

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (5): 20220035.doi: 10.7503/cjcu20220035
收稿日期:2022-01-15
出版日期:2022-05-10
发布日期:2022-03-11
通讯作者:
孙晓甫,韩布兴
E-mail:sunxiaofu@iccas.ac.cn;hanbx@iccas.ac.cn
基金资助:
JIN Xiangyuan, ZHANG Libing, SUN Xiaofu( ), HAN Buxing(
), HAN Buxing( )
)
Received:2022-01-15
Online:2022-05-10
Published:2022-03-11
Contact:
SUN Xiaofu,HAN Buxing
E-mail:sunxiaofu@iccas.ac.cn;hanbx@iccas.ac.cn
Supported by:摘要:
温和条件下以CO2为原料制备高附加值化学品, 是CO2资源化利用的重要方法, 在众多CO2转化方法中, 电催化CO2还原(e-CO2RR)具有绿色、 清洁及条件可控等优势, 可以促进碳中和, 实现可持续发展. 然而, 由于其缓慢的动力学和较低催化剂活性, CO2电催化还原仍然存在低选择性, 低电流密度的问题. 单原子催化剂具有最大的原子利用率和明确定义的催化活性位点, 同时因其良好的配位结构和独特的电子结构极大地促进了CO2电催化还原的动力学过程, 是CO2电还原领域极具发展潜力的催化材料. 本文讨论了过渡金属和主族金属基单原子催化剂用于电催化CO2还原的研究进展, 系统总结了杂原子配位, 双/单原子位点, 金属-载体相互作用, 空间限域和分子桥联等策略调控单原子的微环境进而优化催化的性能, 揭示了单原子催化剂在 e-CO2RR领域内的突出优势和广阔的应用前景. 最后, 分析了单原子催化剂在CO2电催化转化过程中面临的挑战, 并对其未来进行了展望.
中图分类号:
TrendMD:
金湘元, 张礼兵, 孙晓甫, 韩布兴. 单原子催化剂在电催化还原CO2领域的应用. 高等学校化学学报, 2022, 43(5): 20220035.
JIN Xiangyuan, ZHANG Libing, SUN Xiaofu, HAN Buxing. Electrocatalytic CO2 Reduction over Single-atom Catalysts. Chem. J. Chinese Universities, 2022, 43(5): 20220035.
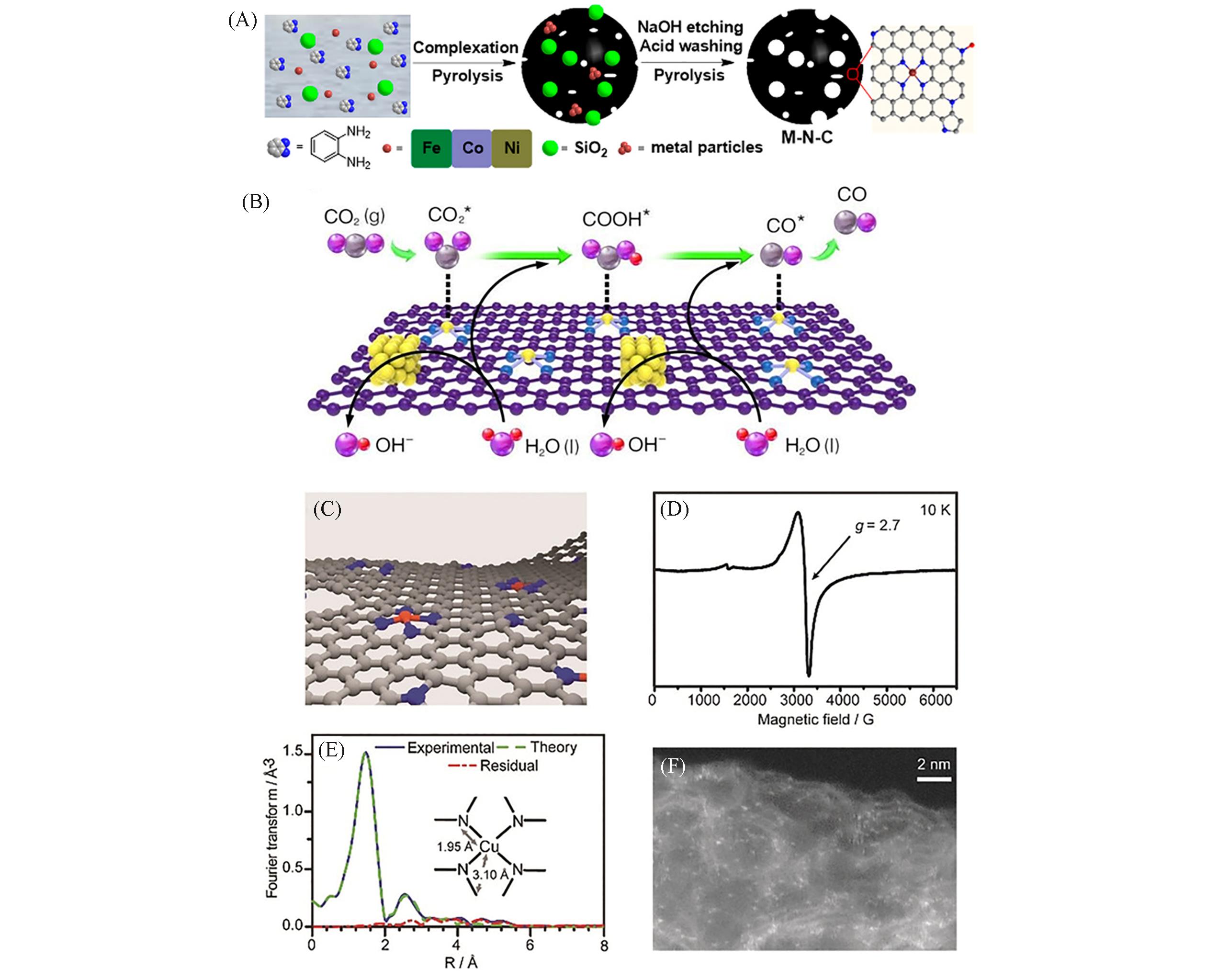
Fig.1 Protocol for the synthesis of M?N?C electrocatalysts(M=Fe, Co, Ni)(A)[32], a proposed reaction mechanism for CO production via ECR of Ni@NiN4CM(Ni yellow, N blue, O pink, C gray, H red)(B)[50], schematic representation(C), EPR spectrum record at 10K of Cu0.5NC diluted in an amorphous silica matrix(D), Cu K?edge EXAFS analysis in the Fourier?transformed space(E) and HAADF?STEM image of Cu0.5NC(F)[54](A) Copyright 2018, American Chemical Society; (B) Copyright 2021, John Wiley and Sons; (C—F) Copyright 2019, John Wiley and Sons.
