2O40(VO)4}; (B) view of the 3D framework of {PNb12O40(VO)6}; (C) polyhedral representation of {PNb10V2O40(VO)4}; (D) photocatalytic hydrogen evolution using different catalysts[41], Copyright 2016, The American Chemical Society; (E) polyhedral representation of {PNb10V2O40(VO)4}.
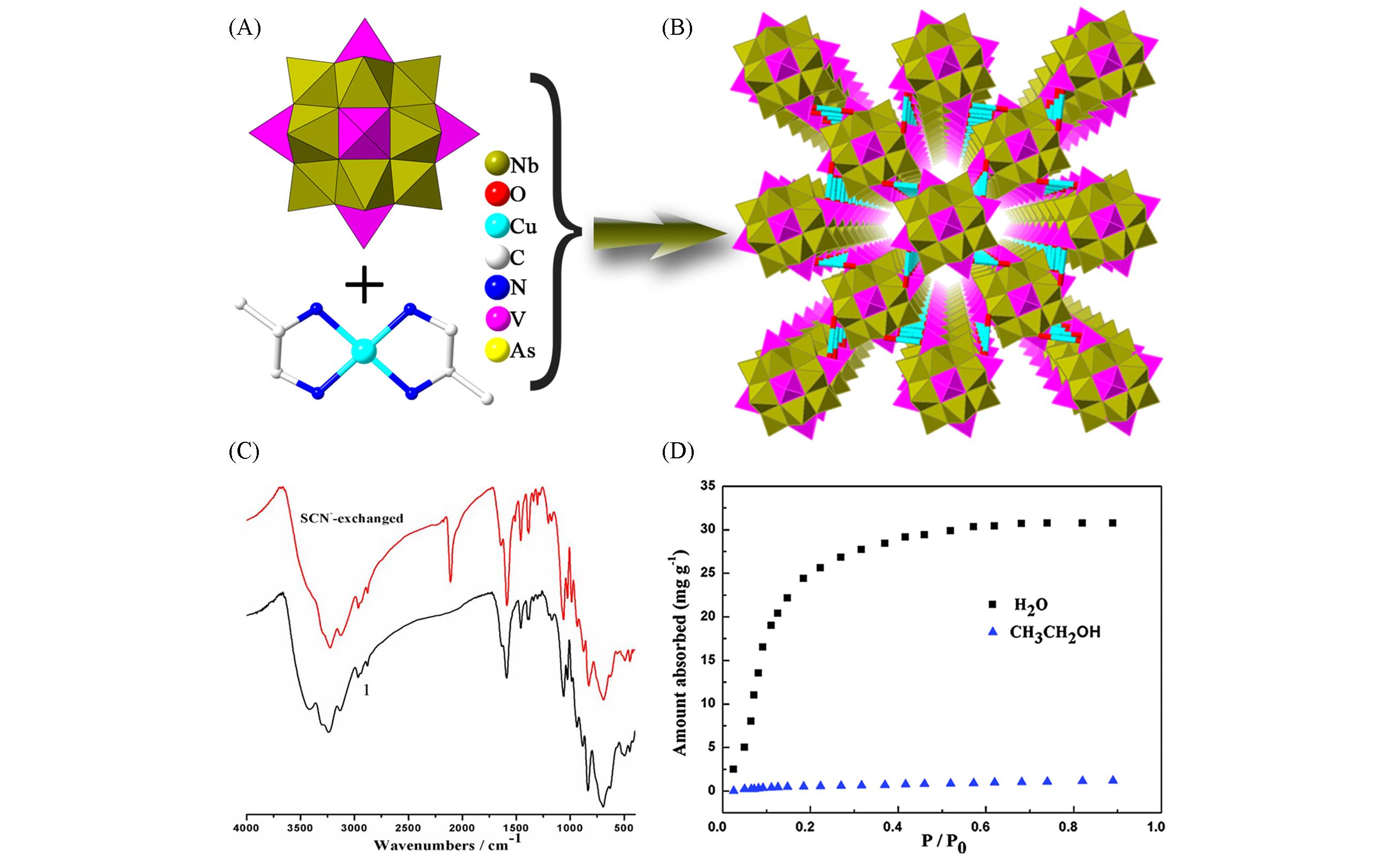
Fig.10 Framework structure based on {AsNb12(VO)4} and the characterization of water and ethanol adsorption(A) Polyhedral representation of {AsNb12(VO)4}; (B) view of the 3D framework of [Cu(dap)2]4[AsNb12O40(VO)4]·(OH)·7H2O; (C) the IR spectra of {AsNb12(VO)4}[42]; (D) water and ethanol adsorption isotherms of {AsNb12(VO)4} at 298 K[42]. Copyright 2016, the Royal Society of Chemistry and the Centre National de la Recherche Scientifique.
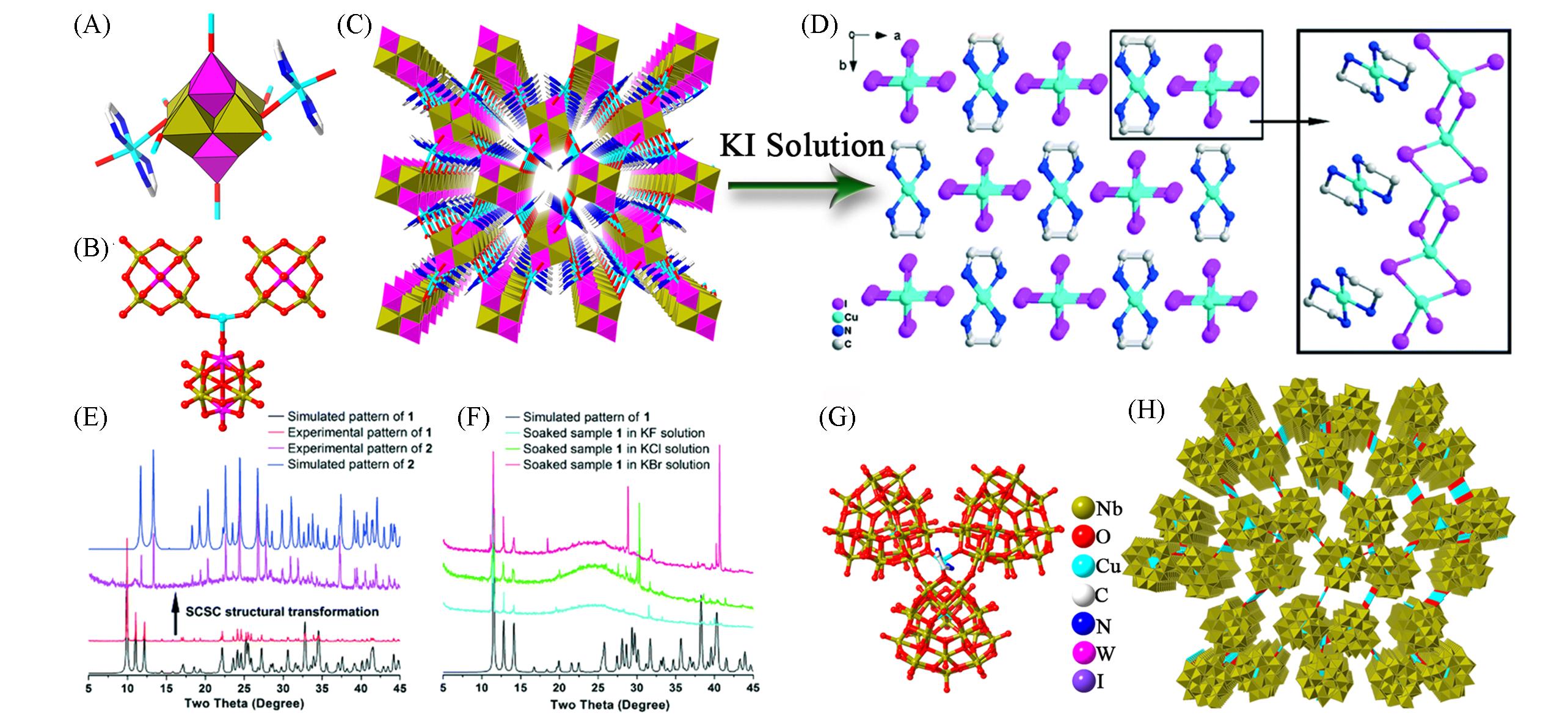
Fig.11 Framework structures based on {Nb3W3O19},{Cu4Nb78} and the structural transformation characterization(A) Polyhedral and stick representations of {Nb3W3O19}; (B) coordination mode of a Cu+ ion with adjacent polyoxoanions; (C) view of the 3D framework of {[Cu(en)2(H2O)]2(Nb3W3O19)][Cu]2}·OH; (D) view of the 1D and 2D framework of [Cu(en)2][CuI2]2[43]; (E) the PXRD spectra of {Nb3W3O19} and [Cu(en)2][CuI2]2[43]; (F) the PXRD patterns of the samples soaked in KF, KCl, and KBr solutions for 45 days[43]; (G) ball-and-stick representation of {Cu4Nb78}[44]; (H) view of the 3D framework of {Cu4Nb78}[44].(D—F) Copyright 2016, the Royal Society of Chemistry; (G, H) Copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
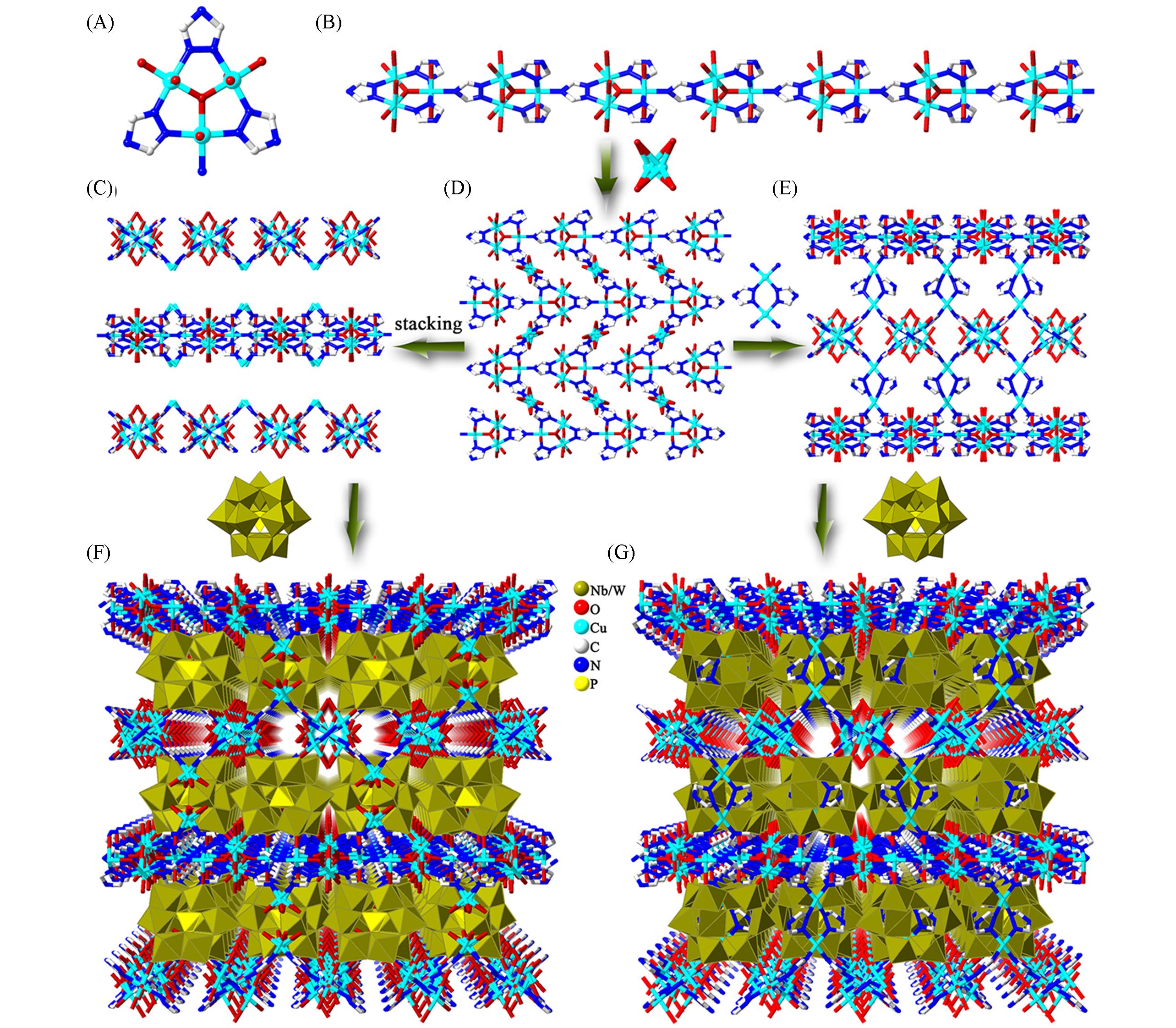
Fig.12 Framework structures based on {PW9Nb3O40}(A) A trinuclear copper unit; (B) the 1D chain constructed by tricopper moieties; (C) view of the 3D framework stacked by the 2D layer; (D) The 2D layer constructed by anti-parallel chains; (E) the 3D network constructed by the 2D layer; (F) view of the 3D framework of [CuICuII3(μ3-OH)(H2O)6(trz)3]2(PW9Nb3O40)·13H2O; (G) view of the 3D framework of [CuICuII3(μ3-OH)(H2O)4(H-trz)(trz)3]2·(PW9Nb3O40)·13H2O.
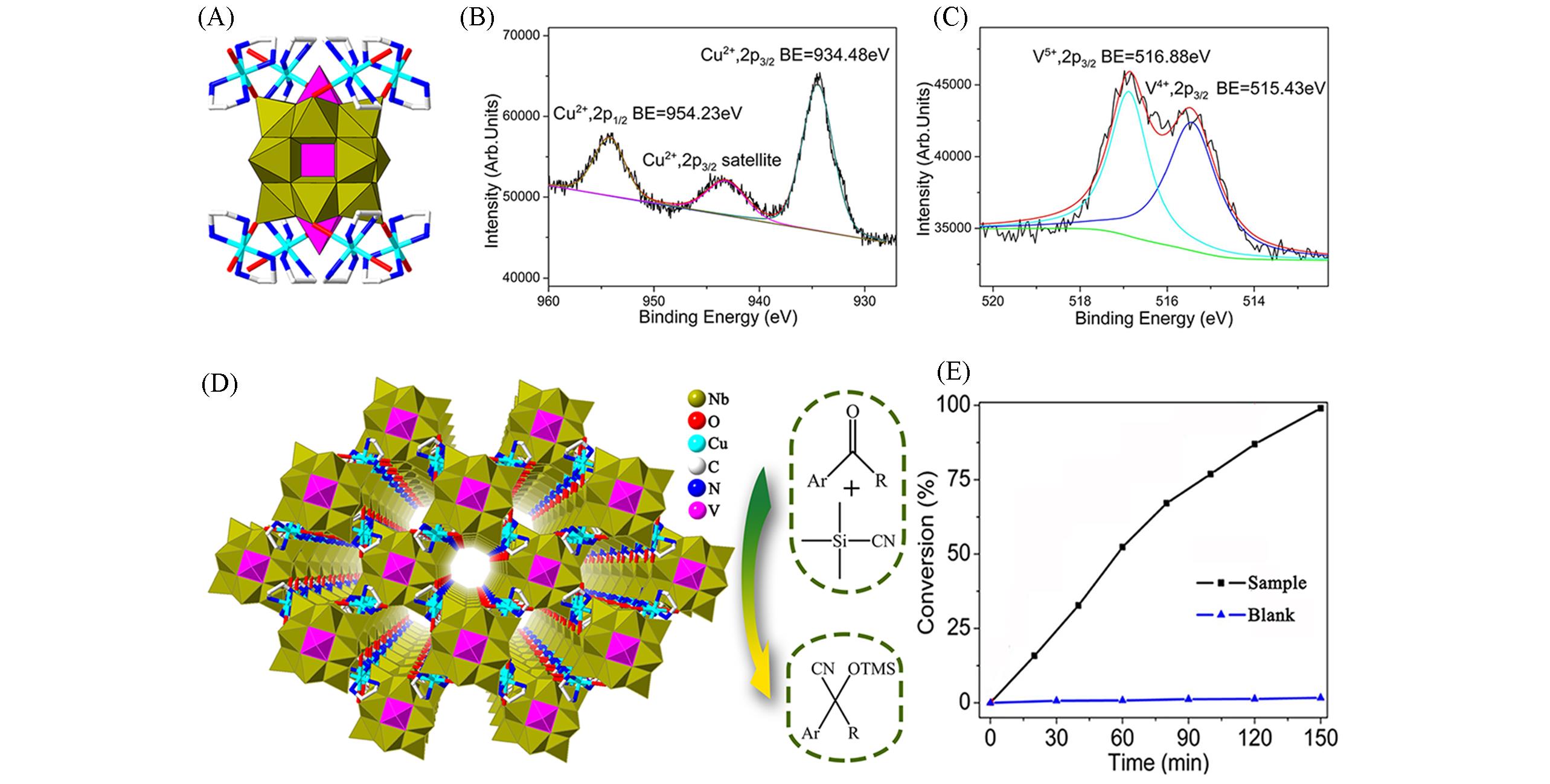
Fig.14 Framework structure based on {VNb12O40V2} and the related characterization(A) Polyhedral and stick representation of H3[Cu(en)2]4[VNb12O40(VO)2]·13H2O; (B, C) the XPS spectra for Cu2p(B) and V2p(C)[46];(D) view of the 3D framework of this compound; (E) cyanosilylation reaction using this compound[46].Copyright 2020, Elsevier B. V.
