

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (9): 20220428.doi: 10.7503/cjcu20220428
收稿日期:2022-06-17
出版日期:2022-09-10
发布日期:2022-07-25
通讯作者:
黄凯,伍晖
E-mail:kai@bupt.edu.cn;huiwu@tsinghua.edu.cn
基金资助:
WANG Ruyue1,2, WEI Hehe3, HUANG Kai1,2( ), WU Hui2(
), WU Hui2( )
)
Received:2022-06-17
Online:2022-09-10
Published:2022-07-25
Contact:
HUANG Kai,WU Hui
E-mail:kai@bupt.edu.cn;huiwu@tsinghua.edu.cn
Supported by:摘要:
近年来, 单原子催化剂因其最大化的金属原子利用效率和高催化性能, 已成为能量存储和转化领域中的研究热点. 单原子催化剂的高活性主要来源于其低配位结构、 量子尺寸效应和原子与载体之间的强相互作用. 因此, 如何根据构-效关系开发通用且简单的制备高效单原子催化剂的方法具有重要的意义. 从实际应用的角度而言, 湿化学法因具有工艺简单和易于大规模生产的特性, 被认为是一种实现工业化制备单原子催化剂的方法, 现已开发了一系列制备负载型单原子催化剂的策略. 本文从独特的抑制反应物前驱体物质形核的角度出发, 总结了冷冻合成方法对形核的抑制机制, 进一步针对不同方面的应用, 探讨了单原子材料的催化机理, 并对其未来的发展进行了展望.
中图分类号:
TrendMD:
王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成. 高等学校化学学报, 2022, 43(9): 20220428.
WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials. Chem. J. Chinese Universities, 2022, 43(9): 20220428.
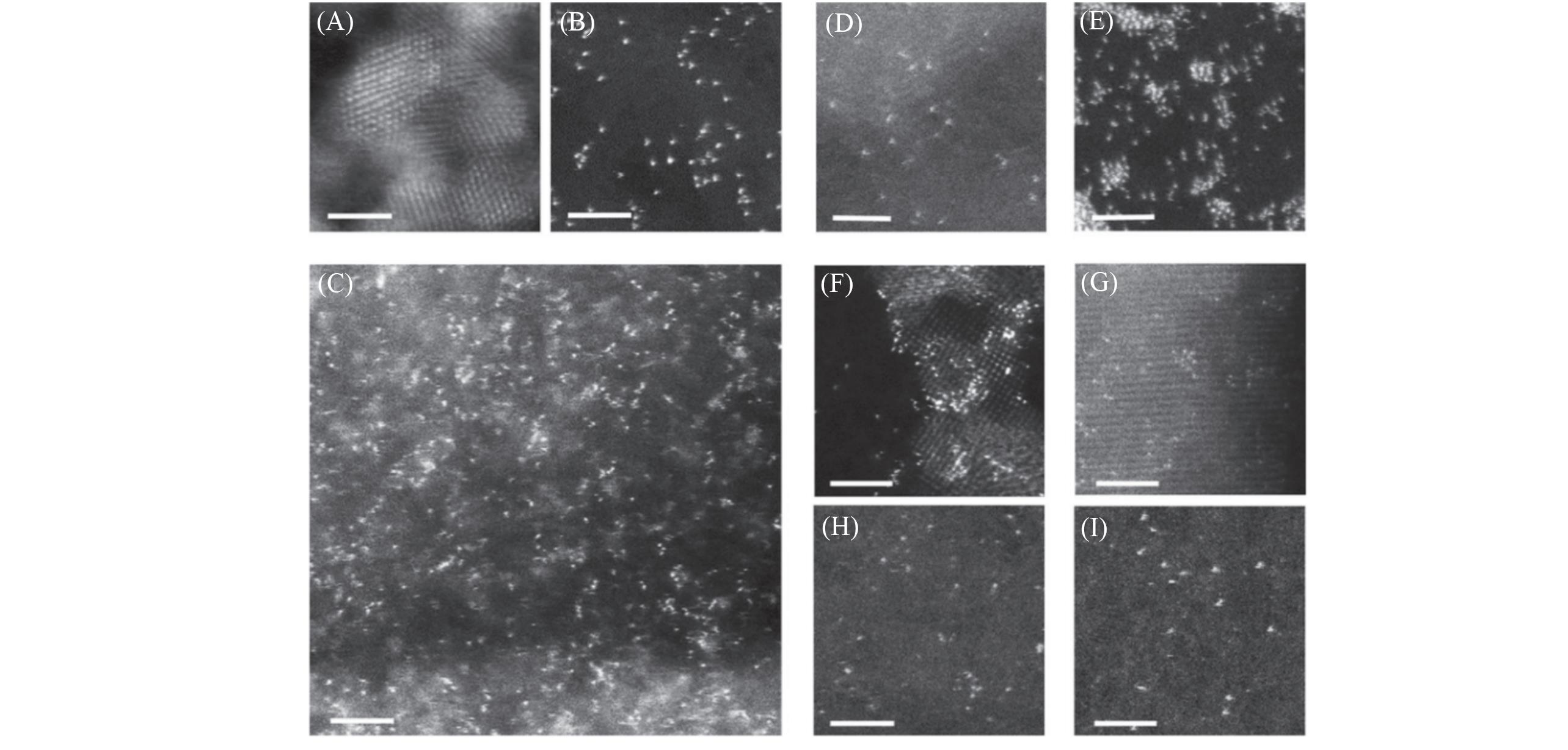
Fig.2 HAADF?STEM images of samples[68](A) Pt nanocrystals formed by normal photochemical reduction. Pt single atoms dispersed on ultrathin carbon film(B), mesoporous carbon(C), and MWCNTs(D). (E) Pt1/graphene, with concomitant Pt single atoms and clusters. Atomically dispersed Pt on titanium oxide nanoparticles(F), zinc oxide nanowires(G). Ag(H) and Au(I) single atoms prepared by a similar iced-photochemical route. Scale bar: 2 nm. Copyright 2017, Springer Nature.
