

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (9): 20220321.doi: 10.7503/cjcu20220321
林高鑫1,2,王家成1,2
收稿日期:2022-05-10
出版日期:2022-09-10
发布日期:2022-08-05
基金资助:
LIN Gaoxin1,2, WANG Jiacheng1,2( )
)
Received:2022-05-10
Online:2022-09-10
Published:2022-08-05
Contact:
WANG Jiacheng
E-mail:jiacheng.wang@mail.sic.ac.cn
Supported by:摘要:
层状二硫化钼由于具有独特的物理化学特性, 在电化学制氢领域受到广泛关注. 二硫化钼的氢惰性表面导致其在酸性和碱性电解液中的析氢活性都比铂差. 将单原子锚定在二硫化钼中能够有效活化惰性的基面,促使其成为先进的析氢电催化剂. 本文从单原子掺杂的二硫化钼的结构出发, 探讨了单原子在提升活性方面的具体机制, 总结了关于单原子掺杂的二硫化钼的制备方法、 表征手段和最新的研究进展, 以及单原子掺杂所产生的缺陷对于活性提升的重要作用. 最后, 基于单原子掺杂二硫化钼在析氢反应中的最新进展, 总结了该领域中相关催化剂的设计思想和主要挑战.
中图分类号:
TrendMD:
林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望. 高等学校化学学报, 2022, 43(9): 20220321.
LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution. Chem. J. Chinese Universities, 2022, 43(9): 20220321.
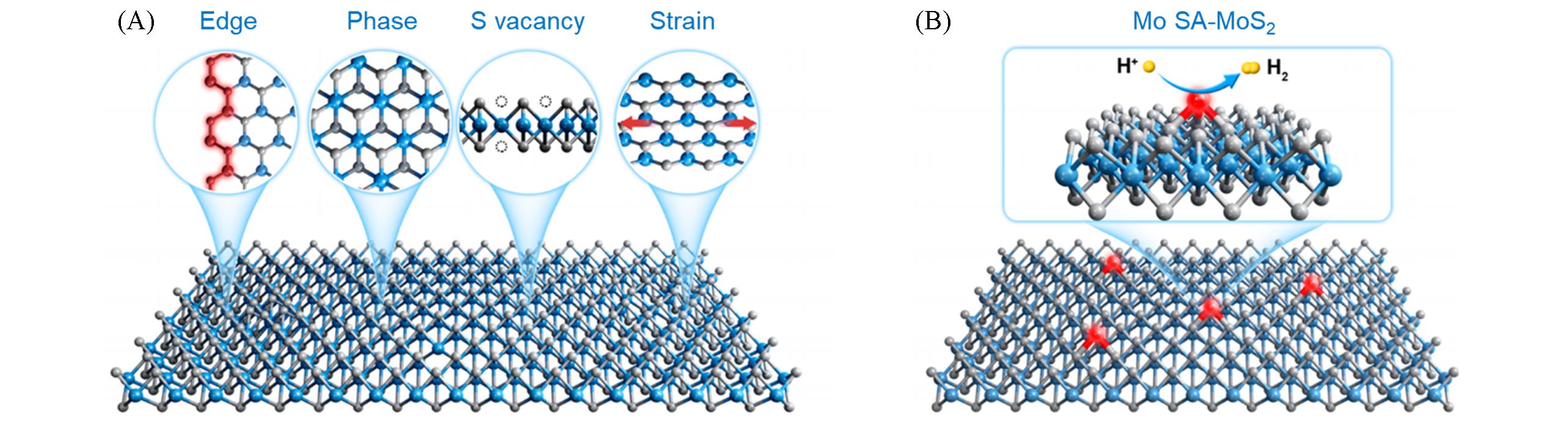
Fig.2 Active sites(edges, 1T phase, S vacancies and strain) of MoS2 for HER(A) and schematic illustration of Mo?SA?MoS2 in which Mo SAs are active sites for HER(B)[32]
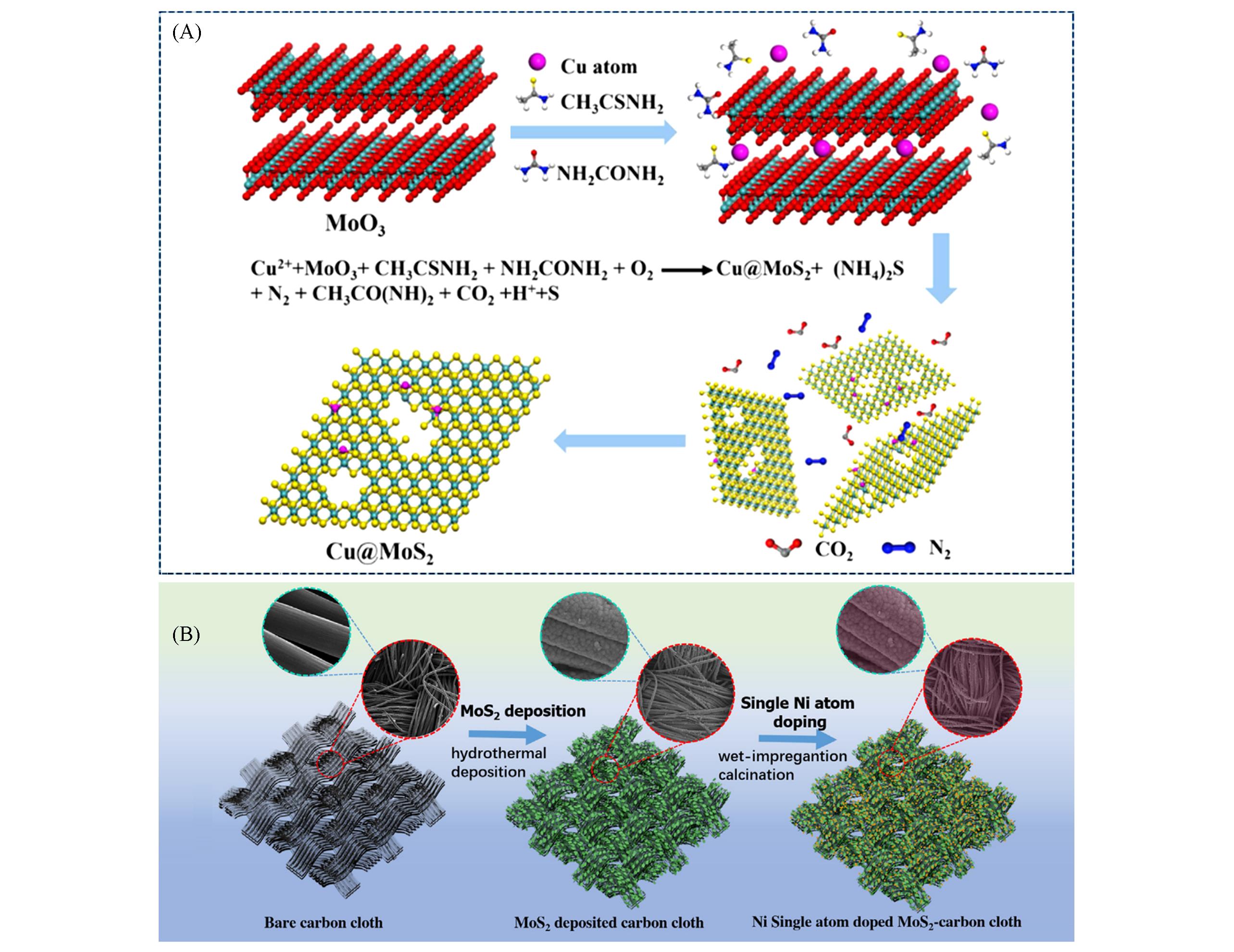
Fig.3 Schematic procedure for syntheszing Cu@MoS2(A)[49] and schematic illustration of the preparation of Ni?SA?MoS2(B)[52](A) Copyright 2019, Elsevier B. V.; (B) Copyright 2018, Elsevier Ltd.
| Catalyst | Synthesis method | Overpotential(at 10 mA/cm2)/mV | Electrolyte | Ref. |
|---|---|---|---|---|
