

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (1): 20230525.doi: 10.7503/cjcu20230525
何扩1,2, 丁彬彬1( ), 马平安1,2(
), 马平安1,2( ), 林君1,2(
), 林君1,2( )
)
收稿日期:2023-12-26
出版日期:2025-01-10
发布日期:2024-02-21
通讯作者:
丁彬彬,马平安,林君
E-mail:bbding@ciac.ac.cn;mapa675@ciac.ac.cn;jlin@ciac.ac.cn
基金资助:
HE Kuo1,2, DING Binbin1( ), MA Ping’an1,2(
), MA Ping’an1,2( ), LIN Jun1,2(
), LIN Jun1,2( )
)
Received:2023-12-26
Online:2025-01-10
Published:2024-02-21
Contact:
DING Binbin, MA Ping’an, LIN Jun
E-mail:bbding@ciac.ac.cn;mapa675@ciac.ac.cn;jlin@ciac.ac.cn
Supported by:摘要:
纳米材料选择性诱导肿瘤细胞发生程序性细胞死亡(PCD)被视为一种很有前途的癌症治疗方法. 铜死亡是一种最近被发现的由细胞内铜离子过载引起、 以脂酰化线粒体酶的聚集和Fe-S蛋白质丢失为特征的全新的PCD模式. 目前, 多种纳米材料已被开发并用于诱导肿瘤细胞铜死亡, 实现癌症治疗. 大量的研究表明, 将铜死亡与其它肿瘤治疗方式联合使用可取得更好的治疗效果, 展现出巨大的潜力. 本文综合评述了细胞铜死亡的相关机制及特征, 总结和概括了纳米材料诱导的肿瘤细胞铜死亡策略及相关机制, 重点分类概述了纳米材料诱导的铜死亡联合治疗的最新研究进展, 并对这一新兴的肿瘤治疗方式的未来前景进行了总结和展望.
中图分类号:
TrendMD:
何扩, 丁彬彬, 马平安, 林君. 纳米材料诱导肿瘤细胞铜死亡的研究进展. 高等学校化学学报, 2025, 46(1): 20230525.
HE Kuo, DING Binbin, MA Ping’an, LIN Jun. Research Progress in Nanomaterial-induced Cuproptosis in Tumor Cells. Chem. J. Chinese Universities, 2025, 46(1): 20230525.
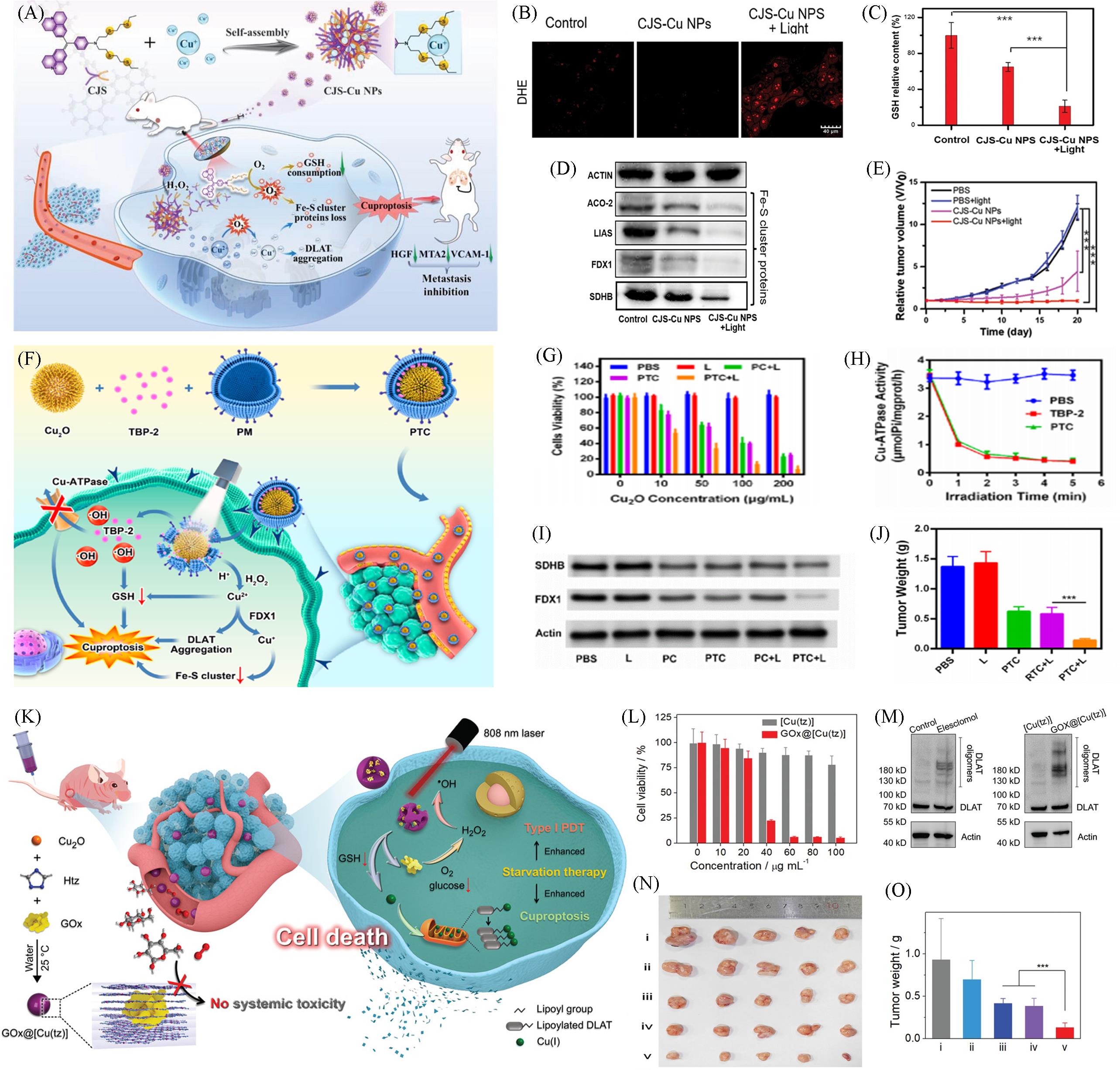
Fig.3 Nanomaterials based on Cuproptosis for PDT combination therapy(A) Schematic of the preparation of CJS-Cu NPs and the underlying mechanism of CJS-Cu NPs in cuproptosis tumor treatment; (B) detections of ·O2-; (C) relative GSH contents in 4T1 cells after various treatments; (D) western blot analysis on the expressions of ACO-2, LIAS, and FDX1; (E) relative tumor volume change during the 20 d therapy[32]; (F) schematic illustration PTC loaded by the biomimetic system for tumor cuproptosis; (G) cell viability of cancer cell safter the indicated treatments with various Cu2O concentrations; (H) changes in Cu-ATPase activity after indicated treatment under white light irradiation; (I) Fe-S cluster protein expression; (J) evolution of the tumor volume in mice after various treatments[23]; (K) schematic illustration of the synthesis of nonporous GOx@[Cu(tz)] and the starvation-augmented cuproptosis and photodynamic synergistic therapy without producing systemic toxicity; (L) the viability of MCF-7 cells treated with [Cu(tz)] and GOx@[Cu(tz)] at different concentrations for 24 h in the dark; (M) oligomerization analysis of DLAT after different treatments; (N, O) photographs of mice tumors(N) and the weight(O) of excised tumors after treatments with PBS(i), [Cu(tz)](ii), [Cu(tz)]/laser(iii), GOx@[Cu(tz)](iv) and GOx@[Cu(tz)]/laser[27].(A—E) Copyright 2023, Wiley-VCH; (F—J) Copyright 2023, American Chemical Society; (K—O) Copyright 2022, Wiley-VCH.