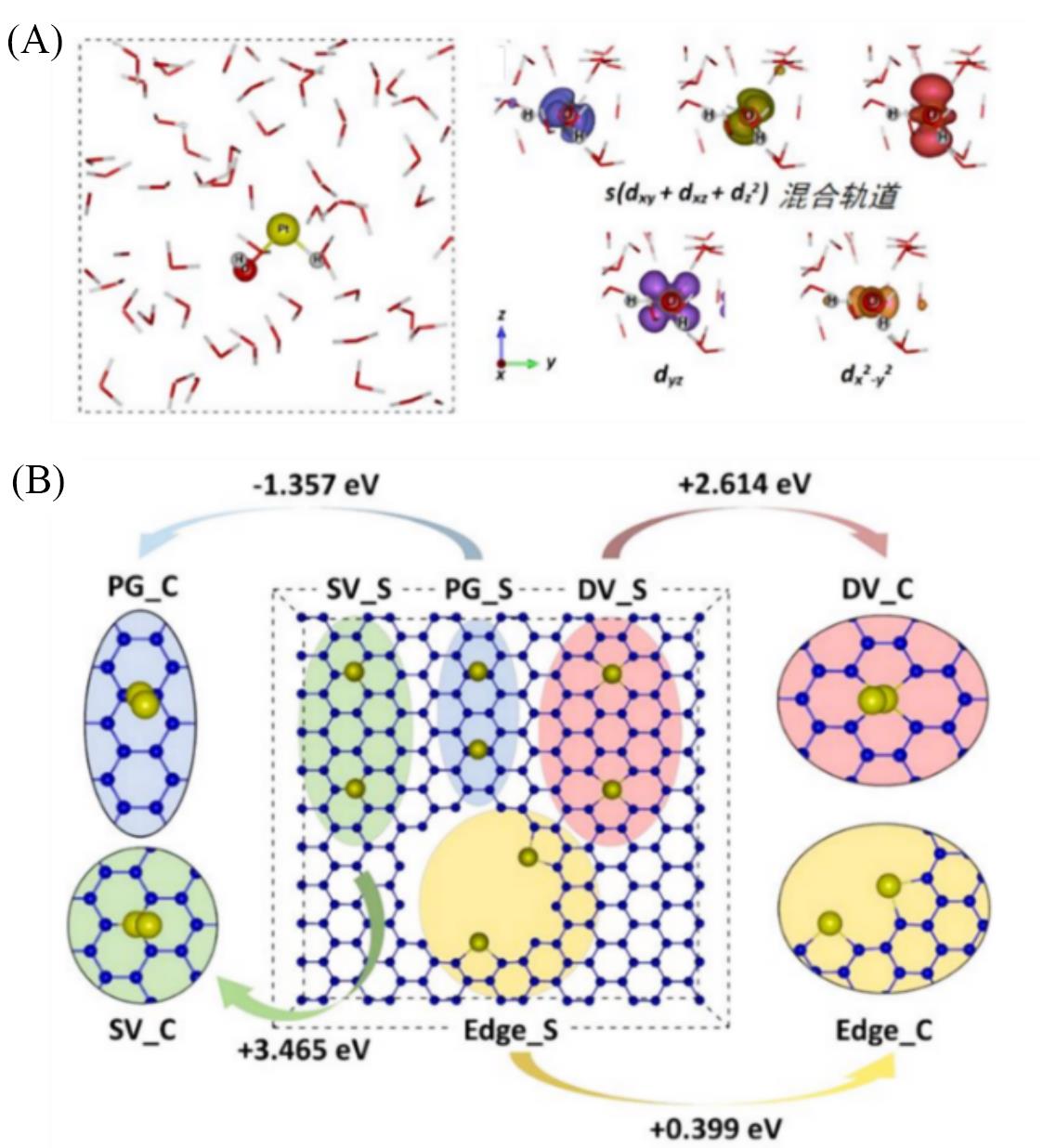
Fig.3 Illustrated structure and partial charge of the Pt single atom in the form of H—Pt—OH in water environment(A), structure and size distribution for Pt single atoms and Pt clusters on mesoporous carbon(B)[68]Copyright 2017, Springer Nature.
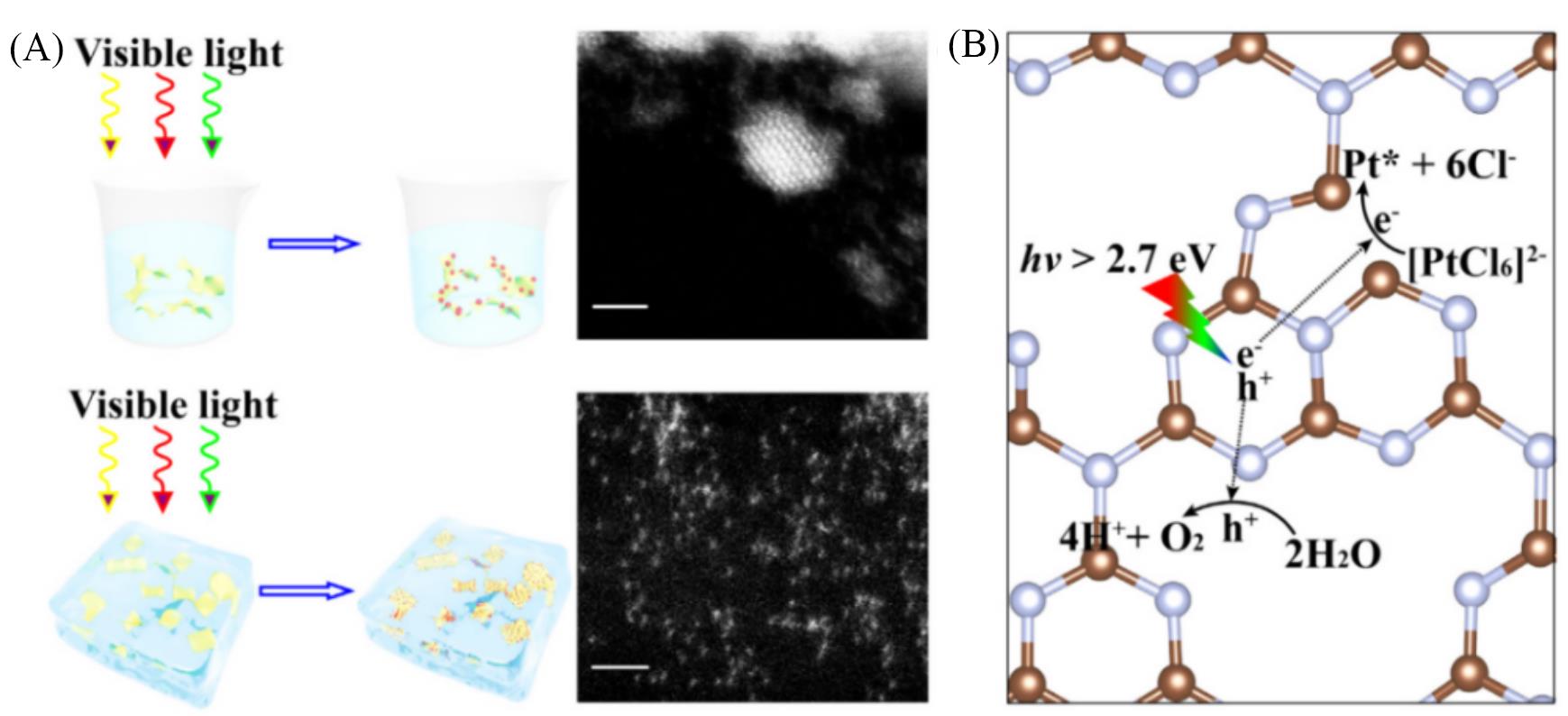
Fig.4 Schematic illustration of the icing?assisted in situ photocatalytic reduction method for preparing the supra?high?density Pt SAs?loaded g?C3N4[70](A) In a typical photocatalytic reduction of H2PtCl6 on g-C3N4 photocatalyst, the adsorbed [PtCl6]2- was reduced into metal Pt species by the photogenerated electrons from the light excitation of g-C3N4. The Pt NP was directly obtained by the diffusion and nucleation of metal Pt species on the surface of g-C3N4(scale bar: 2 nm). (B) Under the icing assistance, only the surface adsorbed [PtCl6]2- at the N vacancies could be in situ reduced into the Pt SAs without aggregation.Copyright 2019, Elsevier.