| Co?MoS2 | Hydrothermal synthesis | 47 | 0.5 mol/L H2SO4 | [ |
| Pt?MoS2 | Hydrothermal synthesis | 180 | 0.5 mol/L H2SO4 | [ |
| Ru?MoS2 | Hydrothermal synthesis | 41 | 1 mol/L KOH | [ |
| N?MoS2 | Hydrothermal synthesis | 168 | 0.5 mol/L H2SO4 | [ |
| Ni?MoS2 | Hydrothermal synthesis | 127 | 0.5 mol/L H2SO4 | [ |
| Ru, Ni?MoS2 | Wet?chemistry method | 31 | 1 mol/L KOH | [ |
| Ru?MoS2 | Wet?chemistry method | 51 | 1 mol/L KOH | [ |
| Ru?MoS2 | Wet?chemistry method | 30 | 1 mol/L KOH | [ |
| Ru?MoS2 | Wet?chemistry method | 76 | 1 mol/L KOH | [ |
| Co?MoS2 | Chemical vapor deposition | 137 | 0.5 mol/L H2SO4 | [ |
| Ru?MoS2/MoP | Galvanostatic deposition | 45 | 1 mol/L KOH | [ |
| Pt?MoS2 | Potential?cycling method | 88.4 | 1 mol/L KOH | [ |
| Pt?MoS2 | Solar irradiation | 44 | 0.5 mol/L H2SO4 | [ |
| U?MoS2 | Pulse voltammetry method | 72 | 1 mol/L KOH | [ |
Table 1 Electrocatalytic HER performance of different SAs doped MoS2
| Catalyst | Synthesis method | Overpotential(at 10 mA/cm2)/mV | Electrolyte | Ref. |
|---|---|---|---|---|
| Co?MoS2 | Hydrothermal synthesis | 47 | 0.5 mol/L H2SO4 | [ |
| Pt?MoS2 | Hydrothermal synthesis | 180 | 0.5 mol/L H2SO4 | [ |
| Ru?MoS2 | Hydrothermal synthesis | 41 | 1 mol/L KOH | [ |
| N?MoS2 | Hydrothermal synthesis | 168 | 0.5 mol/L H2SO4 | [ |
| Ni?MoS2 | Hydrothermal synthesis | 127 | 0.5 mol/L H2SO4 | [ |
| Ru, Ni?MoS2 | Wet?chemistry method | 31 | 1 mol/L KOH | [ |