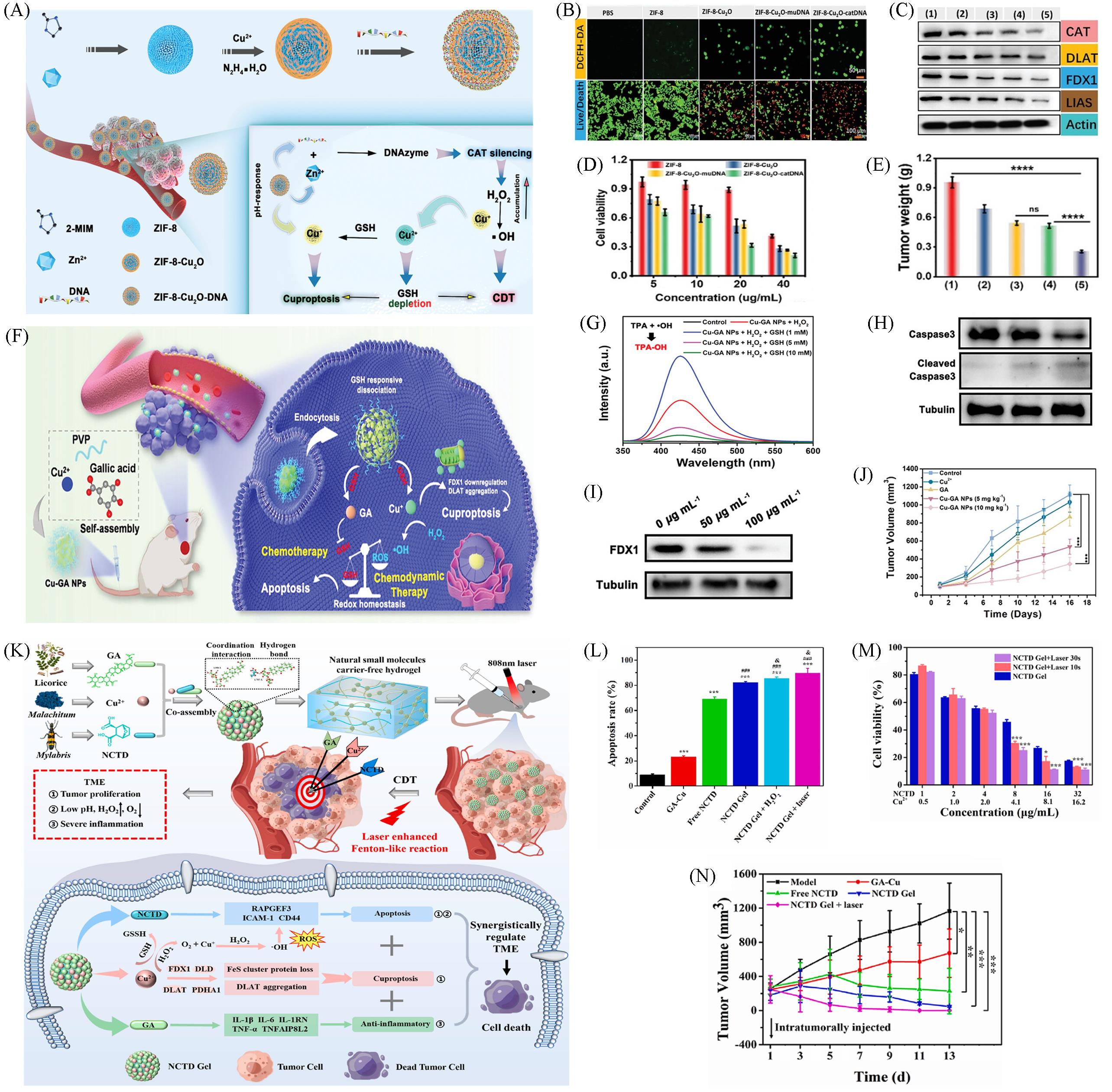
Fig.4 Nanomaterials based on Cuproptosis for CDT combination therapy(A) Schematic illustration of the preparation for ZIF-8-Cu2O-DNA and the mechanism of cascade reactions for cuproptosis and chemodynamic therapy; (B) confocal images of intracellular ROS of PANC-1 cells treated with different composition nanomedicine(Scale bar: 50 μm) and the imaging of PANC-1 cells costained by calcein-AM and PI under various treatments(20 μg/mL, scale bar: 100 μm); (C) western blot analysis of CAT, DLAT, FDX1, and LIAD expression levels in PANC-1 cells after different treatments(ZIF-8, ZIF-8-Cu2O, ZIF-8-Cu2O-mu-DNA, and ZIF-8-Cu2O-cat-DNA, respectively); (D) MTT viability assessment of PANC-1 cells treated with different concentrations of ZIF-8, ZIF-8-Cu2O, ZIF-8-Cu2O-mu-DNA, and ZIF-8-Cu2O-cat-DNA, respectively, for 24 h; (E) photographs of tumor tissues in different groups after 12 d of treatment[39]; (F) schematic diagram of Cu-GA NPs synthesis process and the Cu-GA NPs mediated chemo/chemodynamic synergistic therapy of tumors; (G) TPA assay of Cu-GA NPs under different treatments (TPA as the ·OH trapping agent); (H) western blot analysis on the expressions of Caspase 3 and cleaved Caspase 3; (I) western blot analysis on the expressions of FDX1; (J) time-course change in the 4T1 tumor volume after different treatments, n=5[40]; (K) schematic illustration of NCTD Gel’s self-assembly mechanism and synergistically regulate the tumor microenvironment via apoptosis, cuproptosis and anti-inflammation; (L) flow cytometric analysis of HepG2 cells apoptosis induced by different treatments with Annexin V-FITC/PI staining; (M) cell viability of HepG2 cells treated with a series concentration of NCTD Gel + laser at different time(1.0 W/cm2) for 72 h; (N) curves of tumor growth volume during the treated process[12].(A—E) Copyright 2023, Wiley-VCH; (F—J) Copyright 2023, Wiley-VCH; (K—N) Copyright 2023, Elsevier.