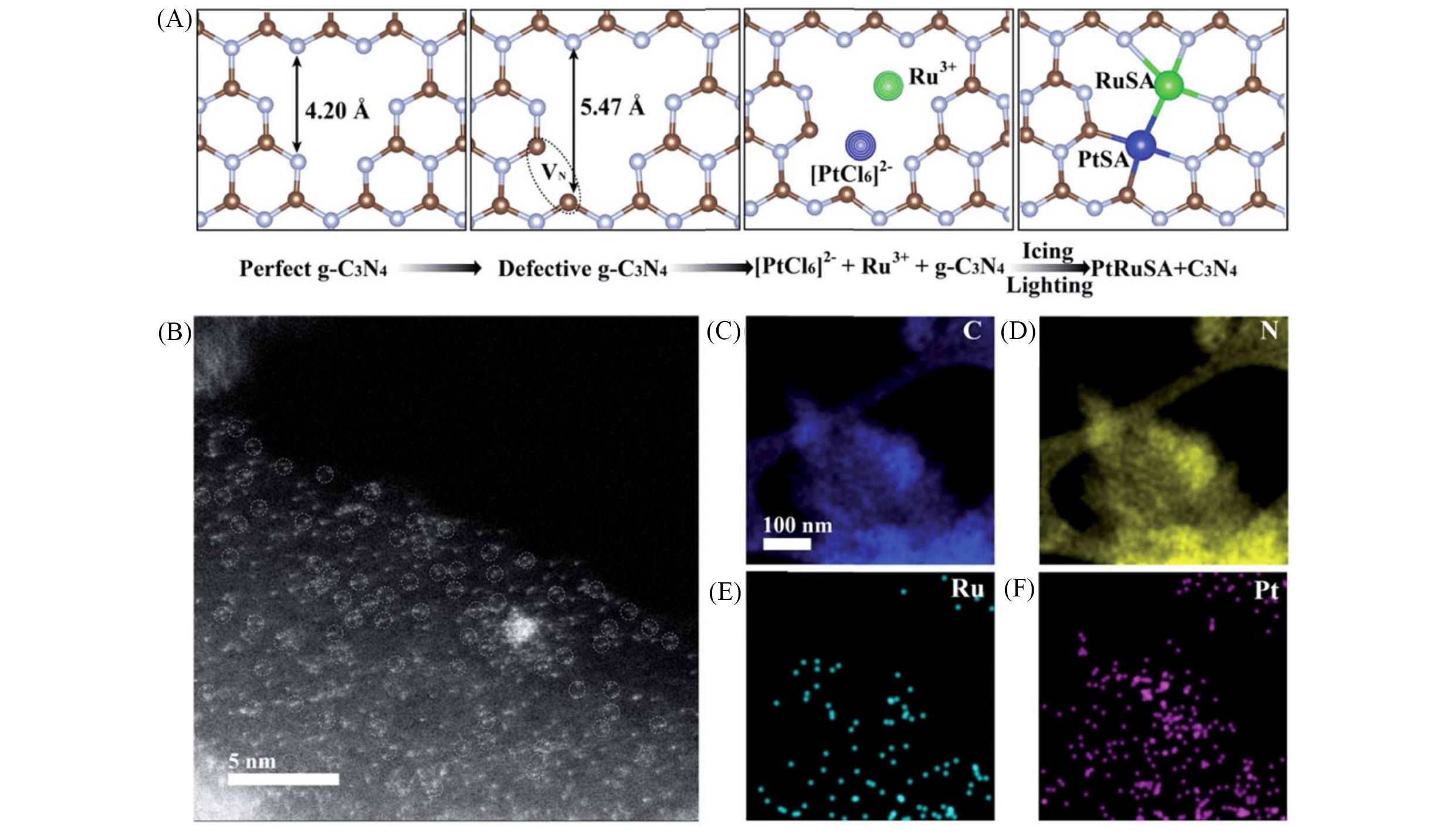
Fig.5 Schematic illustration of the icing?assisted in situ photocatalytic reduction method for preparing PtRu SA?CN620(A), HAADF?STEM images(B) and the corresponding distribution of C(C), N(D), Ru(E) and Pt(F) of PtRu SA?CN620[71]Copyright 2019, the Royal Society of Chemistry.
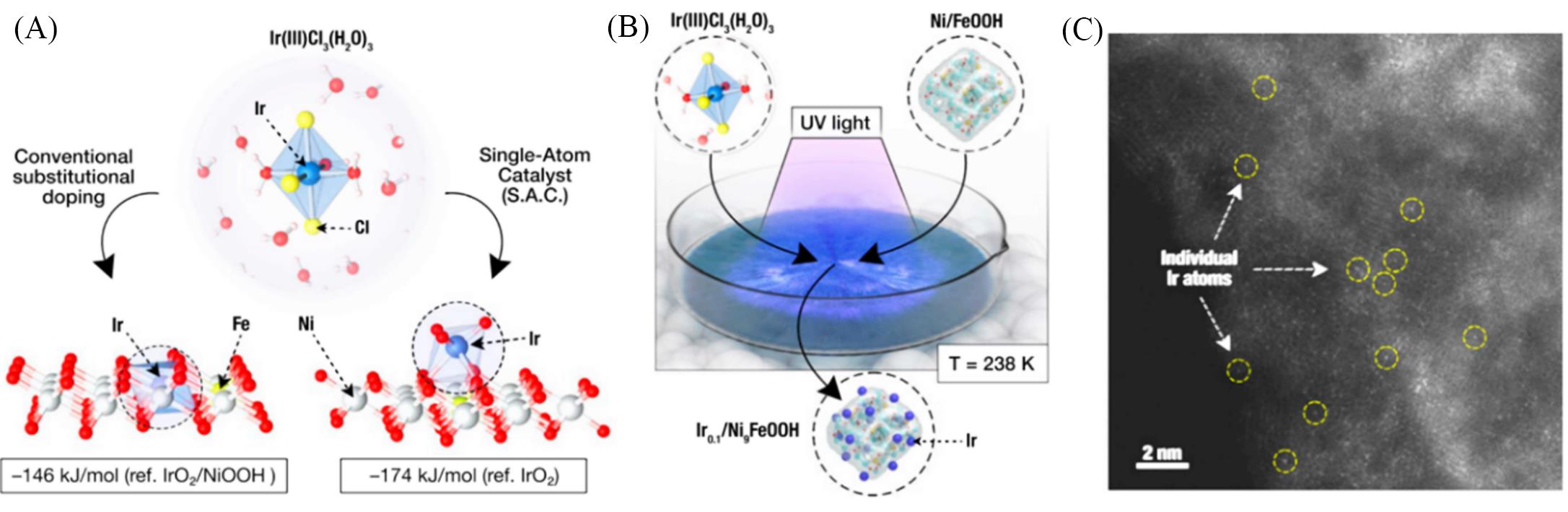
Fig.6 Preparation route to Ir single?atom on NiFe oxyhydroxides and atomic structure characterizations of NiFeIr by HAADF?STEM[72](A) DFT prediction of a preferred Ir atom embedding within the NiFeOOH layered structure under operating conditions; (B) in situ cryogenic-photochemical reduction synthesis of Ir0.1/Ni9Fe samples; (C) HAADF-STEM image of Ir single atoms on Ni9FeOOH supports(Ir0.1/Ni9Fe). Copyright 2021, Proceedings of the National Academy of Sciences.