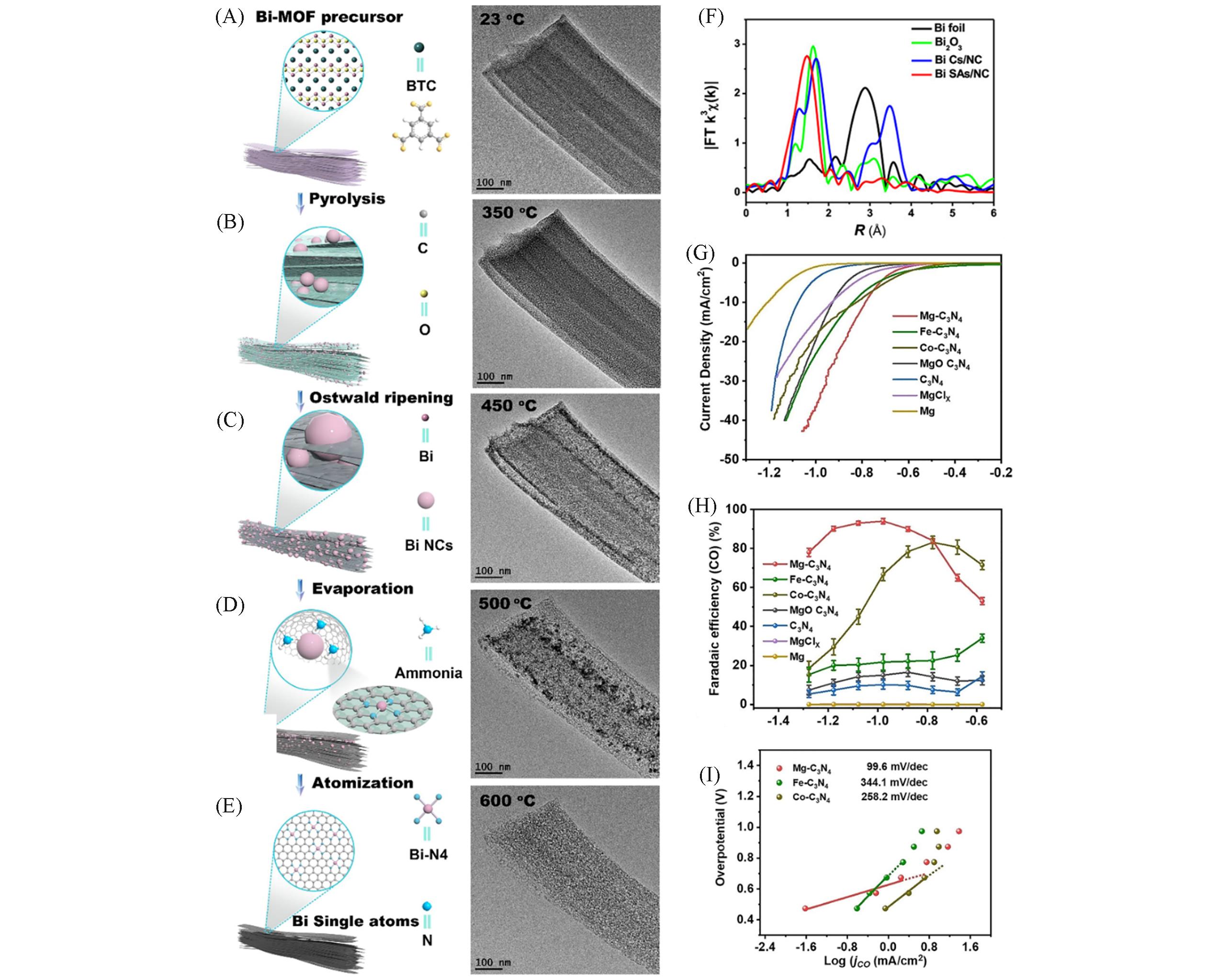
Fig.2 Scheme of the transformation from Bi?MOF to single Bi atoms and corresponding representative TEM images of Bi?MOF pyrolyzed at different temperatures with the assistance of DCD(dicyandiamide) in situ(A—E), k3?weighted χ(k) function of the EXAFS spectra of Bi foil, Bi2O3, BiCs/NC, BiSAs/NC(F)[76], electrochemical CO2RR performance of LSV(Linear sweep voltammetry) curves at scan rate of 10 mV/s in H?cell(G), FECO at different potentials in H?cell(H) and Tafel plots(I) of Mg?C3N4[83](A—F) Copyright 2019, the American Chemical Society; (G—I) Copyright 2021, John Wiley and Sons.
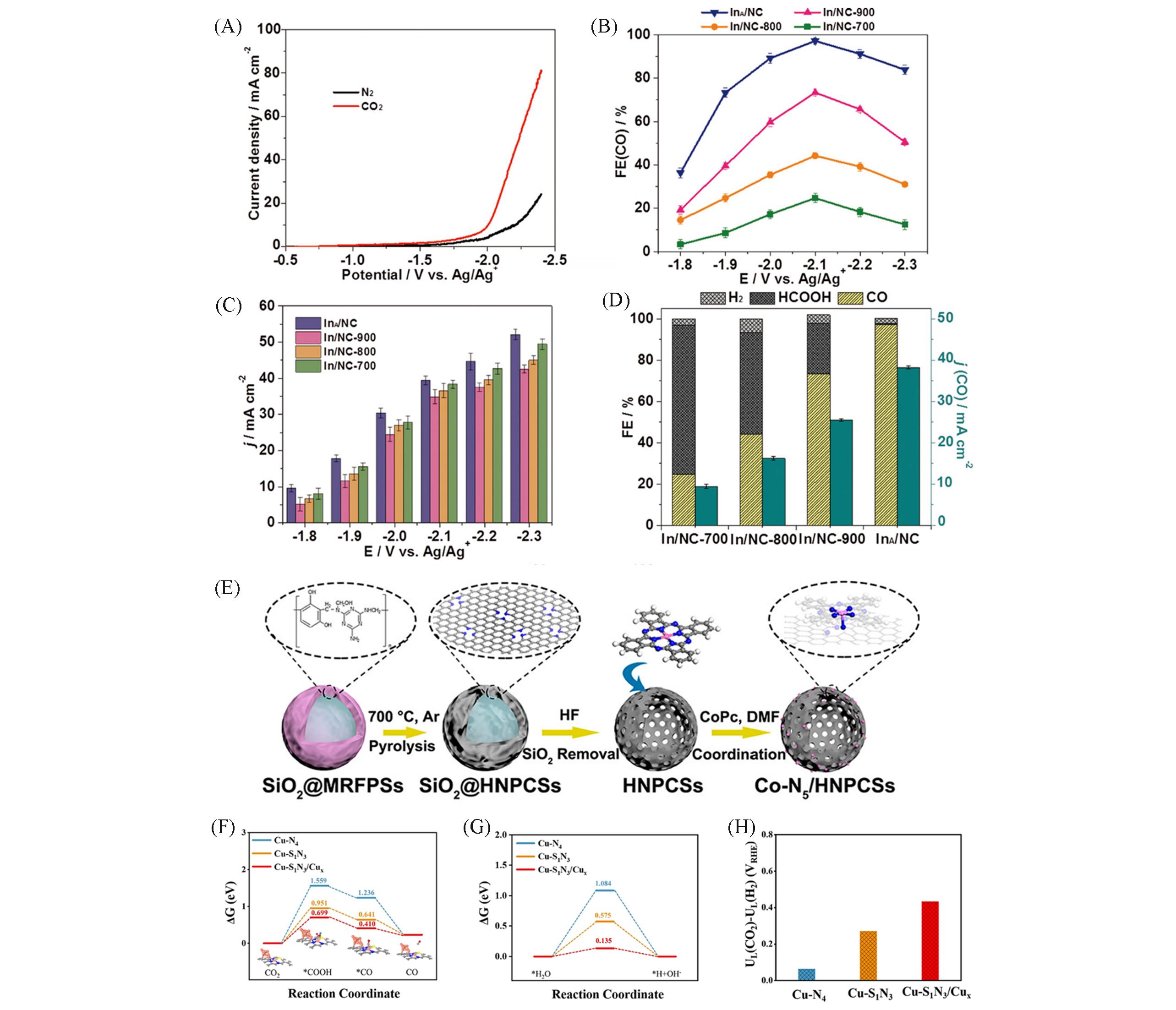
Fig.3 CO2 electroduction in 0.5 mol/L [Bmim]PF6/MeCN electrolyte(A—D)[8], schematic illustration of Co?N5/HNPCSs(E)[87], DFT investigations of Cu?S1N3/Cu x, Cu?S1N3 and Cu?N4(F—H)[51](A) LSV curves of the as-prepared InA/NC electrode in N2 and CO2 saturated electrolyte; (B) the FECO and (C) current density(j) over InA/NC, In/NC-900, In/NC-800, and In/NC-700 at different applied potentials with 2 h electrolysis; (D) the FECO, FEformate, and FEH2 and the partial current densities of CO over InA/NC, In/NC-900, In/NC-800, and In/NC-700 at -2.1 V(vs. Ag/Ag+). Copyright 2021, the American Chemical Society; (E) Copyright 2018, the American Chemical Society; (F) free-energy diagram for the conversion of CO2 into CO at U=0?V(vs. RHE) on the Cu-S1N3/Cu x, Cu-S1N3, and Cu-N4; (G) free energy diagram for dissociative water reaction on Cu-S1N3/Cu x, Cu-S1N3, and Cu-N4 moieties at U=0?V(vs. RHE); (H) the limiting potential difference for CO2 reduction and H2 evolution on different catalyst models at U=0?V(vs. RHE); Copyright 2021, John Wiley and Sons.