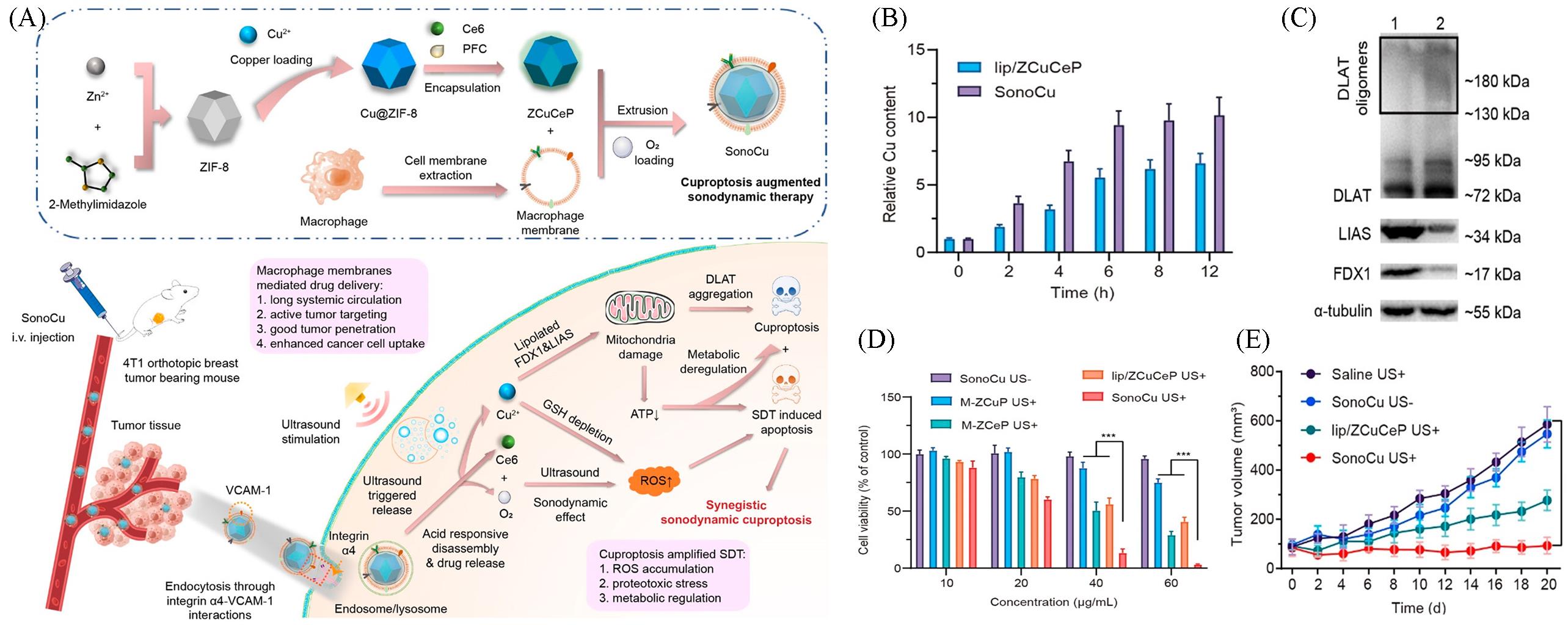
Fig.5 Nanomaterials based on Cuproptosis for SDT combination therapy[46](A) Schematic illustration of the preparation for SonoCu and the mechanism of cascade reactions for synergistically triggering cuproptosis-augmented SDT; (B) measurement of intracellular levels of Cu by ICP-MS after treating with SonoCu for 12 h; (C) western blot analysis of the expressions of DLAT, LIAS, and FDX1 in 4T1 cells after treating with SonoCu plus ultrasound irradiation; (D) in vitro anticancer effects of SonoCu against 4T1 cancer cells under normoxia[4T1 cells were incubated with different concentrations of M-ZCuP(SonoCu without Ce6), M-ZCeP(SonoCu without Cu2+), lip/ZCuCeP, or SonoCu under normoxia for 6 h, followed by treating with ultrasound(1 W/cm2, 1 MHz at 0.45 MPa) for 3 min, the cell viability was determined by MTT assay after a 24 h treatment. SonoCu without ultrasound stimulation was set as the control]; (E) tumor growth curves of 4T1 tumor bearing mice after the treatment of SonoCu[4T1 tumor bearing mice were intravenously treated with saline, lip/ZCuCeP, or SonoCu(8 mg/kg) every 2 d, seven times, followed by ultrasound stimulation (1 W/cm2, 1 MHz at 0.45 MPa) for 3 min 12 h post injection, the tumor growth was monitored for 20 d].Copyright 2023, American Chemical Society.
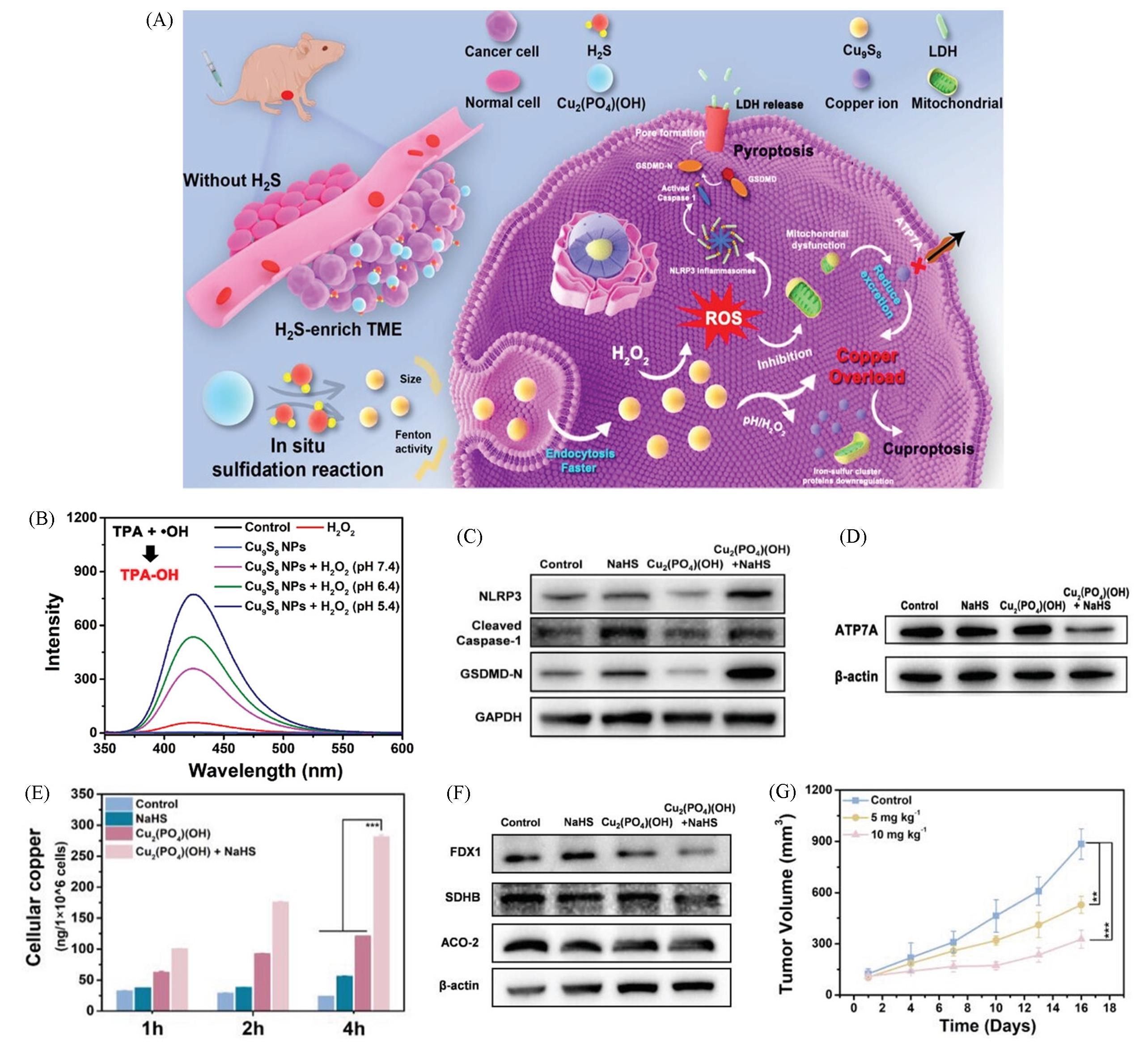
Fig.6 Nanomaterials based on Cuproptosis for pyroptosis combination therapy[22](A) Schematic illustration of Cu2(PO4)(OH) NPs serve as the copper homeostasis disrupter for tumor selective therapy based on copperoverload-mediated cuproptosis and pyroptosis; (B) TPA assay of Cu9S8 NPs under different treatments with TPA as the ·OH trapping agent; (C) western blot analysis on the expressions of NLRP3, cleaved Caspase-1, and GSDMD-N; (D) western blot analysis on the expressions of ATP7A; (E) the intracellular copper levels of HCT116 cells under different conditions for 1, 2, and 4 h, n=3; (F) western blot analysis on the expressions of FDX1, SDHB, and ACO-2; (G) time-dependent HCT116 tumor growth curves after different treatments, n=5.Copyright 2023, Wiley-VCH.
