

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (9): 20220341.doi: 10.7503/cjcu20220341
收稿日期:2022-05-14
出版日期:2022-09-10
发布日期:2022-06-24
通讯作者:
向中华
E-mail:xiangzh@mail.buct.edu.cn
基金资助:
CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua( )
)
Received:2022-05-14
Online:2022-09-10
Published:2022-06-24
Contact:
XIANG Zhonghua
E-mail:xiangzh@mail.buct.edu.cn
Supported by:摘要:
采用微波加热和高温碳化技术, 以ZIF-8为前驱体, 在甲醇-水双溶剂体系中先后引入Fe(NO3)3·9H2O和KSCN, 制备了一系列S掺杂的Fe-N-C催化剂(Fe3C/Fe-SAS@SNC), 并通过X射线粉末衍射、 扫描透射电子显微镜和氮气吸附-脱附测试等表征手段进行分析. 结果表明, Fe和S两种元素的合理掺杂使Fe3C/Fe-SAS@SNC催化剂具有明显的分级多孔结构, 比表面积达到673 m2/g, 在酸、 碱电解质中均表现出了优异的氧还原催化性能. 在0.1 mol/L KOH中, Fe3C/Fe-SAS@SNC催化剂的半波电位达到0.880 V(vs. RHE), 高于商业Pt/C催化剂, 且表现出了比商业Pt/C更优的稳定性. 在0.5 mol/L H2SO4中, Fe3C/Fe-SAS@SNC电催化氧还原的性能也与商业Pt/C催化剂相当.
中图分类号:
TrendMD:
程前, 杨博龙, 吴文依, 向中华. S掺杂Fe-N-C高活性氧还原反应催化剂. 高等学校化学学报, 2022, 43(9): 20220341.
CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions. Chem. J. Chinese Universities, 2022, 43(9): 20220341.
| Sample | Atomic percentage(%) | ||||
|---|---|---|---|---|---|
| Fe2p | S2p | N1s | C1s | O1s | |
| Fe3C/Fe?SAS@SNC | 0.63 | 0.75 | 4.05 | 83.52 | 11.05 |
| Fe?SAS@SNC | 0.42 | 0.46 | 1.84 | 81.85 | 15.44 |
| SNC | — | 0.40 | 2.52 | 82.00 | 15.08 |
| Fe@NC | 0.72 | — | 2.98 | 84.84 | 11.46 |
Table 1 Atomic percentage of the samples as measured by XPS
| Sample | Atomic percentage(%) | ||||
|---|---|---|---|---|---|
| Fe2p | S2p | N1s | C1s | O1s | |
| Fe3C/Fe?SAS@SNC | 0.63 | 0.75 | 4.05 | 83.52 | 11.05 |