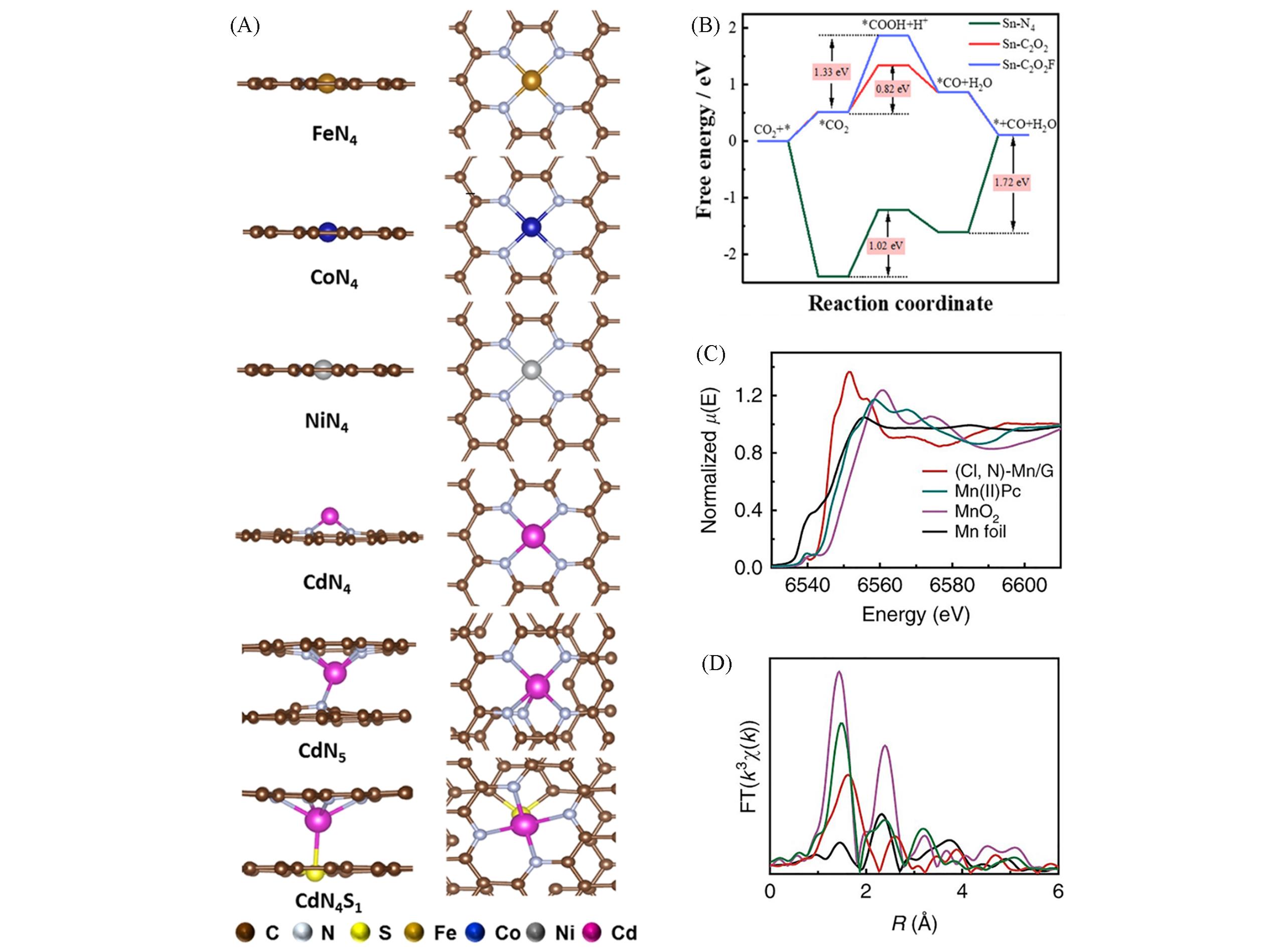
Fig.4 Side and top view of the FeN4, CoN4, NiN4, CdN4, CdN5, and CdN4S1 models(A)[99], Gibbs free energy diagrams for formate formation on Sn?N4, Sn?C2O2, and Sn?C2O2F(B)[100], XANES(C) and EXAFS(D) spectra at Mn K?edge[101](A) Copyright 2021, John Wiley and Sons; (B) Copyright 2021, the American Chemical Society; (C, D) Copyright 2019, Springer Nature.
