

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (6): 1622.doi: 10.7503/cjcu20200874
丁中振1, 李天1, 李长明2, 赵宇飞1( ), 宋宇飞1(
), 宋宇飞1( )
)
收稿日期:2020-12-16
出版日期:2021-06-10
发布日期:2021-06-08
通讯作者:
赵宇飞
E-mail:zhaoyufei@mail.buct.edu.cn;songyf@mail.buct.edu.cn
作者简介:宋宇飞, 男, 博士, 教授, 主要从事多酸功能材料和超分子组装研究. E-mail: 基金资助:
DING Zhongzhen1, LI Tian1, LI Changming2, ZHAO Yufei1( ), SONG Yu⁃Fei1(
), SONG Yu⁃Fei1( )
)
Received:2020-12-16
Online:2021-06-10
Published:2021-06-08
Contact:
ZHAO Yufei
E-mail:zhaoyufei@mail.buct.edu.cn;songyf@mail.buct.edu.cn
Supported by:摘要:
碳纳米材料是一类推动能源存储、 多相催化、 高性能复合和生物医药等领域发展的重要材料, 可控合成碳纳米材料对相关领域的发展具有重要意义. 水滑石(LDHs)材料具有层板金属种类及含量可调等特点, 经焙烧、 还原后可制备出金属种类、 密度和粒径分布各异的高分散、 高稳定金属纳米催化剂, 可实现高效催化生长各种类型的碳纳米材料. 此外, 通过调控反应条件和反应器等, 可以影响LDHs基金属纳米催化剂催化生长的碳纳米材料的结构和性能. 本文总结了LDHs基金属纳米催化剂的可控制备、 碳纳米材料结构调控以及利用LDHs基催化剂制备的碳纳米材料的应用等方面的研究工作, 并阐明了催化剂的可控制备是控制合成碳纳米材料的核心手段, 这为利用LDHs基催化剂进一步合成更高性能碳纳米材料的研究指明了方向. 此外, 本文还结合近些年在光、 电及光热催化方面的研究进展, 展望了基于新型LDHs纳米结构生长碳纳米材料的研究前景.
中图分类号:
TrendMD:
丁中振, 李天, 李长明, 赵宇飞, 宋宇飞. 水滑石基催化剂催化合成碳纳米材料的研究进展. 高等学校化学学报, 2021, 42(6): 1622.
DING Zhongzhen, LI Tian, LI Changming, ZHAO Yufei, SONG Yu⁃Fei. Research Progress of Catalytic Synthesis of Carbon Nanomaterials by Layered Double Hydroxide-based Catalysts. Chem. J. Chinese Universities, 2021, 42(6): 1622.
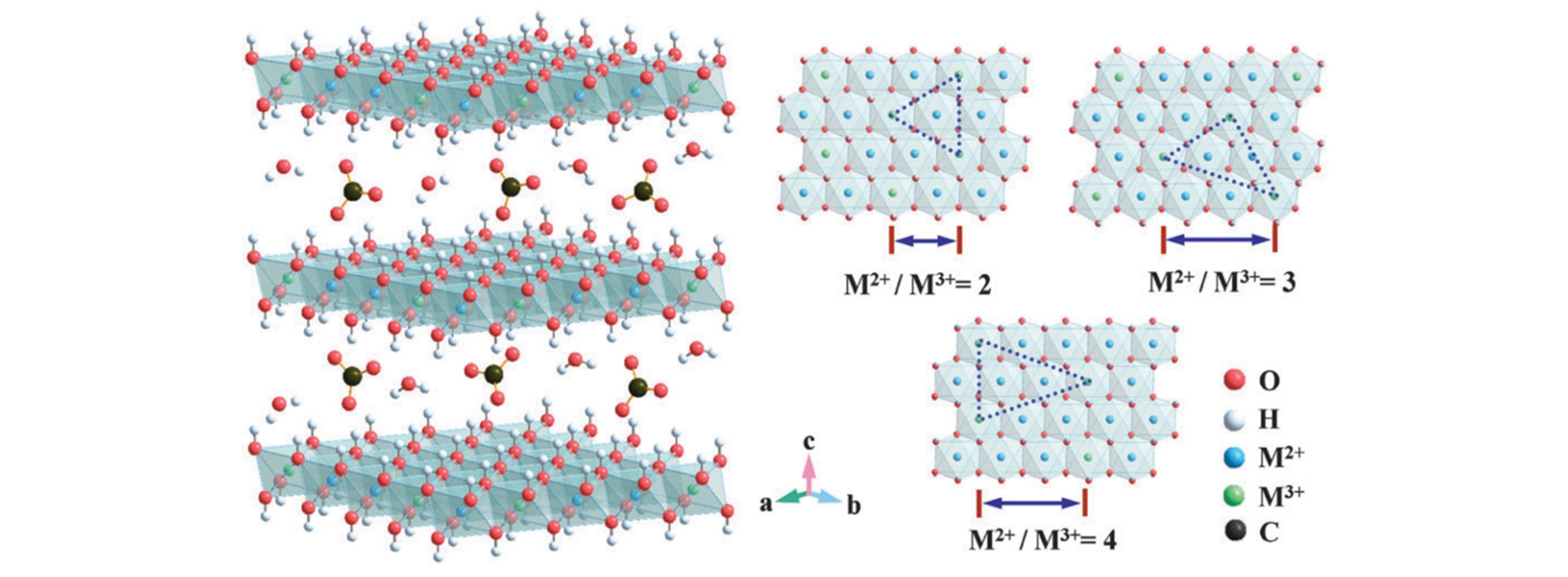
Fig.1 Idealized structure of carbonate?intercalated LDHs with different M2+/M3+ molar ratios, showing the metal hydroxide octahedral stacked along the crystallographic c?axis, as well as water and anions present in the interlayer region[17]Copyright 2014, the Royal Society of Chemistry.