| Fe?SAS@SNC | 0.42 | 0.46 | 1.84 | 81.85 | 15.44 |
| SNC | — | 0.40 | 2.52 | 82.00 | 15.08 |
| Fe@NC | 0.72 | — | 2.98 | 84.84 | 11.46 |
| Species percentage(%, atomic fraction) | ||||||
|---|---|---|---|---|---|---|
| Sample | Total atomic N | Pyridinic N | Graphitic N | Pyrrolic N | Oxidized N | Fe?N |
| Fe3C/Fe?SAS@SNC | 4.05 | 1.05 | 1.20 | 0.62 | 0.89 | 0.29 |
| Fe?SAS@SNC | 1.84 | 0.44 | 0.34 | 0.46 | 0.41 | 0.19 |
| SNC | 2.52 | 0.78 | 0.39 | 0.73 | 0.61 | — |
| Fe@NC | 2.98 | 0.70 | 0.66 | 0.63 | 0.59 | 0.40 |
Table 2 XPS atomic percentage of different types of N of the obtained samples
| Species percentage(%, atomic fraction) | ||||||
|---|---|---|---|---|---|---|
| Sample | Total atomic N | Pyridinic N | Graphitic N | Pyrrolic N | Oxidized N | Fe?N |
| Fe3C/Fe?SAS@SNC | 4.05 | 1.05 | 1.20 | 0.62 | 0.89 | 0.29 |
| Fe?SAS@SNC | 1.84 | 0.44 | 0.34 | 0.46 | 0.41 | 0.19 |
| SNC | 2.52 | 0.78 | 0.39 | 0.73 | 0.61 | — |
| Fe@NC | 2.98 | 0.70 | 0.66 | 0.63 | 0.59 | 0.40 |
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | Vmicro/(cm3·g-1) | Vmeso+macro/(cm3·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| Fe3C/Fe?SAS@SNC | 673 | 0.76 | 0.21 | 0.55 | 12.59 |
| Fe?SAS@SNC | 474 | 0.85 | 0.10 | 0.75 | 14.25 |
Table 3 Pore characteristics of Fe3C/Fe-SAS@SNC and Fe-SAS@SNC products
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | Vmicro/(cm3·g-1) | Vmeso+macro/(cm3·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| Fe3C/Fe?SAS@SNC | 673 | 0.76 | 0.21 | 0.55 | 12.59 |