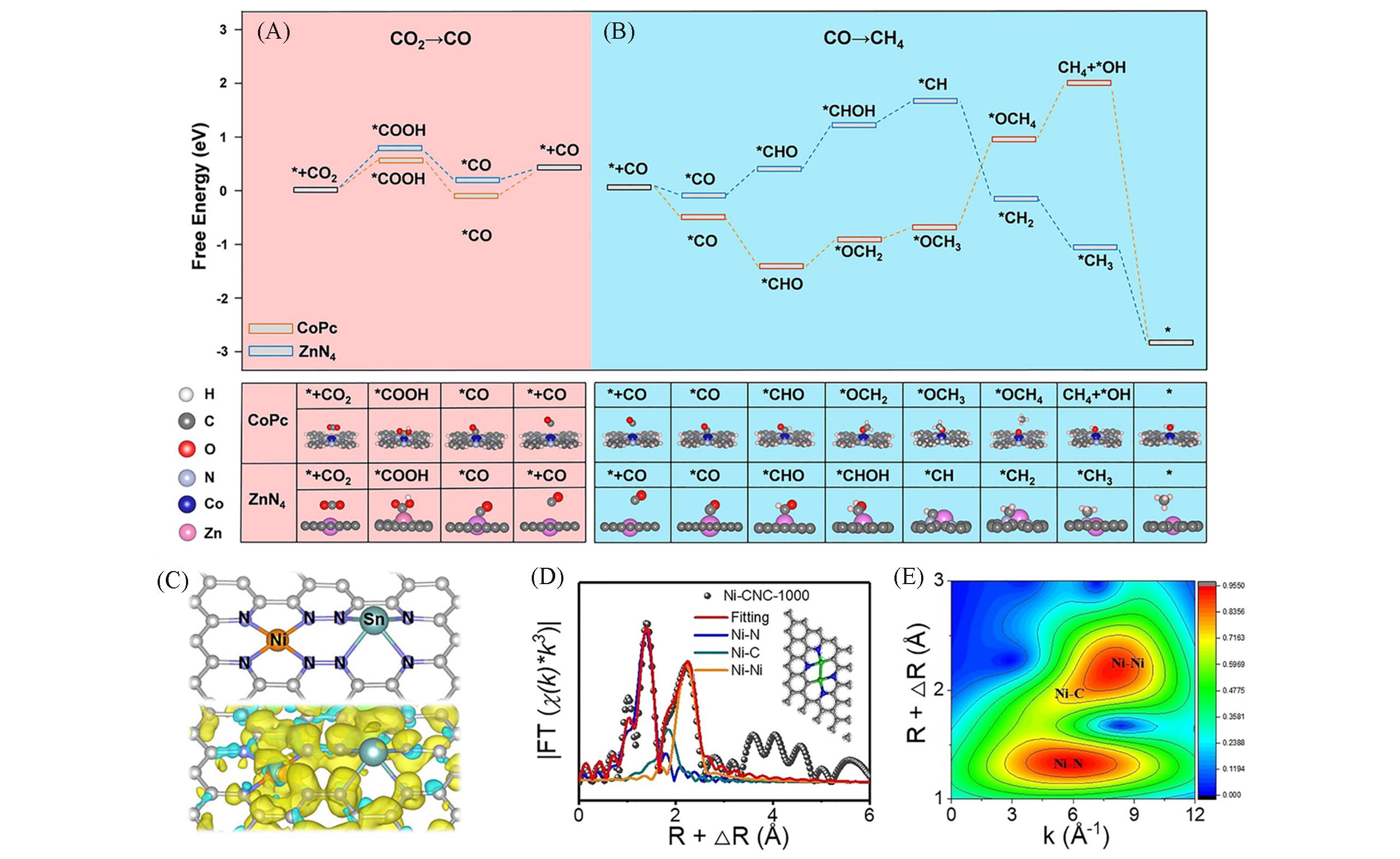
Fig.5 Free?energy profile and optimized configurations of intermediates in?CO2 electroreduction to CO(A), CO electroreduction to CH4 over CoPc and ZnN4(B)[107], a proposed model with spin electron density of NiSn?APC(yellow stands for spin up and green stands for spin down)(C)[112], corresponding EXAFS R space?fitting curves(D) and WT?EXAFS plot for Ni?CNC?1000(E)[116](A, B) Copyright 2021, John Wiley and Sons; (C) Copyright 2020, John Wiley and Sons; (D, E) Copyright 2021, John Wiley and Sons.
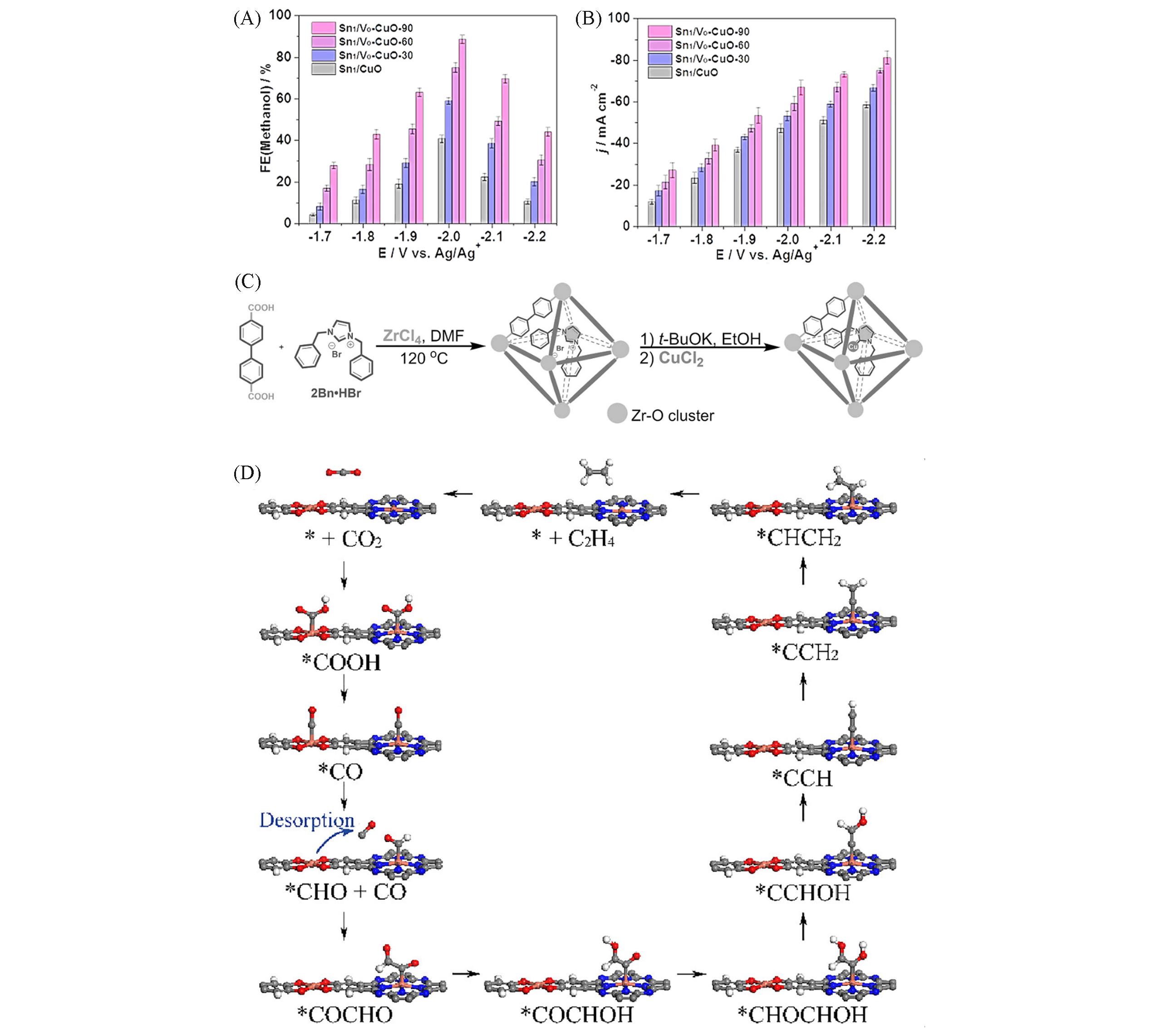
Fig.6 CO2 electroreduction in [Bmim]BF4/H2O electrolyte with a 1:3(molar ratio) on Sn1/VO?CuO?90, Sn1/VO?CuO?60, Sn1/VO?CuO?30 and Sn1/VO(A,B)[4], representation of the synthesis of 2Bn?Cu@UiO?67(C)[123], proposed CO2RR mechanism of PcCu?Cu?O(D)[126]FEmethanol(A) and total current density(j)(B) at different applied potentials with 2?h electrolysis. Copyright 2021, John Wiley and Sons; (C) Copyright 2021, John Wiley and Sons; (D) color codes: carbon(gray), nitrogen(blue), oxygen(red), hydrogen(white), copper(orange). Copyright 2021, the American Chemical Society.