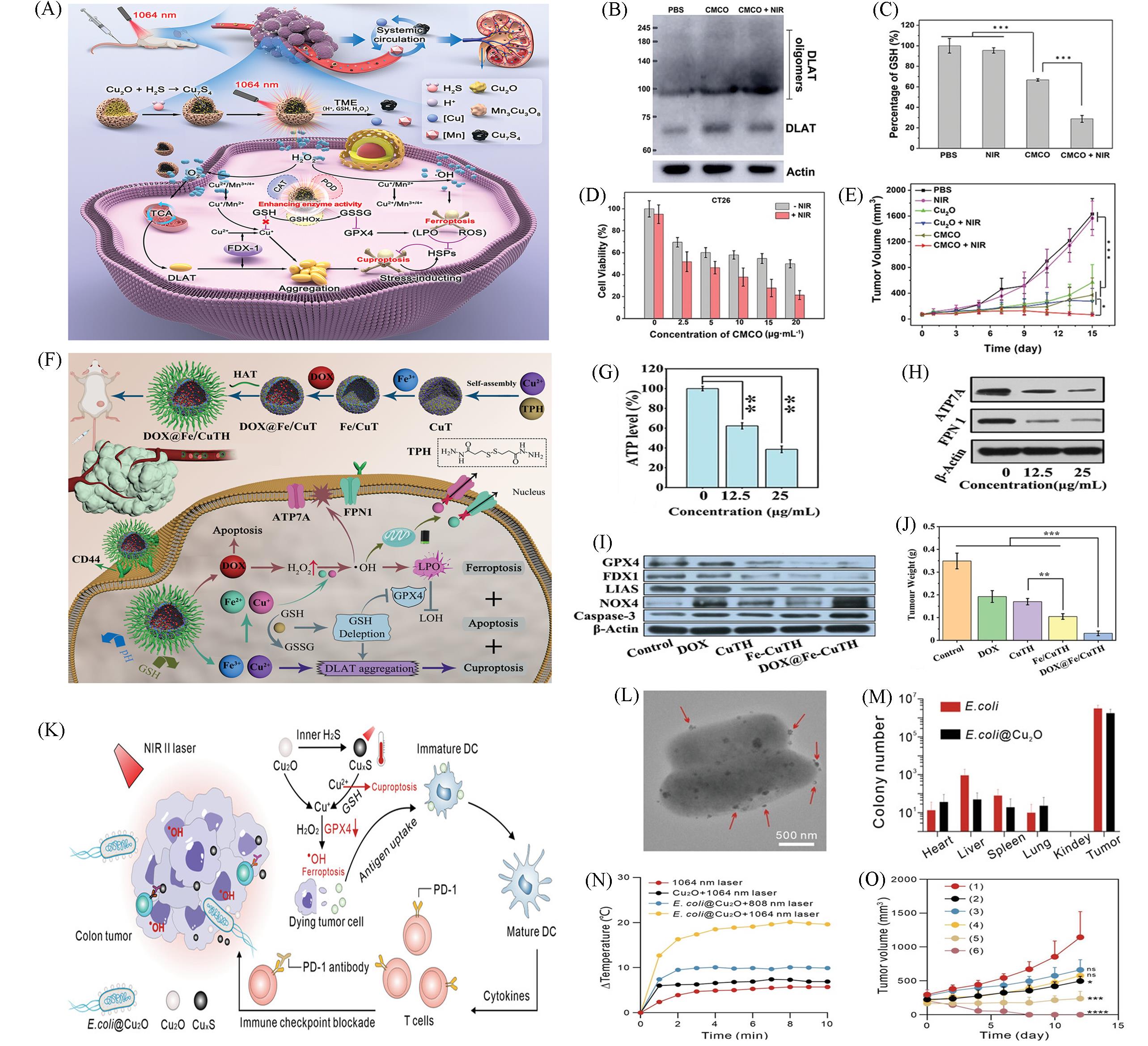
Fig.7 Nanomaterials based on Cuproptosis for Ferroptosis combination therapy(A) The mechanism of high efficiency ferroptosis-boosted-cuproptosis induced by mild-photothermal effect based on Cu2O@Mn3Cu3O8 nanozymes for colorectal cancer therapy; (B) western blot of DLAT and DLAT oligomers after different treatments; (C) the contents of intracellular of GSH on CT26 with different treatments; (D) cytotoxicity assessment on CT26 cells treated with different concentration of CMCO nanozymes with or without 1064 nm laser irradiation; (E) the tumor growth curves of CT26 tumor-bearing mice after different treatments with intratumor injection[59]; (F) schematic illustration of the fabrication process of DOX@Fe/CuTH HaMOF and its application as an oxidative stress amplifier and copper/iron metabolic disrupter for synergistic cuproptosis/ferroptosis/apoptosis antitumor therapy; (G) intracellular ATP levels of 4T1 cells after treatment with different concentrations of DOX@Fe/CuTH; (H) intracellular FPN 1 and ATP7A expression levels of 4T1 cells after treatment with different concentrations of DOX@Fe/CuTH; (I) western blot analysis on the expressions of GPX4, FDX1, LIAS, NOX4, and caspase-3; (J) average weights of the excised tumors[60]; (K) schematic description of E. coli@Cu2O microbial nanohybrid for boosting of antitumor immune responses through tumor microenvironment-activatable NIRII PTT enhanced ferroptosis and cuproptosis; (L) TEM image of E. coli@Cu2O, Arrows indicate the attached Cu2O nanoparticles; (M) quantification of E. coli colonization in tumors and major organs harvested from MC38-bearing mice at 24 h after injection of E. coli or E. coli@Cu2O; (N) temperature changes of mice injected with various formulas under laser illumination(1 W/cm2); (O) tumor growth curves of MC38 tumor-bearing mice with various treatments, PBS without laser irradiation(group 1), PBS with 1064 nm laser irradiation(group 2, 1064 nm laser), E. coli without laser irradiation(group 3, E. coli), Cu2O with 1064 nm laser irradiation(group 4, Cu2O + 1064 nm laser), E. coli@Cu2O with 808 nm laser irradiation(group 5, E. coli@Cu2O + 808 nm laser), and E. coli@Cu2O with 1064 nm laser irradiation (group 6, E. coli@Cu2O + 1064 nm laser)[61].(A—E) Copyright 2023, Wiley-VCH; (F—J) Copyright 2022, Wiley-VCH; (K—O) Copyright 2023, Wiley-VCH.