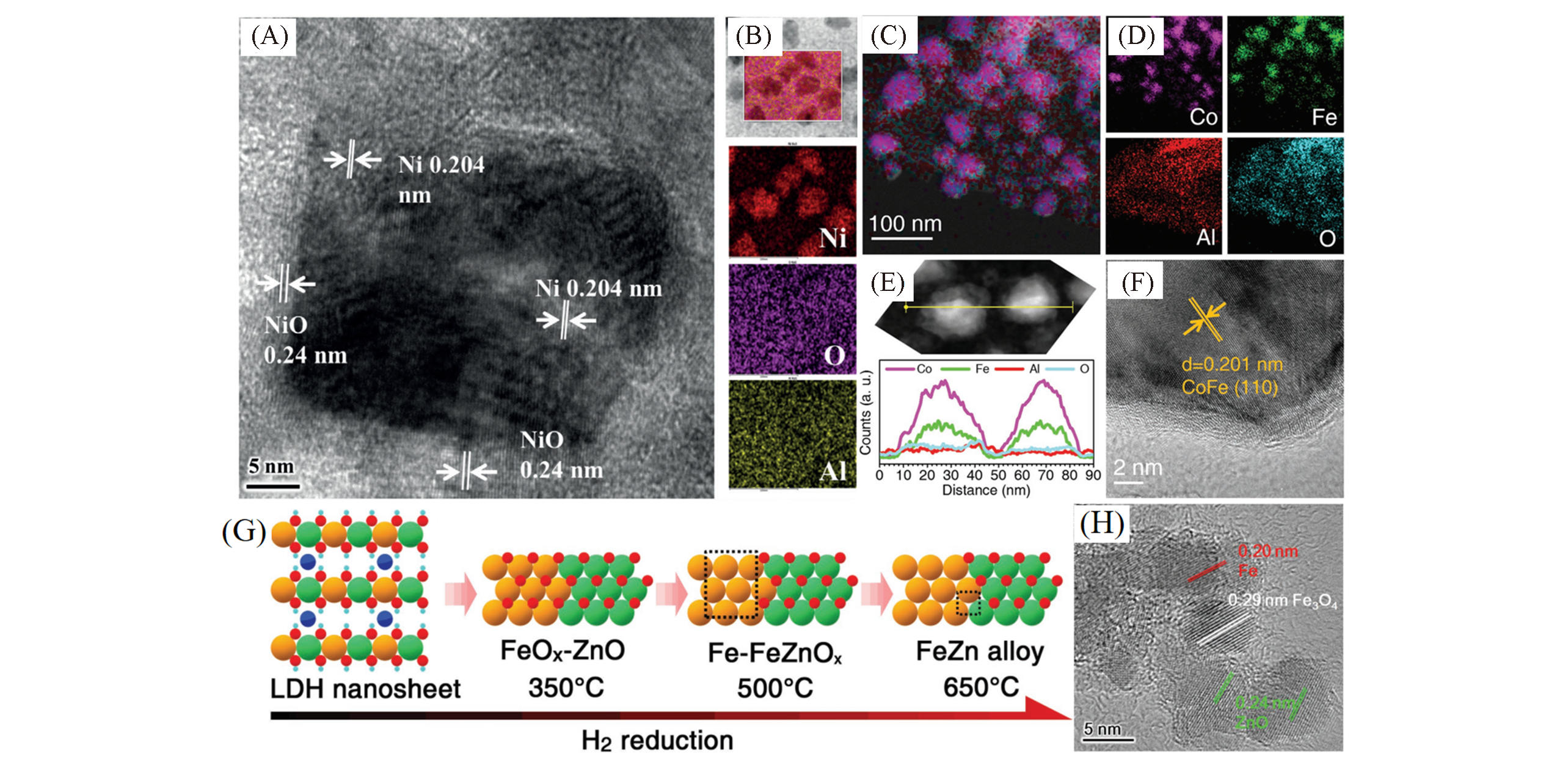
Fig.2 Different element types of LDH?based metal nanocatalysts(A, B) High resolution transmission electron microscopy(HRTEM) image(A) and the element mappings(B) of the Ni@NiO[37]; (C―F) overlayed energy dispersive spectrometer(EDS) element map(C) and individual EDS maps for Co, Fe, Al, and O(D),EDS line-scan element profile over two adjacent nanoparticles(E) and HRTEM image(F) confirming formation of a discrete CoFe alloy nanoparticle[40]; (G, H) preparation of the Fe-x catalysts by hydrogen reduction of ZnFeAl-LDH nanosheets at different temperatures(x)(G) and HRTEM image of Fe-500(H)[39].(A, B) Copyright 2016, Wiley-VCH. (C―F) Copyright 2018, Wiley-VCH. (G, H) Copyright 2018, Wiley-VCH.
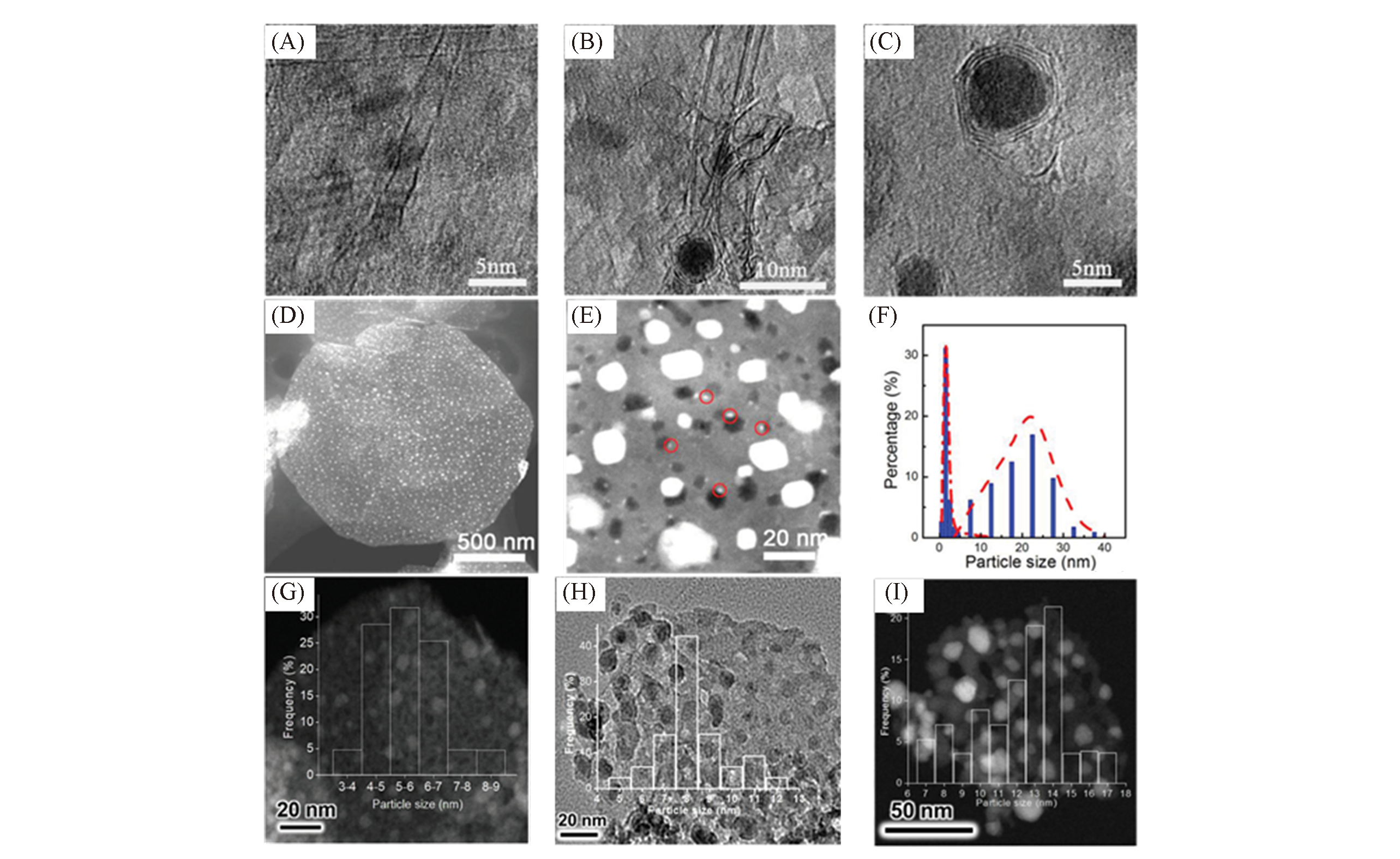
Fig.3 LDH?based metal nanocatalysts of different particle size and special particle size distribution(A—C) TEM images of Fe0.05Mg1.85Al1, Fe0.20Mg1.99Al1 and Fe0.83Mg1.61Al1 LDH-based metal nanocatalysts, respectively[42]; (D―F) scanning TEM(STEM) images(D, E) of the catalyst NPs formed on the FeMoMgAl-LDH flakes reduced by H2 and CH3NO for 5 min at 950 ℃ and their bimodal particle size distribution(F)[21]; (G―I) the supported Co metal nanoparticle catalyst and the particle size distribution of Co metal nanoparticle obtained by reducing ZnCoAl-LDH at 450 ℃(G), 550 ℃(H) and 700 ℃(I)[38].(A―C) Copyright 2018, Wiley-VCH. (D―F) Copyright 2014, Wiley-VCH. (G―I) Copyright 2018, Wiley-VCH.