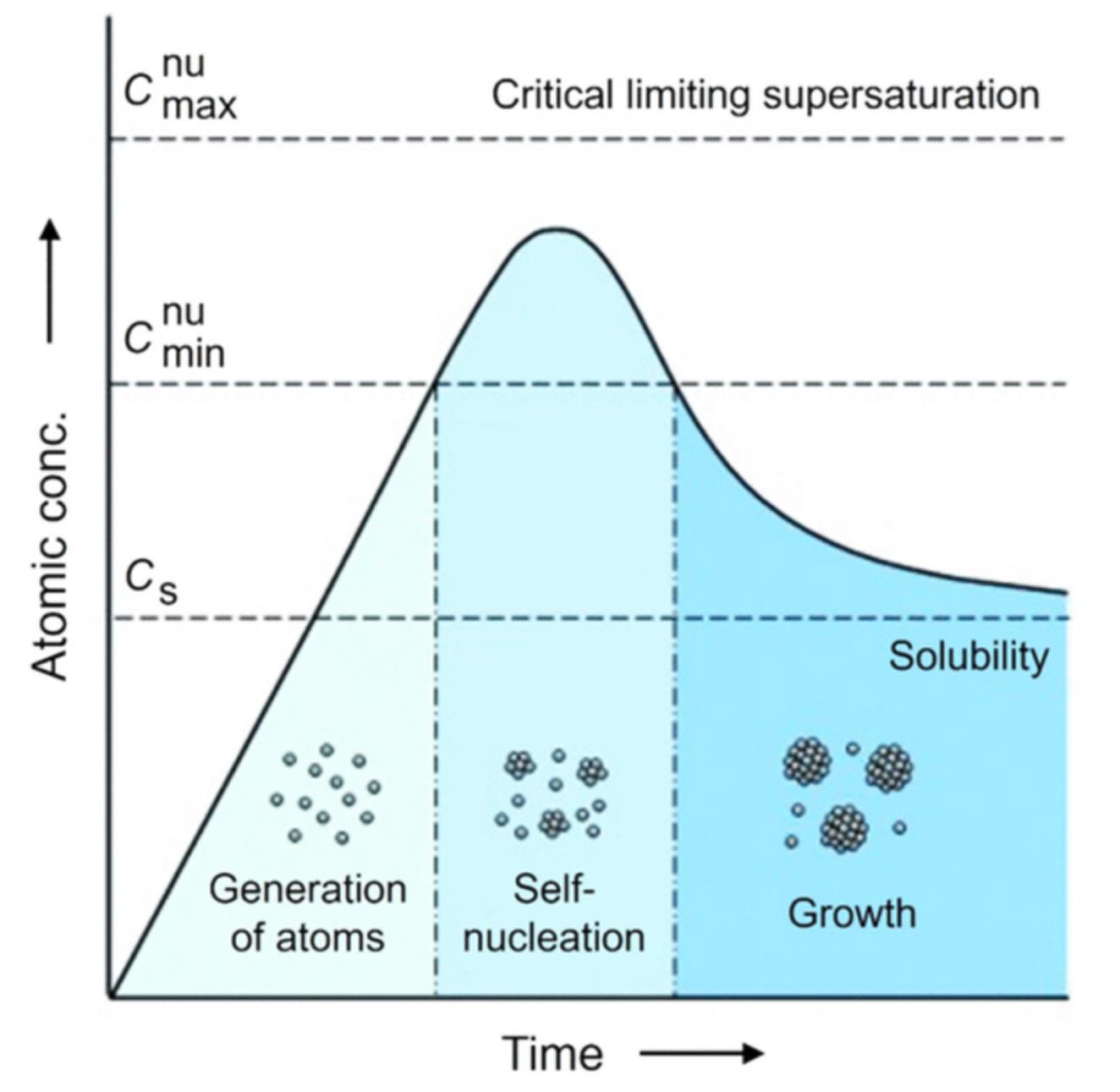
Fig.7 Plot of the concentration of atoms as a function of reaction time illustrating the major steps involved in a synthesis, including the generation of atoms, homogeneous nucleation, and growth[80]Copyright 2021, American Chemical Society.
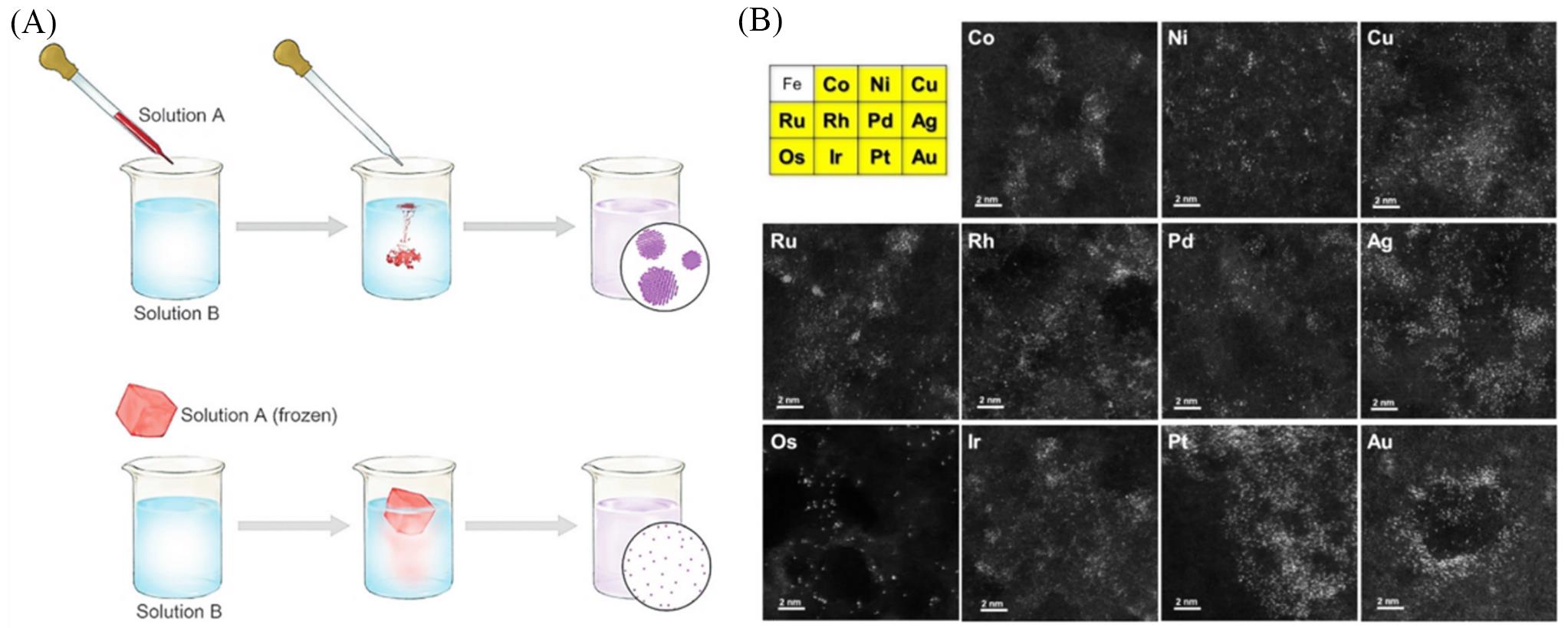
Fig.8 Illustration of the syntheses of atomically dispersed metals(A), periodic table of the elements and STEM images for multiple types of atomically dispersed metals, including Co, Ni, Cu, Ru, Rh, Pd, Ag, Os, Ir, Pt, and Au(B)[82]Copyright 2018, Wiley?VCH.