| Fe?SAS@SNC | 474 | 0.85 | 0.10 | 0.75 | 14.25 |
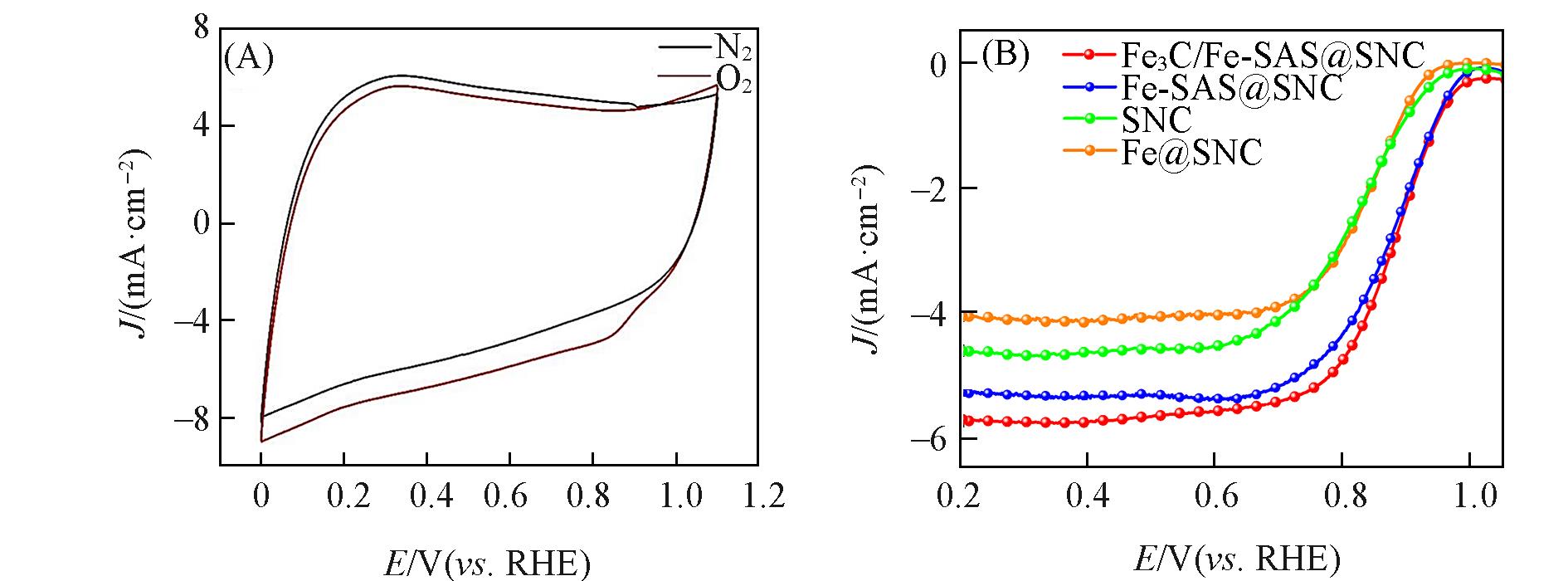
Fig.7 CV curves of Fe3C/Fe?SAS@SNC in N2?saturated and O2?saturated 0.1 mol/L KOH electrolyte(A), ORR polarization curves in O2?saturated 0.1 mol/L KOH electrolyte(B)
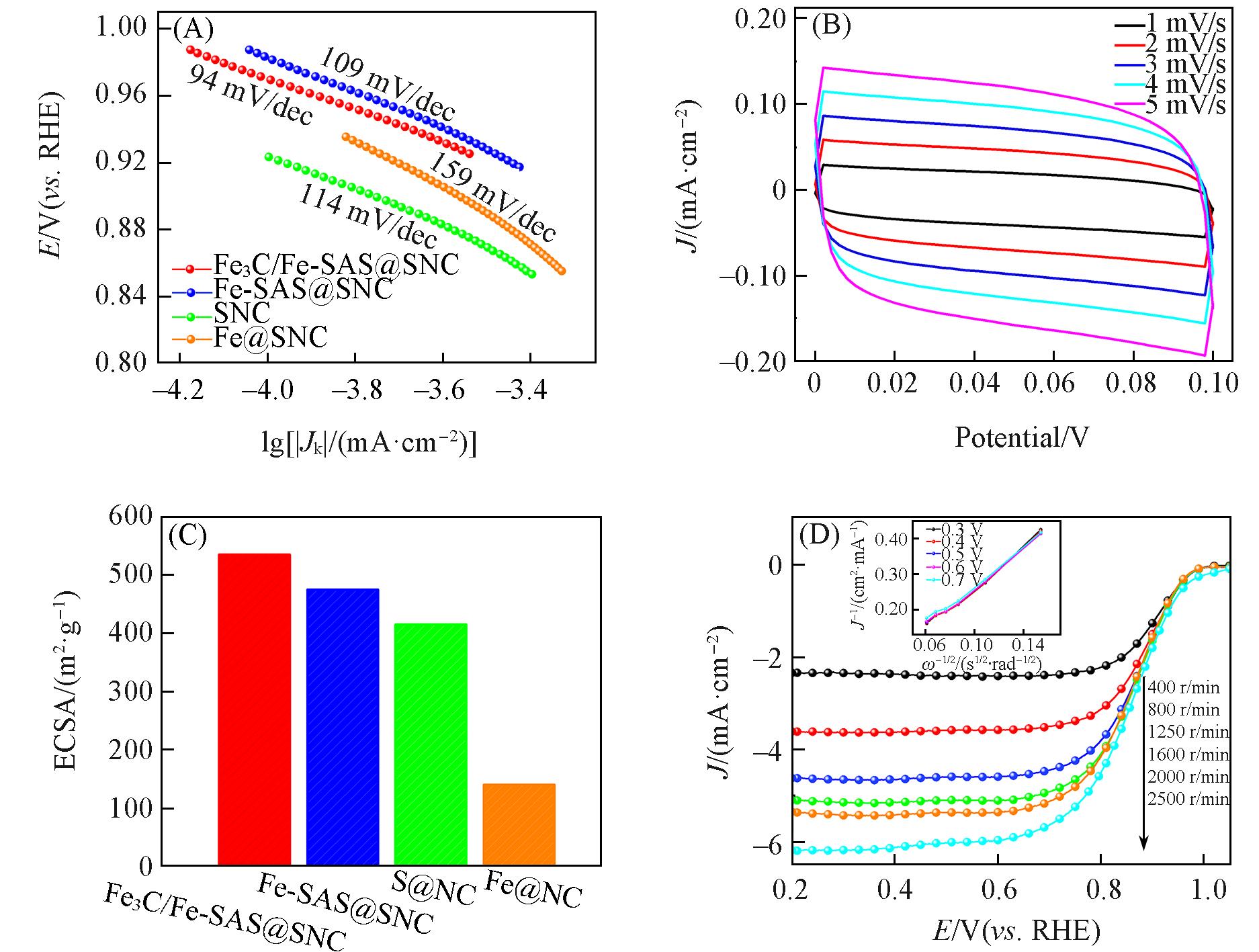
Fig.8 Tafel slope of different catalysts(A), CV curves at different scan rates of Fe3C/Fe?SAS@SNC(B), the corresponding ECSA for ORR of different catalysts(C), LSV curves at different rotating speeds of Fe3C/Fe?SAS@SNC(inset: K?L plots at different potentials)(D)
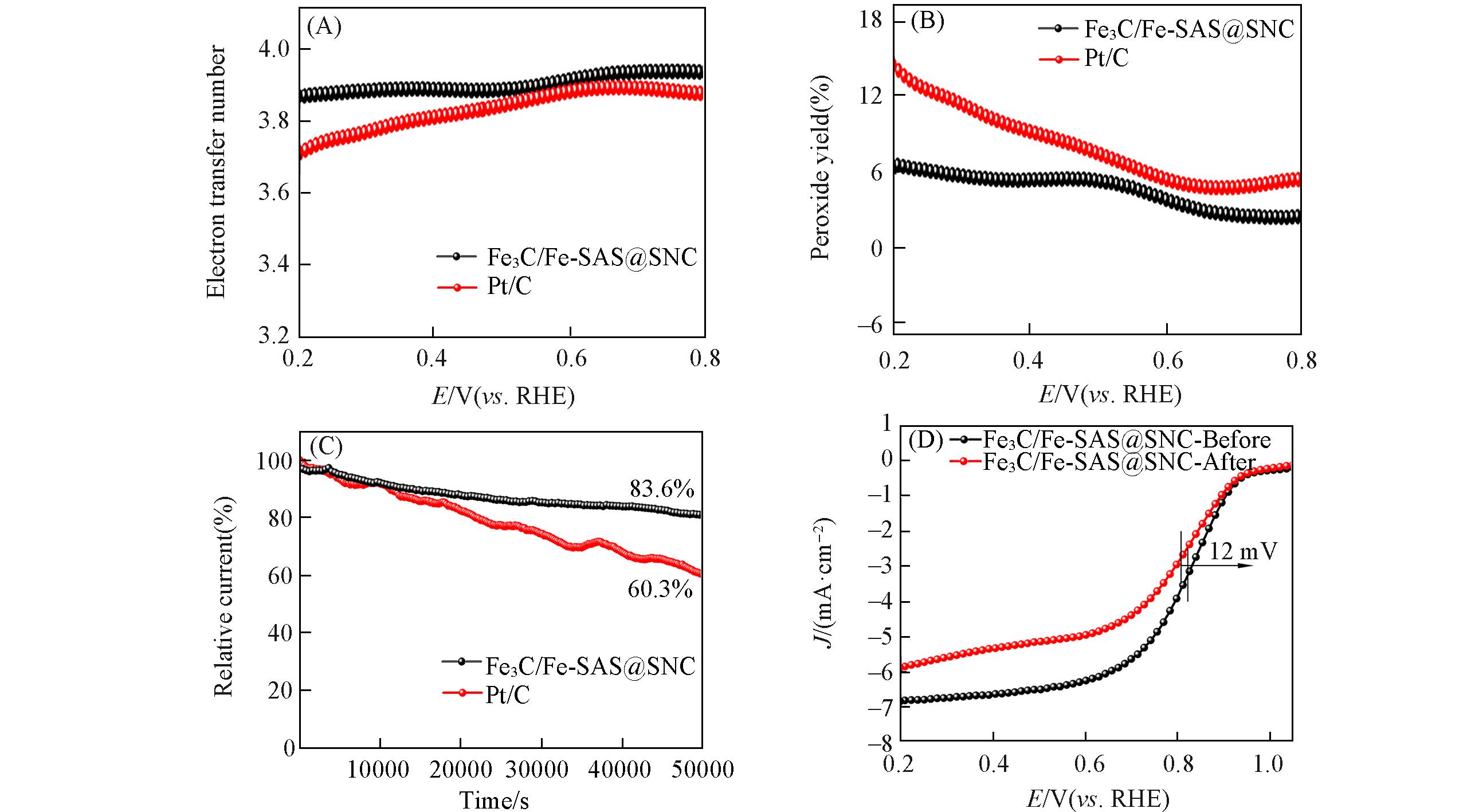
Fig.9 ORR catalytic performance in O2?saturated 0.1 mol/L KOH electrolyte(A) Electron transfer number; (B) H2O2 yield plots; (C) chronoamperometric(i?t) responses of Fe3C/Fe?SAS@SNC and 20%Pt/C; (D) ORR polarization curves of Fe3C/Fe?SAS@SNC before and after chronoamperometric(i?t) test(1600 r/min).
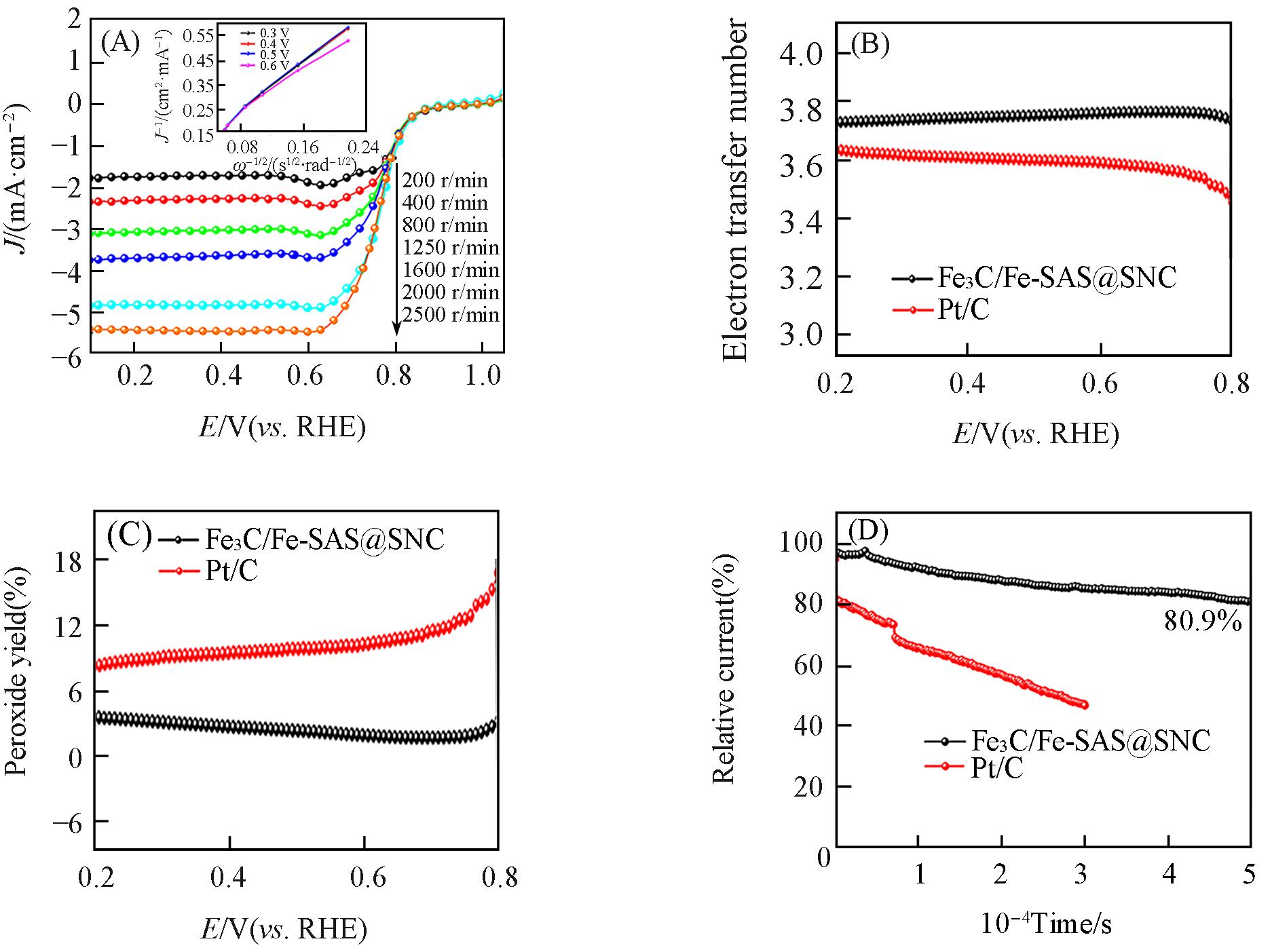
Fig.11 LSV curves of Fe3C/Fe?SAS@SNC at different rotating speeds(inset: K?L plots at diffe?rent potentials)(A), electron transfer number(B), H2O2 yield plots(C), chronoamperometric(i?t) responses of Fe3C/Fe?SAS@SNC and 20%Pt/C in O2?saturated 0.5 mol/L H2SO4 electrolyte(D)
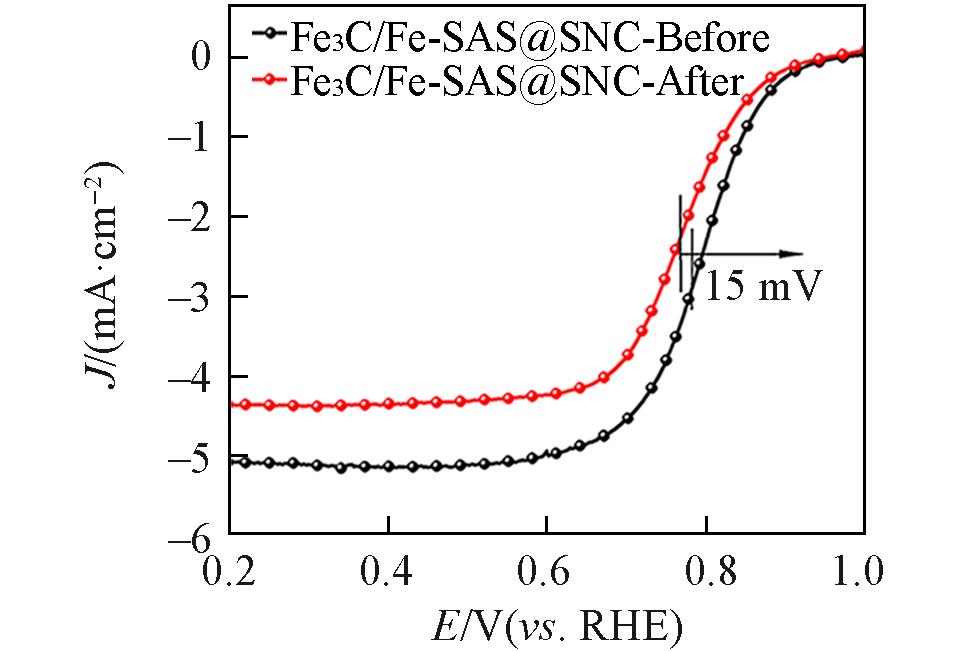
Fig.12 ORR polarization curves of Fe3C/Fe?SAS@SNC before and after Chronoamperometric(i?t) test(1600 r/min)in O2?saturated 0.5 mol/L H2SO4 electrolyte
| Catalyst | Onset potential/V | Half?wavepotential/V | Electrolyte | Ref. |