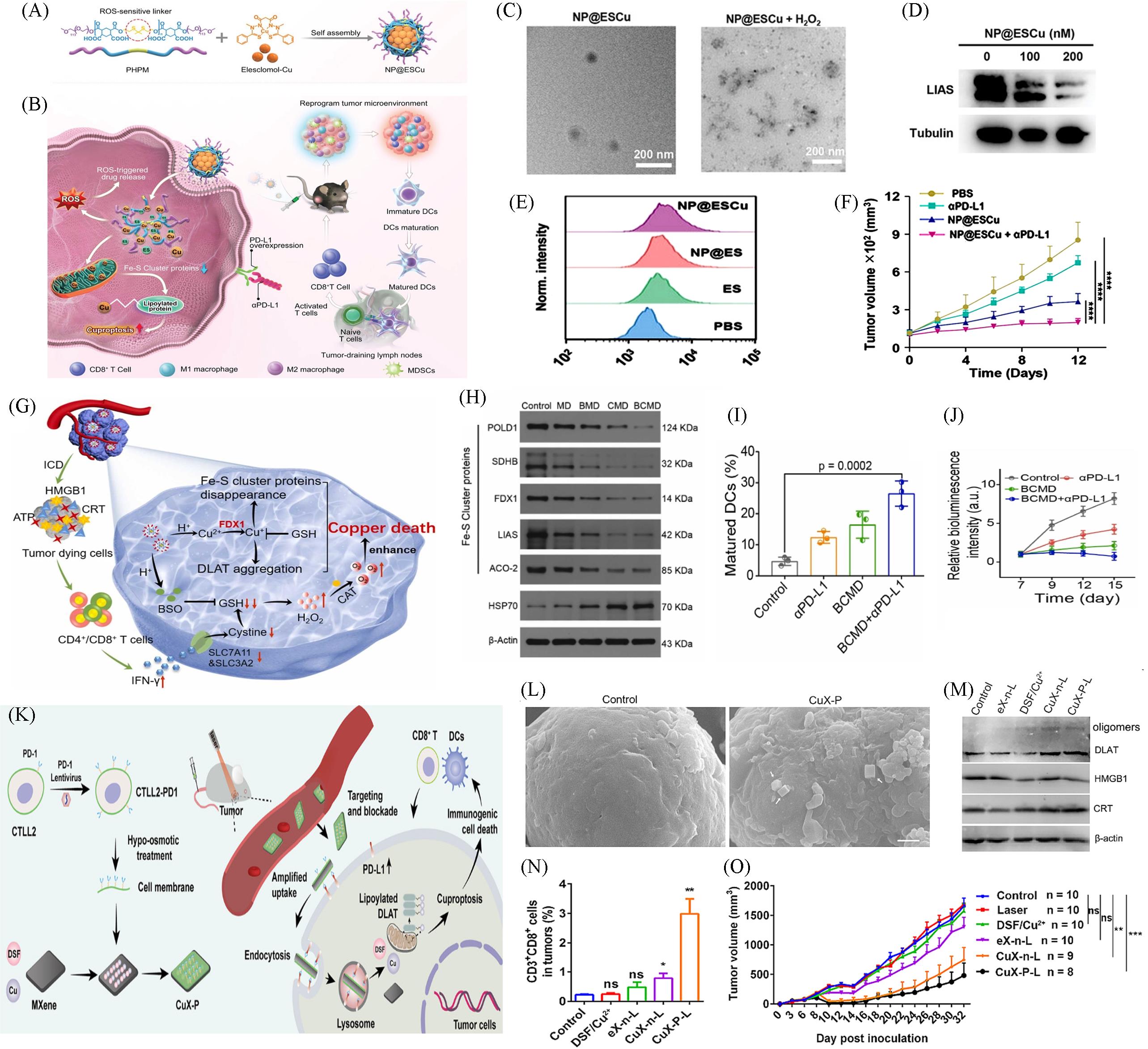
Fig.8 Nanomaterials based on Cuproptosis for αPD⁃L1 combination therapy(A) Design of NP@ESCu to induce cuproptosis; (B) schematic illustration targeting cancer cells by inducing cuproptosis with NP@ESCu combined with αPD-L1 for enhanced cancer therapy; (C) representative TEM images of NP@ES and representative TEM images of NP@ESCu in the presence of 10 mmol/L H2O2; (D) the expression of LIAS by western blot; (E) representative FCM profiles of PD-L1 expression after various treatments; (F) representative FCM profiles of PD-L1 expression after various treatments[66]; (G) schematic of the preparation and therapeutic mechanism of BCMD; (H) western blotting analysis on the expressions of Fe-S cluster protein in GL261 cells after different treatments; (I) quantitative analysis of mature DCs; (J) relative fluorescence intensity of glioblastoma-bearing mice with different treatments[13]; (K) schematic illustration showing the preparation of CuX-P and the proposed mechanism of CuX-P to treat TNBC. CuX-P, DSF/Cu2+-loading MXene with CTLL2-PD1 membrane coating, DLAT, dihydrolipoamide S-acetyltransferase, DSF, disulfiram, TNBC, triple-negative breast cancer; (L) CuX-P outperformed than spherical nanoparticles in blocking PD-L1 and inducing cuproptosis in tumor cells; (M) WB analysis of DLAT, HMGB1 and CRT in 4T1 cells; (N) flow-cytometric analysis of CD3+ CD8+ cells in tumors at 7 d post laser irradiation. n=5; (O) tumor growth curve of mice after different treatments[67].(A—F) Copyright 2023, Wiley-VCH; (G—J) Copyright 2023, Elsevier; (K—O) Copyright 2023, Elsevier.
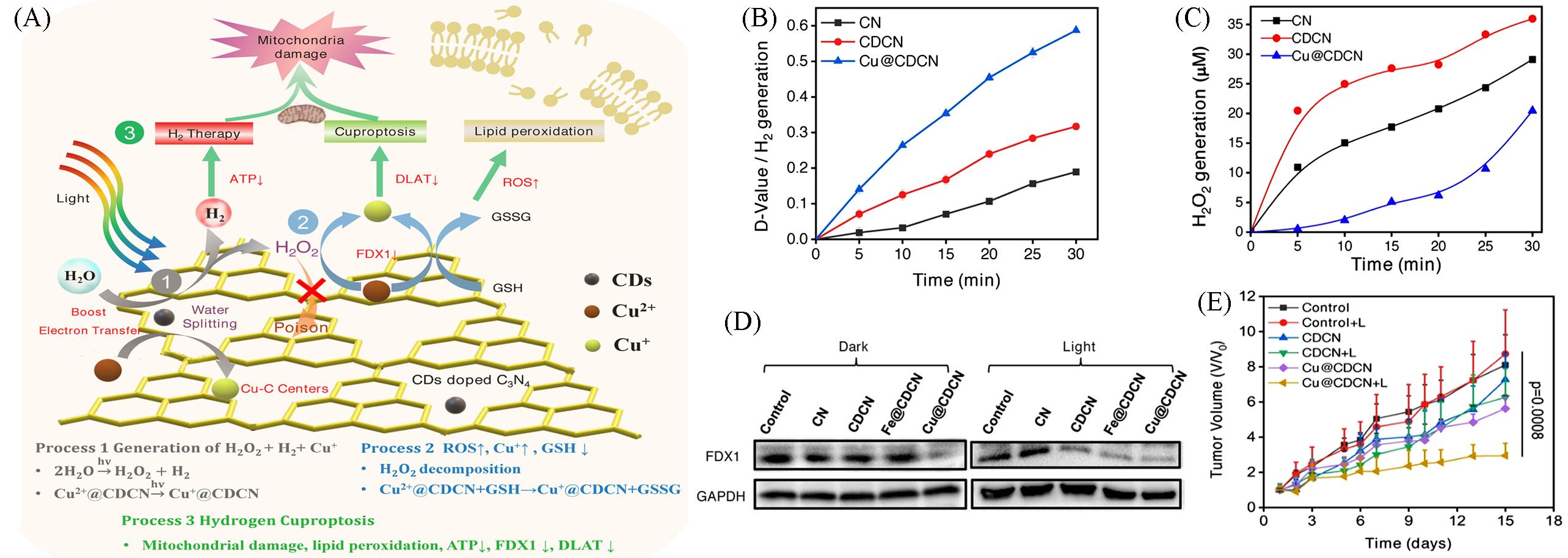
Fig.9 Nanomaterials based on Cuproptosis for hydrogen combination therapy[77](A) Schematic illustration of a Cu(II)-anchored CD-doped C3N4 nanosheet(Cu@CDCN) for the synergistic photocatalytic hydrogen-boosted cuproptosis therapy of cancer; (B) statistical chart of the difference between the 663-nm peaks of different solutions after irradiation; (C) H2O2 generation by CN, CDCN, and Cu@CDCN under LED irradiation; (D) FDX1 protein content of 4T1 cells after different treatments; (E) average tumor growth curves of 4T1-tumor-bearing mice after different 15 d treatments.Copyright 2023, Wiley-VCH.