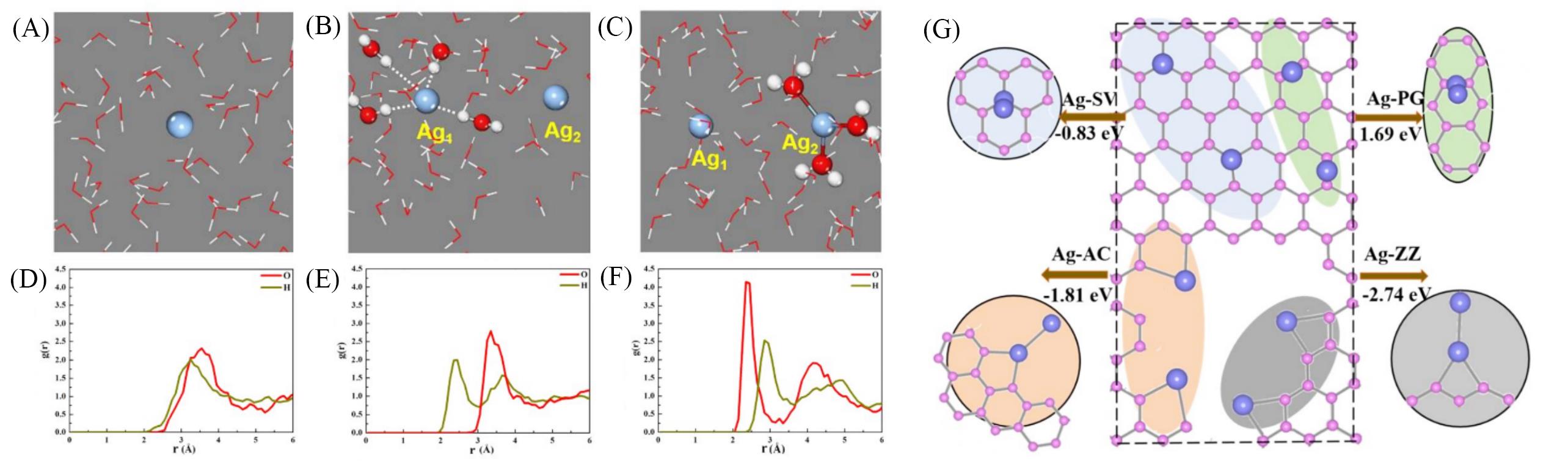
Fig.9 Selected atomic configurations of isolated Ag atoms in an aqueous solution based on first?principles molecular dynamics(FPMD)[82](A, D) Simulated results and corresponding radial distribution functions(RDF) results for a single Ag atom; (B, E) simulated results and corresponding RDF results of two isolated Ag atoms(Ag1 and Ag2) in a water environment at 300 K; (C, F) simulated results and corresponding RDF results of two isolated Ag atoms under the different perspectives of Ag1 and Ag2 in a water environment at the same moment; (G) structure distribution for single Ag atoms and a Ag dimer on carbon films. Copyright 2018, Wiley?VCH.
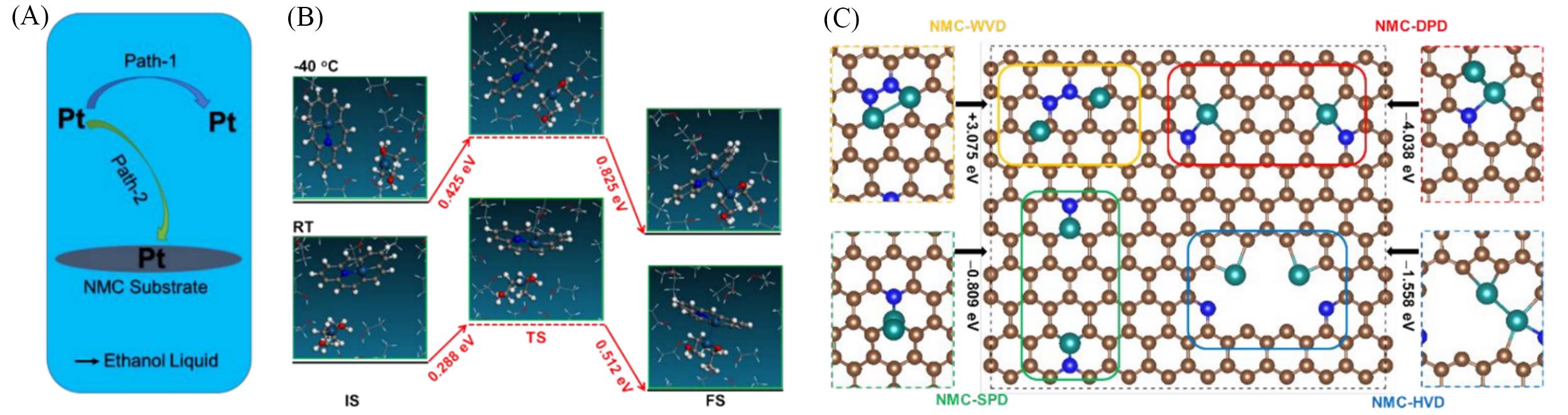
Fig.13 Schematic illustration of the two types of nucleation pathways of Pt atoms in the ethanol and NMC substrate reaction mixture(A), energy diagram of path?2 for Pt?Pt dimer formation at -40 ℃(up panel) and RT(bottom panel)(B), schematic diagrams of structures and distributions for atomically dispersed Pt atoms and Pt?Pt dimers on sites of NMC without vacancy defects(WVD), single point defects(SPD), double point defects(DPD) and hole vacancy defects(HVD)(C)[85](B) The initial state(IS), transition state(TS) and final state(FS) are shown from left to right.Copyright 2019, the Royal Society of Chemistry.