|---|---|---|---|---|
| Fe?N/P?C?700 | 0.941 | 0.867 | 0.1 mol/L KOH | [ |
| S, N?Fe/N/C?CN | — | 0.850 | 0.1 mol/L KOH | [ |
| FeNC?S?FexC/Fe | — | 0.887 | 0.1 mol/L KOH | [ |
| Fe/N/S?CNTs | 0.987 | 0.880 | 0.1 mol/L KOH | [ |
| Fe3N@N?C | 0.995 | 0.849 | 0.1 mol/L KOH | [ |
| Fe3C/Fe?SAS@SNC | 1.020 | 0.880 | 0.1 mol/L KOH | This work |
| Fe SAs/N?C | 0.950 | 0.750 | 0.1 mol/L HClO4 | [ |
| Fe?N/P?C?700 | 0.890 | 0.720 | 0.1 mol/L HClO4 | [ |
| Fe/SNC | — | 0.770 | 0.5 mol/L H2SO4 | [ |
| Fe?N/CNT?2 | — | 0.770 | 0.5 mol/L H2SO4 | [ |
| FeNC?SN?2 | 0.861 | 0.789 | 0.5 mol/L H2SO4 | [ |
| Fe3C/Fe?SAS@SNC | 0.892 | 0.785 | 0.5 mol/L H2SO4 | This work |
Table 4 Fe-based electrode materials for ORR
| Catalyst | Onset potential/V | Half?wavepotential/V | Electrolyte | Ref. |
|---|---|---|---|---|
| Fe?N/P?C?700 | 0.941 | 0.867 | 0.1 mol/L KOH | [ |
| S, N?Fe/N/C?CN | — | 0.850 | 0.1 mol/L KOH | [ |
| FeNC?S?FexC/Fe | — | 0.887 | 0.1 mol/L KOH | [ |
| Fe/N/S?CNTs | 0.987 | 0.880 | 0.1 mol/L KOH | [ |
| Fe3N@N?C | 0.995 | 0.849 | 0.1 mol/L KOH | [ |
| Fe3C/Fe?SAS@SNC | 1.020 | 0.880 | 0.1 mol/L KOH | This work |
| Fe SAs/N?C | 0.950 | 0.750 | 0.1 mol/L HClO4 | [ |
| Fe?N/P?C?700 | 0.890 | 0.720 | 0.1 mol/L HClO4 | [ |
| Fe/SNC | — | 0.770 | 0.5 mol/L H2SO4 | [ |
| Fe?N/CNT?2 | — | 0.770 | 0.5 mol/L H2SO4 | [ |
| FeNC?SN?2 | 0.861 | 0.789 | 0.5 mol/L H2SO4 | [ |
| Fe3C/Fe?SAS@SNC | 0.892 | 0.785 | 0.5 mol/L H2SO4 | This work |