| Ru?MoS2 | Wet?chemistry method | 51 | 1 mol/L KOH | [ |
| Ru?MoS2 | Wet?chemistry method | 30 | 1 mol/L KOH | [ |
| Ru?MoS2 | Wet?chemistry method | 76 | 1 mol/L KOH | [ |
| Co?MoS2 | Chemical vapor deposition | 137 | 0.5 mol/L H2SO4 | [ |
| Ru?MoS2/MoP | Galvanostatic deposition | 45 | 1 mol/L KOH | [ |
| Pt?MoS2 | Potential?cycling method | 88.4 | 1 mol/L KOH | [ |
| Pt?MoS2 | Solar irradiation | 44 | 0.5 mol/L H2SO4 | [ |
| U?MoS2 | Pulse voltammetry method | 72 | 1 mol/L KOH | [ |
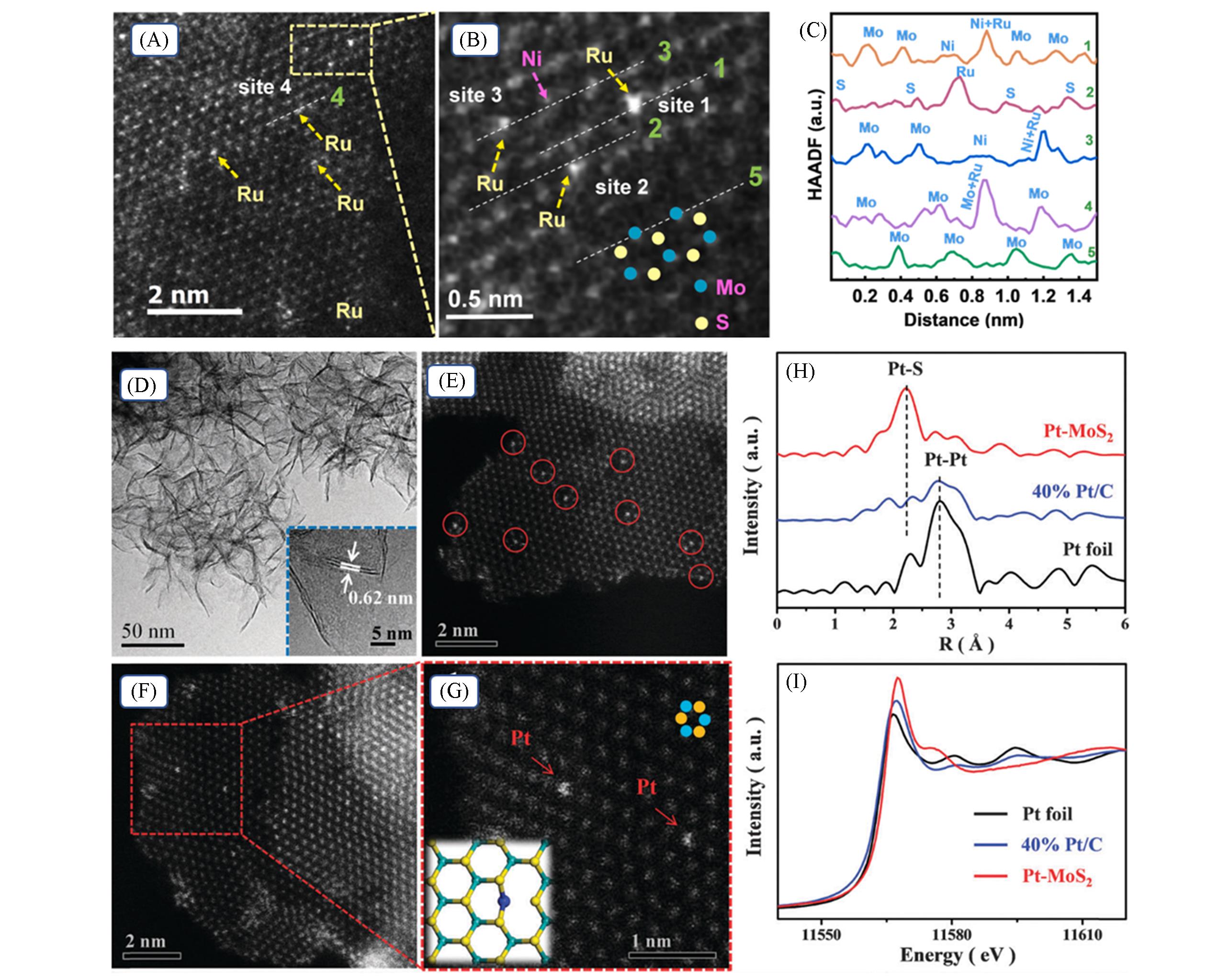
Fig.4 HAADF?STEM image(A) and corresponding enlarged image(B) of Ru/Ni?MoS2, HAADF intensity line profiles taken along the appropriately numbered lines indicated in (A) and (B)(C)[60], TEM image of Pt?MoS2 with the inset showing a typical MoS2 layer distance of 0.62 nm(D), HAADF?STEM images of Pt?MoS2 showing that the single Pt atoms marked by red circles uniformly disperse in the 2D MoS2 plane(E), enlarged image showing a honeycomb arrangement of MoS2(F), and the single Pt atoms occupying the exact positions of the Mo atoms(marked by red arrows)(G), the k2?weighted EXAFS spectra(H) and the normalized Pt L3?edge XANES spectra(I)[68](G) The bottom inset shows the simulated configuration of Pt-MoS2. The green, yellow and blue balls represent Mo, S and Pt, respectively. (A)—(C) Copyright 2021, Elsevier B. V.; (D)—(I) Copyright 2015, Royal Society of Chemistry.