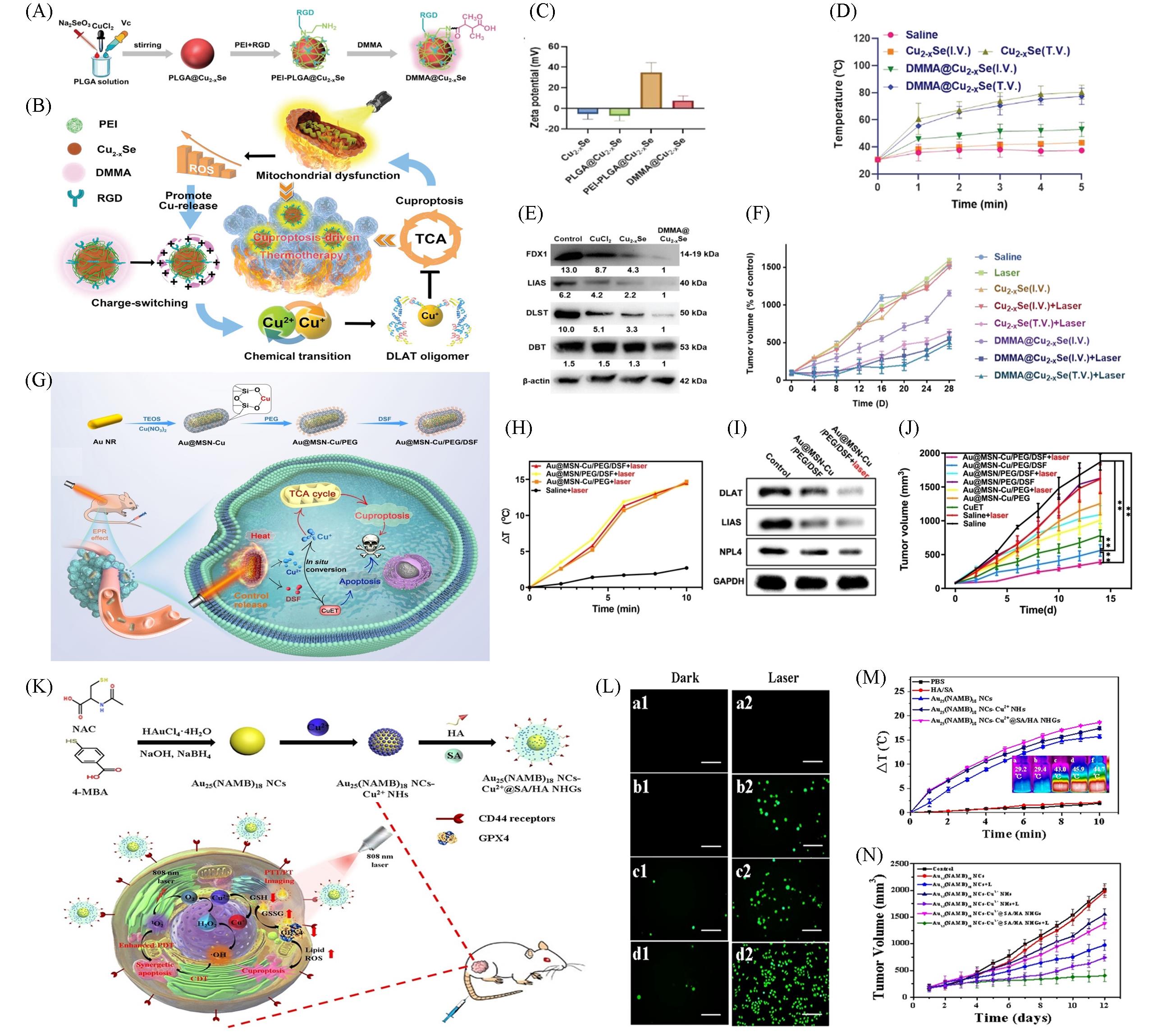
Fig.10 Nanomaterials based on Cuproptosis for PTT combination therapy(A) The design and preparation of DMMA@Cu2‒x Se; (B) schematic illustration of DMMA@Cu2‒x Se for cuproptosis-driven enhancement of thermotherapy; (C) Zeta potential of DMMA@Cu2‒x Se; (D) temperature curves of A375 tumor-bearing nude mice after treatment with Cu2‒x Se and DMMA@Cu2‒x Se (4 mg/kg) under 808 nm laser (2 W/cm2, 5 min) irradiation, tumor after intravenous injection(I.V.) or tumor injection(T.V.); (E) DMMA@Cu2‒xSe down-regulated the proteins related to TAC; (F) tumor volume of A375 tumor-bearing nude mice in 28 d of cotreatment[81]; (G) fabrication process of Au@MSN-Cu/PEG/DSF and its specific functionality for tumor therapy; (H) temperature change at the tumor site; (I) western blot analysis of DLAT, LIAS, NPL4, and GAPDH expression levels in 4T1cells after different treatments; (J) change curves for the tumor volume[82]; (K) schematic diagram for the preparation and simulated antitumor processes of Au25(NAMB)18 NCs-Cu@SA/HA NHGs; (L) fluorescence microscopy images of HepG2 cells incubated with PBS(a1, a2, control groups), Au25(NAMB)18 NCs(b1, b2), Au25(NAMB)18 NCsCu2+ NHs(c1, c2), and Au25(NAMB)18 NCs-Cu2+@SA/HA NHGs (d1, d2), without(1) and with(2) laser irradiation(808 nm, 1 W/cm2), respectively, scale bar: 200 μm; (M) temperature change curves and theramal images of dispersions with different samples irradiated by 808 nm laser at 1 W/cm2 for 10 min; (N) relative tumor volume curves[36].(A—F) Copyright 2023, Wiley-VCH; (G—J) Copyright 2023, Wiley-VCH; (K—N) Copyright 2023, Elsevier.
| 1 | Tsvetkov P., Coy S., Petrova B., Dreishpoon M., Verma A., Abdusamad M., Rossen J., Joesch⁃Cohen L., Humeidi R., Spangler R. D., Eaton J. K., Frenkel E., Kocak M., Corsello S. M., Lutsenko S., Kanarek N., Santagata S., Golub T. R., Science, 2022, 375(6586), 1254—1261 |
| 2 | Wu D., Wang S., Yu G., Chen X., Angew. Chem. Int. Ed., 2021, 60(15), 8018—8034 |
| 3 | Halliwell B., Gutteridge J. M. C., Biochem. J., 1984, 219(1), 1—14 |
| 4 | Chen L., Min J., Wang F., Sig. Transduct. Target Ther., 2022, 7(1), 1—16 |
| 5 | Albalawi F., Hussein M. Z., Fakurazi S., Masarudin M. J., Int. J. Nanomedicine, 2021, 16, 161—184 |
| 6 | Wen J., Yang K., Liu F., Li H., Xu Y., Sun S., Chem. Soc. Rev., 2017, 46(19), 6024—6045 |
| 7 | Irvine D. J., Dane E. L., Nat. Rev. Immunol., 2020, 20(5), 321—334 |
| 8 | Cobine P. A., Moore S. A., Leary S. C., Biochim. Biophys. Acta Mol. Cell Res., 2021, 1868(1), 118867 |
| 9 | Garza N. M., Swaminathan A. B., Maremanda K. P., Zulkifli M., Gohil V. M., Trends Endocrinol. Metab., 2023, 34(1), 21—33 |
| 10 | Liu C., Zhang Y., Sun W., Zhu H., Su M., Wang X., Rong X., Wang K., Yu M., Sheng W., Zhu B., Talanta, 2023, 260, 124567 |
| 11 | Banci L., Bertini I., Ciofi⁃Baffoni S., Kozyreva T., Zovo K., Palumaa P., Nature, 2010, 465(7298), 645—648 |
| 12 | Pi W., Wu L., Lu J., Lin X., Huang X., Wang Z., Yuan Z., Qiu H., Zhang J., Lei H., Wang P., Bioact. Mater., 2023, 29, 98—115 |
| 13 | Huang Q. X., Liang J. L., Chen Q. W., Jin X. K., Niu M. T., Dong C. Y., Zhang X. Z., Nano Today, 2023, 51, 101911 |
| 14 | Bailey H. H., Mulcahy R. T., Tutsch K. D., Arzoomanian R. Z., Alberti D., Tombes M. B., Wilding G., Pomplun M., Spriggs D. R., J. Clin. Oncol., 1994, 12(1), 194—205 |
| 15 | Sainero⁃Alcolado L., Liaño⁃Pons J., Ruiz⁃Pérez M. V., Arsenian⁃Henriksson M., Cell Death Differ., 2022, 29(7), 1304—1317 |
| 16 | Han Y., Ouyang J., Li Y., Wang F., Jiang J. H., ACS Appl. Mater. Interfaces, 2020, 12(1), 288—297 |
| 17 | Li W. P., Su C. H., Chang Y. C., Lin Y. J., Yeh C. S., ACS Nano, 2016, 10(2), 2017—2027 |
| 18 | Sang Y., Cao F., Li W., Zhang L., You Y., Deng Q., Dong K., Ren J., Qu X., J. Am. Chem. Soc., 2020, 142(11), 5177—5183 |
| 19 | McKie A. T., Barrow D., Latunde⁃Dada G. O., Rolfs A., Sager G., Mudaly E., Mudaly M., Richardson C., Barlow D., Bomford A., Peters T. J., Raja K. B., Shirali S., Hediger M. A., Farzaneh F., Simpson R. J., Science, 2001, 291(5509), 1755—1759 |
| 20 | Ohgami R. S., Campagna D. R., McDonald A., Fleming M. D., Blood, 2006, 108(4), 1388—1394 |
| 21 | Puig S., Thiele D. J., Curr. Opin. Chem. Biol., 2002, 6(2), 171—180 |
| 22 | Zhao F., Liang L., Wang H., Wang C., Su D., Ying Y., Li W., Li J., Zheng J., Qiao L., Mou X., Che S., Yu J., Adv. Funct. Materials, 2023, 33(38), 2300941 |