| 1 | Debe M. K., Nature, 2012, 486(7401), 43—51 |
| 2 | Yan Q., Wu J., Energ Convers Manage., 2008, 49(8), 2425—2433 |
| 3 | Al⁃Shahat Eissa A., Kim N. H., Lee J. H., J. Mater. Chem. A, 2020, 8(44), 23436—23454 |
| 4 | Yang C. C., Zai S. F., Zhou Y. T., Du L., Jiang Q., Adv. Funct. Mater., 2019, 29(27), 1901949 |
| 5 | Ren G., Lu X., Li Y., Zhu Y., Dai L., Jiang L., ACS Appl. Mater. Inter., 2016, 8(6), 4118—4125 |
| 6 | Zhao C. X., Li B. Q., Liu J. N., Zhang Q., Angew. Chem. Int. Ed., 2021, 60(9), 4448—4463 |
| 7 | Guo Y., Liu F., Feng L., Wang X., Zhang X., Liang J., Chem. Eng. J., 2022, 429, 132150 |
| 8 | Lu J. D., Yang M. C., J. Power Sources, 2011, 196(20), 8519—8524 |
| 9 | Guha A., Zawodzinski T. A., Schiraldi. D. A. Jr., J. Power Sources, 2010, 195(16), 5167—5175 |
| 10 | Gong K., Du F., Xia Z., Durstock M., Dai L., Science, 2009, 323(5915), 760—764 |
| 11 | Liang J., Jiao Y., Jaroniec M., Qiao S. Z., Angew. Chem. Int. Ed., 2012, 51(46), 11496—11500 |
| 12 | Li Z. X., Yang B. L., Kong L., Yue M. L., Duan H. H., Carbon, 2019, 144, 540—548 |
| 13 | Wang S., Zhang L., Xia Z., Roy A., Chang D. W., Baek J. B., Dai L., Angew. Chem. Int. Ed., 2012, 51(17), 4209—4212 |
| 14 | Wang S., Iyyamperumal E., Roy A., Xue Y., Yu D., Dai L., Angew. Chem. Int. Ed., 2011, 50(49), 11756—11760 |
| 15 | Duan J., Chen S., Jaroniec M., Qiao S. Z., ACS Catal., 2015, 5(9), 5207—5234 |
| 16 | Guan Z., Zhang X., Chen W., Pei J., Liu D., Xue Y., Zhu W., Zhuang Z., Chem. Commun., 2018, 54(85), 12073—12076 |