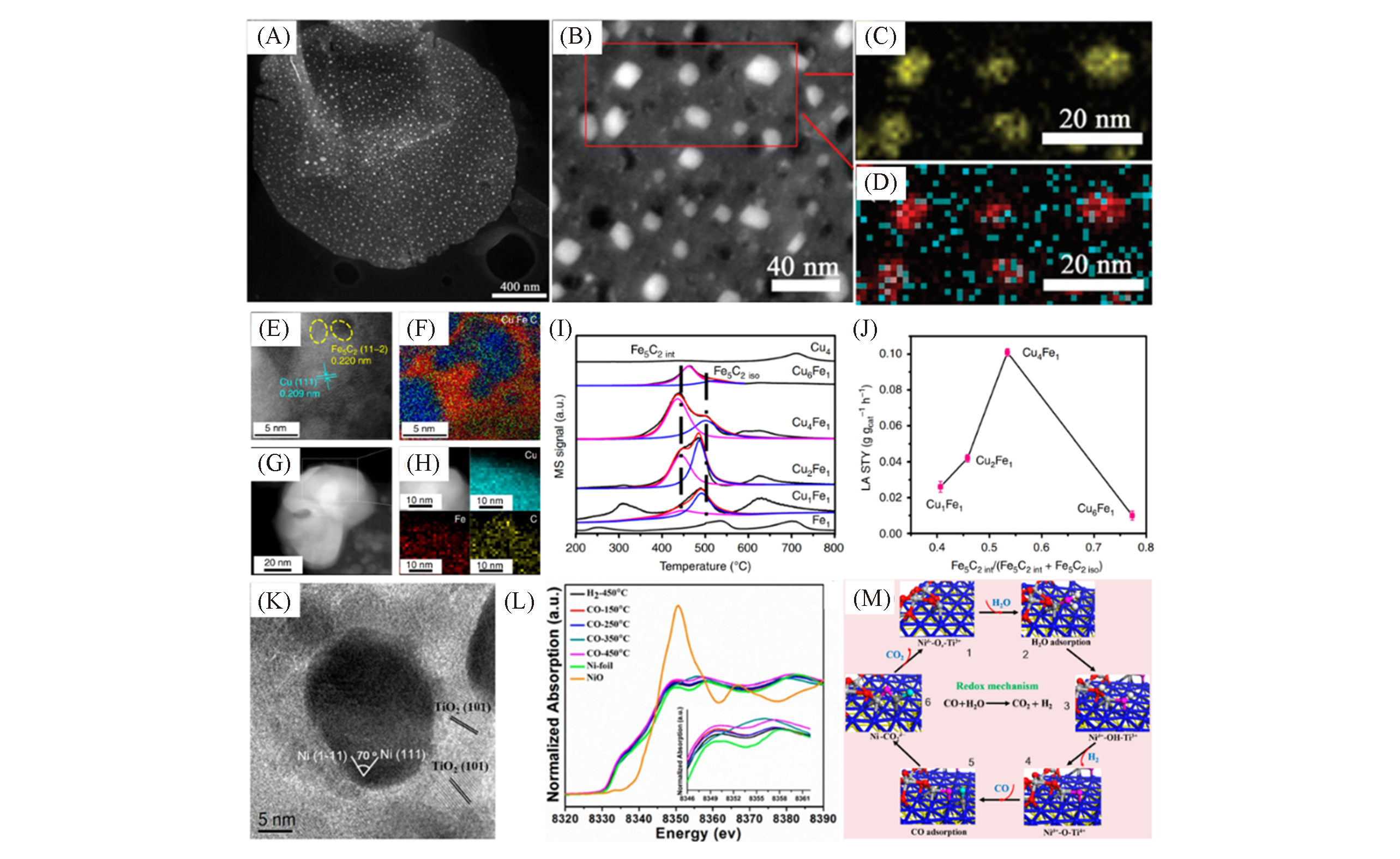
Fig.4 Highly dispersed LDH?based metal nanocatalysts(A) STEM image of the Fe NPs distributed on the reduced FeMgAl layered bimetal oxide(LDO) flake; (B, C) the high-angle annular darkfield scanning TEM(HAADF-STEM) image(B) and Fe map(C) of the NPs formed on the FeMgAl-Mo LDO flakes reduced by H2; (D) the element mapping showing the distribution of Fe(red) and Mo(turquoise) on the reduced FeMgAl-Mo LDO flakes[43]; (E) HRTEM image of Cu4Fe1 sample; (F) EELS mapping of Cu(blue), C(red), and Fe(green) of (E); (G, H) HAADF-STEM image(G) and EDS mapping of the selected region[white box in(G)] showing elemental distribution of Cu, C, and Fe of Cu4Fe1 sample(G); (I) CO-TPD profiles of Fe1, Cu4, and CuxFey samples; (J) long-chain alcohols space time yield as a function of interfacial sites in the catalyst[44]; (K) HRTEM image of the Ni@TiO2-x; (L) in?situ EXAFS spectra at different atmospheres and temperatures; (M) mechanism of WGS reaction catalyzed on Ni@TiO2-x catalyst[45].(A―D) Copyright 2010, American Chemical Society. (E―J) Copyright 2020, Springer Nature. (K―M) Copyright 2018, American Chemical Society.