| 23 | Ning S., Lyu M., Zhu D., Lam J. W. Y., Huang Q., Zhang T., Tang B. Z., ACS Nano, 2023, 17(11), 10206—10217 |
| 24 | Liu Y., Zhao Z. H., Wang T., Yao J. Y., Wei W. Q., Su L. H., Tan S. S., Liu Z. X., Song H., Chen J. Y., Zheng W., Luo W. J., Zheng G., Ecotoxicol. Environ. Saf., 2023, 256, 114861 |
| 25 | Matsui M. S., Petris M. J., Niki Y., Karaman⁃Jurukovska N., Muizzuddin N., Ichihashi M., Yarosh D. B., J. Invest. Dermatol., 2015, 135(3), 834—841 |
| 26 | Porporato P. E., Filigheddu N., Pedro J. M. B. S., Kroemer G., Galluzzi L., Cell Res., 2018, 28(3), 265—280 |
| 27 | Xu Y., Liu S., Zeng L., Ma H., Zhang Y., Yang H., Liu Y., Fang S., Zhao J., Xu Y., Ashby C. R., He Y., Dai Z., Pan Y., Adv. Mater., 2022, 34(43), 2204733 |
| 28 | Zhang Z., Zhou Y., Zhao S., Ding L., Chen B., Chen Y., Adv. Sci., 2022, 9(35), 2203583 |
| 29 | Wang Y., Liu T., Li X., Sheng H., Ma X., Hao L., Front. Pharmacol., 2021, 12, 735965 |
| 30 | Lucky S. S., Soo K. C., Zhang Y., Chem. Rev., 2015, 115(4), 1990—2042 |
| 31 | Rundle P., Biomedicines, 2017, 5(4), 69 |
| 32 | Zheng J., Ge H., Guo M., Zhang T., Hu Q., Yao Q., Long S., Sun W., Fan J., Du J., Peng X., Small, 2023, 20, 2304407 |
| 33 | Liang S., Deng X., Ma P., Cheng Z., Lin J., Adv. Mater., 2020, 32(47), 2003214 |
| 34 | Qian X., Zheng Y., Chen Y., Adv. Mater., 2016, 28(37), 8097—8129 |
| 35 | Tang Z., Liu Y., He M., Bu W., Angew. Chem. Int. Ed., 2019, 58(4), 946—956 |
| 36 | Yang Z., Zhao Z., Cheng H., Shen Y., Xie A., Zhu M., J. Colloid Interface Sci., 2023, 641, 215—228 |
| 37 | Zhou Y., Fan S., Feng L., Huang X., Chen X., Adv. Mater., 2021, 33(48), 2104223 |
| 38 | Li Y., Xiao Y., Lin H. P., Reichel D., Bae Y., Lee E. Y., Jiang Y., Huang X., Yang C., Wang Z., Biomaterials, 2019, 188, 160—172 |
| 39 | Yu Q., Zhou J., Liu Y., Li X. Q., Li S., Zhou H., Kang B., Chen H., Xu J., Adv. Healthc. Mater., 2023, 12, 2301429 |
| 40 | Zhao F., Yu H., Liang L., Wang C., Shi D., Zhang X., Ying Y., Cai W., Li W., Li J., Zheng J., Qiao L., Che S., Yu J., Adv. Healthc. Mater., 2023, 12(29), 2301346 |
| 41 | Li Y., Xiao Y., Lin H. P., Reichel D., Bae Y., Lee E. Y., Jiang Y., Huang X., Yang C., Wang Z., Biomaterials, 2019, 188, 160—172 |
| 42 | Liang S., Deng X., Ma P., Cheng Z., Lin J., Adv. Mater., 2020, 32(47), 2003214 |
| 43 | Qian X., Zheng Y., Chen Y., Adv. Mater., 2016, 28(37), 8097—8129 |
| 44 | Carneiro B. A., El⁃Deiry W. S., Nat. Rev. Clin. Oncol., 2020, 17(7), 395—417 |
| 45 | Fu J., Li T., Zhu Y., Hao Y., Adv. Funct. Mater., 2019, 29(51), 1906195 |
| 46 | Chen K., Zhou A., Zhou X., Liu Y., Xu Y., Ning X., Nano Lett., 2023, 23(7), 3038—3047 |
| 47 | Gao W., Wang X., Zhou Y., Wang X., Yu Y., Sig Transduct. Target Ther., 2022, 7(1), 196 |
| 48 | Ryder C. B., Kondolf H. C., O’Keefe M. E., Zhou B., Abbott D. W., J. Mol. Biol., 2022, 434(4), 167183 |
| 49 | Wei X., Xie F., Zhou X., Wu Y., Yan H., Liu T., Huang J., Wang F., Zhou F., Zhang L., Cell Mol. Immunol., 2022, 19(9), 971—992 |
| 50 | Tan Y., Chen Q., Li X., Zeng Z., Xiong W., Li G., Li X., Yang J., Xiang B., Yi M., J. Exp. Clin. Cancer Res., 2021, 40 (1), 153 |
| 51 | Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X., Sig. Transduct. Target Ther., 2021, 6(1), 128 |
| 52 | Miao R., Jiang C., Chang W. Y., Zhang H., An J., Ho F., Chen P., Zhang H., Junqueira C., Amgalan D., Liang F. G., Zhang J., Evavold C. L., Hafner⁃Bratkovič I., Zhang Z., Fontana P., Xia S., Waldeck⁃Weiermair M., Pan Y., Michel T., Bar⁃Peled L., Wu H., Kagan J. C., Kitsis R. N., Zhang P., Liu X., Lieberman J., Immunity, 2023, 56(11), 2523—2541.e8 |
| 53 | Qiao L., Zhu G., Jiang T., Qian Y., Sun Q., Zhao G., Gao H., Li C., Adv. Mater., 2023, 36, 2308241 |
| 54 | Ma T., Chen J., Zhu P., Zhang C., Zhou Y.,Duan J., Life Sci., 2022, 307, 120868 |
| 55 | Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., Patel D. N., Bauer A. J., Cantley A. M., Yang W. S., Morrison B., Stockwell B. R., Cell, 2012, 149(5), 1060—1072 |
| 56 | Tang D., Kang R., Berghe T. V., Vandenabeele P., Kroemer G., Cell Res., 2019, 29(5), 347—364 |
| 57 | Xue Q., Yan D., Chen X., Li X., Kang R., Klionsky D. J., Kroemer G., Chen X., Tang D., Liu J., Autophagy, 2023, 19(7), 1982—1996 |
| 58 | Wang W., Lu K., Jiang X., Wei Q., Zhu L., Wang X., Jin H., Feng L., J. Exp. Clin. Cancer. Res., 2023, 42(1), 142 |
| 59 | Chen W., Xie W., Gao Z., Lin C., Tan M., Zhang Y., Hou Z., Adv. Sci., 2023, 10, 2303694 |
