

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (11): 2393.doi: 10.7503/cjcu20200427
• 庆祝《高等学校化学学报》复刊40周年专栏 • 上一篇 下一篇
收稿日期:2020-07-06
出版日期:2020-11-10
发布日期:2020-11-06
通讯作者:
刘会贞
E-mail:liuhz@iccas.ac.cn;hanbx@iccas.ac.cn
作者简介:韩布兴, 男, 博士, 研究员, 中国科学院院士, 主要从事生物质及Cl资源转化利用方面的研究. E-mail: 基金资助:
WANG Yanyan1,2, LIU Huizhen1,2( ), HAN Buxing1,2(
), HAN Buxing1,2( )
)
Received:2020-07-06
Online:2020-11-10
Published:2020-11-06
Contact:
LIU Huizhen
E-mail:liuhz@iccas.ac.cn;hanbx@iccas.ac.cn
Supported by:摘要:
近年来, 随着空气中二氧化碳含量的不断升高, 二氧化碳的催化转化在科研界和工业界受到了广泛关注. 非均相催化的二氧化碳加氢合成甲醇是实现二氧化碳资源化利用的重要手段之一, 具有良好的应用前景. 本文系统概述了非均相催化二氧化碳加氢合成甲醇反应的近期研究进展, 重点介绍了金属催化剂和金属氧化物催化剂, 对反应机理进行了阐述, 并对该领域仍待解决的问题和发展前景进行了展望.
中图分类号:
TrendMD:
王艳燕, 刘会贞, 韩布兴. 多相催化剂催化二氧化碳加氢合成甲醇的研究进展. 高等学校化学学报, 2020, 41(11): 2393.
WANG Yanyan, LIU Huizhen, HAN Buxing. Advances in CO2 Hydrogenation to Methanol by Heterogeneous Catalysis. Chem. J. Chinese Universities, 2020, 41(11): 2393.
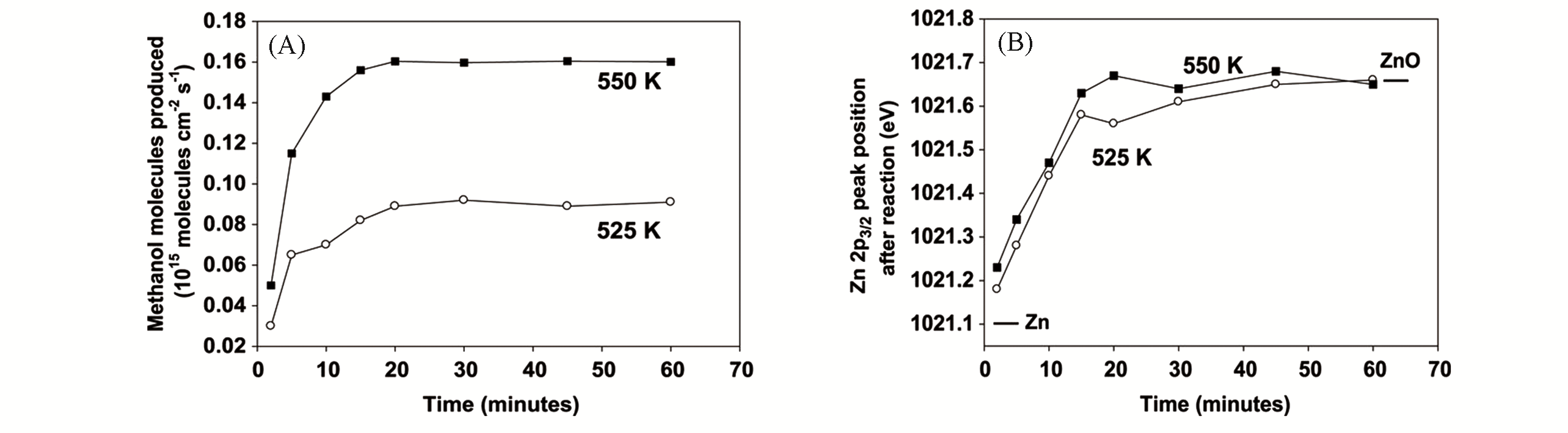
Fig.1 Rate for the conversion of CO2 to methanol on ZnCu(111) as a function of reaction time(A) and Zn2p3/2 XPS binding energies measured after performing the hydrogenation of CO2 on the Zn/Cu(111) catalyst(B)[11](A) The copper substrate was precovered with 0.2 mL of metallic Zn. PH2: 4.5×105 Pa. PCO2: 5×104 Pa. Copyright 2017, Science.
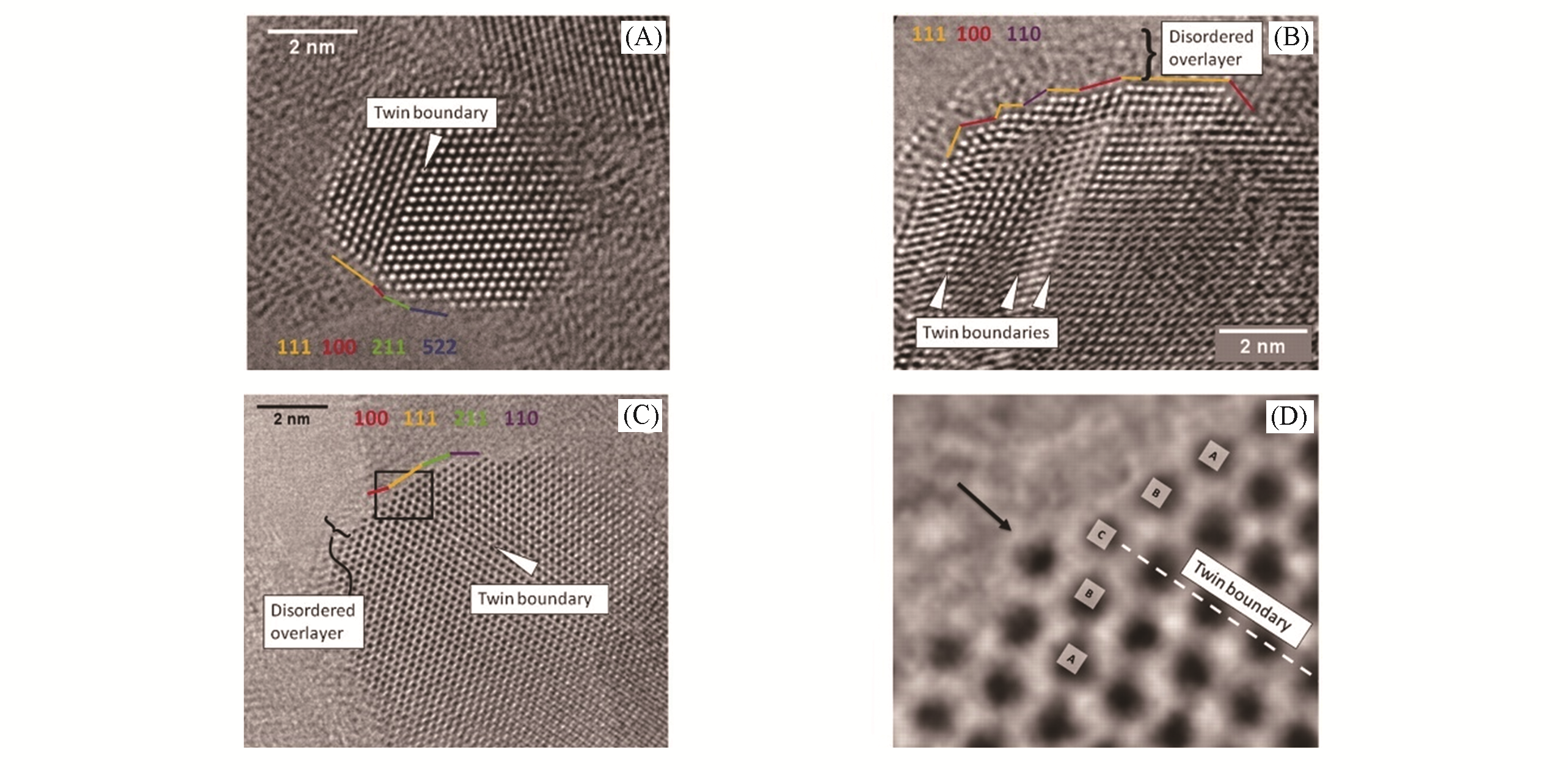
Fig.2 Aberration?corrected HRTEM images of Cu particles in the conventionally prepared, most?active Cu/ZnO/Al2O3 catalyst[12](D) is a close?up of the marked area in (C). Copyright 2012, Science.
| Catalyst | p(H2)/p(CO2) | p/MPa | GHSVa/h-1 | (W/F)b/ (gcat·h·mol-1) | T/K | CO2 conv.(%) | SCH3OH (%) | YCH3OH/ (gCH3OH·g | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Cu/ZnO/ZrO2(coprecipitation | 3 | 3 | 10000 | — | 513 | 16.0 | 48.7 | ca. 0.288 | [ |
| method) | |||||||||
| Cu/ZnO/ZrO2(complexation | 3 | 3 | 10000 | — | 513 | 12.5 | 51.8 | ca. 0.240 | [ |
| method) | |||||||||
| Cu/ZnO/ZrO2(gel oxalate | 3 | 3 | 10000 | — | 513 | 18.0 | 51.2 | ca. 0.340 | [ |
| coprecipitation method) | |||||||||
| Cu?ZnO?ZrO2(surfactant | 3 | 3 | 3600 | — | 513 | 12.1 | 54.1 | 6.5% | [ |
| assistedcoprecipitation) | |||||||||
| CuO?ZnO?ZrO2 | 3 | 3 | — | — | 513 | 18.2 | 41.6 | 0.061 | [ |
| CuO?ZnO?ZrO2?Cr2O3 | 3 | 3 | — | 9.33 | 513 | 18.1 | 40.0 | 0.058 | [ |