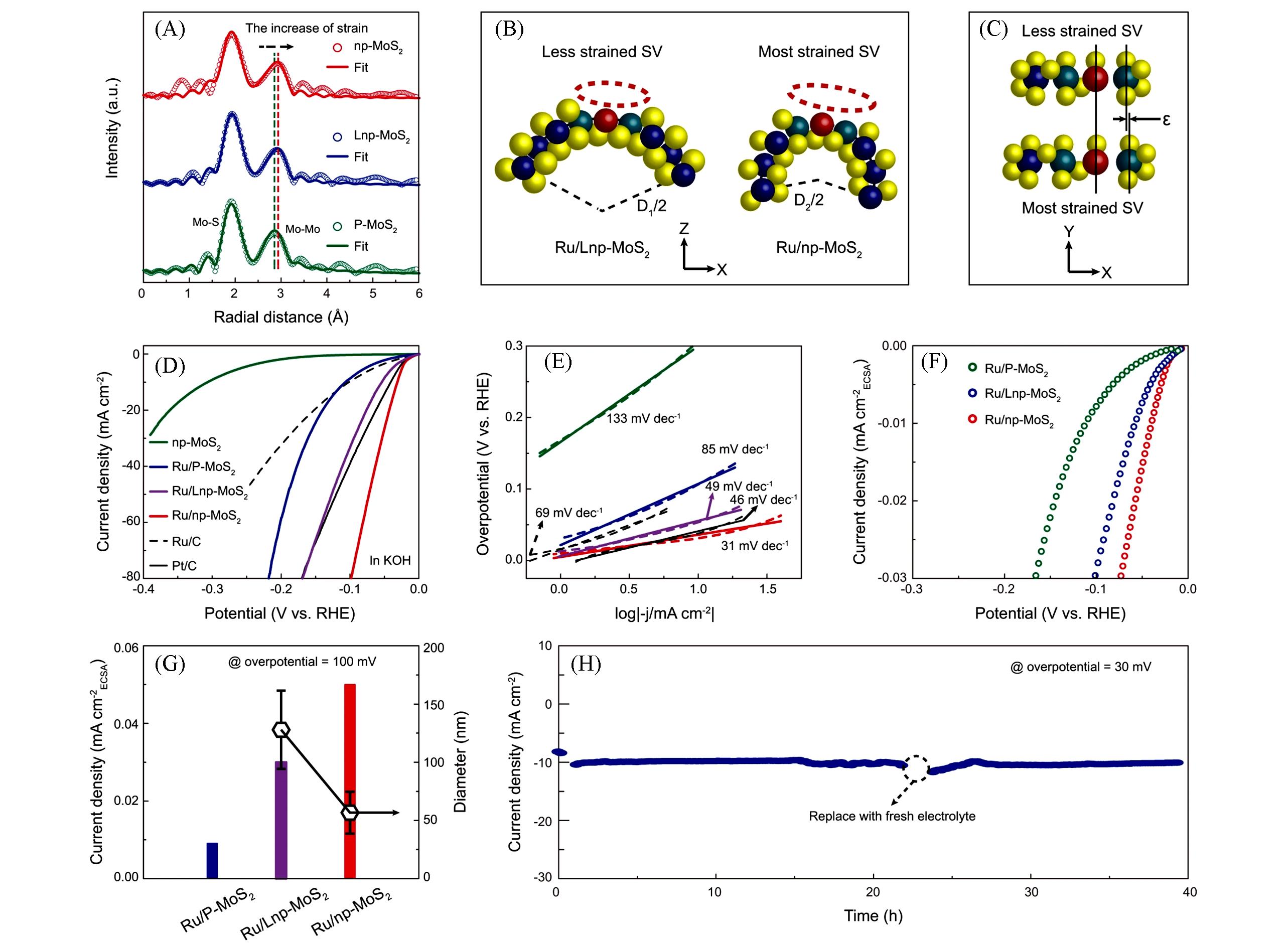
Fig.5 FT?EXAFS spectra(A), schematic of the atomic structure of Ru/Lnp?MoS2 and Ru/np?MoS2 derived from(A)(B, C)[? in (C) represents the amount of deformation], polarization curves(D), corresponding Tafel plots derived from (D)(E), ECSA?normalized polarization curves(F), ECSA?normalized current density at an overpotential of 100 mV(G)(the average diameters of ligaments for Ru/LnpMoS2 and Ru/np?MoS2 were also shown), and stability measurement of Ru/np?MoS2 at an overpotential of 30 mV(H)[29]
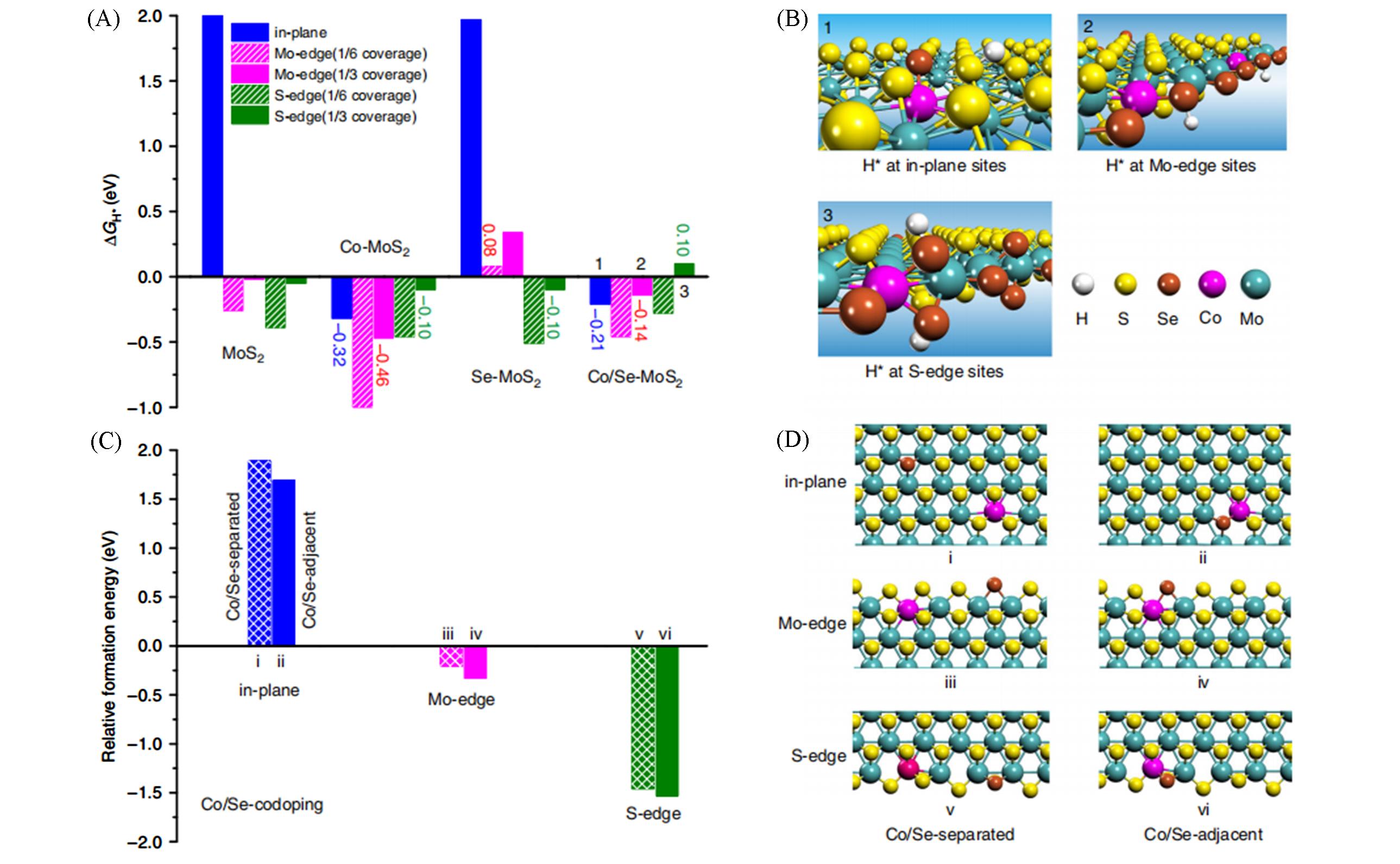
Fig.6 Adsorption free energies of H*(ΔGH*) at different catalysts(A), adsorption models of H* at different sites of Co/Se?MoS2(B), relative formation energies of the Co/Se?co?doped MoS2 with different doping configurations of Co and Se being separated(Co/Se?separated, grid bars) or adjacent (Co/Se?adjacent, solid bars) to each other(C), structures of Co/Se?co?doped basal plane, Mo?edge, and S?edge with Co/Se?separated and Co/Se?adjacent configurations(D)[86]
| 1 | Wang X. X., Swihart M. T., Wu G., Nat. Catal., 2019, 2(7), 578―589 |
| 2 | Seh Z. W., Kibsgaard J., Dickens C. F., Chorkendorff I., Nørskov J. K., Jaramillo T. F., Science, 2017, 355(6321), eaad4998 |
| 3 | Oener S. Z., Foster M. J., Boettcher S. W., Science, 2020, 369(6507), 1099―1103 |
| 4 | Zou X. X., Wu Y. Y., Liu Y. P., Liu D. P., Li W., Gu L., Liu H., Wang P. W., Sun L., Zhang Y., Chem, 2018, 4(5), 1139―1152 |
| 5 | Zhang B. W., Zhu C. Q., Wu Z. S., Stavitski E., Lui Y. H., Kim T. H., Liu H., Huang L., Luan X. C., Zhou L., Jiang K., Huang W. Y., Hu S., Wang H. L., Francisco J. S., Nano Lett., 2020, 20(1), 136―144 |
| 6 | Liu Q., Ding J., Ji G. J., Hu J. M., Gu H., Zhong Q., J. Inorg. Mater., 2021, 36(10), 1053―1058 |
| 7 | Dong M. Y., Xu W., Zhao J., Di L. B., Zhang X. L., J. Inorg. Mater., 2020, 35(5), 567―572 |
| 8 | Li Z, Ma R., Ju Q., Liu Q., Liu L., Zhu Y., Yang M., Wang J., The Innovation, 2022, 3(4), 100268 |
| 9 | Ma S., Deng J., Xu Y., Tao W., Wang X., Lin Z., Zhang Q., Gu L., Zhong W., J. Energy Chem., 2022, 3(4), 100268 |
| 10 | Wu Q. L., Luo M., Han J. H., Peng W., Zhao Y., Chen D. C., Peng M., Liu J., de Groot F. M. F., Tan Y. W., ACS Energy Lett., 2019, 5(1), 192―199 |
| 11 | Hu C., Zhang L., Gong J., Energy Environ. Sci., 2019, 12, 2620―2645 |
| 12 | Sun H. C., Tung C. W., Qiu Y., Zhang W., Wang Q., Li Z. S., Tang J., Chen H. C., Wang C. D., Chen H. M., J. Am. Chem. Soc., 2022, 144(3), 1174―1186 |
| 13 | Subbaraman R., Tripkovic D., Strmcnik D., Chang K. C., Uchimura M., Paulikas A. P., Stamenkovic V., Markovic N. D., Science, 2011, 334(6060), 1256―1260 |