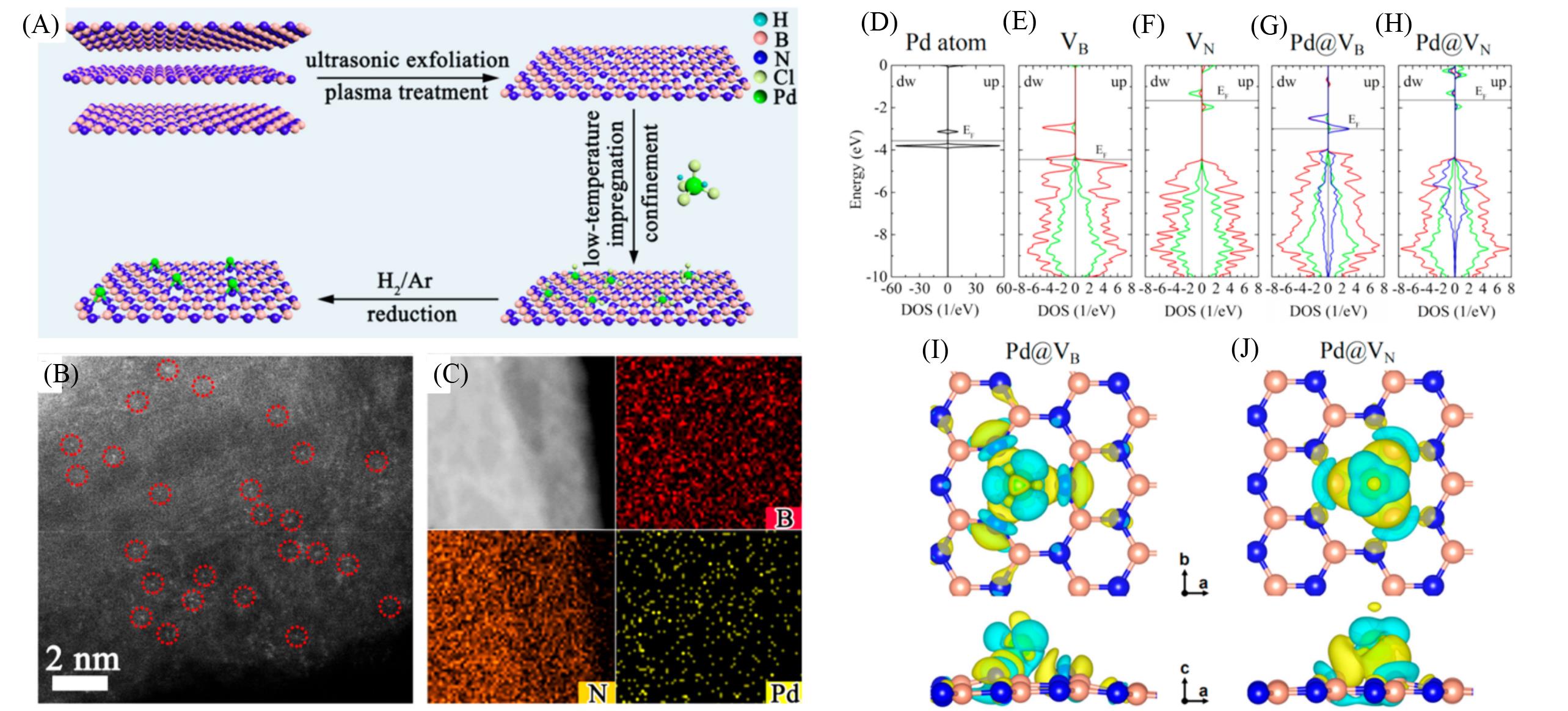
Fig.14 Synthetic route for the construction of Pd1/h?BN(A), AC HAADF?STEM image(B), energy?dispersive X?ray elemental mapping(C), spin up and spin down density of states for Pd atom(D), h?BN with VB(E), h?BN with VN(F), Pd adsorbed h?BN with VB(G), and Pd adsorbed h?BN with VN(H), top view and side view of difference charge density of Pd adsorbed h?BN with VB(I), and Pd adsorbed h?BN with VN(J)[86]Copyright 2021, American Chemical Society.
| 33 | Haruta M., Catal. Today, 1997, 36, 153—166 |
| 34 | Yoon B., Hakkinen H., Landman U., Wörz A. S., Antonietti J. M., Abbet S., Judai K., Heiz U., Science, 2005, 307, 403—407 |
| 35 | Tauster S. J., Fung S. C., Baker R. T. K., Horsley J. A., Science, 1981, 211, 1121—1125 |
| 36 | Campbell C. T., Nat. Chem., 2012, 4, 597—598 |
| 37 | Hu P., Huang Z., Amghouz Z., Makkee M., Xu F., Kapteijn F., Dikhtiarenko A., Chen Y., Gu X., Tang X., Angew. Chem. Int. Ed., 2014, 53, 3418 |
| 38 | Jones J., Xiong H., DeLaRiva A. T., Peterson E. J., Pham H., Challa S. R., Qi G., Oh S., Wiebenga M. H., Hernández X. I. P., Wang Y., Datye A. K., Science, 2016, 353, 150 |
| 39 | Su J., Ge R., Dong Y., Hao F., Chen L., J. Mater. Chem. A, 2018, 6, 14025 |
| 40 | Fei H., Dong J., Feng Y., Allen C. S., Wan C., Volosskiy B., Li M., Zhao Z., Wang Y., Sun H., An P., Chen W., Guo Z., Lee C., Chen D., Shakir I., Liu M., Hu T., Li Y., Kirkland A. I., Duan X., Huang Y., Nat. Catal., 2018, 1, 63—72 |
| 41 | Quan Z., Wang Y., Fang J., Acc. Chem. Res., 2013, 46, 191—202 |
| 42 | Feng L. L., Yu G., Wu Y., Li G. D., Li H., Sun Y., Asefa T., Chen W., Zou X., J. Am. Chem. Soc., 2015, 137, 14023—14026 |
| 43 | Ji S. F., Chen Y. J., Wang X. L., Zhang Z. D., Wang D. S., Li Y. D., Chem. Rev., 2020, 120, 11900—11955 |
| 44 | Chen Y., Ji S., Chen C., Peng Q., Wang D. S., Li Y. D., Joule, 2018, 2, 1242—1264 |
| 45 | Zhang W., Zheng W., Adv. Funct. Mater., 2016, 26(18), 2988—2993 |
| 46 | Xing L., Jin Y., Weng Y., Feng R., Ji Y., Gao H., Chen X., Zhang X., Jia D., Wang G., Matter, 2022, 5(3), 788—807 |
| 47 | Li Z. J., Wang D. H., Wu Y., Li Y. D., Natl. Sci. Rev., 2018, 5, 673—689 |
| 48 | Wang Y., Hu F. L., Mi Y., Yan C., Zhao S., Chem. Eng. J., 2021, 406(15), 127135 |
| 49 | Nikam A. V., Prasad B. L. V., Kulkarni A. A., CrystEngComm, 2018, 20, 5091—5107 |
| 50 | Guan J., Bai X., Tang T., Nano Res., 2022, 15, 818—837 |
| 51 | Liu W., Zhang H., Li C., Wang X., Liu J., Zhang X., J. Energy Chem., 2020, 47, 333—345 |
| 52 | Han Y., Wang Y. G., Chen W., Xu R., Zheng L., Zhang J., Luo J., Shen R. A., Zhu Y., Cheong W. C., Chen C., Peng Q., Wang D. S., Li Y. D., J. Am. Chem. Soc., 2017, 139, 17269—17272 |
| 53 | Chen P., Zhou T., Xing L., Xu K., Tong Y., Xie H., Zhang L., Yan W., Chu W., Wu C., Xie Y., Angew. Chem. Int. Ed., 2017, 56, 610—614 |
| 54 | Sa Y. J., Seo D. J., Woo J., Lim J. T., Cheon J. Y., Yang S. Y., Lee J. M., Kang D., Shin T. J., Shin H. S., Jeong H. Y., Kim C. S., Kim M. G., Kim T. Y., Joo S. H., J. Am. Chem. Soc., 2016, 138, 15046—15056 |
| 55 | Liu P., Zhao Y., Qin R., Mo S., Chen G., Gu L., Chevrier D. M., Zhang P., Guo Q., Zang D., Wu B., Fu G., Zheng N. F., Science, 2016, 352, 797—801 |
| 56 | Zhang J., Wu X., Cheong W. C., Chen W., Lin R., Li J., Zheng L., Yan W., Gu L., Chen C., Peng Q., Wang D. S., Li Y. D., Nat. Commun., 2018, 9, 1002 |
| 57 | Ida S., Kim N., Ertekin E., Takenaka S., Ishihara T., J. Am. Chem. Soc., 2015, 137, 239—244 |
| 58 | Zhang S., Nguyen L., Liang J. X., Shan J., Liu J. J., Frenkel A. I., Patlolla A., Huang W., Li J., Tao F. F., Nat. Commun., 2015, 6, 7938 |
| 59 | Liu G. L., Robertson A. W., Li M. M. J., Kuo W. C. H., Darby M. T., Muhieddine M. H., Lin Y. C., Suenaga K., Stamatakis M., Warner J. H., Tsang S. C. E., Nat. Chem., 2017, 9, 810—816 |
| 60 | Wan J. W., Chen W. X., Jia C. Y., Zheng L. R., Dong J. C., Zheng X. S., Wang Y., Yan W. S., Chen C., Peng Q., Wang D. S., Li Y. D., Adv. Mater., 2018, 30, 1705369 |