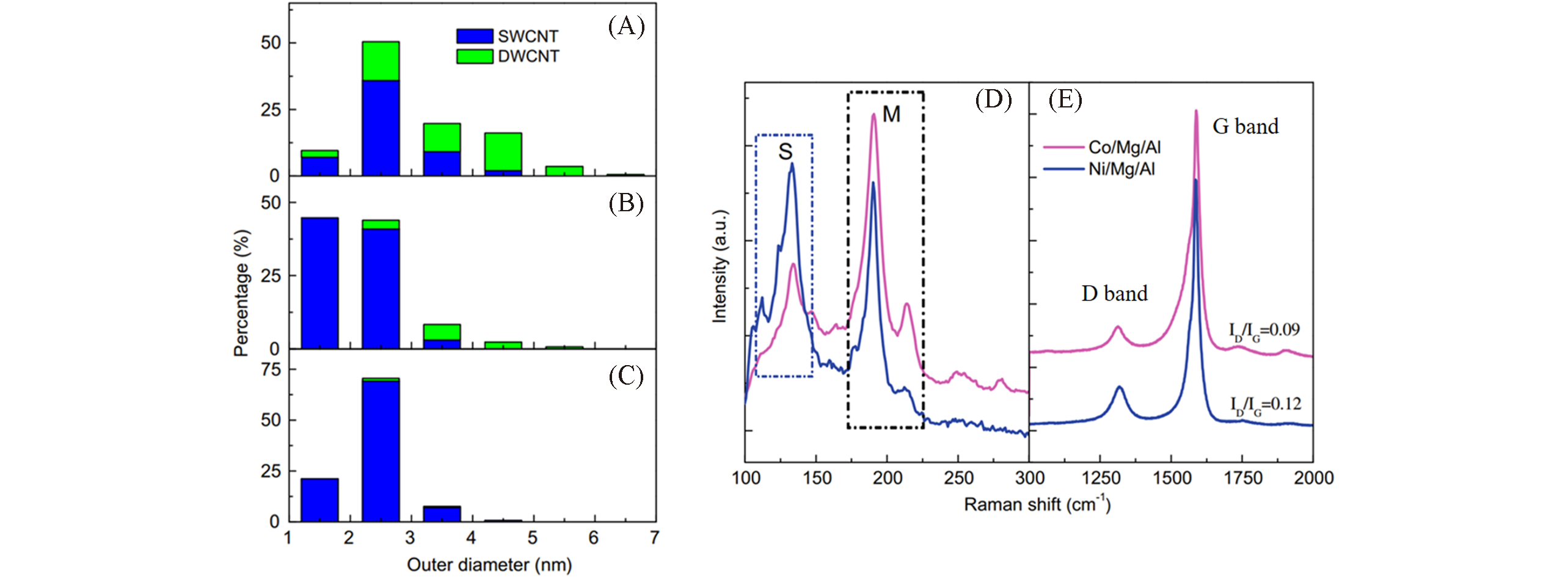
Fig.6 Wall number, diameter distribution and graphitization degree of CNTs grown on different LDH?based catalysts[41](A―C) The wall number, diameter distribution of CNTs grown on FeMgAl(A), CoMgAl(B) and NiMgAl(C) LDH-based catalysts; (D, E) Raman spectra of SWCNTs grown on CoMgAl and NiMgAl LDH-based catalysts: (D) RBM peak and (E) D peak and G peak. Copyright 2010, Elsevier.
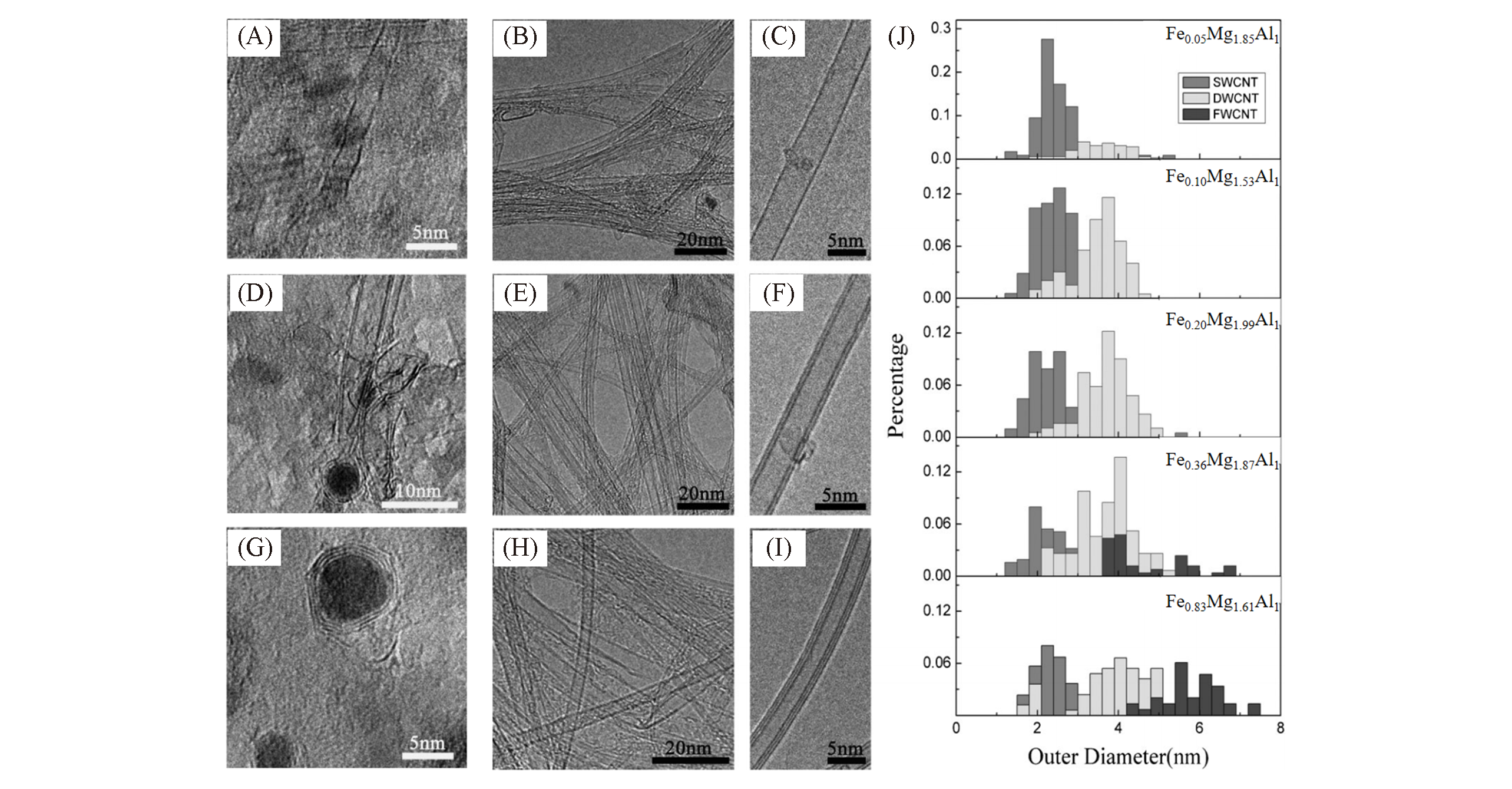
Fig.7 Preparation of CNTs with different wall numbers and diameters by LDH?based metal namocatalysts of different particle sizes[42](A―I) TEM images of three kinds of LDH-based metal nanocatalysts of Fe0.05Mg1.85Al1-LDH(A―C), Fe0.20Mg1.99Al1-LDH(D―F), Fe0.83Mg1.61Al1-LDH(G―I) and the CNTs grown on corresponding catalyst; (J) outer diameter distribution of CNTs grown on LDH-based metal nanocatalysts with different Fe content. Copyright 2010, Wiley-VCH.