| 1 | Li S., Zhao S., Lu X., Ceccato M., Hu X. M., Roldan A., Catalano J., Liu M., Skrydstrup T., Daasbjerg K., Angew. Chem. Int. Ed., 2021, 60(42), 22826—22832 |
| 2 | Peng Y., Chen J., Song A. Y., Catrysse P. B., Hsu P. C., Cai L., Liu B., Zhu Y., Zhou G., Wu D. S., Lee H. R., Fan S., Cui Y., Nature Sustain., 2018, 1(2), 105—112 |
| 3 | Chen J., Huang Y., Zhang N., Zou H., Liu R., Tao C., Fan X., Wang Z. L., Nat. Energy, 2016, 1(10), 16138 |
| 4 | Guo W., Liu S., Tan X., Wu R., Yan X., Chen C., Zhu Q., Zheng L., Ma J., Zhang J., Huang Y., Sun X., Han B., Angew. Chem. Int. Ed., 2021, 60(40), 21979—21987 |
| 5 | Wang Y., Wang Z., Dinh C. T., Li J., Ozden A., Golam Kibria M., Seifitokaldani A., Tan C. S., Gabardo C. M., Luo M., Zhou H., Li F., Lum Y., McCallum C., Xu Y., Liu M., Proppe A., Johnston A., Todorovic P., Zhuang T. T., Sinton D., Kelley S., O., Sargent E. H., Nat. Catal., 2020, 3(2), 98—106 |
| 6 | Cheng Q., Mao K., Ma L., Yang L., Zou L., Zou Z., Hu Z., Yang H., ACS Energy Lett., 2018, 3(5), 1205—1211 |
| 7 | Qiao B., Wang A., Yang X., Allard L. F., Jiang Z., Cui Y., Liu J., Li J., Zhang T., Nat. Chem., 2011, 3(8), 634—641 |
| 8 | Guo W., Tan X., Bi J., Xu L., Yang D., Chen C., Zhu Q., Ma J., Tayal A., Ma J., Huang Y., Sun X., Liu S., Han B., J. Am. Chem. Soc., 2021, 143(18), 6877—6885 |
| 9 | Lu X., Gao S., Lin H., Shi J., Small, 2021, 17(16), 2004467 |
| 10 | Hoang S., Guo Y., Binder A. J., Tang W., Wang S., Liu J., Tran H., Lu X., Wang Y., Ding Y., Kyriakidou E. A., Yang J., Toops T. J., Pauly T., R., Ramprasad R., Gao P. X., Nat. Commun., 2020, 11(1), 1062 |
| 11 | Franco F., Rettenmaier C., Jeon H. S., Roldan Cuenya B., Chem. Soc. Rev., 2020, 49(19), 6884—6946 |
| 12 | Shang H., Wang T., Pei J., Jiang Z., Zhou D., Wang Y., Li H., Dong J., Zhuang Z., Chen W., Wang D., Zhang J., Li Y., Angew. Chem. Int. Ed., 2020, 59(50), 22465—22469 |
| 13 | Yang H. B., Hung S. F., Liu S., Yuan K., Miao S., Zhang L., Huang X., Wang H. Y., Cai W., Chen R., Gao J., Yang X., Chen W., Huang Y., Chen H. M., Li C. M., Zhang T., Liu B., Nat. Energy, 2018, 3(2), 140—147 |
| 14 | Zhang W., Hu Y., Ma L., Zhu G., Wang Y., Xue X., Chen R., Yang S., Jin Z., Adv. Sci., 2018, 5(1), 1700275 |
| 15 | Birdja Y. Y., Pérez⁃Gallent E., Figueiredo M. C., Göttle A. J., Calle⁃Vallejo F., Koper M. T. M., Nat. Energy, 2019, 4(9), 732—745 |
| 16 | Xing Y., Kong X., Guo X., Liu Y., Li Q., Zhang Y., Sheng Y., Yang X., Geng Z., Zeng J., Adv. Sci., 2020, 7(22), 1902989 |
| 17 | Feng X., Zou H., Zheng R., Wei W., Wang R., Zou W., Lim G., Hong J., Duan L., Chen H., Nano Lett., 2022, 22(4), 1656— 1664 |
| 18 | Liang Z. Q., Zhuang T. T., Seifitokaldani A., Li J., Huang C. W., Tan C. S., Li Y., De Luna P., Dinh C. T., Hu Y., Xiao Q., Hsieh P. L., Wang Y., Li F., Quintero⁃Bermudez R., Zhou Y., Chen P., Pang Y., Lo S. C., Chen L. J., Tan H., Xu Z., Zhao S., Sinton D., Sargent E. H., Nat. Commun., 2018, 9(1), 3828 |
| 19 | Ning H., Fei X., Tan Z., Wang W., Yang Z., Wu M., ACS Appl. Nano Mater., 2022, 5(2), 2335—2342 |
| 20 | Ma L., Hu W., Mei B., Liu H., Yuan B., Zang J., Chen T., Zou L., Zou Z., Yang B., Yu Y., Ma J., Jiang Z., Wen K., Yang H., ACS Catal., 2020, 10(8), 4534—4542 |
| 21 | Luo H., Li B., Ma J., G., Cheng P., Angew. Chem. Int. Ed., 2022, e202116736 |
| 22 | Yan C., Li H., Ye Y., Wu H., Cai F., Si R., Xiao J., Miao S., Xie S., Yang F., Li Y., Wang G., Bao X., Energy Environ. Sci., 2018, 11(5), 1204—1210 |
| 23 | Huan T. N., Ranjbar N., Rousse G., Sougrati M., Zitolo A., Mougel V., Jaouen F., Fontecave M., ACS Catal., 2017, 7(3), 1520—1525 |
| 24 | Li Y., Zhou W., Wang H., Xie L., Liang Y., Wei F., Idrobo J., C., Pennycook S. J., Dai H., Nat. Nanotechnol., 2012, 7(6), 394—400 |
| 25 | Pan F., Deng W., Justiniano C., Li Y., Appl. Catal. B, 2018, 226, 463—472 |
| 26 | Gu J., Hsu C. S., Bai L., Chen H. M., Hu X., Science, 2019, 364(6445), 1091—1094 |
| 27 | Li X., Zeng Y., Tung C. W., Lu Y. R., Baskaran S., Hung S. F., Wang S., Xu C. Q., Wang J., Chan T. S., Chen H. M., Jiang J., Yu Q., Huang Y., Li J., Zhang T., Liu B., ACS Catal., 2021, 11(12), 7292—7301 |
| 28 | Ju W., Bagger A., Wang X., Tsai Y., Luo F., Möller T., Wang H., Rossmeisl J., Varela A. S., Strasser P., ACS Energy Lett., 2019, 4(7), 1663—1671 |
| 29 | Varela A. S., Kroschel M., Leonard N. D., Ju W., Steinberg J., Bagger A., Rossmeisl J., Strasser P., ACS Energy Lett., 2018, 3(4), 812—817 |
| 30 | Pan F., Zhang H., Liu K., Cullen D., More K., Wang M., Feng Z., Wang G., Wu G., Li Y., ACS Catal., 2018, 8(4), 3116—3122 |
| 31 | Qin X., Zhu S., Xiao F., Zhang L., Shao M., ACS Energy Lett., 2019, 4(7), 1778—1783 |
| 32 | Hu X. M., Hval H. H., Bjerglund E. T., Dalgaard K. J., Madsen M. R., Pohl M. M., Welter E., Lamagni P., Buhl K. B., Bremholm M., Beller M., Pedersen S. U., Skrydstrup T., Daasbjerg K., ACS Catal., 2018, 8(7), 6255—6264 |
| 33 | Zhu Y., Li X., Wang X., Lv K., Xiao G., Feng J., Jiang X., Fang M., Zhu Y., ChemistrySelect, 2020, 5(4), 1282—1287 |