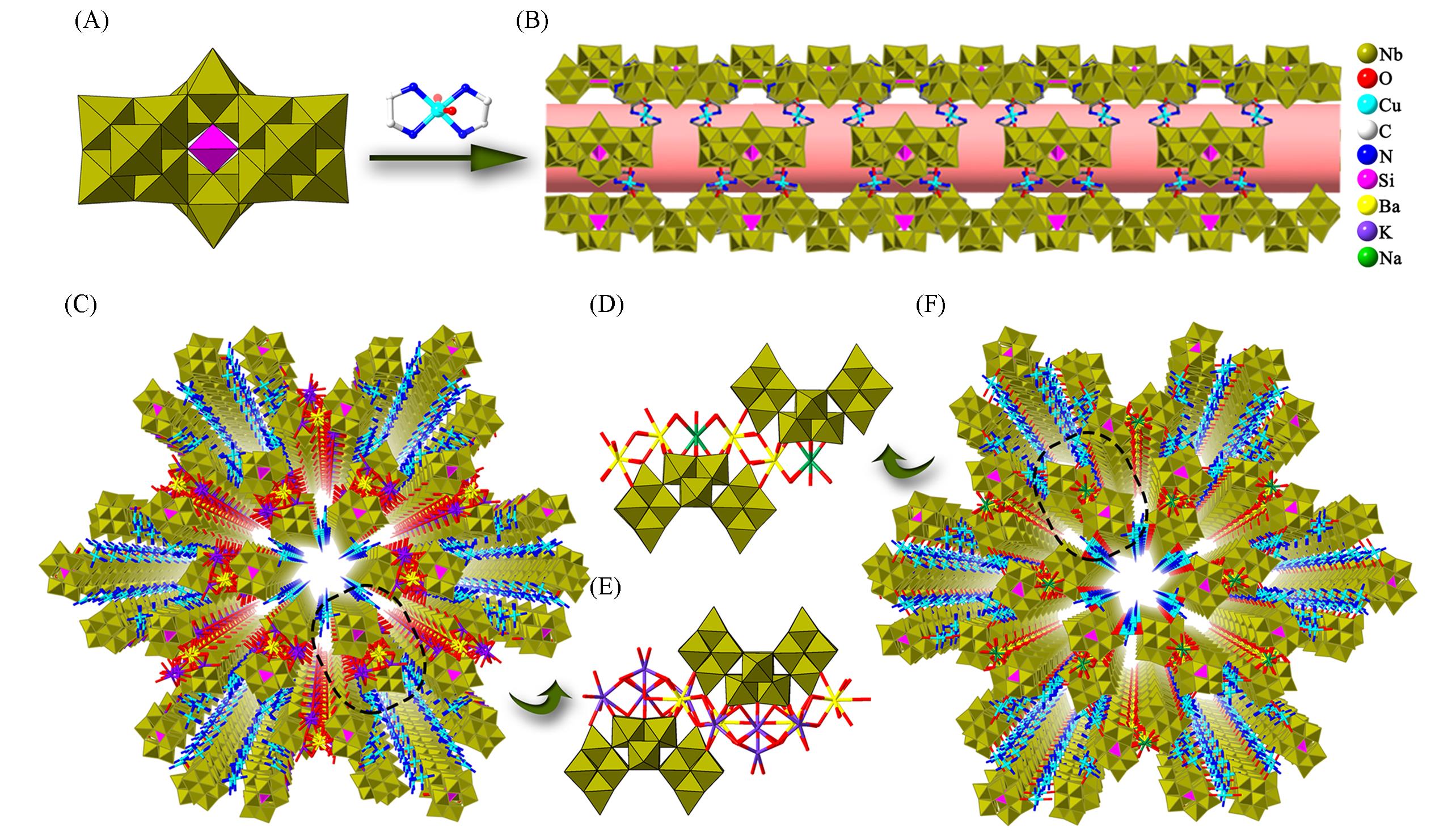
Fig.15 Framework structures based on {SiNb18O54}(A) Polyhedral representation of {SiNb18}; (B) view of the nanotube; (C) view of the 3D framework of [Cu(en)2]2-{[Cu(en)2]2Ba2K4· (H2O)13(SiNb18O54)}2·3en·52H2O; (D, E) polyhedron and ball-and-stick view of the linkage between {SiNb18O54} cluster in [Cu(en)2]2·{[Cu(en)2]2Ba2K4(H2O)13(SiNb18O54)}2·3en·52H2O and H6[Cu(en)2]2{[Cu(en)2]2Ba2Na(H2O)7(SiNb18O54)}2·3en·50H2O; (F) view of the 3D framework ofH6[Cu(en)2]2{[Cu(en)2]2Ba2Na(H2O)7(SiNb18O54)}2·3en·50H2O .
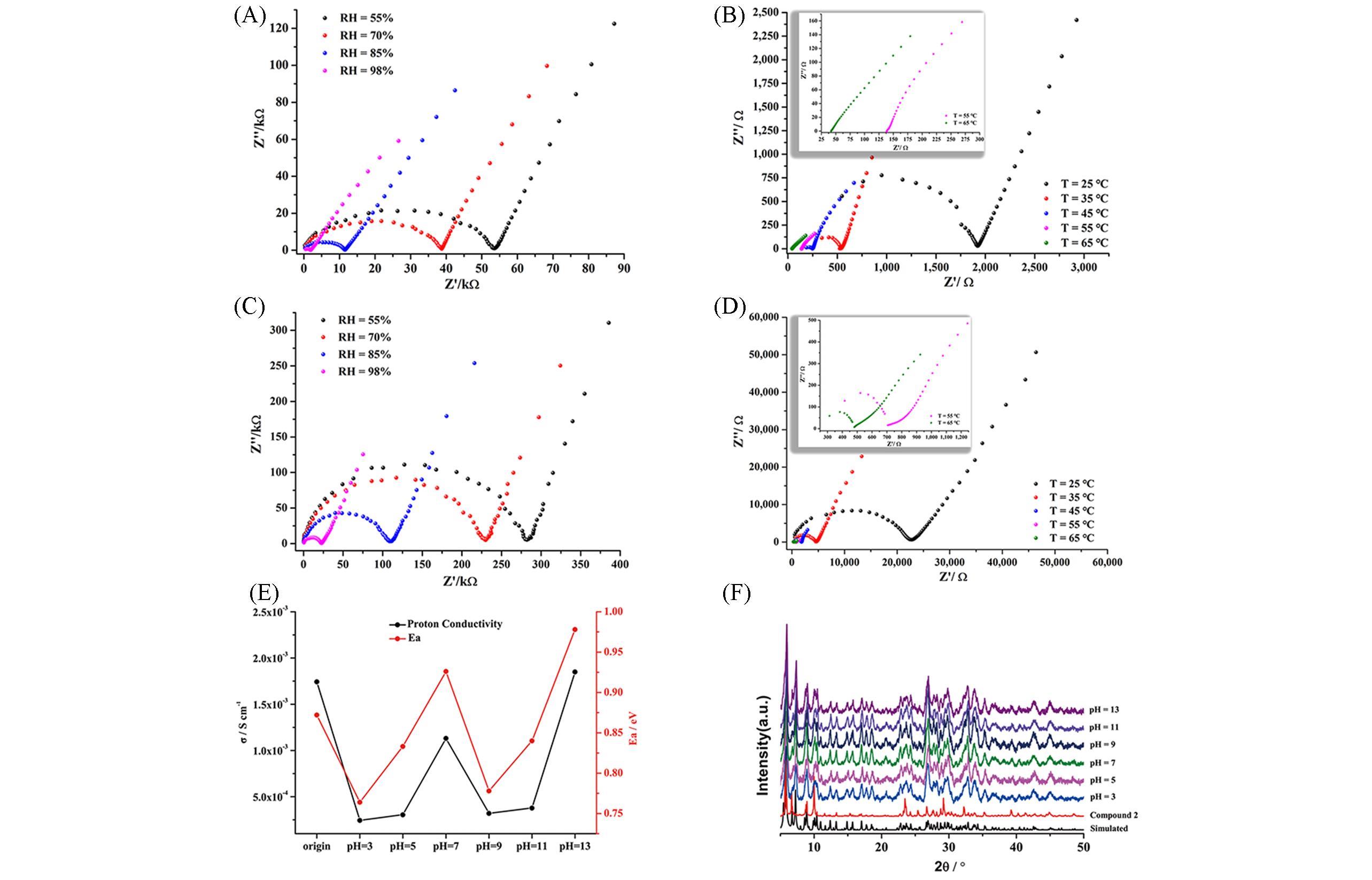
Fig.16 Proton conductivity of [Cu(en)2]2{[Cu(en)2]2Ba2K4(H2O)13(SiNb18O54)}2·3en·52H2O and H6[Cu(en)2]2{[Cu(en)2]2Ba2Na(H2O)7(SiNb18O54)}2·3en·50H2O[47](A) Nyquist plots for [Cu(en)2]2{[Cu(en)2]2Ba2K4(H2O)13(SiNb18O54)}2·3en·52H2O at different RH conditions with 25℃; (B) Nyquist plots for [Cu(en)2]2{[Cu(en)2]2Ba2K4(H2O)13(SiNb18O54)}2·3en·52H2O at different temperature conditions with 98% RH; (C) Nyquist plots for H6[Cu(en)2]2{[Cu(en)2]2Ba2Na(H2O)7(SiNb18O54)}2·3en·50H2O at different RH conditions with 25℃; (D) Nyquist plots for H6[Cu(en)2]2-{[Cu(en)2]2Ba2Na(H2O)7(SiNb18O54)}2·3en·50H2O at different temperature conditions with 98% RH; (E) the proton conductivity and Ea value of H6[Cu(en)2]2{[Cu(en)2]2Ba2Na(H2O)7(SiNb18O54)}2·3en·50H2O; (F) the PXRD patterns of H6[Cu(en)2]2· {[Cu(en)2]2Ba2Na(H2O)7(SiNb18O54)}2·3en·50H2O after soaking in different pH aqueous solution. Copyright 2021, Elsevier B. V.