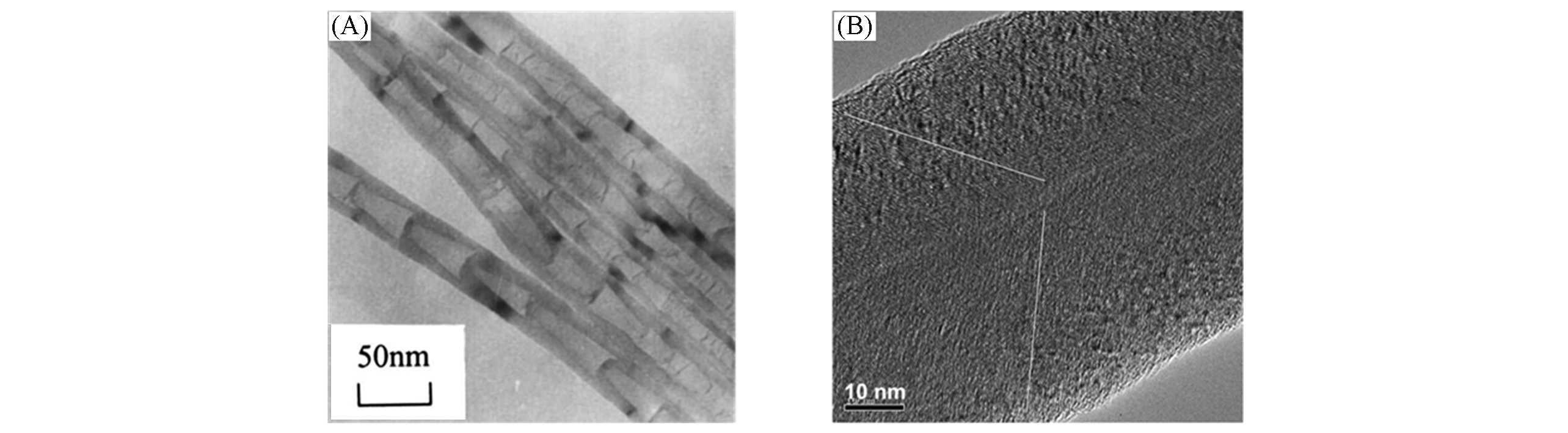
Fig.9 Controlled preparation of CNFs with different structures(A) Bamboo-shaped CNFs[55]; (B) fishbone structure CNFs[56]. (A) Copyright 2001, Elsevier. (B) Copyright 2005, Elsevier.
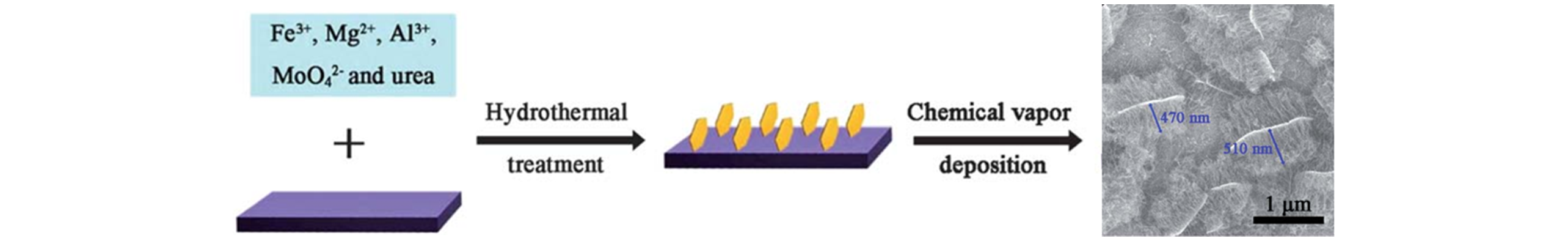
Fig.13 Schematic illustration of the procedure of the bulk preferential growth of short aligned SWCNTs from FeMoMgAl?LDH film[63]Copyright 2012, The Royal Society of Chemistry.
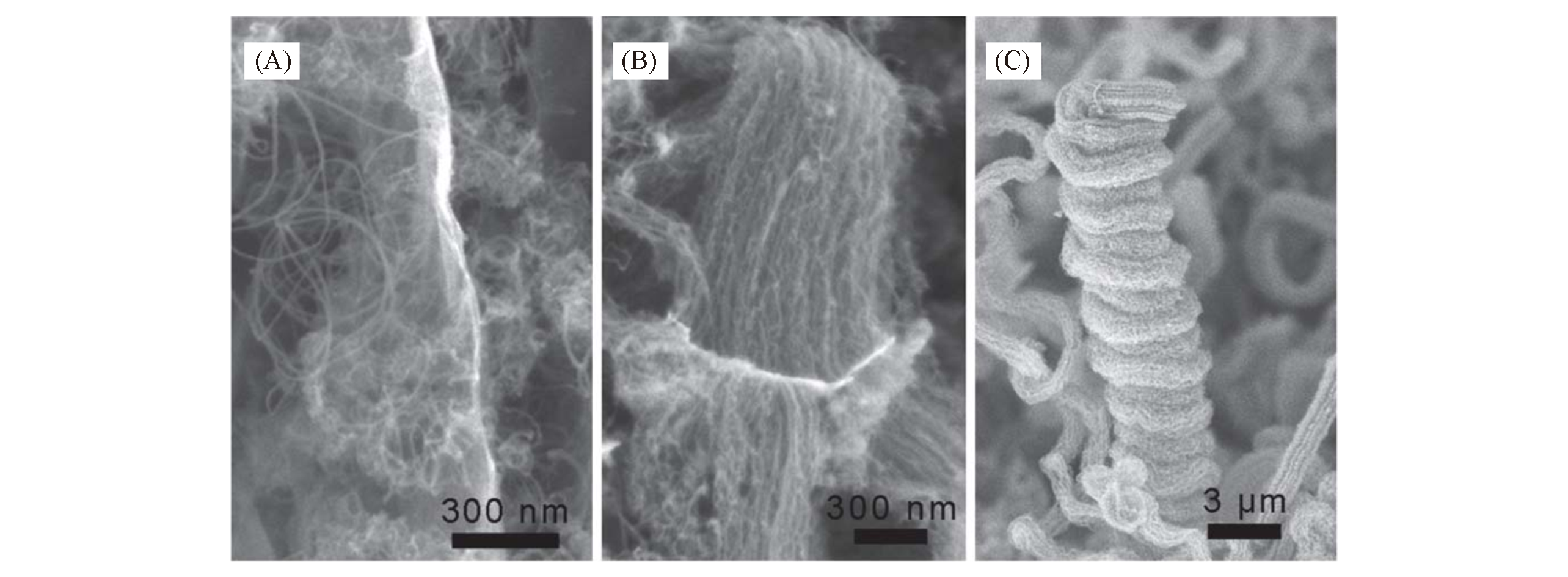
Fig.14 SEM images of CNTs with different aggregation forms[64](A) Entangled CNTs grown on Mg0.64Fe0.02Al0.34-LDH-based catalyst; (B) aligned CNTs grown on Mg0.62Fe0.06Al0.32-LDH-based catalyst; (C) double helical aligned CNTs grown on Mg0.47Fe0.24Al0.29-LDH-based catalyst. Copyright 2014, The Royal Society of Chemistry.