| 34 | Liu C., Wu Y., Sun K., Fang J., Huang A., Pan Y., Cheong W. C., Zhuang Z., Zhuang Z., Yuan Q., Xin H. L., Zhang C., Zhang J., Xiao H., Chen C., Li Y., Chem, 2021, 7(5), 1297—1307 |
| 35 | Ni W., Liu Z., Zhang Y., Ma C., Deng H., Zhang S., Wang S., Adv. Mater., 2021, 33(1), e2003238 |
| 36 | Lin L., Li H., Wang Y., Li H., Wei P., Nan B., Si R., Wang G., Bao X., Angew. Chem. Int. Ed., 2021, 60(51), 26582—26586 |
| 37 | Li J., Pršlja P., Shinagawa T., Martín Fernández A. J., Krumeich F., Artyushkova K., Atanassov P., Zitolo A., Zhou Y., García⁃Muelas R., López N., Pérez⁃Ramírez J., Jaouen F., ACS Catal., 2019, 9(11), 10426—10439 |
| 38 | Wang C., Liu Y., Ren H., Guan Q., Chou S., Li W., ACS Catal., 2022, 12(4), 2513—2521 |
| 39 | Wang C., Ren H., Wang Z., Guan Q., Liu Y., Li W., Appl. Catal. B, 2022, 304, 120958 |
| 40 | Zhao C., Dai X., Yao T., Chen W., Wang X., Wang J., Yang J., Wei S., Wu Y., Li Y., J. Am. Chem. Soc., 2017, 139(24), 8078—8081 |
| 41 | Yang J., Qiu Z., Zhao C., Wei W., Chen W., Li Z., Qu Y., Dong J., Luo J., Li Z., Wu Y., Angew. Chem. Int. Ed., 2018, 57(43), 14095—14100 |
| 42 | Li X., Bi W., Chen M., Sun Y., Ju H., Yan W., Zhu J., Wu X., Chu W., Wu C., Xie Y., J. Am. Chem. Soc., 2017, 139(42), 14889—14892 |
| 43 | Zhao S., Chen G., Zhou G., Yin L. C., Veder J. P., Johannessen B., Saunders M., Yang S. Z., De Marco R., Liu C., Jiang S. P., Adv. Func. Mater., 2020, 30(6), 1906157 |
| 44 | Chen B., Li B., Tian Z., Liu W., Liu W., Sun W., Wang K., Chen L., Jiang J., Adv. Energy Mater., 2021, 11(40), 2102152 |
| 45 | Li Y., Zhang S. L., Cheng W., Chen Y., Luan D., Gao S., Lou X. W., Adv. Mater., 2021, e2105204 |
| 46 | Liu S., Yang H. B., Hung S. F., Ding J., Cai W., Liu L., Gao J., Li X., Ren X., Kuang Z., Huang Y., Zhang T., Liu B., Angew. Chem. Int. Ed., 2020, 59(2), 798—803 |
| 47 | Jiao L., Yang W., Wan G., Zhang R., Zheng X., Zhou H., Yu S. H., Jiang H. L., Angew. Chem. Int. Ed., 2020, 59(46), 20589—20595 |
| 48 | Yang H., Lin Q., Zhang C., Yu X., Cheng Z., Li G., Hu Q., Ren X., Zhang Q., Liu J., He C., Nat. Commun., 2020, 11(1), 593 |
| 49 | Guo Y., Yao S., Xue Y., Hu X., Cui H., Zhou Z., Appl. Catal. B, 2022, 304, 120997 |
| 50 | Wang X., Sang X., Dong C. L., Yao S., Shuai L., Lu J., Yang B., Li Z., Lei L., Qiu M., Dai L., Hou Y., Angew. Chem. Int. Ed., 2021, 60(21), 11959—11965 |
| 51 | Chen D., Zhang L. H., Du J., Wang H., Guo J., Zhan J., Li F., Yu F., Angew. Chem. Int. Ed., 2021, 60(45), 24022—24027 |
| 52 | Wang X., Feng S., Lu W., Zhao Y., Zheng S., Zheng W., Sang X., Zheng L., Xie Y., Li Z., Yang B., Lei L., Wang S., Hou Y., Adv. Func. Mater., 2021, 31(50), 2104243 |
| 53 | Ju W., Bagger A., Hao G. P., Varela A. S., Sinev I., Bon V., Roldan Cuenya B., Kaskel S., Rossmeisl J., Strasser P., Nat. Commun., 2017, 8(1), 944 |
| 54 | Karapinar D., Huan N. T., Ranjbar Sahraie N., Li J., Wakerley D., Touati N., Zanna S., Taverna D., Galvao Tizei L. H., Zitolo A., Jaouen F., Mougel V., Fontecave M., Angew. Chem. Int. Ed., 2019, 58(42), 15098—15103 |
| 55 | Xu H., Rebollar D., He H., Chong L., Liu Y., Liu C., Sun C. J., Li T., Muntean J. V., Winans R. E., Liu D. J., Xu T., Nat. Energy, 2020, 5(8), 623—632 |
| 56 | Zhao K., Nie X., Wang H., Chen S., Quan X., Yu H., Choi W., Zhang G., Kim B., Chen J. G., Nat. Commun., 2020, 11(1), 2455 |
| 57 | Bao H., Qiu Y., Peng X., Wang J. A., Mi Y., Zhao S., Liu X., Liu Y., Cao R., Zhuo L., Ren J., Sun J., Luo J., Sun X., Nat. Commun., 2021, 12(1), 238 |
| 58 | Han L., Song S., Liu M., Yao S., Liang Z., Cheng H., Ren Z., Liu W., Lin R., Qi G., Liu X., Wu Q., Luo J., Xin H. L., J. Am. Chem. Soc., 2020, 142(29), 12563—12567 |
| 59 | Li S., Zhao S., Lu X., Ceccato M., Hu X. M., Roldan A., Catalano J., Liu M., Skrydstrup T., Daasbjerg K., Angew. Chem. Int. Ed., 2021, 60(42), 22826—22832 |
| 60 | Chen J., Li Z., Wang X., Sang X., Zheng S., Liu S., Yang B., Zhang Q., Lei L., Dai L., Hou Y., Angew. Chem. Int. Ed., 2021, e202111683 |
| 61 | Ma M., Trzesniewski B. J., Xie J., Smith W. A., Angew. Chem. Int. Ed., 2016, 55(33), 9748—9752 |
| 62 | Ringe S., Morales⁃Guio C. G., Chen L. D., Fields M., Jaramillo T. F., Hahn C., Chan K., Nat. Commun., 2020, 11(1), 33 |
| 63 | Zhang N., Zhang X., Tao L., Jiang P., Ye C., Lin R., Huang Z., Li A., Pang D., Yan H., Wang Y., Xu P., An S., Zhang Q., Liu L., Du S., Han X., Wang D., Li Y., Angew. Chem. Int. Ed., 2021, 60(11), 6170—6176 |
| 64 | Bok J., Lee S. Y., Lee B. H., Kim C., Nguyen D. L. T., Kim J. W., Jung E., Lee C. W., Jung Y., Lee H. S., Kim J., Lee K., Ko W., Kim Y. S., Cho S. P., Yoo J. S., Hyeon T., Hwang Y. J., J. Am. Chem. Soc., 2021, 143(14), 5386—5395 |
| 65 | Huang P., Cheng M., Zhang H., Zuo M., Xiao C., Xie Y., Nano Energy, 2019, 61, 428—434 |
| 66 | Sun X., Lu L., Zhu Q., Wu C., Yang D., Chen C., Han B., Angew. Chem. Int. Ed., 2018, 57(9), 2427—2431 |
| 67 | Yang D., Zhu Q., Sun X., Chen C., Guo W., Yang G., Han B., Angew. Chem. Int. Ed., 2020, 59(6), 2354—2359 |
| 68 | Ma W., Xie S., Zhang X. G., Sun F., Kang J., Jiang Z., Zhang Q., Wu D. Y., Wang Y., Nat. Commun., 2019, 10(1), 892 |