| CuO?ZnO?ZrO2?MoO3 | 3 | 3 | — | 9.33 | 513 | 19.0 | 46.7 | 0.071 | [ |
| CuO?ZnO?ZrO2?WO3 | 3 | 3 | — | 9.33 | 513 | 19.4 | 47.8 | 0.074 | [ |
| CuZnZr | 3 | 3 | — | 10 | 503 | 19.6 | 44.4 | 0.073 | [ |
| CuZnZrLa | 3 | 3 | — | 10 | 503 | 20.5 | 49.8 | 0.086 | [ |
| CuZnZrCe | 3 | 3 | — | 10 | 503 | 22.8 | 53.0 | 0.102 | [ |
| CuZnZrNd | 3 | 3 | — | 10 | 503 | 19.0 | 40.5 | 0.064 | [ |
| CuZnZrPr | 3 | 3 | — | 10 | 503 | 19.3 | 42.0 | 0.070 | [ |
| Cu/SiO2 | 3 | 2.5 | — | — | 503 | <10 | ca. 51.9 | ca. 0.011 | [ |
| Cu2.4%(mass fraction)/Al2O3 | 3 | 2.5 | — | — | 503 | <10 | ca. 18.6 | ca. 0.008 | [ |
| Cu/Zr@SiO2 | 3 | 2.5 | — | — | 503 | <10 | 73 | 0.052 | [ |
| Cu/Ti@SiO2 | 3 | 2.5 | — | — | 503 | <10 | 85 | 0.093 | [ |
| Cu/ZrO2(Ⅲ) | 3 | 8 | 3600 | — | 533 | 15.0 | 86.0 | ca. 0.206 | [ |
| Cu/ZrO2(Ⅳ) | 3 | 8 | 3600 | — | 533 | 8.6 | 92.0 | ca. 0.144 | [ |
| Pd/Ga2O3 | 3 | 5 | — | 1.24 | 523 | 19.6 | 51.5 | ca. 0.649 | [ |
| Pd/Al2O3 | 3 | 5 | — | 1.24 | 523 | 3.4 | 29.9 | ca. 0.064 | [ |
| Pd/Cr2O3 | 3 | 5 | — | 1.24 | 523 | 2.1 | 22.4 | ca. 0.030 | [ |
| Pd/SiO2 | 3 | 5 | — | 1.24 | 523 | 0.05 | 100 | ca. 0.003 | [ |
| Pd/TiO2 | 3 | 5 | — | 1.24 | 523 | 15.5 | 3.9 | ca. 0.040 | [ |
| Pd/ZnO | 3 | 5 | — | 1.24 | 523 | 13.8 | 37.5 | ca. 0.333 | [ |
| Pd/ZrO2 | 3 | 5 | — | 1.24 | 523 | 0.4 | 4.3 | ca. 0.001 | [ |
| Pd/ZnO?3.93Al | 3 | 3 | — | 3.73 | 523 | 14.2 | 51.6 | ca. 0.144 | [ |
| Pd/ZnO | 3 | 3 | — | 3.73 | 523 | 5.8 | 69.7 | ca. 0.080 | [ |
| Pd/CNTs?in | 3 | 2 | — | — | 523 | 0.77 | 48.8 | 0.002 | [ |
| Pd/CNTs?out | 3 | 2 | — | — | 523 | 0.61 | 13.4 | 0.0004 | [ |
| Pd?Cu/SiO2 | 3 | 4.1 | — | 6.2 | 523 | 6.6 | 34.0 | 0.036 | [ |
| Catalyst | p(H2)/p(CO2) | p/MPa | GHSVa/h-1 | (W/F)b/ (gcat·h·mol-1) | T/K | CO2 conv.(%) | SCH3OH (%) | YCH3OH/ (gCH3OH·g | Ref. |
| Pd?Cu/P25 | 3 | 4.1 | — | 6.2 | 523 | 16.4 | 25.7 | 0.058 | [ |
| Pd?Cu/CeO2 | 3 | 4.1 | — | 6.2 | 523 | 9.9 | 28.4 | 0.044 | [ |
| Pd?Cu/ZrO2 | 3 | 4.1 | — | 6.2 | 523 | 15.8 | 26.8 | 0.060 | [ |
| Pd?Cu/Al2O3 | 3 | 4.1 | — | 6.2 | 523 | 12.4 | 31.4 | 0.054 | [ |
| PdZn(1∶1)/CeO2 | 3 | 2 | 2400 | — | 493 | 14.07 | 97.2 | 0.166 | [ |
| Ni5Ga3/SiO2/Al2O3/Al?fiber | 3 | 0.1 | — | 7.47 | 483 | ca. 2.3 | 86.7 | 0.020 | [ |
| PdZnAl | 3 | 3 | — | ca. 1.49 | 523 | 0.6 | 60.0 | 0.018 | [ |
| PdMgGa | 3 | 3 | — | ca. 1.49 | 523 | 1.0 | 47.0 | 0.020 | [ |