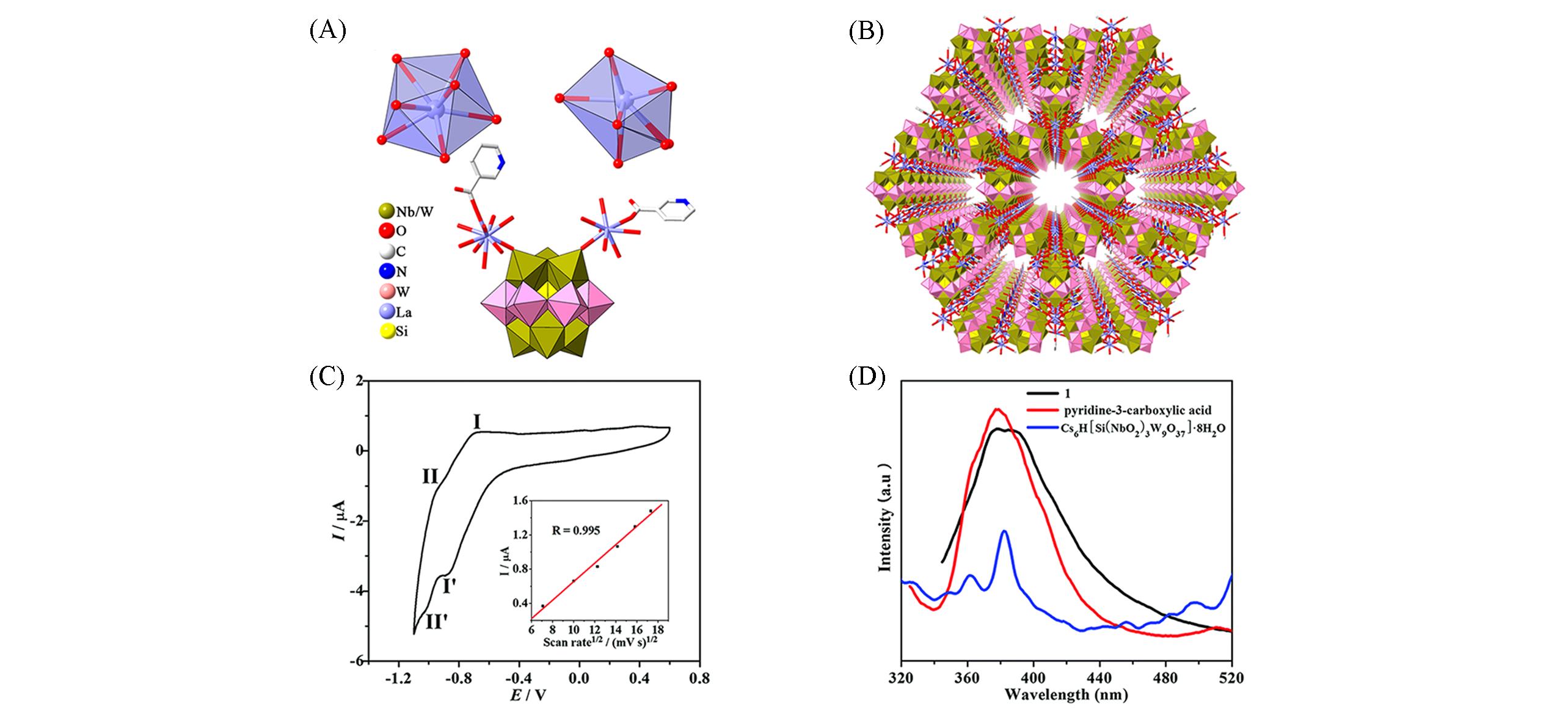
Fig.17 Framework structure based on {Nb3W3O19} and its characterization(A) Coordination mode of a La3+ ion(top), polyhedral and stick representations of {Nb3W3O19}; (B) view of the 3D framework of {[Cu(en)2(H2O)]2(Nb3W3O19)][Cu]2}·OH; (C) the CV of sample in the 0.5 mol/L HAc-NaAc(pH=4.7) aqueous solution(scan rate: 100 mV/s)[48]; (D) emission spectrum of samples, Cs6H[Si(NbO2)3W9O37]·8H2O, and pyridine-3-carboxylic aci[48].Copyright 2017, the Royal Society of Chemistry and the Centre National de la Recherche Scientifique.
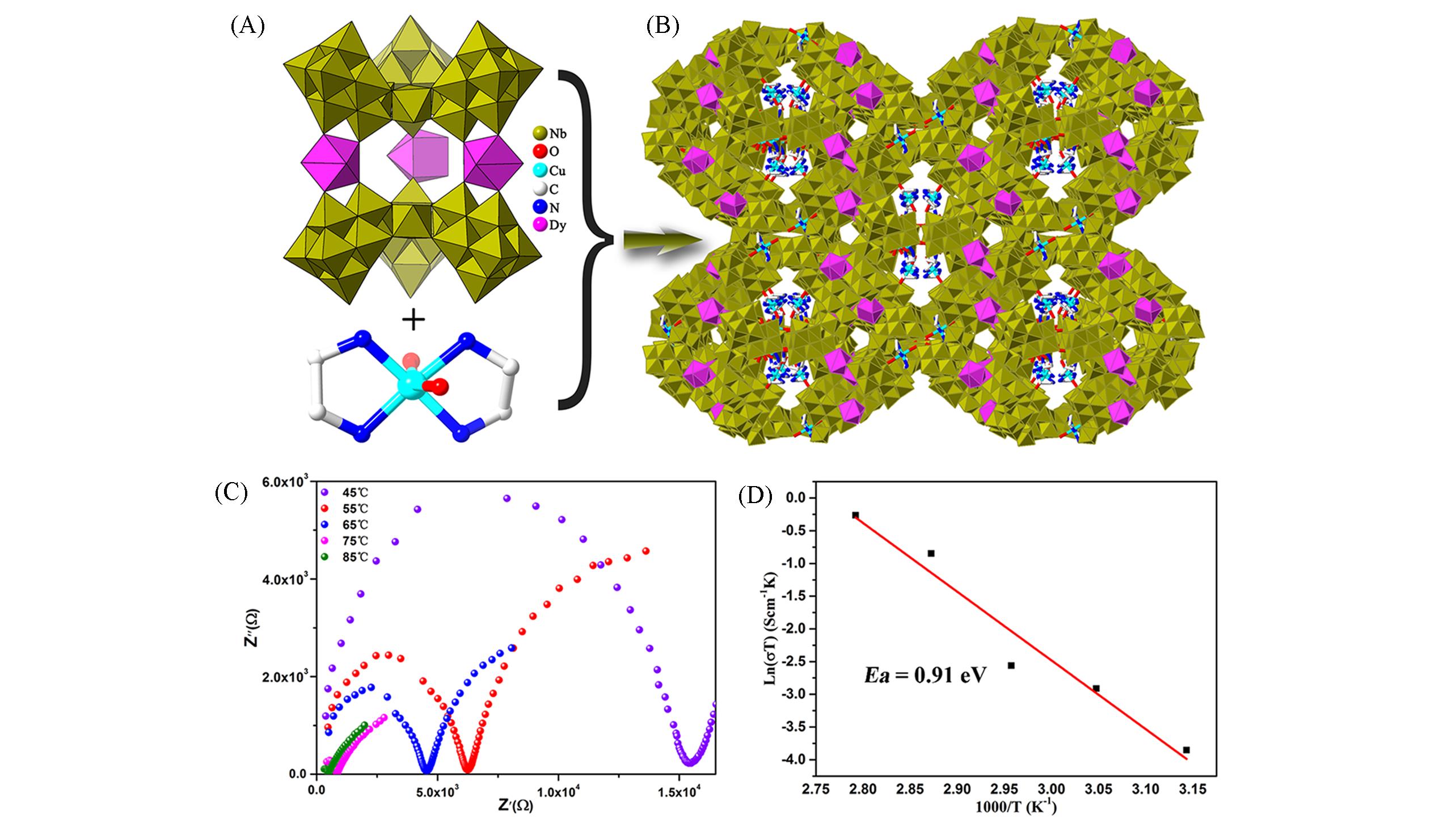
Fig.18 Framework structure based on {[Dy(H2O)4]3[Nb24O69(H2O)3]2} and the characterization of proton conduction(A) Polyhedral representation of {[Dy(H2O)4]3[Nb24O69(H2O)3]2}; (B) view of the 3D framework of H9[Cu(en)(H2O)2]?[Cu(en)2]8[Dy(H2O)4]3[Nb24O69(H2O)3]2?36H2O; (C) nyquist plots at 98% RH under different temperature[49];(D) Ea value of H9[Cu(en)?(H2O)2][Cu(en)2]8[Dy(H2O)4]3[Nb24O69(H2O)3]2?36H2O[49].