| 17 | Liu P., Cheng Q. Q., Chen C., Zou L. L., Zou Z. Q., Yang H., Chem. J. Chinese Universities, 2018, 39(11), 2492—2499 |
| 刘培, 程庆庆, 陈驰, 邹亮亮, 邹志青, 杨辉. 高等学校化学学报, 2018, 39(11), 2492—2499 | |
| 18 | Chen G., Liu P., Liao Z., Sun F., He Y., Zhong H., Zhang T., Zschech E., Chen M., Wu G., Zhang J., Feng X., Adv. Mater., 2020, 32(8), 1907399 |
| 19 | Zhang N., Zhou T., Chen M., Feng H., Yuan R., Zhong C. A., Yan W., Tian Y., Wu X., Chu W., Wu C., Xie Y., Energ. Environ. Sci., 2020, 13(1), 111—118 |
| 20 | Sun X., Wei P., Gu S., Zhang J., Jiang Z., Wan J., Chen Z., Huang L., Xu Y., Fang C., Li Q., Han J., Huang Y., Small, 2020, 16(6), 1906057 |
| 21 | Zheng L., Dong Y., Chi B., Cui Z., Deng Y., Shi X., Du L., Liao S., Small, 2019, 15(4), 1803520 |
| 22 | Yin Y., Wang J., Li T., Hill J. P., Rowan A., Sugahara Y., Yamauchi Y., ACS Nano, 2021, 13240—13248 |
| 23 | Wang Y., Wang D., Li Y., Adv. Mater., 2021, 33(34), e2008151 |
| 24 | Xiao F., Wang Y. C., Wu Z. P., Chen G., Yang F., Zhu S., Siddharth K., Kong Z., Lu A., Li J. C., Zhong C. J., Zhou Z. Y., Shao M., Adv. Mater., 2021, 33(50), e2006292 |
| 25 | Su S., Huang L., Su S., Meng C., Zhou H., Zhang L., Bian T., Yuan A., ACS Appl. Nano Mater., 2020, 3(11), 11574—11580 |
| 26 | Li Z., Gao Q., Liang X., Zhang H., Xiao H., Xu P., Liu Z., Carbon, 2019, 150, 93—100 |
| 27 | Zhai X., Lin W., Liu J., Chen X., Yong J., Yang W., J. Electroanal. Chem., 2020, 866, 114170 |