| In2O3/ZrO2 | 4 | 5 | 16000 | — | 573 | 5.2 | 99.8 | 0.295 | [ |
| In2O3/ZrO2 | 4 | 5 | 16000 | — | 503 | — | 100 | ca. 0.042 | [ |
| In2O3 | 4 | 5 | 16000 | — | 573 | — | 100 | ca. 0.200 | [ |
| In2O3 | 4 | 5 | 16000 | — | 503 | — | 100 | ca. 0.025 | [ |
| Pd?P/In2O3 | 4 | 5 | — | 1.1 | 573 | 20 | 70 | 0.890 | [ |
| Pd?P/In2O3 | 4 | 5 | — | 1.1 | 498 | ca. 3 | ca. 95 | 0.192 | [ |
| Pd?I/In2O3 | 4 | 5 | — | 1.1 | 573 | ca. 18 | ca. 70 | ca. 0.800 | [ |
| Pd?I/In2O3 | 4 | 5 | — | 1.1 | 498 | ca. 2 | ca. 92 | 0.085 | [ |
| Pt/film/In2O3 | 3 | 0.1 | — | 4.67 | 303 | 37 | 62.6 | 0.355 | [ |
| In∶Pd(2∶1)/SiO2 | 4 | 4 | — | 2.99 | 573 | — | 61 | 18.36c | [ |
| CuIn?350 | 3 | 3 | — | 2.99 | 553 | 11.4 | 80.5 | 0.196 | [ |
| 1.5YIn2O3/ZrO2 | 4 | 4 | — | 0.43 | 573 | 7.6 | 69.0 | 0.420 | [ |
| 3La10In/ZrO2 | 4 | 4 | — | 0.43 | 573 | 7.7 | 66.0 | 0.420 | [ |
| Pd?In2O3 CP | 4 | 5 | — | 0.47 | 553 | — | 78 | 1.010 | [ |
| Pd?In2O3 CP | 4 | 5 | — | 0.93 | 553 | — | 75 | 0.610 | [ |
| ZnO?ZrO2 | 3 | 2 | — | 0.93 | 573 | 3.4 | 87.0 | 0.248 | [ |
| ZnO?ZrO2 | 3 | 5 | — | 0.93 | 593 | 10 | ca. 86 | ca. 0.737 | [ |
| CdZrOx | 3 | 2 | 24000 | — | 573 | 5.4 | 80 | — | [ |
| GaZrOx | 3 | 2 | 24000 | — | 573 | 2.4 | 75 | — | [ |
Table 1 Summary of research on CO2 hydrogenation to methanol via heterogenous catalysts
| Catalyst | p(H2)/p(CO2) | p/MPa | GHSVa/h-1 | (W/F)b/ (gcat·h·mol-1) | T/K | CO2 conv.(%) | SCH3OH (%) | YCH3OH/ (gCH3OH·g | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Cu/ZnO/ZrO2(coprecipitation | 3 | 3 | 10000 | — | 513 | 16.0 | 48.7 | ca. 0.288 | [ |
| method) | |||||||||
| Cu/ZnO/ZrO2(complexation | 3 | 3 | 10000 | — | 513 | 12.5 | 51.8 | ca. 0.240 | [ |
| method) | |||||||||
| Cu/ZnO/ZrO2(gel oxalate | 3 | 3 | 10000 | — | 513 | 18.0 | 51.2 | ca. 0.340 | [ |
| coprecipitation method) | |||||||||
| Cu?ZnO?ZrO2(surfactant | 3 | 3 | 3600 | — | 513 | 12.1 | 54.1 | 6.5% | [ |
| assistedcoprecipitation) | |||||||||
| CuO?ZnO?ZrO2 | 3 | 3 | — | — | 513 | 18.2 | 41.6 | 0.061 | [ |
| CuO?ZnO?ZrO2?Cr2O3 | 3 | 3 | — | 9.33 | 513 | 18.1 | 40.0 | 0.058 | [ |
| CuO?ZnO?ZrO2?MoO3 | 3 | 3 | — | 9.33 | 513 | 19.0 | 46.7 | 0.071 | [ |
| CuO?ZnO?ZrO2?WO3 | 3 | 3 | — | 9.33 | 513 | 19.4 | 47.8 | 0.074 | [ |
| CuZnZr | 3 | 3 | — | 10 | 503 | 19.6 | 44.4 | 0.073 | [ |
| CuZnZrLa | 3 | 3 | — | 10 | 503 | 20.5 | 49.8 | 0.086 | [ |