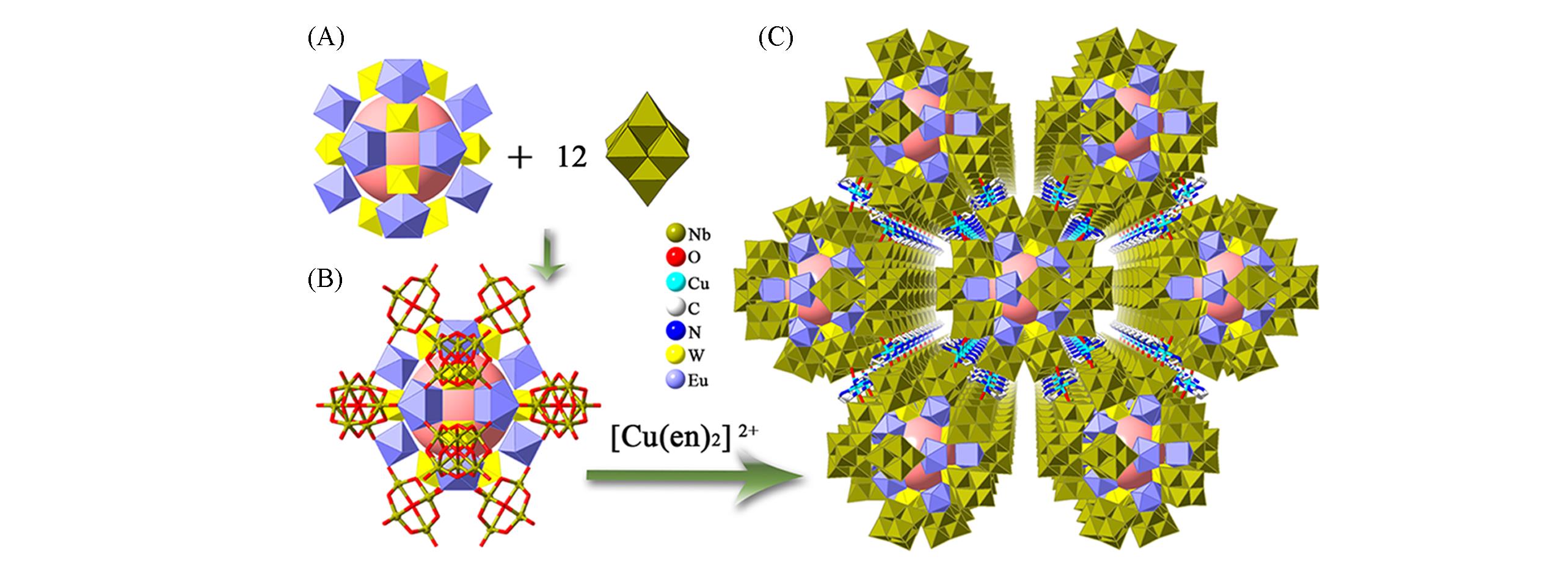
Fig.19 Framework structure based on {Eu12W12O36}(A) Polyhedral representation of {Eu12W12O36}(left) and [Nb6O19]8-(right); (B) polyhedral and ball-and-stick representations of {Eu12W12O36(H2O)24(Nb6O19)12}; (C) view of the 3D framework of Na6K10[Cu(en)2]4[Eu12W12O36(H2O)24(H3Nb6O19)12]·solv.
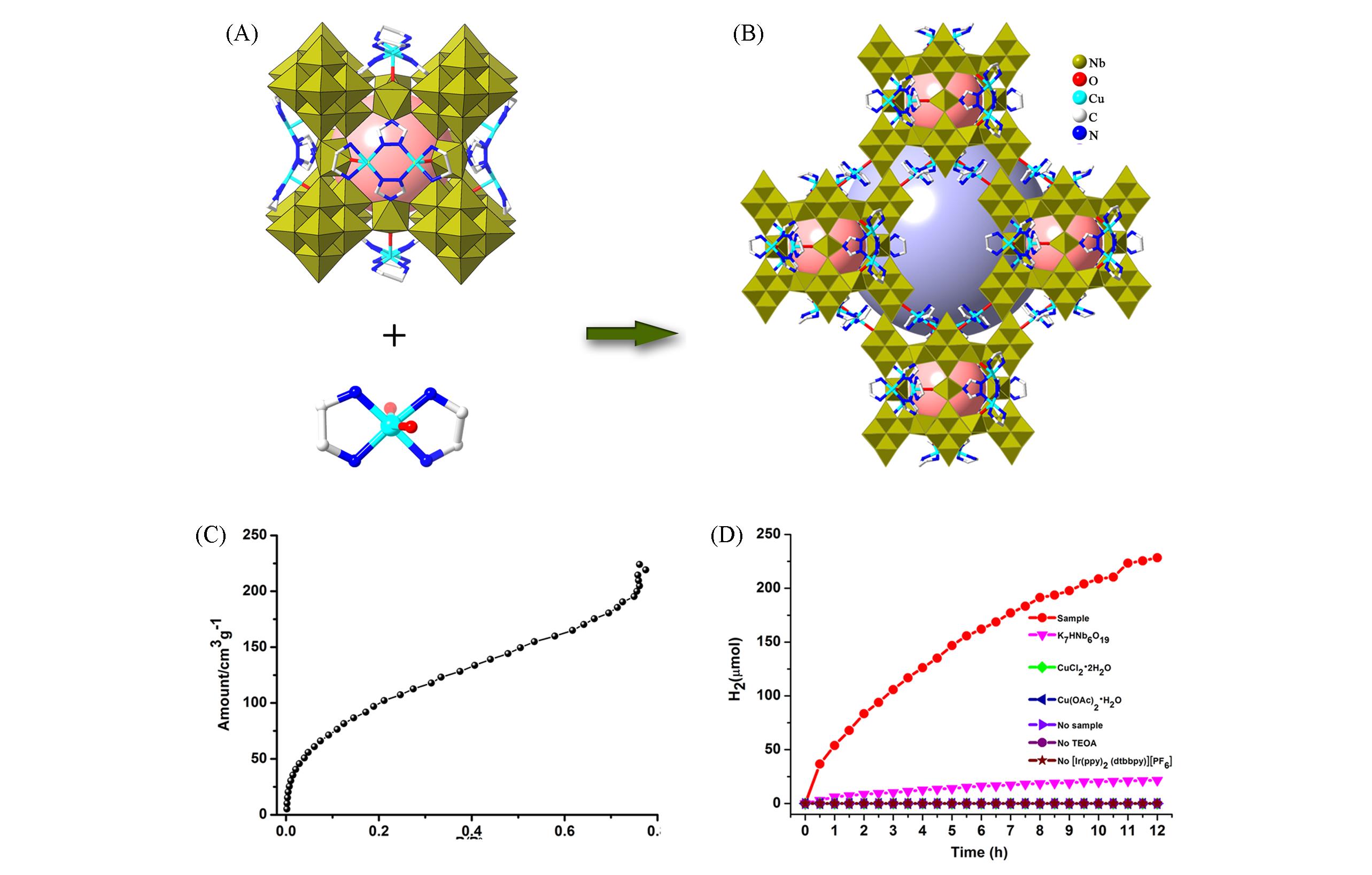
Fig.20 Framework structure of {[Cu(en)2]@{[Cu2(trz)2(en)2]6[H10Nb68O188]}} and the related photocatalytic characterization(A) Polyhedral and stick representations of {Nb68} cage; (B) view of the 3D framework of {[Cu(en)2]@{[Cu2 (trz)2(en)2]6·[H10Nb68O188]}}; (C) vapor sorption isotherms[51]; (D) time courses of photocatalytic H2 evolution over different photocatalytic systems[51].Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
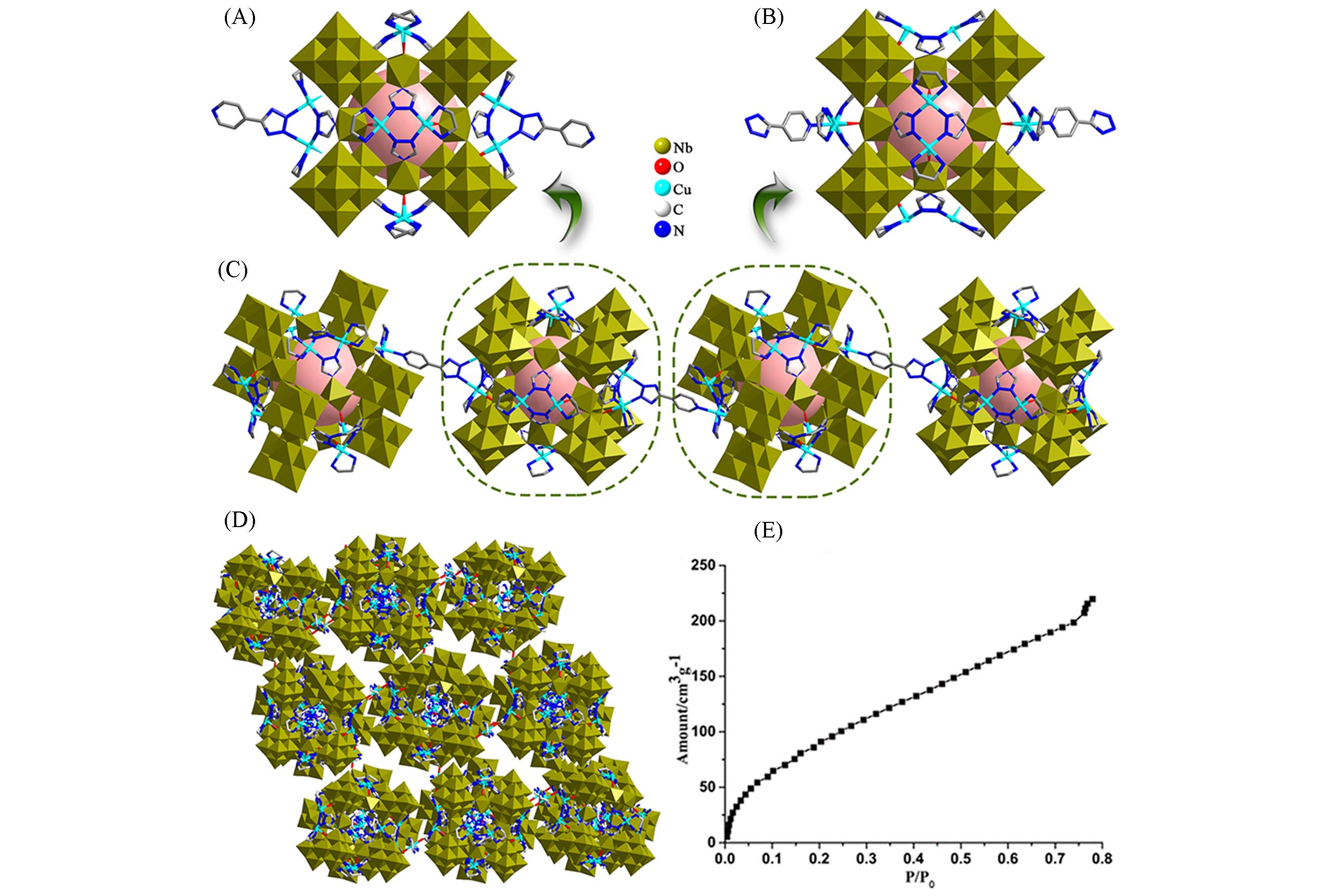
Fig.21 Framework structure of H20Cu(en)[Cu(en)2]11{[{[Cu(en)2]@{[Cu2(en)2(trz)2]6(Nb68O188)}}][4?Tzp]}2·22en·130H2O and the characterization of vapor sorption(A) The coordination environment of cage 1; (B) the coordination environment of cage 2; (C) 1D pillar-like chain formed by two diffe-rent cubic cages and 4-Tzp ligands; (D) view of the 3D framework of H20Cu(en)[Cu(en)2]11{[{[Cu(en)2]@{[Cu2(en)2(trz)2]6(Nb68O188)}}]·[4-Tzp]}2·22en·130H2O; (E) vapor sorption isotherms[52], Copyright 2020, American Chemical Society.