| 28 | He Y., Guo H., Hwang S., Yang X., He Z., Braaten J., Karakalos S., Shan W., Wang M., Zhou H., Feng Z., More K. L., Wang G., Su D., Cullen D. A., Fei L., Litster S., Wu G., Adv. Mater., 2020, 32(46), 2003577 |
| 29 | Yuan K., Luetzenkirchen⁃Hecht D., Li L., Shuai L., Li Y., Cao R., Qiu M., Zhuang X., Leung M. K. H., Chen Y., Scherf U., J. Am. Chem. Soc., 2020, 142(5), 2404—2412 |
| 30 | Chen P., Zhou T., Xing L., Xu K., Tong Y., Xie H., Zhang L., Yan W., Chu W., Wu C., Xie Y., Angew. Chem. Int. Ed., 2017, 56(2), 610—614 |
| 31 | Qiao Y., Yuan P., Hu Y., Zhang J., Mu S., Zhou J., Li H., Xia H., He J., Xu Q., Adv. Mater., 2018, 30(46), 1804504 |
| 32 | Jin H., Zhou H., Li W., Wang Z., Yang J., Xiong Y., He D., Chen L., Mu S., J. Mater. Chem. A, 2018, 6(41), 20093—20099 |
| 33 | Li T., Li M., Zhang M., Li X., Liu K., Zhang M., Liu X., Sun D., Xu L., Zhang Y., Tang Y., Carbon, 2019, 153, 364—371 |
| 34 | Wang J., Huang Z., Liu W., Chang C., Tang H., Li Z., Chen W., Jia C., Yao T., Wei S., Wu Y., Lie Y., J. Am. Chem. Soc., 2017, 139(48), 17281—17284 |
| 35 | Shen H., Gracia⁃Espino E., Ma J., Zang K., Luo J., Wang L., Gao S., Mamat X., Hu G., Wagberg T., Guo S., Angew. Chem. Int. Edit., 2017, 56(44), 13800—13804 |
| 36 | Xia D., Yang X., Xie L., Wei Y., Jiang W., Dou M., Li X., Li J., Gan L., Kang F., Adv. Funct. Mater., 2019, 29(49), 1906174 |
| 37 | Shao C., Wu L., Zhang H., Jiang Q., Xu X., Wang Y., Zhuang S., Chu H., Sun L., Ye J., Li B., Wang X., Adv. Funct. Mater., 2021, 31(25), 2100833 |