| CuZnZrCe | 3 | 3 | — | 10 | 503 | 22.8 | 53.0 | 0.102 | [ |
| CuZnZrNd | 3 | 3 | — | 10 | 503 | 19.0 | 40.5 | 0.064 | [ |
| CuZnZrPr | 3 | 3 | — | 10 | 503 | 19.3 | 42.0 | 0.070 | [ |
| Cu/SiO2 | 3 | 2.5 | — | — | 503 | <10 | ca. 51.9 | ca. 0.011 | [ |
| Cu2.4%(mass fraction)/Al2O3 | 3 | 2.5 | — | — | 503 | <10 | ca. 18.6 | ca. 0.008 | [ |
| Cu/Zr@SiO2 | 3 | 2.5 | — | — | 503 | <10 | 73 | 0.052 | [ |
| Cu/Ti@SiO2 | 3 | 2.5 | — | — | 503 | <10 | 85 | 0.093 | [ |
| Cu/ZrO2(Ⅲ) | 3 | 8 | 3600 | — | 533 | 15.0 | 86.0 | ca. 0.206 | [ |
| Cu/ZrO2(Ⅳ) | 3 | 8 | 3600 | — | 533 | 8.6 | 92.0 | ca. 0.144 | [ |
| Pd/Ga2O3 | 3 | 5 | — | 1.24 | 523 | 19.6 | 51.5 | ca. 0.649 | [ |
| Pd/Al2O3 | 3 | 5 | — | 1.24 | 523 | 3.4 | 29.9 | ca. 0.064 | [ |
| Pd/Cr2O3 | 3 | 5 | — | 1.24 | 523 | 2.1 | 22.4 | ca. 0.030 | [ |
| Pd/SiO2 | 3 | 5 | — | 1.24 | 523 | 0.05 | 100 | ca. 0.003 | [ |
| Pd/TiO2 | 3 | 5 | — | 1.24 | 523 | 15.5 | 3.9 | ca. 0.040 | [ |
| Pd/ZnO | 3 | 5 | — | 1.24 | 523 | 13.8 | 37.5 | ca. 0.333 | [ |
| Pd/ZrO2 | 3 | 5 | — | 1.24 | 523 | 0.4 | 4.3 | ca. 0.001 | [ |
| Pd/ZnO?3.93Al | 3 | 3 | — | 3.73 | 523 | 14.2 | 51.6 | ca. 0.144 | [ |
| Pd/ZnO | 3 | 3 | — | 3.73 | 523 | 5.8 | 69.7 | ca. 0.080 | [ |
| Pd/CNTs?in | 3 | 2 | — | — | 523 | 0.77 | 48.8 | 0.002 | [ |
| Pd/CNTs?out | 3 | 2 | — | — | 523 | 0.61 | 13.4 | 0.0004 | [ |
| Pd?Cu/SiO2 | 3 | 4.1 | — | 6.2 | 523 | 6.6 | 34.0 | 0.036 | [ |
| Catalyst | p(H2)/p(CO2) | p/MPa | GHSVa/h-1 | (W/F)b/ (gcat·h·mol-1) | T/K | CO2 conv.(%) | SCH3OH (%) | YCH3OH/ (gCH3OH·g | Ref. |
| Pd?Cu/P25 | 3 | 4.1 | — | 6.2 | 523 | 16.4 | 25.7 | 0.058 | [ |
| Pd?Cu/CeO2 | 3 | 4.1 | — | 6.2 | 523 | 9.9 | 28.4 | 0.044 | [ |
| Pd?Cu/ZrO2 | 3 | 4.1 | — | 6.2 | 523 | 15.8 | 26.8 | 0.060 | [ |
| Pd?Cu/Al2O3 | 3 | 4.1 | — | 6.2 | 523 | 12.4 | 31.4 | 0.054 | [ |
| PdZn(1∶1)/CeO2 | 3 | 2 | 2400 | — | 493 | 14.07 | 97.2 | 0.166 | [ |
| Ni5Ga3/SiO2/Al2O3/Al?fiber | 3 | 0.1 | — | 7.47 | 483 | ca. 2.3 | 86.7 | 0.020 | [ |
| PdZnAl | 3 | 3 | — | ca. 1.49 | 523 | 0.6 | 60.0 | 0.018 | [ |
| PdMgGa | 3 | 3 | — | ca. 1.49 | 523 | 1.0 | 47.0 | 0.020 | [ |
| In2O3/ZrO2 | 4 | 5 | 16000 | — | 573 | 5.2 | 99.8 | 0.295 | [ |
| In2O3/ZrO2 | 4 | 5 | 16000 | — | 503 | — | 100 | ca. 0.042 | [ |
| In2O3 | 4 | 5 | 16000 | — | 573 | — | 100 | ca. 0.200 | [ |
| In2O3 | 4 | 5 | 16000 | — | 503 | — | 100 | ca. 0.025 | [ |