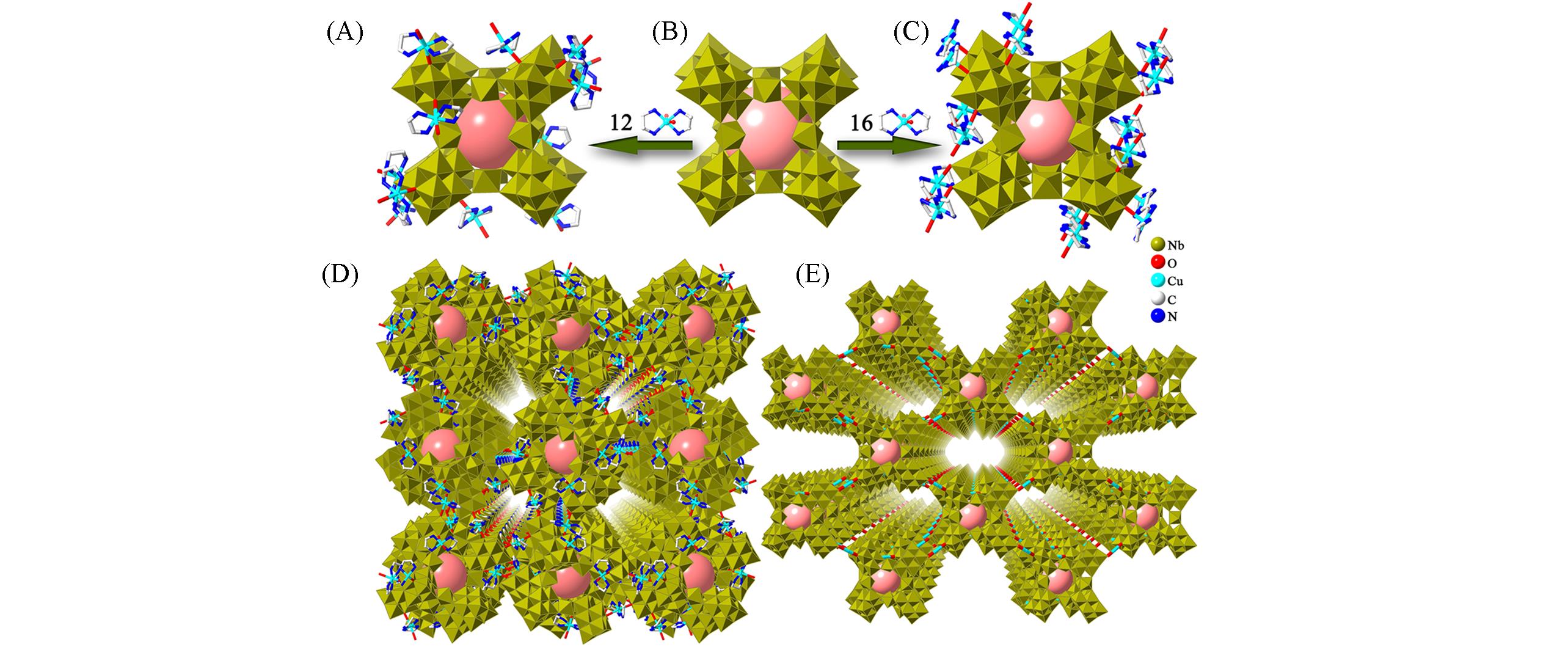
Fig.22 Framework structures of H12{[Cu(en)2]6[Nb68O176(OH)12(H2O)12]}·52H2O and H12{[Cu(en)2]10[Nb68O182(OH)8(H2O)10]}·54H2O(A) Polyhedral and ball-and-stick representations of H12{[Cu(en)2]6[Nb68O176(OH)12(H2O)12]}·52H2O; (B) polyhedral representation of {Nb68} cage; (C) polyhedral and ball-and-stick representations of H12{[Cu(en)2]10[Nb68O182(OH)8(H2O)10]}·54H2O; (D) view of the 3D framework of H12{[Cu(en)2]6[Nb68O176(OH)12(H2O)12]}·52H2O; (E) view of the 3D framework of H12{[Cu(en)2]10[Nb68O182(OH)8(H2O)10]}·54H2O.
| No. | Formular | SBU | Property | Ref. |
|---|---|---|---|---|
| 1 | [Cu(en)2]3[Cu(en)2(H2O)]9[{H2Nb6O19}@{[({KNb24O72H10.25}· {Cu(en)2})2{Cu3(en)3(H2O)3}{Na1.5Cu1.5(H2O)8}{Cu(en)2}4]6}]· 144H2O | {KNb24O72Nb6O19} | / | [ |
| 2 | [Cu(en)2]3{[Cu(en)2][H2V4Nb6O30]}·12H2O | {V4Nb6O30} | / | [ |
| 3 | [Cu(1, 2dap)2]4[H2V4Nb6O30]·16H2O | {V4Nb6O30} | / | [ |
| 4 | Na4[Cu(en)2(H2O)2]5[Na6Ge8Nb32O108H8(OH)4]·41H2O | {Ge4Nb16} | Pa | [ |
| 5 | [Cu(TETA)]4[VNb12(VO)4O40][OH]·10H2O | {VNb12(VO)4} | / | [ |
| 6 | [Cu(TETA)]4[VNb12(VO)6O40][OH]5·5H2O | {VNb12(VO)6} | / | [ |
| 7 | [Cu(en)2]4[PNb12O40(VO)6]·(OH)5·8H2O | {VPNb12(VO)6} | Pa | [ |
| 8 | [Cu(dap)2]4[PNb12O40(VO)6]·(OH)5·6H2O | {VPNb12(VO)6} | Pa | [ |
| 9 | [Cu(en)2(H2O)][Cu(en)2]4H{AsNb8V8O44}·11H2O | {AsNb8V8} | Pa | [ |
| 10 | [Co(dap)2]4[HPNb10VIV2O40(VIVO)4]·17H2O | {PNb10V2O40(VO)4} | Pa | [ |
| 11 | [Co(dap)2]5[PNb12O40(VIVO)6](OH)7·15H2O | {PNb12O40(VO)6} | Pa | [ |
| 12 | Na6K10[Cu(en)2]4[Eu12W12O36(H2O)24(H3Nb6O19)12]·solv | {Eu12W12O36(Nb6O19)12} | / | [ |
| 13 | {[Cu(en)2(H2O)]2(Nb3W3O19)][Cu]2}·OH | {Nb3W3O19} | Sb | [ |
| 14 | [Cu(dap)2]4[AsNb12O40(VO)4]·(OH)·7H2O | {AsNb12(VO)4} | Wc | [ |
| 15 | Na4K2H16[Cu(en)2]0.5{[Cu(en)2]9.5(K?H3Cu4(en)Nb78O222)} | {Cu4Nb78} | / | [ |
| 16 | [CuICuII3(μ3?OH)(H2O)6(trz)3]2(PW9Nb3O40)·13H2O | {PW9Nb3} | Md | [ |
| 17 | [CuICuII3(μ3?OH)(H2O)4(H?trz)(trz)3]2(PW9Nb3O40)·13H2O | {PW9Nb3} | Md | [ |
| 18 | [H3La8(H2O)32(C6H5NO2)6][SiW9Nb3O40]3·8H2O | {SiW9Nb3} | PLe | [ |
| 19 | H2Li5Na5K5[Cu(en)2][Nb47O128(OH)6(CO3)2]·20H2O | {Nb47} | Df | [ |
| 20 | {[Cu(en)2]@{[Cu2(trz)2(en)2]6[H10Nb68O188]}} | {[Cu2(trz)2(en)2]6Nb68} | Pa | [ |
| Vg | ||||
| 21 | H3[Cu(en)2]4[VNb12O40(VO)2]·13H2O | {VNb12O40(VO)2} | Ch | [ |
| 22 | {[Ni(cyclam)]2H4Nb6O19}·12H2O | {Nb6O19} | / | [ |
| 23 | H9[Cu(en)(H2O)2][Cu(en)2]8[Dy(H2O)4]3[Nb24O69(H2O)3]2?36H2O | {[Dy(H2O)4]3[Nb24O69(H2O)3]2} | PCi | [ |
| 24 | H20Cu(en)[Cu(en)2]11{[{[Cu(en)2]@ {[Cu2(en)2(trz)2]6(Nb68O188)}}][4?Tzp]}2·22en·130H2O | {[Cu(en)2]@{[Cu2(en)2(trz)2]6(Nb68O188)}} | Vg | [ |
| 25 | [Cu(en)2(H2O)]2{[Cu(en)2]4[Cu(en)2]5{[Cu(en)2KNb24O72H10]2}·6en·70H2O. | {Cu(en)2KNb24O72H10} | Vg | [ |
| 26 | H12{[Cu(en)2]6[Nb68O176(OH)12(H2O)12]}·52H2O | {Nb68O200} | PCi | [ |
| 27 | H12{[Cu(en)2]10[Nb68O182(OH)8(H2O)10]}·54H2O | {Nb68O200} | PCi | [ |
| 28 | Ca2TMA2Nb10O28?5H2O | Ca?{Nb10O28} | / | [ |
| 29 | Sr2TMA2Nb10O28?4H2O | Sr?{Nb10O28} | / | [ |
| 30 | Ba2TMA2Nb10O28?9H2O | Ba?{Nb10O28} | / | [ |
| 31 | [Cu(en)2]2{[Cu(en)2]2Ba2K4(H2O)13(SiNb18O54)}2·3en·52H2O | {SiNb18O54} | PCi | [ |
| 32 | H6[Cu(en)2]2{[Cu(en)2]2Ba2Na(H2O)7(SiNb18O54)}2·3en·50H2O | {SiNb18O54} | PCi | [ |
Table 1 Summary of reported three-dimensional PONb frameworks
| No. | Formular | SBU | Property | Ref. |
|---|---|---|---|---|
| 1 | [Cu(en)2]3[Cu(en)2(H2O)]9[{H2Nb6O19}@{[({KNb24O72H10.25}· {Cu(en)2})2{Cu3(en)3(H2O)3}{Na1.5Cu1.5(H2O)8}{Cu(en)2}4]6}]· 144H2O | {KNb24O72Nb6O19} | / | [ |
| 2 | [Cu(en)2]3{[Cu(en)2][H2V4Nb6O30]}·12H2O | {V4Nb6O30} | / | [ |
| 3 | [Cu(1, 2dap)2]4[H2V4Nb6O30]·16H2O | {V4Nb6O30} | / | [ |
| 4 | Na4[Cu(en)2(H2O)2]5[Na6Ge8Nb32O108H8(OH)4]·41H2O | {Ge4Nb16} | Pa | [ |
| 5 | [Cu(TETA)]4[VNb12(VO)4O40][OH]·10H2O | {VNb12(VO)4} | / | [ |
| 6 | [Cu(TETA)]4[VNb12(VO)6O40][OH]5·5H2O | {VNb12(VO)6} | / | [ |
| 7 | [Cu(en)2]4[PNb12O40(VO)6]·(OH)5·8H2O | {VPNb12(VO)6} | Pa | [ |
| 8 | [Cu(dap)2]4[PNb12O40(VO)6]·(OH)5·6H2O | {VPNb12(VO)6} | Pa | [ |
| 9 | [Cu(en)2(H2O)][Cu(en)2]4H{AsNb8V8O44}·11H2O | {AsNb8V8} | Pa | [ |
| 10 | [Co(dap)2]4[HPNb10VIV2O40(VIVO)4]·17H2O | {PNb10V2O40(VO)4} | Pa | [ |
| 11 | [Co(dap)2]5[PNb12O40(VIVO)6](OH)7·15H2O | {PNb12O40(VO)6} | Pa | [ |
| 12 | Na6K10[Cu(en)2]4[Eu12W12O36(H2O)24(H3Nb6O19)12]·solv | {Eu12W12O36(Nb6O19)12} | / | [ |
| 13 | {[Cu(en)2(H2O)]2(Nb3W3O19)][Cu]2}·OH | {Nb3W3O19} | Sb | [ |
| 14 | [Cu(dap)2]4[AsNb12O40(VO)4]·(OH)·7H2O | {AsNb12(VO)4} | Wc | [ |
| 15 | Na4K2H16[Cu(en)2]0.5{[Cu(en)2]9.5(K?H3Cu4(en)Nb78O222)} | {Cu4Nb78} | / | [ |