| [1] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [2] | 谷雨, 奚宝娟, 李江潇, 熊胜林. 单原子催化剂在氧还原反应中的分子级调控[J]. 高等学校化学学报, 2022, 43(5): 20220036. |
| [3] | 张小玉, 薛冬萍, 杜宇, 蒋粟, 魏一帆, 闫文付, 夏会聪, 张佳楠. MOF衍生碳基电催化剂限域催化O2还原和CO2还原反应[J]. 高等学校化学学报, 2022, 43(3): 20210689. |
| [4] | 何宇婧, 李佳乐, 王东洋, 王福玲, 肖作旭, 陈艳丽. 锌活化Fe/Co/N掺杂的生物质碳基高效氧还原催化剂[J]. 高等学校化学学报, 2022, 43(11): 20220475. |
| [5] | 马骏, 钟洋, 张珊珊, 黄仪珺, 张利鹏, 李亚平, 孙晓明, 夏振海. 高效催化氧还原及氧析出反应的掺杂石墨炔的设计与理论计算[J]. 高等学校化学学报, 2021, 42(2): 624. |
| [6] | 王跃民, 孟庆磊, 王显, 葛君杰, 刘长鹏, 邢巍. 铜,硫掺杂对铁氮碳氧还原催化剂性能的提升作用[J]. 高等学校化学学报, 2020, 41(8): 1843. |
| [7] | 殷雯婧, 刘啸, 钱汇东, 邹志青. 高活性位点密度Fe-N共掺杂碳纳米片的制备及氧还原性能[J]. 高等学校化学学报, 2019, 40(7): 1480. |
| [8] | 徐朝权, 马俊红, 石旻慧, 冯超, 谢亚红, 米红宇. 基于天然产物的新型铁氮共掺杂碳电催化剂的制备及氧还原性能[J]. 高等学校化学学报, 2018, 39(7): 1532. |
| [9] | 王秀利, 何兴权. 氮/硫双掺多孔碳负载Fe9S10纳米粒子的氧还原电催化性能[J]. 高等学校化学学报, 2018, 39(7): 1524. |
| [10] | 黄骥培, 李毅, 杨申辉, 周亚洲, 程晓农, 朱佳, 杨娟. 三维多孔结构Pt-Ag气凝胶的制备及电催化氧还原反应性能[J]. 高等学校化学学报, 2018, 39(5): 1063. |
| [11] | 狄沐昕, 肖国正, 黄鹏, 曹翊寰, 朱英. 钴/氮掺杂碳纳米管/石墨烯复合材料的构筑及氧还原催化性能[J]. 高等学校化学学报, 2018, 39(2): 343. |
| [12] | 刘培, 程庆庆, 陈驰, 邹亮亮, 邹志青, 杨辉. 包覆氮化铁的Fe, N掺杂碳纳米纤维的制备及对氧还原反应的催化性能[J]. 高等学校化学学报, 2018, 39(11): 2492. |
| [13] | 康欢, 李赏, 刘畅, 郭伟, 潘牧. 自组装合成Fe-N-C-PANI有序介孔结构催化剂及其在酸性条件下的氧还原活性[J]. 高等学校化学学报, 2017, 38(8): 1423. |
| [14] | 徐凯, 李毅, 赵南, 杜文修, 曾炜炜, 高帅, 程晓农, 杨娟. 中空铂镍/三维石墨烯催化剂的制备及电化学性能[J]. 高等学校化学学报, 2016, 37(8): 1476. |
| [15] | 李赏, 李沛, 赵伟, 康欢, 潘牧. 石墨烯负载Fe-N/C复合型氧还原催化剂[J]. 高等学校化学学报, 2015, 36(9): 1737. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||