| 14 | Yu Z. Y., Duan Y., Feng X. Y., Yu X. X., Gao M. R., Yu S. H., Adv. Mater., 2021, 33(31), 2007100 |
| 15 | An L., Wei C., Lu M., Liu H. W., Chen Y. B., Scherer G. G., Fisher A. C., Xi P. X., Xu Z. J., Yan C. H., Adv. Mater., 2021, 33(20), 2006328 |
| 16 | Raja Sulaiman R. R., Wong W. Y., Loh K. S., Int. J. Energy Res., 2021, 46(3), 2241―2276 |
| 17 | Wang D., Wang X., Lu Y., Song C. S., Pan J., Li C. L., Sui M. L., Zhao W., Huang F. Q., J. Mater. Chem. A, 2017, 5(43), 22618―22624 |
| 18 | Chen P. Z., Zhang N., Wang S. B., Zhou T. P., Tong Y., Ao C. C., Yan W. S., Zhang L. D., Chu W. S., Wu C. Z., Xie J., Proc. Natl. Acad. Sci. USA, 2019, 116(14), 6635―6640 |
| 19 | Li J. C., Zhang C., Ma H. J., Wang T. H., Guo Z., Yang Y., Wang Y. Y., Ma H. X., Chem. Eng. J., 2021, 414, 128834 |
| 20 | Liu Y. W., Xiao C., Huang P. C., Cheng M., Xie Y., Chem, 2018, 4(6), 1263―1283 |
| 21 | Luo Y. T., Li X., Cai X. K., Zhou X. L., Kang H. M., Liu B. L., ACS Nano, 2018, 12(5), 4565―4573 |
| 22 | Luo Z., Ouyang Y., Zhang H., Xiao M., Ge J., Jiang Z., Wang J., Tang D., Cao X., Liu C., Xing W., Nat. Commun., 2018, 9(2120), 1―8 |
| 23 | Guan J., Bai X., Tang T., Nano Res., 2022, 15(2), 818―837 |
| 24 | Wang Y., Mao J., Meng X. G., Yu L., Deng D. H., Bao X. H., Chem. Rev., 2019, 119(3), 1806―1854 |
| 25 | Wu W. Z., Niu C. Y., Wei C., Jia Y., Li C., Xu Q., Angew. Chem. Int. Ed., 2019, 58(7), 2029―2033 |
| 26 | Fu H. Q., Zhou M., Liu P. F., Yin H. J., Sun K. Z., Yang H. G., Mamun M., Hu P. J., Wang H. F., Zhou H. J., J. Am. Chem. Soc., 2022, 144(13), 6028―6039 |
| 27 | Joo J., Kim T., Lee J., Choi S., Lee K., Adv. Mater., 2019, 31(14), 1806682 |
| 28 | Yu Y., Nam G. H., He Q., Wu X. J., Zhang K., Yang Z., Chen J., Ma Q., Zhao M., Liu Z., Ran F. R., Wang X. Z., Li H., Huang X., Li B., Xiong Q., Zhang Q., Liu Z., Gu L., Du Y. H., Huang W., Zhang H., Nat. Chem., 2018, 10(6), 638―643 |
| 29 | Jiang K., Luo M., Liu Z. X., Peng M., Chen D. C., Lu Y. R., Chan T. S., de Groot F. K. M., Tan Y. W., Nat. Commun., 2021, 12(1), 1687 |
| 30 | Shang B., Ma P., Fan J., Jiao L., Liu Z., Zhang Z., Chen N., Cheng Z., Cui X., Zheng W., Nanoscale, 2018, 10(26), 12330―12336 |
| 31 | Guo Y., Yao Z., Timmer B. J. J., Sheng X., Fan L., Li Y., Zhang F., Sun L., Nano Energy, 2019, 62, 282―288 |
| 32 | Luo Y., Zhang S., Pan H., Xiao S., Guo Z., Tang L., Khan U., Ding B. F., Li M., Caai Z., Zhao Y., Lv W., Feng Q., Zou X., Lin J., Cheng H. M., Liu B., ACS Nano, 2020, 14(1), 767―776 |
| 33 | Qin Q., Chen L., Wei T., Liu X., Small, 2019, 15(29), e1803639 |
| 34 | Li Y., Gu Q., Johannessen B., Zheng Z., Li C., Luo Y., Zhang Z., Zhang Q., Fan H., Luo W., Liu B., Dou S., Liu H., Nano Energy, 2021, 84, 105898 |