| Pd?P/In2O3 | 4 | 5 | — | 1.1 | 573 | 20 | 70 | 0.890 | [ |
| Pd?P/In2O3 | 4 | 5 | — | 1.1 | 498 | ca. 3 | ca. 95 | 0.192 | [ |
| Pd?I/In2O3 | 4 | 5 | — | 1.1 | 573 | ca. 18 | ca. 70 | ca. 0.800 | [ |
| Pd?I/In2O3 | 4 | 5 | — | 1.1 | 498 | ca. 2 | ca. 92 | 0.085 | [ |
| Pt/film/In2O3 | 3 | 0.1 | — | 4.67 | 303 | 37 | 62.6 | 0.355 | [ |
| In∶Pd(2∶1)/SiO2 | 4 | 4 | — | 2.99 | 573 | — | 61 | 18.36c | [ |
| CuIn?350 | 3 | 3 | — | 2.99 | 553 | 11.4 | 80.5 | 0.196 | [ |
| 1.5YIn2O3/ZrO2 | 4 | 4 | — | 0.43 | 573 | 7.6 | 69.0 | 0.420 | [ |
| 3La10In/ZrO2 | 4 | 4 | — | 0.43 | 573 | 7.7 | 66.0 | 0.420 | [ |
| Pd?In2O3 CP | 4 | 5 | — | 0.47 | 553 | — | 78 | 1.010 | [ |
| Pd?In2O3 CP | 4 | 5 | — | 0.93 | 553 | — | 75 | 0.610 | [ |
| ZnO?ZrO2 | 3 | 2 | — | 0.93 | 573 | 3.4 | 87.0 | 0.248 | [ |
| ZnO?ZrO2 | 3 | 5 | — | 0.93 | 593 | 10 | ca. 86 | ca. 0.737 | [ |
| CdZrOx | 3 | 2 | 24000 | — | 573 | 5.4 | 80 | — | [ |
| GaZrOx | 3 | 2 | 24000 | — | 573 | 2.4 | 75 | — | [ |
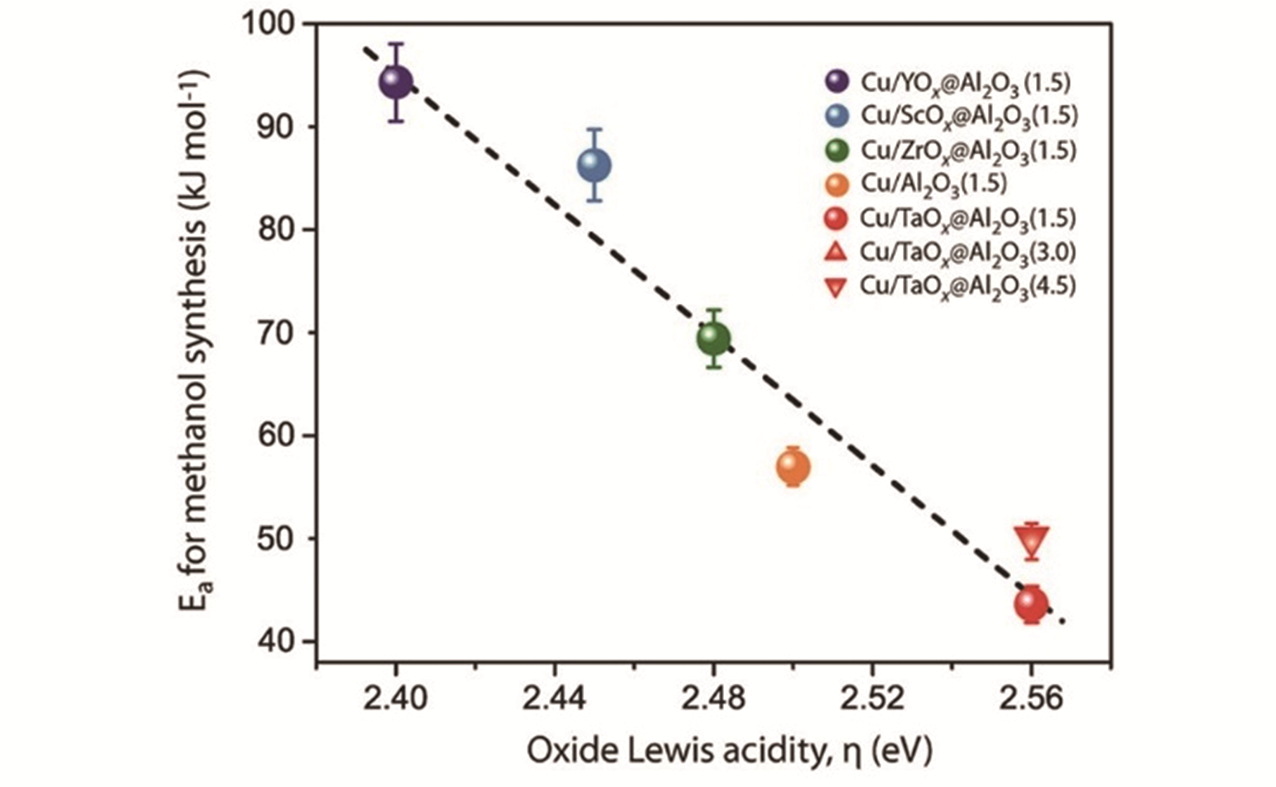
Fig.3 Dependence of the apparent activation energy for methanol synthesis with the Lewis acidity of cus exposed on the surface of the oxide overlay interfaced with Cu nanoparticles, as described with the spectroscopic parameter η, for the series of Cu/MOx@Al2O3 catalyst with surface copper contents of 1.5 Cu·nm-2(●), 3.0 Cu·nm-2(▲), 4.5 Cu·nm-2(▼), respectively[47]Reaction conditions: H2/CO2 volume ratio: 3.0, T=433—493 K, p=63×105 Pa, CO2 conversion < 5%. Error bars for Ea correspond to the standard error as determined from 3 independent tests with selected catalysts. Error bars for η are smaller than the symbol. The dotted line is a guide to the eye. Copyright 2019, American Chemical Society.
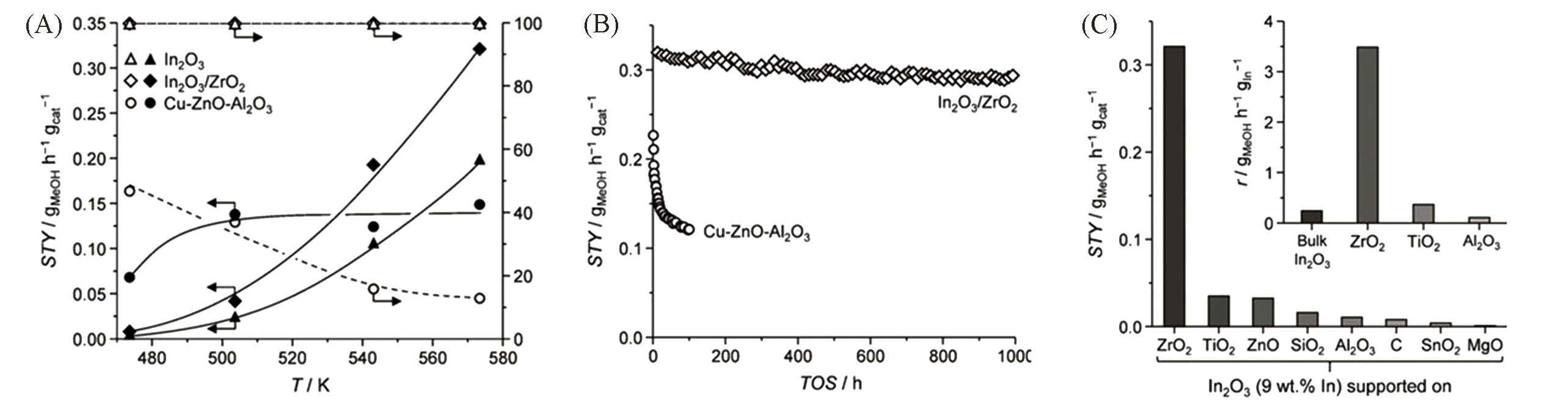
Fig.4 Methanol STY and selectivity for CO2 hydrogenation over bulk In2O3, In2O3/ZrO2(9% In, mass fraction), and the benchmark Cu?ZnO?Al2O3 catalyst at various temperatures(A), evolution of the methanol STY with time on stream(TOS) over In2O3/ZrO2 and Cu?ZnO?Al2O3(B) and methanol STY for different supported catalysts after 4 h on stream(C)[31]Reaction conditions: p=5.0 MPa, H2/CO2 volume ratio 4∶1, and GHSV=16000 h-1, 573 K for (B) and (C). The inset of (C) compares the TOF of different supports based on the In amount. Copyright 2016, John Wiley & Sons.