| 69 | Ju L., Tan X., Mao X., Gu Y., Smith S., Du A., Chen Z., Chen C., Kou L., Nat. Commun., 2021, 12(1), 5128 |
| 70 | Li F., Chen L., Knowles G. P., MacFarlane D. R., Zhang J., Angew. Chem. Int. Ed., 2017, 56(2), 505—509 |
| 71 | Wen G., Ren B., Park M. G., Yang J., Dou H., Zhang Z., Deng Y. P., Bai Z., Yang L., Gostick J., Botton G. A., Hu Y., Chen Z., Angew. Chem. Int. Ed., 2020, 59(31), 12860—12867 |
| 72 | Tan X., Yu C., Ren Y., Cui S., Li W., Qiu J., Energy Environ. Sci., 2021, 14(2), 765—780 |
| 73 | Chang S., Xuan Y., Duan J., Zhang K., Appl. Catal. B, 2022, 306, 121135 |
| 74 | Wu Z. Z., Gao F. Y., Gao M. R., Energy Environ. Sci., 2021, 14(3), 1121—1139 |
| 75 | Rong X., Wang H. J., Lu X. L., Si R., Lu T. B., Angew. Chem. Int. Ed., 2020, 59(5), 1961—1965 |
| 76 | Zhang E., Wang T., Yu K., Liu J., Chen W., Li A., Rong H., Lin R., Ji S., Zheng X., Wang Y., Zheng L., Chen C., Wang D., Zhang J., Li Y., J. Am. Chem. Soc., 2019, 141(42), 16569—16573 |
| 77 | Wang Z., Wang C., Hu Y., Yang S., Yang J., Chen W., Zhou H., Zhou F., Wang L., Du J., Li Y., Wu Y., Nano Res., 2021, 14(8), 2790—2796 |
| 78 | Lu P., Tan X., Zhao H., Xiang Q., Liu K., Zhao X., Yin X., Li X., Hai X., Xi S., Wee A. T. S., Pennycook S. J., Yu X., Yuan M., Wu J., Zhang G., Smith S. C., Yin Z., ACS Nano, 2021, 15(3), 5671—5678 |
| 79 | Zu X., Li X., Liu W., Sun Y., Xu J., Yao T., Yan W., Gao S., Wang C., Wei S., Xie Y., Adv. Mater., 2019, 31(15), e1808135 |
| 80 | Jia M., Hong S., Wu T. S., Li X., Soo Y. L., Sun Z., Chem. Commun., 2019, 55(80), 12024—12027 |
| 81 | Jiang Z., Wang T., Pei J., Shang H., Zhou D., Li H., Dong J., Wang Y., Cao R., Zhuang Z., Chen W., Wang D., Zhang J., Li Y., Energy Environ. Sci., 2020, 13(9), 2856—2863 |
| 82 | Hulva J., Meier M., Bliem R., Jakub Z., Kraushofer F., Schmid M., Diebold U., Franchini C., Parkinson G. S., Science, 2021, 371(6527), 375—379 |
| 83 | Wang Q., Liu K., Fu J., Cai C., Li H., Long Y., Chen S., Liu B., Li H., Li W., Qiu X., Zhang N., Hu J., Pan H., Liu M., Angew. Chem. Int. Ed., 2021, 60(48), 25241—25245 |
| 84 | Li X., Liu L., Ren X., Gao J., Huang Y., Liu B., Sci. Adv., 2020, 6(39), eabb6833 |
| 85 | Chen Z., Zhang X., Liu W., Jiao M., Mou K., Zhang X., Liu L., Energy Environ. Sci., 2021, 14(4), 2349—2356 |
| 86 | Zhang H., Li J., Xi S., Du Y., Hai X., Wang J., Xu H., Wu G., Zhang J., Lu J., Wang J., Angew. Chem. Int. Ed., 2019, 58(42), 14871—14876 |
| 87 | Pan Y., Lin R., Chen Y., Liu S., Zhu W., Cao X., Chen W., Wu K., Cheong W. C., Wang Y., Zheng L., Luo J., Lin Y., Liu Y., Liu C., Li J., Lu Q., Chen X., Wang D., Peng Q., Chen C., Li Y., J. Am. Chem. Soc., 2018, 140(12), 4218—4221 |
| 88 | Geng Z., Cao Y., Chen W., Kong X., Liu Y., Yao T., Lin Y., Appl. Catal. B, 2019, 240, 234—240 |
| 89 | Jiang K., Siahrostami S., Zheng T., Hu Y., Hwang S., Stavitski E., Peng Y., Dynes J., Gangisetty M., Su D., Attenkofer K., Wang H., Energy Environ. Sci., 2018, 11(4), 893—903 |
| 90 | Wang X., Chen Z., Zhao X., Yao T., Chen W., You R., Zhao C., Wu G., Wang J., Huang W., Yang J., Hong X., Wei S., Wu Y., Li Y., Angew. Chem. Int. Ed., 2018, 57(7), 1944—1948 |
| 91 | Gong Y. N., Jiao L., Qian Y., Pan C. Y., Zheng L., Cai X., Liu B., Yu S. H., Jiang H. L., Angew. Chem. Int. Ed., 2020, 59(7), 2705—2709 |
| 92 | Jia C., Li S., Zhao Y., Hocking R. K., Ren W., Chen X., Su Z., Yang W., Wang Y., Zheng S., Pan F., Zhao C., Adv. Func. Mater., 2021, 31(51), 2107072 |
| 93 | Zhao X., Liu Y., J. Am. Chem. Soc., 2020, 142(12), 5773—5777 |
| 94 | Zheng W., Yang J., Chen H., Hou Y., Wang Q., Gu M., He F., Xia Y., Xia Z., Li Z., Yang B., Lei L., Yuan C., He Q., Qiu M., Feng X., Adv. Func. Mater., 2020, 30(4), 1907658 |
| 95 | Fan Q., Hou P., Choi C., Wu T. S., Hong S., Li F., Soo Y. L., Kang P., Jung Y., Sun Z., Adv. Energy Mater., 2020, 10(5), 1903068 |
| 96 | Zhang Y., Dong L. Z., Li S., Huang X., Chang J. N., Wang J. H., Zhou J., Li S. L., Lan Y. Q., Nat. Commun., 2021, 12(1), 6390 |
| 97 | Jia C., Tan X., Zhao Y., Ren W., Li Y., Su Z., Smith S. C., Zhao C., Angew. Chem. Int. Ed., 2021, 60(43), 23342—23348 |
| 98 | Guo J., Zhang W., Zhang L. H., Chen D., Zhan J., Wang X., Shiju N. R., Yu F., Adv. Sci., 2021, 8(23), e2102884 |
| 99 | Wu Y., Chen C., Yan X., Sun X., Zhu Q., Li P., Li Y., Liu S., Ma J., Huang Y., Han B., Angew. Chem. Int. Ed., 2021, 60(38), 20803—20810 |
| 100 | Ni W., Gao Y., Lin Y., Ma C., Guo X., Wang S., Zhang S., ACS Catal., 2021, 11(9), 5212—5221 |
| 101 | Zhang B., Zhang J., Shi J., Tan D., Liu L., Zhang F., Lu C., Su Z., Tan X., Cheng X., Han B., Zheng L., Zhang J., Nat. Commun., 2019, 10(1), 2980 |
| 102 | Chen Z., Huang A., Yu K., Cui T., Zhuang Z., Liu S., Li J., Tu R., Sun K., Tan X., Zhang J., Liu D., Zhang Y., Jiang P., Pan Y., Chen C., Peng Q., Li Y., Energy Environ. Sci., 2021, 14(6), 3430—3437 |
| 103 | Sun X., Tuo Y., Ye C., Chen C., Lu Q., Li G., Jiang P., Chen S., Zhu P., Ma M., Zhang J., Bitter J., H., Wang D., Li Y., Angew. Chem. Int. Ed., 2021, 60(44), 23614—23618 |