| 35 | Alarawi A., Ramalingam V., He J. H., Mater. Today Energy, 2019, 11, 1―23 |
| 36 | Zhao W., Pan J., Fang Y., Che X., Wang D., Bu K., Huang f., Chem⁃Eur. J., 2018, 24(60), 15942―15954 |
| 37 | Lin G. X., Ju Q. J., Guo X. W., Zhao W., Adimi S., Ye J. Y., Bi Q. Y., Wang J. C., Yang M. H., Huang F. Q., Adv. Mater., 2021, 33(32), 2007509 |
| 38 | Jin W., Yeh P. C., Zaki N., Zhang D., Sadowski J. T., Al⁃Mahboob A., van der Zande A. M., Chenet D. A., Dadap J. I., Herman I. P., Sutter P., Hone J., Osgood R. M., Phys. Rev. Lett., 2013, 111(10), 106801 |
| 39 | Voiry D., Mohite A., Chhowalla M., Chem. Soc. Rev., 2015, 44(9), 2702―2712 |
| 40 | Kappera R., Voiry D., Yalcin S. E., Branch B., Gupta G., Mohite A. D., Chhowalla M., Nat. Mater., 2014, 13(12), 1128―1134 |
| 41 | Yan Y., Xia B. Y., Zhao B., Wang X., J. Mater. Chem. A, 2016, 4(45), 17587―17603 |
| 42 | Sheng W., Myint M., Chen J. G., Yan Y., Energy Environ. Sci., 2013, 6(5), 1509―1512 |
| 43 | Greeley J., Jaramillo T. F., Bonde J., Chorkendorff I. B., Nørskov J. K., Nat. Mater., 2006, 5(11), 909―913 |
| 44 | Hinnemann B., Moses P. G., Bonde J., Jørgensen K. P., Nielsen J. H., Horch S., Chorkendorff I., Nørskov J. K., J. Am. Chem. Soc., 2005, 127(15), 5308―5309 |
| 45 | Kang Y., Surf. Sci., 2021, 704, 121759 |
| 46 | Cui Z., Sa R., Du W., Xiao C., Li Q., Ma Z., Appl. Surf. Sci., 2021, 542, 148535 |
| 47 | Hao Y., Wang Y. T., Xu L. C., Yang Z., Liu R. P., Li X. Y., Appl. Surf. Sci., 2019, 469, 292―297 |
| 48 | Ma Z., Niu L., Jiang W., Dong C., Liu G., Qu D., An L., Sun Z., J. Phys. Mater., 2021, 4(4), 042002 |
| 49 | Ji L., Yan P., Zhu C., Ma C., Wu W., Wei C., Shen Y., Chu S., Wang J., Du Y., Chen J., Yang X., Xu Q., Appl. Catal. B: Environ., 2019, 251, 87―93 |
| 50 | Wu C., Li D., Ding S., Rehman Z. Liu Q., Chen S., Zhang B., Song L., J. Phys. Chem. Lett., 2019, 10(20), 6081―6087 |
| 51 | Zhang J., Xu X., Yang L., Cheng D., Cao D., Small Methods, 2019, 3(12), 1900653 |
| 52 | Wang Q., Zhao Z. L., Dong S., He D., Lawrence M. J., Han S., Cai C., Xiang S., Rodriguez P., Xiang B., Wang Z., Liang Y., Gu M., Nano Energy, 2018, 53, 458―467 |
| 53 | Zheng F., Huang N., Peng R., Ding Y., Li G., Xia Z., Sun P., Sun X., Geng J., Electrochim. Acta, 2018, 263, 328―337 |
| 54 | Zhu J., Tu Y., Cai L., Ma H., Chai Y., Zhang L., Zhang W., Small, 2022, 18(4), 2104824 |
| 55 | Liu M., Chen H., Tang X., Liu H., Tu B., Guo W., Zheng Y., Liu Y., Tang Y., He R., Zhu W., Small, 2022, 18(11), 2107444 |
| 56 | Qiao W., Xu W., Xu X., Wu L., Yan S., Wang D., ACS Appl. Energy Mater., 2020, 3(3), 2315―2322 |