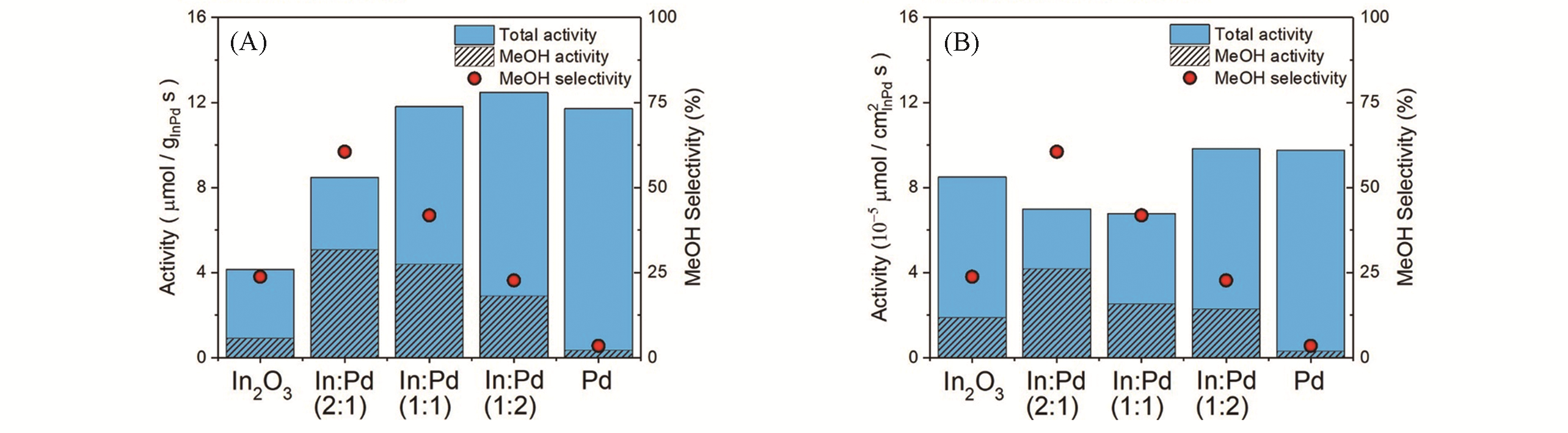
Fig.5 Steady?state activity and methanol selectivity of In?Pd/SiO2 catalysts, averaged from multiple runs and normalized to either the mass of In?Pd(A) or the estimated surface area from TEM(B) [34]Catalysts were reduced at 300 ℃ in 10% H2?N2 prior to CO2 hydrogenation at 4×106 Pa, 300 ℃, and 25 sccm(mL/min at standard condition) of H2/CO2(volume ratio 4∶1). Copyright 2019, American Chemical Society.
| 1 | Sanz⁃Perez E. S., Murdock C. R., Didas S. A., Jones C. W., Chem. Rev., 2016, 116(19), 11840—11876 |
| 2 | Ye R. P., Ding J., Gong W., Argyle M. D., Zhong Q., Wang Y., Russell C. K., Xu Z., Russell A. G., Li Q., Fan M., Yao Y. G., Nat. Commun., 2019, 10(1), 5698 |
| 3 | Kattel S., Liu P., Chen J. G., J. Am. Chem. Soc., 2017, 139(29), 9739—9754 |
| 4 | Guo L., Sun J., Ge Q., Tsubaki N., J. Mater. Chem. A, 2018, 6(46), 23244—23262 |
| 5 | Ma Z., Porosoff M. D., ACS Catal., 2019, 9(3), 2639—2656 |
| 6 | Zhou W., Cheng K., Kang J., Zhou C., Subramanian V., Zhang Q., Wang Y., Chem. Soc. Rev., 2019, 48(12), 3193—3228 |
| 7 | Song C., Catal. Today, 2006, 115(1), 2—32 |
| 8 | Jiang X., Nie X., Guo X., Song C., Chen J. G., Chem. Rev., 2020, doi: 10.1021/acs.chemrev.9b00723 |
| 9 | Wang W. H., Himeda Y., Muckerman J. T., Manbeck G. F., Fujita E., Chem. Rev., 2015, 115(23), 12936—12973 |
| 10 | Alvarez A., Bansode A., Urakawa A., Bavykina A. V., Wezendonk T. A., Makkee M., Gascon J., Kapteijn F., Chem. Rev., 2017, 117(14), 9804—9838 |
| 11 | Kattel S., Ramirez P. J., Chen J. G., Rodriguez J. A., Liu P., Science, 2017, 355(6331), 1296—1299 |
| 12 | Behrens M., Studt F., Kasatkin I., Kuhl S., Havecker M., Abildpedersen F., Zander S., Girgsdies F., Kurr P., Kniep B., Science, 2012, 336(6083), 893—897 |
| 13 | Karelovic A., Ruiz P., Catal. Sci. Technol., 2015, 5(2), 869—881 |
| 14 | Li K., Chen J. G., ACS Catal., 2019, 9(9), 7840—7861 |
| 15 | Bonura G., Cordaro M., Cannilla C., Arena F., Frusteri F., Appl. Catal. B: Environ., 2014, 152/153, 152—161 |
| 16 | Li L., Mao D., Yu J., Guo X., J. Power Sources, 2015, 279, 394—404 |
| 17 | Wang G., Mao D., Guo X., Yu J., Int. J. Hydrogen Energy, 2019, 44(8), 4197—4207 |
| 18 | Ban H., Li C., Asami K., Fujimoto K., Catal. Commun., 2014, 54, 50—54 |
| 19 | Lam E., Corral‐Pérez J. J., Larmier K., Noh G., Wolf P., Comas‐Vives A., Urakawa A., Copéret C., Angew. Chem. Int. Ed., 2019, 58(39), 13989—13996 |
| 20 | Lam E., Larmier K., Wolf P., Tada S., Safonova O. V., Copéret C., J. Am. Chem. Soc., 2018, 140(33), 10530—10535 |
| 21 | Noh G., Lam E., Alfke J. L., Larmier K., Searles K., Wolf P., Copéret C., ChemSusChem, 2019, 12(5), 968—972 |
| 22 | Samson K., Śliwa M., Socha R. P., Góra⁃Marek K., Mucha D., Rutkowska⁃Zbik D., Paul J. F., Ruggiero⁃Mikołajczyk M., Grabowski R., Słoczyński J., ACS Catal., 2014, 4(10), 3730—3741 |
| 23 | Fujitani T., Saito M., Kanai Y., Watanabe T., Nakamura J., Uchijima T., Appl. Catal. A: Gen., 1995, 125(2), L199—L202 |
| 24 | Song J., Liu S., Yang C., Wang G., Tian H., Zhao Z. J., Mu R., Gong J., Appl. Catal. B: Environ., 2020, 263, 118367 |
| 25 | Wang J., Lu S. M., Li J., Li C., Chem. Commun., 2015, 51(99), 17615—17618 |
| 26 | Jiang X., Koizumi N., Guo X., Song C., Appl. Catal. B: Environ., 2015, 170/171, 173—185 |
| 27 | Lin F., Jiang X., Boreriboon N., Wang Z., Song C., Cen K., Appl. Catal. A: Gen., 2019, 585, 117210 |
| 28 | Ojelade O. A., Zaman S. F., Daous M. A., Al⁃Zahrani A. A., Malik A. S., Driss H., Shterk G., Gascon J., Appl. Catal. A: Gen., 2019, 584, 117185 |