| 16 | [CuICuII3(μ3?OH)(H2O)6(trz)3]2(PW9Nb3O40)·13H2O | {PW9Nb3} | Md | [ |
| 17 | [CuICuII3(μ3?OH)(H2O)4(H?trz)(trz)3]2(PW9Nb3O40)·13H2O | {PW9Nb3} | Md | [ |
| 18 | [H3La8(H2O)32(C6H5NO2)6][SiW9Nb3O40]3·8H2O | {SiW9Nb3} | PLe | [ |
| 19 | H2Li5Na5K5[Cu(en)2][Nb47O128(OH)6(CO3)2]·20H2O | {Nb47} | Df | [ |
| 20 | {[Cu(en)2]@{[Cu2(trz)2(en)2]6[H10Nb68O188]}} | {[Cu2(trz)2(en)2]6Nb68} | Pa | [ |
| Vg | ||||
| 21 | H3[Cu(en)2]4[VNb12O40(VO)2]·13H2O | {VNb12O40(VO)2} | Ch | [ |
| 22 | {[Ni(cyclam)]2H4Nb6O19}·12H2O | {Nb6O19} | / | [ |
| 23 | H9[Cu(en)(H2O)2][Cu(en)2]8[Dy(H2O)4]3[Nb24O69(H2O)3]2?36H2O | {[Dy(H2O)4]3[Nb24O69(H2O)3]2} | PCi | [ |
| 24 | H20Cu(en)[Cu(en)2]11{[{[Cu(en)2]@ {[Cu2(en)2(trz)2]6(Nb68O188)}}][4?Tzp]}2·22en·130H2O | {[Cu(en)2]@{[Cu2(en)2(trz)2]6(Nb68O188)}} | Vg | [ |
| 25 | [Cu(en)2(H2O)]2{[Cu(en)2]4[Cu(en)2]5{[Cu(en)2KNb24O72H10]2}·6en·70H2O. | {Cu(en)2KNb24O72H10} | Vg | [ |
| 26 | H12{[Cu(en)2]6[Nb68O176(OH)12(H2O)12]}·52H2O | {Nb68O200} | PCi | [ |
| 27 | H12{[Cu(en)2]10[Nb68O182(OH)8(H2O)10]}·54H2O | {Nb68O200} | PCi | [ |
| 28 | Ca2TMA2Nb10O28?5H2O | Ca?{Nb10O28} | / | [ |
| 29 | Sr2TMA2Nb10O28?4H2O | Sr?{Nb10O28} | / | [ |
| 30 | Ba2TMA2Nb10O28?9H2O | Ba?{Nb10O28} | / | [ |
| 31 | [Cu(en)2]2{[Cu(en)2]2Ba2K4(H2O)13(SiNb18O54)}2·3en·52H2O | {SiNb18O54} | PCi | [ |
| 32 | H6[Cu(en)2]2{[Cu(en)2]2Ba2Na(H2O)7(SiNb18O54)}2·3en·50H2O | {SiNb18O54} | PCi | [ |
| 1 | Gouzerh P., Proust A., Chem. Rev., 1998, 98(1), 77—111 |
| 2 | Long D. L., Tsunashima R., Cronin L., Angew. Chem. Int. Ed., 2010, 49(10), 1736—1758 |
| 3 | Zheng S. T., Yang G. Y., Chem. Soc. Rev., 2012, 41(22), 7623—7646 |
| 4 | Zhao H. Y., Li Y. Z., Zhao J. W., Wang L., Yang G. Y., Coord. Chem. Rev., 2021, 443, 213966 |
| 5 | Lu J. K., He P. P., Niu J. Y., Wang J. P., Inorg. Chem. Front., 2019, 6(11), 3041—3056 |
| 6 | Song F. Y., Ding Y., Zhao C. C., Acta Chim. Sinica, 2014, 72(2), 133—144 |
| 7 | Li P. C., Ye S. H., Chen S. H., Xiong J. P., Wang L., Song Y. H., Asian J. Chem., 2013, 25(3), 1420—1424 |
| 8 | Chen Z. F., An H. Y., Zhang H., Hu Y., Crystengcomm, 2013, 15(23), 4711—4720 |
| 9 | Lv C. L., Khan R. N. N., Zhang J., Hu J. J., Hao J., Wei Y. G., Chem. Eur. J., 2013, 19(4), 1174—1178 |
| 10 | Chen W. L., Li Y. G., Wang Y. H., Wang E. B., Eur. J. Inorg. Chem., 2007, 15, 2216—2220 |
| 11 | Pang H. J., Zhang C. J., Chen Y. G., Hu M. X., Li J., J. Cluster Sci., 2008, 19(4), 631—640 |
| 12 | Kong Q. J., Zhang C. J., Chen Y. G., J. Mol. Struct., 2010, 964(1—3), 82—87 |
| 13 | Du J., Cao M. D., Feng S. L., Su F., Sang X. J., Zhang L. C., You W. S., Yang M., Zhu Z. M., Chem. Eur. J., 2017, 23(58), 14614—14622 |
| 14 | Skjaervo S. L., Ong G. K., Grendal O. G., Wells K. H., Van Beek W., Ohara K., Milliron D. J., Tominaka S., Grande T., Einarsrud M. A., Inorg. Chem., 2021, 60(11), 7632—7640 |
| 15 | Dopta J., Mahnke L. K., Bensch W., CrystEngComm, 2020, 22(19), 3254—3268 |
| 16 | Liang Z. J., Wu H. C., Singh V., Qiao Y. Y., Li M. M., Ma P. T., Niu J. Y., Wang J. P., Inorg. Chem., 2019, 58(19), 13030—13036 |
| 17 | Yuan L. B., He Y. P., Zhang L., Zhang J., Inorg. Chem., 2018, 57(8), 4226—4229 |
| 18 | Li X. X., Zhao D., Zheng S. T., Coord. Chem. Rev., 2019, 397, 220—240 |
| 19 | Martin N. P., Nyman M., Angew. Chem. Int. Ed., 2021, 60(2), 954—960 |
| 20 | Chen G., Wang C. Z., Ma P. T., Wang J. P., Niu J. Y., J. Cluster Sci., 2010, 21(2), 121—131 |
| 21 | Wang J. P., Niu H. Y., Niu J. Y., Inorg. Chem. Commun., 2008, 11(1), 63—65 |
| 22 | Guo G. L., Xu Y. Q., Hu C. W., J. Coord. Chem., 2010, 63(18), 3137—3145 |
| 23 | Niu J. Y., Ma P. T., Niu H. Y., Li J., Zhao J. W., Song Y., Wang J. P., Chem. Eur. J., 2007, 13(31), 8739—8748 |
| 24 | Wu Y. L., Li X. X., Qi Y. J., Yu H., Jin L., Zheng S. T., Angew. Chem. Int. Ed., 2018, 57(28), 8572—8576 |
| 25 | Tsunashima R., Long D. L., Miras H. N., Gabb D., Pradeep C. P., Cronin L., Angew. Chem. Int. Ed., 2010, 49(1), 113—116 |
| 26 | Zhu Z. K., Lin L. D., Zhang J., Li X. X., Sun Y. Q., Zheng S. T., Inorg. Chem. Front., 2020, 7(20), 3919—3924 |
| 27 | Müscher Polzin P., Näther C., Bensch W., Z. Naturforsch. B: Chem. Sci., 2020, 75(6/7), 583—588 |
| 28 | Wu P. X., Lai R. D., Ge R., Sun C., Wang G. Q., Li X. X., Zheng S. T., Inorg. Chem., 2021, 60(9), 6162—6166 |
| 29 | Zhang Z. Y., Lin Q. P., Kurunthu D., Wu T., Zuo F., Zheng S. T., Bardeen C. J., Bu X. H., Feng P. Y., J. Am. Chem. Soc., 2011, 133(18), 6934—6937 |
| 30 | Lin Y., Zhang T., Yan L. K., Su Z. M., Chem. J. Chinese Universities, 2013, 34(3), 615—620(林燕, 张婷, 颜力楷, 苏忠民. 高等学校化学学报, 2013, 34(3), 615—620) |