| 57 | Wang D., Li Q., Han C., Xing Z., Yang X., Appl. Catal. B: Environ., 2019, 249, 91―97 |
| 58 | Li R., Yang L., Xiong T., Wu Y., Cao L., Yuan D., Zhou W., J. Power Sources, 2017, 356, 133―139 |
| 59 | Luo R., Luo M., Wang Z., Liu P., Song S., Wang X., Chen M., Nanoscale, 2019, 11(15), 7123―7128 |
| 60 | Ge J., Zhang D., Qin Y., Dou T., Jiang M., Zhang F., Lei X., Appl. Catal. B: Environ., 2021, 298, 120557 |
| 61 | Wang J., Fang W., Hu Y., Zhang Y., Dang J., Wu Y., Chen B., Zhao H., Li Z., Appl. Catal. B: Environ., 2021, 298, 120490 |
| 62 | Duan H., Wang C., Li G., Tan H., Hu W., Cai L., Liu W., Li N., Ji Q., Wang Y., Lu Y., Yan W., Hu F., Zhang W., Sun Z., Qi Z., Song L., Wei S., Angew. Chem. Int. Ed., 2021, 60(13), 7251―7258 |
| 63 | Besenbacher F., Lauritsen J. V., Linderoth T. R., Lægsgaard E., Vang R. T., Wendt S., Surf. Sci., 2009, 603(10), 1315―1327 |
| 64 | Asokan C., DeRita L., Christopher P., Chinese J. Catal., 2017, 38(9), 1473 |
| 65 | Rahmatullah, Qamar S., Phys. Lett. A, 2013, 377(25), 1587―1592 |
| 66 | Wei J., Qin S. N., Yang J., Ya H. L., Huang W. H., Zhuang H., Hwang B. J., Tian Z. Q., Li J. F., Angew. Chem., 2021, 133(17), 9392―9396 |
| 67 | MacArthur K. E., Pennycook T. J., Okunishi E., D'Alfonso A. J., Lugg N. R., Allen L. J., Nellist P. D., Ultramicroscopy, 2013, 133, 109―119 |
| 68 | Deng J., Li H., Xiao J., Tu Y., Deng D., Yang H., Tian H., Li J., Ren P., Bao X., Energy Environmenal Sci., 2015, 8(5), 1594―1601 |
| 69 | van Gastel R., Somfai E., van Saarloos W., Frenken J. W. M., Nature, 2000, 408(6813), 665―665 |
| 70 | Lauritsen J. V., Kibsgaard J., Olesen G. H., Moses P. G., Hinnemann B., Helveg S., Nørskov J. K., Clausen B. S., Topsøe H., Lægsgaard E., Besenbacher F., J. Catal., 2007, 249(2), 220―233 |
| 71 | Pető J., Ollár T., Vancsó P., Popov Z. I., Magda G. Z., Dobrik G., Hwang C., Sorokin P. B., Tapasztó L., Nat. Chem., 2018, 10(12), 1246―1251 |
| 72 | Fang L., Seifert S., Winans R. E., Li T., Small Methods, 2021, 5(5), 2001194 |
| 73 | Zhang T., Chen Z., Walsh A. G., Li Y., Zhang P., Adv. Mater., 2020, 32(44), 2002910 |
| 74 | Li J., Chen S., Quan F., Zhan G., Jia F., Ai Z., Zhang L., Chem, 2020, 6(4), 885―901 |
| 75 | Zhang K., Bersch B. M., Joshi J., Addou R., Cormier C. R., Zhang C., Xu K., Briggs N. C., Wang K., Subramanian S., Cho K., Fullerton⁃Shirey S., Wallace R. M., Vora P. M, Robinson J. A., Adv. Funct. Mater., 2018, 28(16), 1706950 |
| 76 | Gao X., Zhou Y., Cheng Z., Tan Y., Yuan T., Shen Z., Appl. Surf. Sci., 2021, 547, 149113 |
| 77 | Meng X., Ma C., Jiang L., Si R., Meng X., Tu Y., Yu L., Bao X., Deng D., Angew. Chem. Int. Ed., 2020, 59(26), 10502―10507 |