| 29 | Chen P., Zhao G., Liu Y., Lu Y., Appl. Catal. A: Gen., 2018, 562, 234—240 |
| 30 | Ota A., Kunkes E. L., Kasatkin I., Groppo E., Ferri D., Poceiro B., Navarro Yerga R. M., Behrens M., J. Catal., 2012, 293, 27—38 |
| 31 | Martin O., Martin A. J., Mondelli C., Mitchell S., Segawa T. F., Hauert R., Drouilly C., Curulla⁃Ferre D., Perez⁃Ramirez J., Angew. Chem. Int. Ed., 2016, 55(21), 6261—6265 |
| 32 | Rui N., Wang Z., Sun K., Ye J., Ge Q., Liu C. J., Appl. Catal. B: Environ., 2017, 218, 488—497 |
| 33 | Men Y. L., Liu Y., Wang Q., Luo Z. H., Shao S., Li Y. B., Pan Y. X., Chem. Eng. Sci., 2019, 200, 167—175 |
| 34 | Snider J. L., Streibel V., Hubert M. A., Choksi T. S., Valle E., Upham D. C., Schumann J., Duyar M. S., Gallo A., Abild⁃Pedersen F., Jaramillo T. F., ACS Catal., 2019, 9(4), 3399—3412 |
| 35 | Shi Z., Tan Q., Tian C., Pan Y., Sun X., Zhang J., Wu D., J. Catal., 2019, 379, 78—89 |
| 36 | Chou C. Y., Lobo R. F., Appl. Catal. A: Gen., 2019, 583, 117144—117153 |
| 37 | Frei M. S., Mondelli C., Garcia⁃Muelas R., Kley K. S., Puertolas B., Lopez N., Safonova O. V., Stewart J. A., Curulla Ferre D., Perez⁃Ramirez J., Nat. Commun., 2019, 10(1), 3377 |
| 38 | Wang J., Li G., Li Z., Tang C., Feng Z., An H., Liu H., Liu T., Li C., Sci. Adv., 2017,(3), e1701290 |
| 39 | Wang J., Tang C., Li G., Han Z., Li Z., Liu H., Cheng F., Li C., ACS Catal., 2019, 9(11), 10253—10259 |
| 40 | Dong X., Li F., Zhao N., Xiao F., Wang J., Tan Y., Appl. Catal. B: Environ., 2016, 191, 8—17 |
| 41 | Wang Y., Kattel S., Gao W., Li K., Liu P., Chen J. G., Wang H., Nat. Commun., 2019, 10(1), 1166 |
| 42 | Wang G., Mao D., Guo X., Yu J., Appl. Surf. Sci., 2018, 456, 403—409 |
| 43 | Phongamwong T., Chantaprasertporn U., Witoon T., Numpilai T., Pooarporn Y., Limphirat W., Donphai W., Dittanet P., Chareon⁃ panich M., Limtrakul J.,Chem. Eng. J.,2017, 316, 692—703 |
| 44 | Li M. M., Zeng Z., Liao F., Hong X., Tsang S. C., J. Catal., 2016, 343, 157—167 |
| 45 | Deng K., Hu B., Lu Q., Hong X., Catal. Commun., 2017, 100, 81—84 |
| 46 | Witoon T., Numpilai T., Phongamwong T., Donphai W., Boonyuen C., Warakulwit C., Chareonpanich M., Limtrakul J., Chem. Eng. J., 2018, 334, 1781—1791 |
| 47 | Kim J., Sarma B. B., Andres E., Pfander N., Concepcion P., Prieto G., ACS Catal., 2019, 9(11), 10409—10417 |
| 48 | Ferrah D., Haines A. R., Galhenage R. P., Bruce J. P., Babore A. D., Hunt A., Waluyo I., Hemminger J. C., ACS Catal., 2019, 9(8), 6783—6802 |
| 49 | Tada S., Kayamori S., Honma T., Kamei H., Nariyuki A., Kon K., Toyao T., Shimizu K., Satokawa S., ACS Catal., 2018, 8(9), 7809—7819 |
| 50 | Ouyang B., Tan W., Liu B., Catal. Commun., 2017, 95, 36—39 |
| 51 | Zhou X., Qu J., Xu F., Hu J., Foord J. S., Zeng Z., Hong X., Tsang S. C., Chem. Commun., 2013, 49(17), 1747—1749 |
| 52 | Oyolarivera O., Baltanas M. A., Cardonamartinez N., J. CO2 Util., 2015, 9, 8—15 |
| 53 | Iwasa N., Suzuki H., Terashita M., Arai M., Takezawa N., Catal. Lett., 2004, 96(1), 75—78 |
| 54 | Bahruji H., Bowker M., Hutchings G. J., Dimitratos N., Wells P. P., Gibson E. K., Jones W., Brookes C., Morgan D. J., Lalev G., J. Catal., 2016, 343, 133—146 |
| 55 | Díez⁃Ramírez J., Valverde J. L., Sánchez P., Dorado F., Catal. Lett., 2016, 146(2), 373—382 |
| 56 | Liao F., Wu X. P., Zheng J., Li M. M. J., Kroner A., Zeng Z., Hong X., Yuan Y., Gong X. Q., Tsang S. C. E., Green Chem., 2017, 19(1), 270—280 |
| 57 | Jiang X., Jiao Y., Moran C., Nie X., Gong Y., Guo X., Walton K. S., Song C., Catal. Commun., 2019, 118, 10—14 |