| 60 | Xu W., Qian J., Hou G., Wang T., Wang J., Wang Y., Yang L., Cui X., Suo A., Adv. Funct. Mater., 2022, 32(40), 2205013 |
| 61 | Ruan Y., Zhuang H., Zeng X., Lin L., Wang X., Xue P., Xu S., Chen Q., Yan S., Huang W., Adv. Healthc. Mater., 2023, 13, 2302537 |
| 62 | Li X., Zhen Y., Li S., Mater. Des., 2021, 209, 109958 |
| 63 | Keir M. E., Butte M. J., Freeman G. J., Sharpe A. H., Annu. Rev. Immunol., 2008, 26(1), 677—704 |
| 64 | Xu⁃Monette Z. Y., Zhou J., Young K. H., Blood, 2018, 131(1), 68—83 |
| 65 | Voli F., Valli E., Lerra L., Kimpton K., Saletta F., Giorgi F. M., Mercatelli D., Rouaen J. R. C., Shen S., Murray J. E., Ahmed⁃Cox A., Cirillo G., Mayoh C., Beavis P. A., Haber M., Trapani J. A., Kavallaris M., Vittorio O., Cancer Res., 2020, 80(19), 4129—4144 |
| 66 | Guo B., Yang F., Zhang L., Zhao Q., Wang W., Yin L., Chen D., Wang M., Han S., Xiao H., Xing N., Adv. Mater., 2023, 35(22), 2212267 |
| 67 | Liu T., Zhou Z., Zhang M., Lang P., Li J., Liu Z., Zhang Z., Li L., Zhang L., J. Control. Release, 2023, 362, 502—512 |
| 68 | Szabo C., Nat. Rev. Drug Discov., 2016, 15(3), 185—203 |
| 69 | Jin Q., Deng Y., Jia F., Tang Z., Ji J., Adv. Ther., 2018, 1(6), 1800084 |
| 70 | Li S., Liao R., Sheng X., Luo X., Zhang X., Wen X., Zhou J., Peng K., Front. Oncol., 2019, 9, 696 |
| 71 | Ji P., Yang K., Xu Q., Qin G., Zhu Q., Qian Y., Yao W., Pharmaceuticals, 2023, 16(10), 1394 |
| 72 | Opoku⁃Damoah Y., Zhang R., Ta H. T., Xu Z. P., Exploration, 2022, 2, 20210181 |
| 73 | Wu Y., Yuan M., Song J., Chen X., Yang H., ACS Nano, 2019, 13(8), 8505—8511 |
| 74 | Liu J., Liu Y., Liu N., Han Y., Zhang X., Huang H., Lifshitz Y., Lee S. T., Zhong J., Kang Z., Science, 2015, 347(6225), 970—974 |
| 75 | Wu Y., Su L., Yuan M., Chen T., Ye J., Jiang Y., Song J., Yang H., Angew. Chem. Int. Ed., 2021, 60(23), 12868—12875 |
| 76 | Liu J., Zhang Y., Lu L., Wu G., Chen W., Chem. Commun., 2012, 48(70), 8826—8828 |
| 77 | Ding H., Ren F., Liu P., Feng Y., Ma X., Shen Z., Shi Q., Xu M., Li W., Chen H., Angew. Chem. Int. Ed., 2023, 62(44), e202311549 |
| 78 | Tang Z., Zhao P., Ni D., Liu Y., Zhang M., Wang H., Zhang H., Gao H., Yao Z., Bu W., Mater. Horizons., 2018, 5(5), 946—952 |
| 79 | Shevtsov M., Balogi Z., Khachatryan W., Gao H., Vígh L., Multhoff G., Cells, 2020, 9(5), 1263 |
| 80 | Zang P., Du Y., Yu C., Yang D., Gai S., Feng L., Liu S., Yang P., Lin J., Chem. Mater., 2023, 35(18), 7770—7780 |
| 81 | Chan L., Liu Y., Chen M., Su Y., Guo J., Zhu L., Zhan M., Chen T., Lu L., Adv. Funct. Mater., 2023, 33(33), 2302054 |
| 82 | Zhou J., Yu Q., Song J., Li S., Li X., Kang B., Chen H., Xu J., Angew. Chem. Int. Ed., 2023, 62(12), e202213922 |
| 83 | Gao W., Wang X., Zhou Y., Wang X., Yu Y., Sig. Transduct. Target Ther., 2022, 7(1), 1—26 |
| 84 | Hanahan D., Cancer Discov., 2022, 12(1), 31—46 |
| 85 | Steinbrueck A., Sedgwick A. C., Brewster J. T., Yan K. C., Shang Y., Knoll D. M., Vargas⁃Zúñiga G. I., He X. P., Tian H., Sessler J. L., Chem. Soc. Rev., 2020, 49(12), 3726—3747 |
| [1] | 张荡, 孙小敏, 杨海跃, 宋勃翰, 丛萌, 王宇新, 丁锋, 徐珊珊, 毕赛, 王磊. 基于纳米酶的微纳米马达在智能药物递送中的应用[J]. 高等学校化学学报, 2025, 46(1): 76. |
| [2] | 陈俊年, 张海峰, 王海兵, 杨火诚, 罗忠. 基于超分子纳米递送系统的精准医疗研究进展[J]. 高等学校化学学报, 2025, 46(1): 50. |
| [3] | 张璐, 邹云鹤, 徐中胜, 刘云. 中空结构纳米材料在生物医学领域的应用: 现状与展望[J]. 高等学校化学学报, 2023, 44(8): 20230134. |
| [4] | 陶淞源, 夏春雷, 杨柏. 碳点的结构与发光起源[J]. 高等学校化学学报, 2023, 44(10): 20230241. |
| [5] | 张钤, 刘雅薇, 王帆, 刘凯, 张洪杰. 稀土纳米材料在高分辨活体成像及诊疗中的应用[J]. 高等学校化学学报, 2022, 43(12): 20220552. |
| [6] | 章丽玲, 刘浏, 郑明秋, 方文凯, 刘达, 唐宏武. 基于上转换发光共振能量转移的CRISPR/Cas12a生物传感系统用于HPV16 DNA双信号检测[J]. 高等学校化学学报, 2022, 43(11): 20220412. |
| [7] | 温炜, 黄达锭, 鲍劲霄, 张增辉. PD-1与单克隆抗体残基特异性结合机制的计算丙氨酸扫描研究[J]. 高等学校化学学报, 2021, 42(7): 2161. |
| [8] | 丁中振, 李天, 李长明, 赵宇飞, 宋宇飞. 水滑石基催化剂催化合成碳纳米材料的研究进展[J]. 高等学校化学学报, 2021, 42(6): 1622. |
| [9] | 杨瑞琪, 于欣, 刘宏. 四氧化三锡基光催化纳米材料的研究进展[J]. 高等学校化学学报, 2021, 42(5): 1340. |
| [10] | 王常耀, 王帅, 段林林, 朱晓航, 张兴淼, 李伟. 原位限域生长策略制备有序介孔碳负载的超小MoO3纳米颗粒[J]. 高等学校化学学报, 2021, 42(5): 1589. |
| [11] | 葛浩英, 杜健军, 龙飒然, 孙文, 樊江莉, 彭孝军. 功能化金纳米材料在肿瘤诊疗中的研究与应用[J]. 高等学校化学学报, 2021, 42(4): 1202. |
| [12] | 佘沛鸿, 徐文洲, 关卜源. 大孔道介孔氧化硅/碳基纳米材料的设计合成与应用[J]. 高等学校化学学报, 2021, 42(3): 671. |
| [13] | 郑海娇, 姜丽艳, 贾琼. 精氨酸功能化磁性纳米材料的制备及在磷酸化肽富集中的应用[J]. 高等学校化学学报, 2021, 42(3): 717. |
| [14] | 万月, 宋美娜, 赵美廷. 二维金属有机框架纳米片的合成及在超电容和电催化领域的应用[J]. 高等学校化学学报, 2021, 42(2): 575. |
| [15] | 史江维, 孟楠楠, 郭亚梅, 于一夫, 张兵 . 二维材料用于电催化析氢的研究进展[J]. 高等学校化学学报, 2021, 42(2): 492. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||