| 61 | Rivero⁃Crespo M. A., Mon M., Ferrando⁃Soria J., Lopes C. W., Boronat M., Leyva⁃Pérez A., Corma A., Hernández⁃Garrido J. C., López⁃Haro M., Calvino J. J., Ramos⁃Fernandez E. V., Armentano D., Pardo E., Angew. Chem. Int. Ed., 2018, 57, 17094—17099 |
| 62 | Shen K., Chen X. D., Chen J. Y., Li Y., ACS Catal., 2016, 6, 5887—5903 |
| 63 | Wang N., Sun Q.M., Bai R.S., Li X., Guo G.Q., Yu J. H., J. Am. Chem. Soc., 2016, 138, 7484—7487 |
| 64 | Jiao L., Wan G., Zhang R., Zhou H., Yu S. H., Jiang H. L., Angew. Chem. Int. Ed., 2018, 57, 8525—8529 |
| 65 | Kistler J. D., Chotigkrai N., Xu P., Enderle B., Praserthdam P., Chen C. Y., Browning N. D., Gates B. C., Angew. Chem. Int. Ed., 2014, 53, 8904—8907 |
| 66 | Kim Y. H., Heo J. S., Kim T. H., Park S., Yoon M. H., Kim J., Oh M. S., Yi G. R., Noh Y. Y., Park S. K., Nature, 2012, 489, 128—132 |
| 67 | Karkas M. D., Porco J. A. Jr., Stephenson C. R. J., Chem. Rev., 2016, 116, 9683—9747 |
| 68 | Wei H., Huang K., Wang D., Zhang R., Ge B., Ma J., Wen B., Zhang S., Li Q., Lei M., Zhang C., Irawan J., Liu L. M., Wu H., Nat. Commun., 2017, 8, 1490 |
| 69 | Guo Z., Wang T., Wei H., Long Y., Yang C., Wang D., Lang J., Huang K., Hussain N., Song C., Guan B., Ge B., Zhang Q., Wu H., Angew. Chem. Int. Ed., 2019, 58(36), 12569—12573 |
| 70 | Zhou P., Lv F., Li N., Zhang Y., Mu Z., Tang Y., Lai J., Chao Y., Luo M., Lin F., Zhou J., Su D., Guo S., Nano Energy, 2019, 56, 127—137 |
| 71 | Zhou P., Hou X., Chao Y., Yang W., Zhang W., Mu Z., Lai J., Lv F., Yang K., Liu Y., Li J., Ma J., Luo J., Guo S., Chem. Sci., 2019, 10, 5898—5905 |
| 72 | Zheng X., Tang J., Gallo A., Torres J. A. G., Yu X., Athanitis C. J., Been E M., Ercius P., Mao H., Fakra S. C., Song C., Davis R. C., Reimer J. A., Vinson J., Bajdich M., Cui Y., Proc. Natl. Acad. Sci. USA, 2021, 118(36), 2101817118 |
| 73 | Xia Y., Gilroy K. D., Peng H. C., Xia X., Angew. Chem. Int. Ed., 2017, 56, 60—95 |
| 74 | Maxwell J. C., Philosophical Magazine, 1908, 16, 818—824 |
| 75 | Thanh N. T., Maclean N., Mahiddine S., Chem. Rev., 2014, 114, 7610—7630 |
| 76 | Sleutel M., Lutsko J., Van Driessche A. E., Durán⁃Olivencia M. A., Maes D., Nat. Commun., 2014, 5, 5598 |
| 77 | Xia Y., Xiong Y., Lim B., Skrabalak S. E., Angew. Chem. Int. Ed., 2009, 48(1), 60—103 |
| 78 | Liu M., Wang K., Wang L., Han S., Fan H., Rowell N., Ripmeester J. A., Renoud R., Bian F., Zeng J., Yu K., Nat. Commun., 2017, 8,15467 |
| 79 | LaMer V. K., Dinegar R. H., J. Am. Chem. Soc., 1950, 72, 4847—4854 |
| 80 | Shi Y., Lyu Z., Zhao M., Chen R., Nguyen Q. N., Xia Y. N., Chem. Rev., 2021, 121(2), 649—735 |
| 81 | Loh N. D., Sen S., Bosman M., Tan S. F., Zhong J., Nijhuis C. A., Král P., Matsudaira P., Mirsaidov U., Nat. Chem., 2017, 9, 77—82 |
| 82 | Wei H., Huang K., Zhang L., Ge B., Wang D., Lang J., Ma J., Wang D., Zhang S., Li Q., Zhang R., Hussain N., Lei M., Liu L. M., Wu H., Angew. Chem. Int. Ed., 2018, 130(13), 3412—3417 |
| 83 | Wei H., Wu H., Huang K., Ge B., Ma J., Lang J., Zu D., Lei M., Yao Y., Guo W., Wu H., Chem. Sci., 2019, 10, 2830—2836 |
| 84 | Huang K., Zhang L., Xu T., Wei H., Zhang R., Zhang X., Ge B., Lei M., Ma J., Liu L. M., Wu H., Nat. Commun., 2019, 10, 606 |
| 85 | Huang K., Wang R., Wu H., Wang H., He X., Wei H., Wang S., Zhang R., Lei M., Guo W., Ge B., Wu H., J. Mater. Chem. A, 2019, 7, 25779—25784 |
| 1 | Seh Z. W., Kibsgaard J., Dickens C. F., Chorkendorff I., Norskov J. K., Jaramillo T. F., Science, 2017, 355, 146 |
| 2 | Benck J. D., Hellstern T. R., Kibsgaard J., Chakthranont P., Jaramillo T. F., ACS Catal., 2014, 4, 3957—3971 |
| 3 | Zhu C., Fu S., Shi Q., Du D., Lin Y., Angew. Chem. Int. Ed., 2017, 129(45), 14132—14148 |
| 4 | Wang J., Li Z., Wu Y., Li Y. D., Adv. Mater., 2018, 30(48), 1801649 |
| 5 | Mitchell S., Vorobyeva E., Pérez⁃Ramírez J., Angew. Chem. Int. Ed., 2018, 57, 15316—15329 |