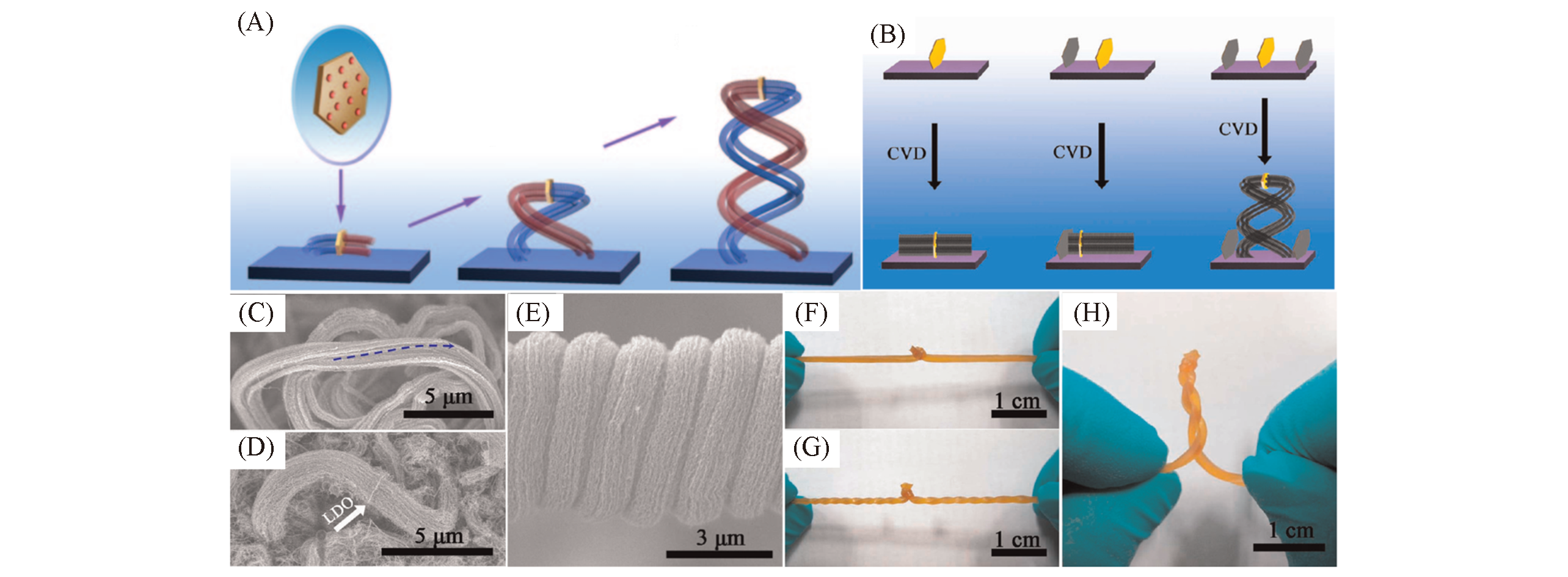
Fig.15 Growth process, morphology characterization and growth mechanism of carbon?nanotube?array double helices(A) The growth diagram of carbon-nanotube-array double helices[22]; (B) schematic illustration showing the three different evolution processes for CNT array growth from both sides of a LDH flake(space confinement is indicated by the gray flakes); (C―E) SEM images showing a long CNT bundle with obvious rotation(C), two CNT bundles grown oppositely from a LDO flake with the same right-handed rotation(D) and the nonrotation of the CNT bundles in the CNT-array double helix(E); (F―H) photo images showing evolution process of the self-organization of a twisted rubber band with a node into a double-helical structure[65].(A) Copyright 2010, Wiley-VCH. (B―H) Copyright 2012, American Chemical Society.
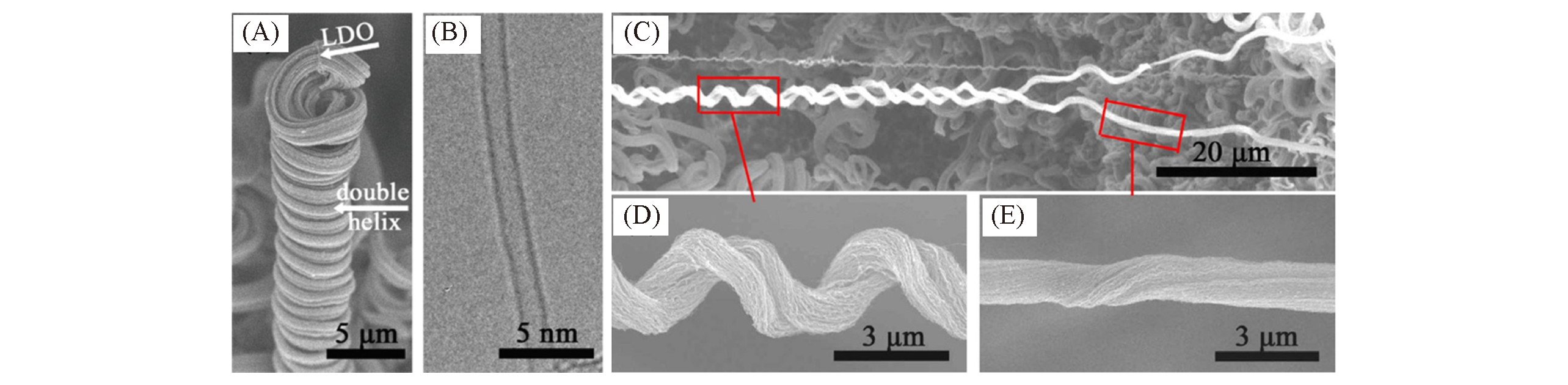
Fig.16 Stretchable single?walled carbon?nanotube?array double helices(A, B) SEM image(A) and HRTEM image(B) of single-walled carbon-nanotube-array double helices[43]; (C―E) SEM images of stretchable single-walled carbon-nanotube-array double helices[66]. (A, B) Copyright 2010, American Chemical Society. (C―E) Copyright 2011, Elsevier.