| 104 | Leow W. R., Lum Y., Ozden A., Wang Y., Nam D. H., Chen B., Wicks J., Zhuang T. T., Li F., Sinton D., Sargent E. H., Science, 2020, 368(6496), 1228—1233 |
| 105 | Lin L., Li H., Yan C., Li H., Si R., Li M., Xiao J., Wang G., Bao X., Adv. Mater., 2019, 31(41), 1903470 |
| 106 | Morales⁃Guio C. G., Cave E. R., Nitopi S. A., Feaster J. T., Wang L., Kuhl K. P., Jackson A., Johnson N. C., Abram D. N., Hatsukade T., Hahn C., Jaramillo T. F., Nature Catal., 2018, 1(10), 764—771 |
| 107 | Lin L., Liu T., Xiao J., Li H., Wei P., Gao D., Nan B., Si R., Wang G., Bao X., Angew. Chem. Int. Ed., 2020, 59(50), 22408—22413 |
| 108 | Luo G., Jing Y., Li Y., J. Mater. Chem. A, 2020, 8(31), 15809—15815 |
| 109 | Ren W., Tan X., Yang W., Jia C., Xu S., Wang K., Smith S. C., Zhao C., Angew. Chem. Int. Ed., 2019, 58(21), 6972—6976 |
| 110 | Jiao L., Zhu J., Zhang Y., Yang W., Zhou S., Li A., Xie C., Zheng X., Zhou W., Yu S. H., Jiang H. L., J. Am. Chem. Soc., 2021, 143(46), 19417—19424 |
| 111 | Zeng Z., Gan L. Y., Bin Yang H., Su X., Gao J., Liu W., Matsumoto H., Gong J., Zhang J., Cai W., Zhang Z., Yan Y., Liu B., Chen P., Nat. Commun., 2021, 12(1), 4088 |
| 112 | Xie W., Li H., Cui G., Li J., Song Y., Li S., Zhang X., Lee J., Y., Shao M., Wei M., Angew. Chem. Int. Ed., 2021, 60(13), 7382—7388 |
| 113 | Liang Z., Song L., Sun M., Huang B., Du Y., Sci. Adv., 2021, 7(47), eabl4915 |
| 114 | Jiao J., Lin R., Liu S., Cheong W. C., Zhang C., Chen Z., Pan Y., Tang J., Wu K., Hung S. F., Chen H. M., Zheng L., Lu Q., Yang X., Xu B., Xiao H., Li J., Wang D., Peng Q., Chen C., Li Y., Nat. Chem., 2019, 11(3), 222—228 |
| 115 | Ding T., Liu X., Tao Z., Liu T., Chen T., Zhang W., Shen X., Liu D., Wang S., Pang B., Wu D., Cao L., Wang L., Liu T., Li Y., Sheng H., Zhu M., Yao T., J. Am. Chem. Soc., 2021, 143(30), 11317—11324 |
| 116 | Cao X., Zhao L., Wulan B., Tan D., Chen Q., Ma J., Zhang J., Angew. Chem. Int. Ed., 2022, 61(6), e202113918 |
| 117 | Jia M., Fan Q., Liu S., Qiu J., Sun Z., Curr. Opin. Green Sustain. Chem., 2019, 16, 1—6 |
| 118 | Jiao Y., Zheng Y., Chen P., Jaroniec M., Qiao S. Z., J. Am. Chem. Soc., 2017, 139(49), 18093—18100 |
| 119 | Jiang Y., Choi C., Hong S., Chu S., Wu T. S., Soo Y. L., Hao L., Jung Y., Sun Z., Cell Rep. Phys. Sci., 2021, 2(3), 100356 |
| 120 | Sun X., Chen C., Liu S., Hong S., Zhu Q., Qian Q., Han B., Zhang J., Zheng L., Angew. Chem. Int. Ed., 2019, 58(14), 4669—4673 |
| 121 | Zhang M., Zhang Z., Zhao Z., Huang H., Anjum D. H., Wang D., He J. H., Huang K. W., ACS Catal., 2021, 11(17), 11103—11108 |
| 122 | Zheng T., Liu C., Guo C., Zhang M., Li X., Jiang Q., Xue W., Li H., Li A., Pao C. W., Xiao J., Xia C., Zeng J., Nat. Nanotechnol., 2021, 16(12), 1386—1393 |
| 123 | Chen S., Li W. H., Jiang W., Yang J., Zhu J., Wang L., Ou H., Zhuang Z., Chen M., Sun X., Wang D., Li Y., Angew. Chem. Int. Ed., 2022, 61(4), e202114450 |
| 124 | Dubed Bandomo G. C., Mondal S. S., Franco F., Bucci A., Martin⁃Diaconescu V., Ortuño M. A., van Langevelde P. H., Shafir A., López N., Lloret⁃Fillol J., ACS Catal., 2021, 11(12), 7210—7222 |
| 125 | Lu M., Zhang M., Liu C. G., Liu J., Shang L. J., Wang M., Chang J. N., Li S. L., Lan Y. Q., Angew. Chem. Int. Ed., 2021, 60(9), 4864—4871 |
| 126 | Qiu X. F., Zhu H. L., Huang J. R., Liao P. Q., Chen X. M., J. Am. Chem. Soc., 2021, 143(19), 7242—7246 |
| 127 | Yi J. D., Si D. H., Xie R., Yin Q., Zhang M. D., Wu Q., Chai G. L., Huang Y. B., Cao R., Angew. Chem. Int. Ed., 2021, 60(31), 17108—17114 |
| 128 | Wang Y. R., Ding H. M., Ma X. Y., Liu M., Yang Y. L., Chen Y., Li S. L., Lan Y. Q., Angew. Chem. Int. Ed., 2022, 61(5), e202114648 |
| 129 | Yang D., Zuo S., Yang H., Zhou Y., Lu Q., Wang X., Adv. Mater., 2022, 34(7), e2107293 |
| [1] | 杨静怡, 李庆贺, 乔波涛. 铱单原子和纳米粒子在N2O分解反应中的协同催化[J]. 高等学校化学学报, 2022, 43(9): 20220388. |
| [2] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [3] | 任诗杰, 谯思聪, 刘崇静, 张文华, 宋礼. 铂单原子催化剂同步辐射X射线吸收谱的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220466. |
| [4] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [5] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [6] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [7] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [8] | 江博文, 陈敬轩, 成永华, 桑微, 寇宗魁. 单原子材料在电化学生物传感中的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220334. |
| [9] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [10] | 吴玉, 李轩, 杨恒攀, 何传新. 钴单原子的双重限域制备策略及高效CO2电还原性能[J]. 高等学校化学学报, 2022, 43(9): 20220343. |
| [11] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [12] | 杨静怡, 施思齐, 彭怀涛, 杨其浩, 陈亮. Ga-C3N4单原子催化剂高效光驱动CO2环加成[J]. 高等学校化学学报, 2022, 43(9): 20220349. |
| [13] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| [14] | 王新天, 李攀, 曹越, 洪文浩, 耿忠璇, 安志洋, 王昊宇, 王桦, 孙斌, 朱文磊, 周旸. 单原子材料在二氧化碳催化中的技术经济分析与产业化应用前景[J]. 高等学校化学学报, 2022, 43(9): 20220347. |
| [15] | 唐全骏, 刘颖馨, 孟蓉炜, 张若天, 凌国维, 张辰. 单原子催化在海洋能源领域的应用[J]. 高等学校化学学报, 2022, 43(9): 20220324. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||