| 6 | Qiao B., Wang A., Yang X., Allard L. F., Jiang Z., Cui Y., Liu J., Li J., Zhang T., Nat. Chem., 2011, 3, 634—641 |
| 7 | Yang X. F., Wang A., Qiao B., Li J., Liu J., Zhang T., Acc. Chem. Res., 2013, 46, 1740—1748 |
| 8 | Liu J., ACS Catal., 2017, 7, 34—59 |
| 9 | Yang M., Allard L. F., Flytzani⁃Stephanopoulos M., J. Am. Chem. Soc., 2013, 135, 3768—3771 |
| 10 | Flytzani⁃Stephanopoulos M., Acc. Chem. Res., 2014, 47, 783—792 |
| 11 | Jiang K., Back S., Akey A. J., Xia C., Hu Y., Liang W., Schaak D., Stavitski, E., Nørskov J. K., Siahrostami S., Wang H., Nat. Commun., 2019, 10, 3997 |
| 12 | Najam T., Shah S. S. A., Ding W., Jiang J., Jia L., Yao W., Li L., Wei Z. D., Angew. Chem. Int. Ed., 2018, 57, 15101—15106 |
| 13 | Yan H., Su C., He J., Chen W., J. Mater. Chem. A, 2018, 6, 8793—8814 |
| 14 | Liang Z., Qu C., Xia D., Zou R., Xu Q., Angew. Chem. Int. Ed., 2018, 57, 9604—9633 |
| 15 | Yang H. B., Hung S. F., Liu S., Yuan K., Miao S., Zhang L., Huang X., Wang H. Y., Cai W., Chen R., Gao J., Yang X., Chen W., Huang Y., Chen H. M., Li C. M., Zhang T., Liu B., Nat. Energy, 2018, 3, 140 |
| 16 | Cao Y., Chen S., Luo Q., Yan H., Lin Y., Liu W., Cao L., Lu J., Yang J., Yao T., Wei S., Angew. Chem. Int. Ed., 2017, 56, 12191 |
| 17 | Mistry H., Reske R., Zeng Z., Zhao Z. J., Greeley J., Strasser P., Cuenya B. R., J. Am. Chem. Soc., 2014, 136, 16473—16476 |
| 18 | Reske R., Mistry H., Behafarid F., Roldan Cuenya B., Strasser P., J. Am. Chem. Soc., 2014, 136, 6978—6986 |
| 19 | Xia B. Y., Yan Y., Li N., Wu H. B., Lou X. W., Wang X., Nat. Energy, 2016, 1, 15006 |
| 20 | Yang Y., Lun Z. Y., Xia G. L., Zheng F. C., He M. N., Chen Q. W., Energy Environ. Sci., 2015, 8, 3563—3571 |
| 21 | Yang F., Deng D. H., Pan X. L., Fu Q., Bao X. H., Natl. Sci. Rev., 2015, 2, 183—201 |
| 22 | van Hardeveld R., Hartog F., Surf. Sci., 1969, 15, 189—230 |
| 23 | Gates B. C., Chem. Rev., 1995, 95, 511—522 |
| 24 | Lopez N., Janssens T. V. W., Clausen B. S., Xu Y., Mavrikakis M., Bligaard T., Nørskov J. K., J. Catal., 2004, 223, 232—235 |
| 25 | Cuenya B. R., Thin Solid Films, 2010, 518, 3127—3150 |
| 26 | Kubo R., J. Phys. Soc. Jpn., 1962, 17, 975—986 |
| 86 | Li Z., Wei W., Li H., Li S., Leng L., Zhang M., Horton J. H., Wang D., Sun W., Guo C., Wu W., Wang J., ACS Nano, 2021, 15, 10175—10184 |
| 27 | Johansson M. P., Sundholm D., Vaara J., Angew. Chem. Int. Ed., 2004, 116, 2732—2735 |
| 28 | Von Issendorff B., Cheshnovsky O., Annu. Rev. Phys. Chem., 2005, 56, 549—580 |
| 29 | Roduner E., Chem. Soc. Rev., 2006, 35, 583—592 |
| 30 | Valden M., Lai X., Goodman D. W., Science, 1998, 281, 1647-1650 |
| 31 | Claus P., Brückner A., Mohr C., Hofmeister H. J., J. Am. Chem. Soc., 2000, 122, 11430—11439 |
| 32 | Li J., Li X., Zhai H. J., Wang L. S., Science, 2003, 299, 864—867 |
| [1] | 杨静怡, 李庆贺, 乔波涛. 铱单原子和纳米粒子在N2O分解反应中的协同催化[J]. 高等学校化学学报, 2022, 43(9): 20220388. |
| [2] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [3] | 任诗杰, 谯思聪, 刘崇静, 张文华, 宋礼. 铂单原子催化剂同步辐射X射线吸收谱的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220466. |
| [4] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [5] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [6] | 杨静怡, 施思齐, 彭怀涛, 杨其浩, 陈亮. Ga-C3N4单原子催化剂高效光驱动CO2环加成[J]. 高等学校化学学报, 2022, 43(9): 20220349. |
| [7] | 王新天, 李攀, 曹越, 洪文浩, 耿忠璇, 安志洋, 王昊宇, 王桦, 孙斌, 朱文磊, 周旸. 单原子材料在二氧化碳催化中的技术经济分析与产业化应用前景[J]. 高等学校化学学报, 2022, 43(9): 20220347. |
| [8] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [9] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [10] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [11] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [12] | 程前, 杨博龙, 吴文依, 向中华. S掺杂Fe-N-C高活性氧还原反应催化剂[J]. 高等学校化学学报, 2022, 43(9): 20220341. |
| [13] | 唐全骏, 刘颖馨, 孟蓉炜, 张若天, 凌国维, 张辰. 单原子催化在海洋能源领域的应用[J]. 高等学校化学学报, 2022, 43(9): 20220324. |
| [14] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [15] | 黄秋红, 李文军, 李鑫. 有机催化靛红衍生酮亚胺与噁唑酮的不对称Mannich型加成反应[J]. 高等学校化学学报, 2022, 43(8): 20220131. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||