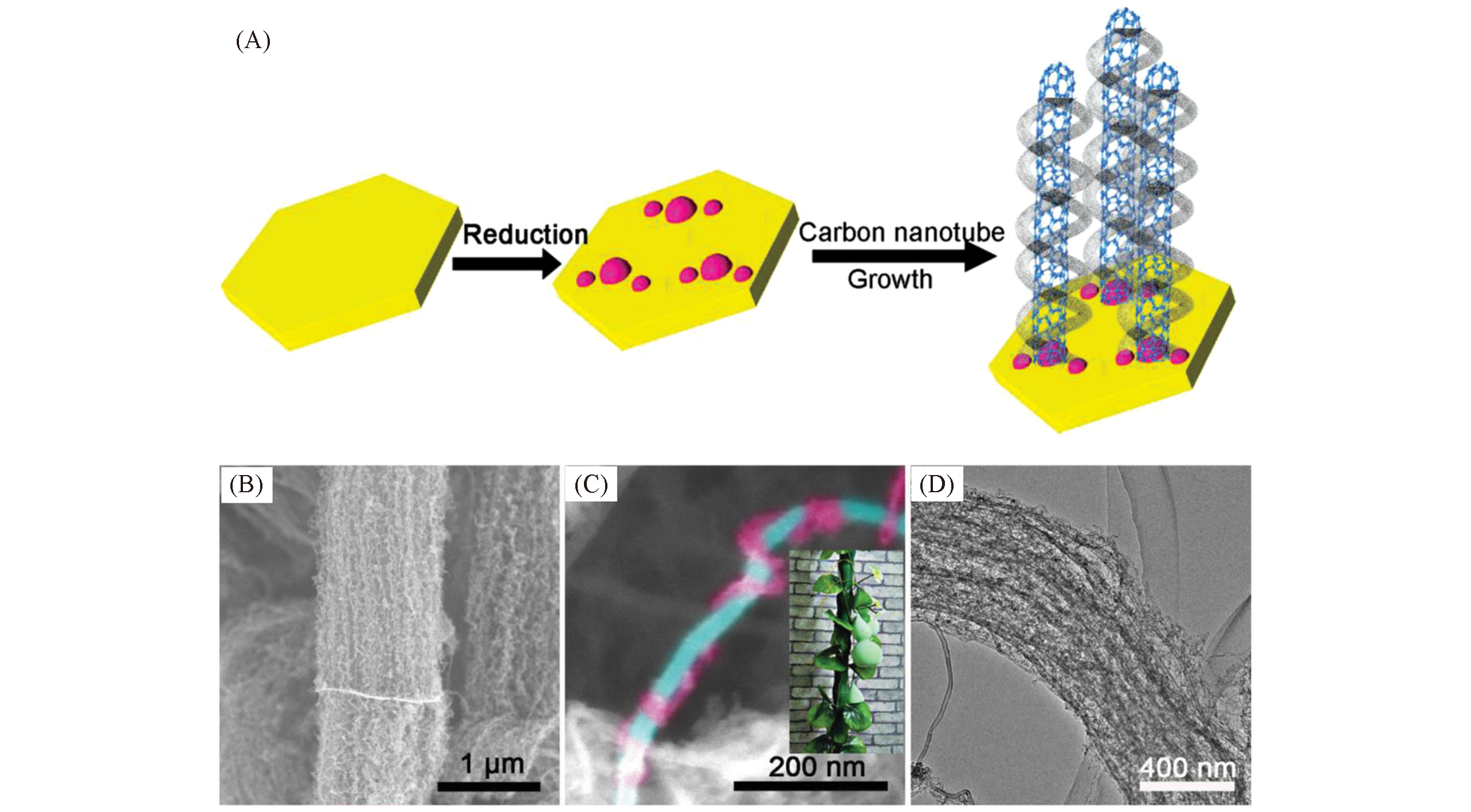
Fig.17 Growth diagram and microscopic characterization the vine?tree?like CNTs[21](A) Schematic illustration of the CCVD self-assembly of CNTs into a vine-tree-like structure on flake catalysts; (B, C) SEM ima-ges of the CNT bundles(B) and an obvious vine-tree-like CNT(C), the inserted image in (C) shows an optical image of the gourd vines wrapping around a bamboo; (D) TEM image of the as-grown VT-CNTs assembly. Copyright 2014, Wiley-VCH.
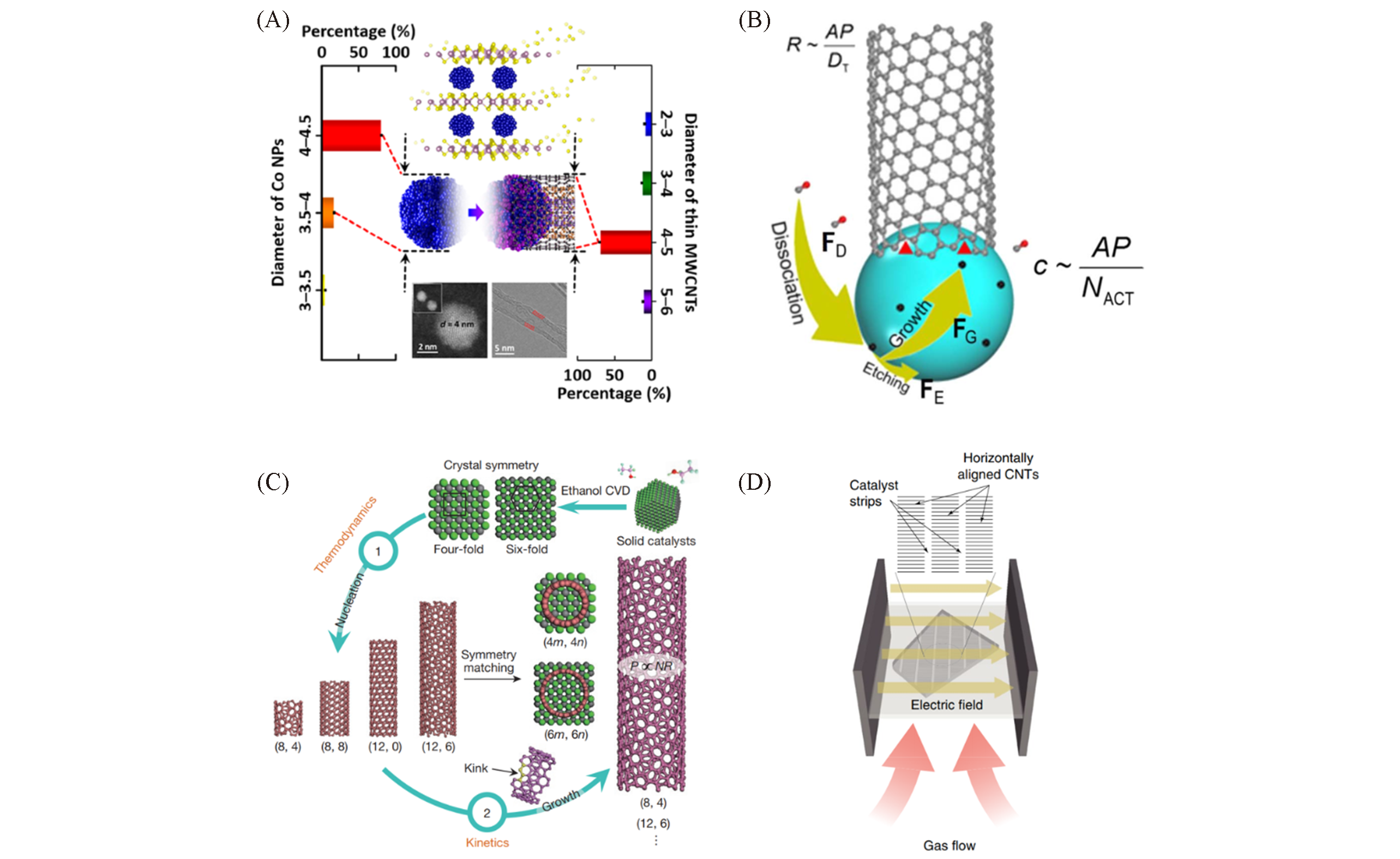
Fig.18 The latest development of CNTs preparation(A) Schematic diagram of MoS2 used to prepare MWCNTs with narrow diameter distribution[67]; (B) growth model for SWCNT growth in the absence of sufficient etching agents[68]; (C) two-step control of the chirality of SWNTs in ethanol chemical vapour deposition[69]; (D) schematic diagram of the electro-renucleation(ERN) system preparing horizontally aligned CNTs[70].(A) Copyright 2020, American Chemical Society. (B) Copyright 2019, American Association for the Advancement of Science. (C) Copyright 2017, Springer Nature. (D) Copyright 2018, Springer Nature.