| 78 | Lau T. H., Wu S., Kato R., Wu T. S., Kulhavy J., Mo J., Zheng J., Foord J. S., Soo Y. L, Suenaga K., Darby M. T., Tsang S. E., ACS Catal., 2019, 9(8), 7527―7534 |
| 79 | Zhang H., Yu L., Chen T., Zhou W., Lou X. W., Adv. Funct. Mater., 2018, 28(51), 1807086 |
| 80 | Wang Y., Wang M., Lu Z., Ma D., Jia Y., Nanoscale, 2021, 13(31), 13390―13400 |
| 81 | Qi K., Cui X., Gu L., Yu S., Fan X., Luo M., Xu S., Li N., Zheng L., Zhang Q., Ma J., Gong Y., Lv F., Wang K., Huang H., Zhang W., Guo S., Zheng W., Liu P., Nat. Commun., 2019, 10(1), 5231 |
| 82 | Pan J., Song C., Wang X., Yuan X., Fang Y., Guo C., Huang F., Inorg. Chem. Front., 2017, 4(11), 1895―1899 |
| 83 | Lau T. H., Lu X., Kulhavý J., Wu S., Lu L., Wu T. S., Kato R., Foord J. S., Soo Y. L., Suenaga K., Tsang S. C. E., Chem. Sci., 2018, 9(21), 4769―4776 |
| 84 | Ren X., Ma Q., Fan H., Pang L., Zhang Y., Yao Y., Ren X., Liu S. F., Chem. Commun., 2015, 51(88), 15997―16000 |
| 85 | Liu P., Zhu J., Zhang J., Xi P., Tao K., Gao D., Xue D., ACS Energy Lett., 2017, 2(4), 745―752 |
| 86 | Zheng Z., Yu L., Gao M., Gao M., Chen X., Zhou W., Ma C., Wu L., Zhu J., Meng X., Hu J., Tu Y., Wu S., Mao J., Tian Z., Deng D., Nat. Commun., 2020, 11(1), 3315 |
| 87 | Huang Y., Sun Y., Zheng X., Aoki T., Pattengale B., Huang J., He X., Bian W., Younan S., Williams N., Hu J., Ge J., Pu N., Yan X., Pan X., Zhang L., Wei Y., Gu J., Nat. Commun., 2019, 10(1), 982 |
| [1] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [2] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [3] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| [4] | 杨静怡, 李庆贺, 乔波涛. 铱单原子和纳米粒子在N2O分解反应中的协同催化[J]. 高等学校化学学报, 2022, 43(9): 20220388. |
| [5] | 任诗杰, 谯思聪, 刘崇静, 张文华, 宋礼. 铂单原子催化剂同步辐射X射线吸收谱的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220466. |
| [6] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [7] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [8] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [9] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [10] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [11] | 韩付超, 李福进, 陈良, 贺磊义, 姜玉南, 徐守冬, 张鼎, 其鲁. CoSe2/C复合电催化材料修饰隔膜对高载量锂硫电池性能的影响[J]. 高等学校化学学报, 2022, 43(8): 20220163. |
| [12] | 赵润瑶, 纪桂鹏, 刘志敏. 吡咯氮配位单原子铜催化剂的电催化二氧化碳还原性能[J]. 高等学校化学学报, 2022, 43(7): 20220272. |
| [13] | 王茹涵, 贾顺涵, 吴丽敏, 孙晓甫, 韩布兴. CO2参与电化学构筑C—N键制备重要化学品[J]. 高等学校化学学报, 2022, 43(7): 20220395. |
| [14] | 彭奎霖, 李桂林, 江重阳, 曾少娟, 张香平. 电解液调控CO2电催化还原性能微观机制的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220238. |
| [15] | 王丽君, 李欣, 洪崧, 詹新雨, 王迪, 郝磊端, 孙振宇. 调节氧化镉-炭黑界面高效电催化CO2还原生成CO[J]. 高等学校化学学报, 2022, 43(7): 20220317. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||