| 31 | Petel B. E., Matson E. M., Inorg. Chem., 2021, 60(10), 6855—6864 |
| 32 | Schreiber E., Hartley N. A., Brennessel W. W., Cook T. R., Mckone J. R., Matson E. M., Acs Appl. Energ. Mater., 2019, 2(12), 8985—8993 |
| 33 | Guo G. L., Xu Y. Q., Cao J., Hu C. W., Chem. Eur. J., 2012, 18(12), 3493—3497 |
| 34 | Shen J. Q., Yao S., Zhang Z. M., Wu H. H., Zhang T. Z., Wang E. B., Dalton Trans., 2013, 42(16), 5812—5817 |
| 35 | An W. J., Xu L., Chem. J. Chinese Universities, 2011, 32(3), 783—786(安文佳, 许林. 高等学校化学学报, 2011, 32(3), 783—786) |
| 36 | Wang X. N., Liu S. X., Li S. J., Xie R. H., Zhang X., Liu Y. W., Chem. J. Chinese Universities, 2013, 34(5), 1047—1051(王雪娜, 刘术侠, 李书军, 谢瑞红, 张鑫, 刘艺伟. 高等学校化学学报, 2013, 34(5), 1047—1051) |
| 37 | Han N., Xie R. H., Wang X. N., Liu S. X., Chem. J. Chinese Universities, 2018, 39(7), 1378—1383(韩宁, 谢瑞红, 王雪娜, 刘术侠. 高等学校化学学报, 2018, 39(7), 1378—1383) |
| 38 | Zhang X., Liu S. X., Li S. J., Wang X. N., Jin H. Y., Cui J. L., Liu Y. W., Liu C. Z., Chem. J. Chinese Universities, 2013, 34(9), 2046—2050(张鑫, 刘术侠, 李书军, 王雪娜, 金海燕, 崔佳丽, 刘艺伟, 刘成站. 高等学校化学学报, 2013, 34(9), 2046—2050) |
| 39 | Shen J. Q., Zhang Y., Zhang Z. M., Li Y. G., Gao Y. Q., Wang E. B., Chem. Commun., 2014, 50(45), 6017—6019 |
| 40 | Lan Q., Zhang Z. M., Li Y. G., Wang E. B., Rsc Adv., 2015, 5(55), 44198—44203 |
| 41 | Hu J. F., Wang Y., Zhang X. N., Chi Y. N., Yang S., Li J. K., Hu C. W., Inorg. Chem., 2016, 55(15), 7501—7507 |
| 42 | Li N., Liu Y. W., Lu Y., He D. F., Liu S. M., Wang X. Q., Li Y. G., Liu S. X., New J. Chem., 2016, 40(3), 2220—2224 |
| 43 | Jin L., Li X. X., Zhao D., Li H. H., Zheng S. T., CrystEngComm, 2016, 18(10), 1705—1708 |
| 44 | Jin L., Zhu Z. K., Wu Y. L., Qi Y. J., Li X. X., Zheng S. T., Angew. Chem. Int. Ed., 2017, 56(51), 16288—16292 |
| 45 | Weng Z. W., Ren Y. H., Gu M., Yue B., He H. Y., Dalton Trans., 2017, 47(1), 233—239 |
| 46 | Li S. J., Ji P. P., Han S. N., Hao Z. M., Chen X. N., Inorg. Chem. Commun., 2020, 111, 107666 |
| 47 | Zhong Z. H., Jing J. X., Sun Y. Q., Li X. X., Zheng S. T., Inorg. Chem. Commun., 2021, 132, 108813 |
| 48 | Yang M. S., Chen W. C., Qin C., Yao W., Yin Y. X., Su Z. M., New J. Chem., 2017, 41(19), 10532—10536 |
| 49 | Zhang J., Lai R. D., Wu Y. L., Zhu Z. K., Sun Y. Q., Zeng Q. X., Li X. X., Zheng S. T., Chem. Asian J., 2020, 15(10), 1574—1579 |
| 50 | Jin L., Li X. X., Qi Y. J., Niu P. P., Zheng S. T., Angew. Chem. Int. Ed., 2016, 55(44), 13793—13797 |
| 51 | Zhu Z. K., Lin Y. Y., Yu H., Li X. X., Zheng S. T., Angew. Chem. Int. Ed., 2019, 58(47), 16864—16868 |
| 52 | Huang P., Qin C., Su Z. M., Xing Y., Wang X. L., Shao K. Z., Lan Y. Q., J. Am. Chem. Soc., 2012, 134(34), 14004—14010 |
| 53 | Zhu Z. K., Lin Y. Y., Lin L. D., Li X. X., Sun Y. Q., Zheng S. T., Inorg. Chem., 2020, 59(17), 11925—11929 |
| 54 | Lin Y. D., Zhu Z. K., Ge R., Yu H., Li Z., Sun C., Sun Y. Q., Li X. X., Zheng S. T., Chem. Commun., 2021, 57(38), 4702—4705 |
| 55 | Li J. R., Xie Z. L., He X. W., Li L. H., Huang X. Y., Angew. Chem. Int. Ed., 2011, 50(48), 11395—11399 |
| 56 | Shen N. N., Cai M. L., Song Y., Wang Z. P., Huang F. Q., Li J. R., Huang X. Y., Inorg. Chem., 2018, 57(9), 5282—5291 |
| 57 | Chen X., Addicoat M., Irle S., Nagai A., Jiang D. L., J. Am. Chem. Soc., 2013, 135(2), 546—549 |
| 58 | Xiong W. W., Zhang Q. C., Angew. Chem. Int. Ed., 2015, 54(40), 11616—11623 |
| [1] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [2] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [3] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [4] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [5] | 程前, 杨博龙, 吴文依, 向中华. S掺杂Fe-N-C高活性氧还原反应催化剂[J]. 高等学校化学学报, 2022, 43(9): 20220341. |
| [6] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [7] | 杨静怡, 施思齐, 彭怀涛, 杨其浩, 陈亮. Ga-C3N4单原子催化剂高效光驱动CO2环加成[J]. 高等学校化学学报, 2022, 43(9): 20220349. |
| [8] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| [9] | 王新天, 李攀, 曹越, 洪文浩, 耿忠璇, 安志洋, 王昊宇, 王桦, 孙斌, 朱文磊, 周旸. 单原子材料在二氧化碳催化中的技术经济分析与产业化应用前景[J]. 高等学校化学学报, 2022, 43(9): 20220347. |
| [10] | 唐全骏, 刘颖馨, 孟蓉炜, 张若天, 凌国维, 张辰. 单原子催化在海洋能源领域的应用[J]. 高等学校化学学报, 2022, 43(9): 20220324. |
| [11] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [12] | 杨静怡, 李庆贺, 乔波涛. 铱单原子和纳米粒子在N2O分解反应中的协同催化[J]. 高等学校化学学报, 2022, 43(9): 20220388. |
| [13] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [14] | 任诗杰, 谯思聪, 刘崇静, 张文华, 宋礼. 铂单原子催化剂同步辐射X射线吸收谱的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220466. |
| [15] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||