| 58 | Choi E. J., Lee Y. H., Lee D. W., Moon D. J., Lee K. Y., Mol. Catal., 2017, 434, 146—153 |
| 59 | Malik A. S., Zaman S. F., Alzahrani A. A., Daous M. A., Driss H., Petrov L. A., Appl. Catal. A: Gen., 2018, 560, 42—53 |
| 60 | Díez⁃Ramírez J., Díaz J. A., Sánchez P., Dorado F., J. CO2 Util., 2017, 22, 71—80 |
| 61 | Studt F., Sharafutdinov I., Abild⁃Pedersen F., Elkjaer C. F., Hummelshoj J. S., Dahl S., Chorkendorff I., Nørskov J. K., Nat. Chem.,2014, 6(4), 320—324 |
| 62 | Tang Q., Ji W., Russell C. K., Cheng Z., Zhang Y., Fan M., Shen Z., Appl. Energ.,2019, 253, 113623 |
| 63 | Ahmad K., Upadhyayula S.,Sustain. Energ. Fuels, 2019, 3(9), 2509—2520 |
| 64 | Tang Q., Shen Z., Huang L., He T., Adidharma H., Russell A. G., Fan M., Phys. Chem. Chem. Phys., 2017, 19(28), 18539—18555 |
| 65 | Collins S. E., Delgado J. J., Mira C., Calvino J. J., Bernal S., Chiavassa D. L., Baltanás M. A., Bonivardi A. L., J. Catal., 2012, 292, 90—98 |
| 66 | Fiordaliso E. M., Sharafutdinov I., Carvalho H. W. P., Grunwaldt J. D., Hansen T. W., Chorkendorff I., Wagner J. B., Damsgaard C. D., ACS Catal., 2015, 5(10), 5827—5836 |
| 67 | Singh J. A., Cao A., Schumann J., Wang T., Nørskov J. K., Abild⁃Pedersen F., Bent S. F., Catal. Lett., 2018, 148(12), 3583—3591 |
| 68 | Frei M. S., Capdevila⁃Cortada M., García⁃Muelas R., Mondelli C., López N., Stewart J. A., Curulla Ferré D., Pérez⁃Ramírez J., J. Catal., 2018, 361, 313—321 |
| 69 | Tsoukalou A., Abdala P. M., Stoian D., Huang X., Willinger M. G., Fedorov A., Muller C. R., J. Am. Chem. Soc., 2019, 141(34), 13497—13505 |
| 70 | Frei M. S., Mondelli C., Cesarini A., Krumeich F., Hauert R., Stewart J. A., Curulla Ferré D., Pérez⁃Ramírez J., ACS Catal., 2019, 10(2), 1133—1145 |
| 71 | Chen T. Y., Cao C., Chen T. B., Ding X., Huang H., Shen L., Cao X., Zhu M., Xu J., Gao J., Han Y. F., ACS Catal., 2019, 9(9), 8785—8797 |
| 72 | Ye J., Liu C. J., Mei D., Ge Q., J. Catal., 2014, 317, 44—53 |
| 73 | Ye J., Ge Q., Liu C. J., Chem. Eng. Sci., 2015, 135, 193—201 |
| [1] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [2] | 吴玉, 李轩, 杨恒攀, 何传新. 钴单原子的双重限域制备策略及高效CO2电还原性能[J]. 高等学校化学学报, 2022, 43(9): 20220343. |
| [3] | 王新天, 李攀, 曹越, 洪文浩, 耿忠璇, 安志洋, 王昊宇, 王桦, 孙斌, 朱文磊, 周旸. 单原子材料在二氧化碳催化中的技术经济分析与产业化应用前景[J]. 高等学校化学学报, 2022, 43(9): 20220347. |
| [4] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [5] | 崔伟, 赵德银, 白文轩, 张晓东, 余江. CO2在非质子溶剂与铁基离子液体复合体系中的吸收[J]. 高等学校化学学报, 2022, 43(8): 20220120. |
| [6] | 郭志强, 杨博如, 席婵娟. 硼氢化试剂在二氧化碳还原官能化反应中的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220199. |
| [7] | 张昕昕, 许狄, 王艳秋, 洪昕林, 刘国亮, 杨恒权. CO2加氢制低碳醇CuFe基催化剂中的Mn助剂效应[J]. 高等学校化学学报, 2022, 43(7): 20220187. |
| [8] | 周紫璇, 杨海艳, 孙予罕, 高鹏. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220235. |
| [9] | 邱丽琪, 姚向阳, 何良年. 可见光驱动丰产金属卟啉类配合物催化的二氧化碳选择性还原反应[J]. 高等学校化学学报, 2022, 43(7): 20220064. |
| [10] | 王征文, 高凤翔, 曹瀚, 刘顺杰, 王献红, 王佛松. 基于二氧化碳共聚物的紫外光固化高分子材料的制备与性能[J]. 高等学校化学学报, 2022, 43(7): 20220236. |
| [11] | 黄孝舜, 马海英, 柳淑娟, 王斌, 王红利, 钱波, 崔新江, 石峰. 二氧化碳间接转化制化学品的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220222. |
| [12] | 宋德文, 汪明旺, 王亚旎, 焦振梅, 宁汇, 吴明铂. 二氧化碳电还原制草酸研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220248. |
| [13] | 赵润瑶, 纪桂鹏, 刘志敏. 吡咯氮配位单原子铜催化剂的电催化二氧化碳还原性能[J]. 高等学校化学学报, 2022, 43(7): 20220272. |
| [14] | 彭奎霖, 李桂林, 江重阳, 曾少娟, 张香平. 电解液调控CO2电催化还原性能微观机制的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220238. |
| [15] | 张振, 邓煜, 张琴芳, 余达刚. 可见光促进二氧化碳参与的羧基化反应[J]. 高等学校化学学报, 2022, 43(7): 20220255. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||