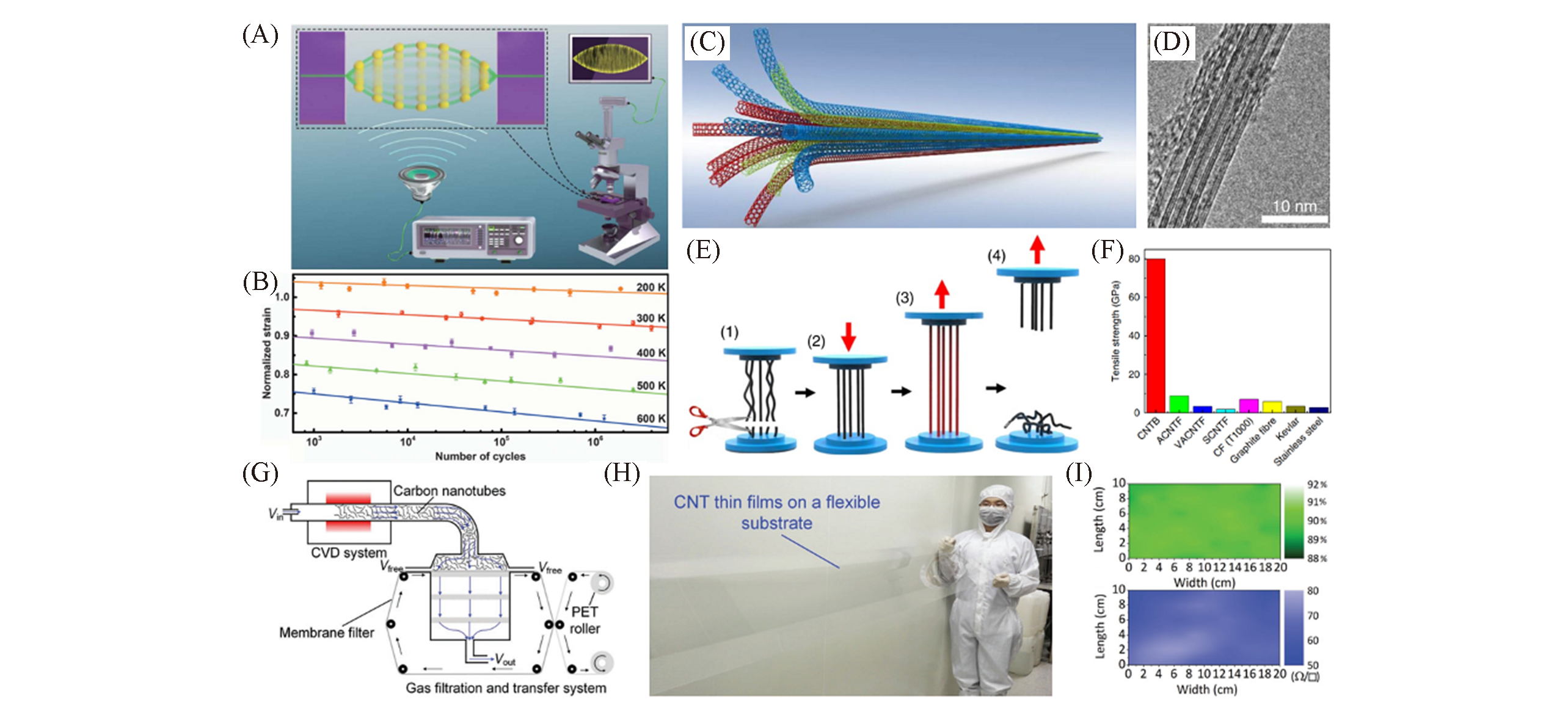
Fig.19 CNTs property testing and CNTs device preparation(A) Schematic illustration of ART system; (B) fatigue behavior of CNTs at different temperatures[70]; (C) schematic illustration of CNTB; (D) HRTEM image of CNTB; (E) illustration of the stretching and breaking process for a CNTB with uniform initial strains after the STR treatment; (F) strength comparison between CNTBs, high-performance commercial materials and other CNT fibres made by different methods[10]; (G) schematic showing the apparatus designed for the synthesis, deposition, and transfer of SWCNT films; (H) a SWCNT thin film transferred on a flexible PET substrate with a length of more than 2 m; (I) transmittance and sheet resistance mapping of the as-prepared SWCNT thin films[71]. (A, B) Copyright 2020, American Association for the Advancement of Science. (C―F) Copyright 2018, American Chemical Society. (G―I) Copyright 2018, Wiley-VCH.
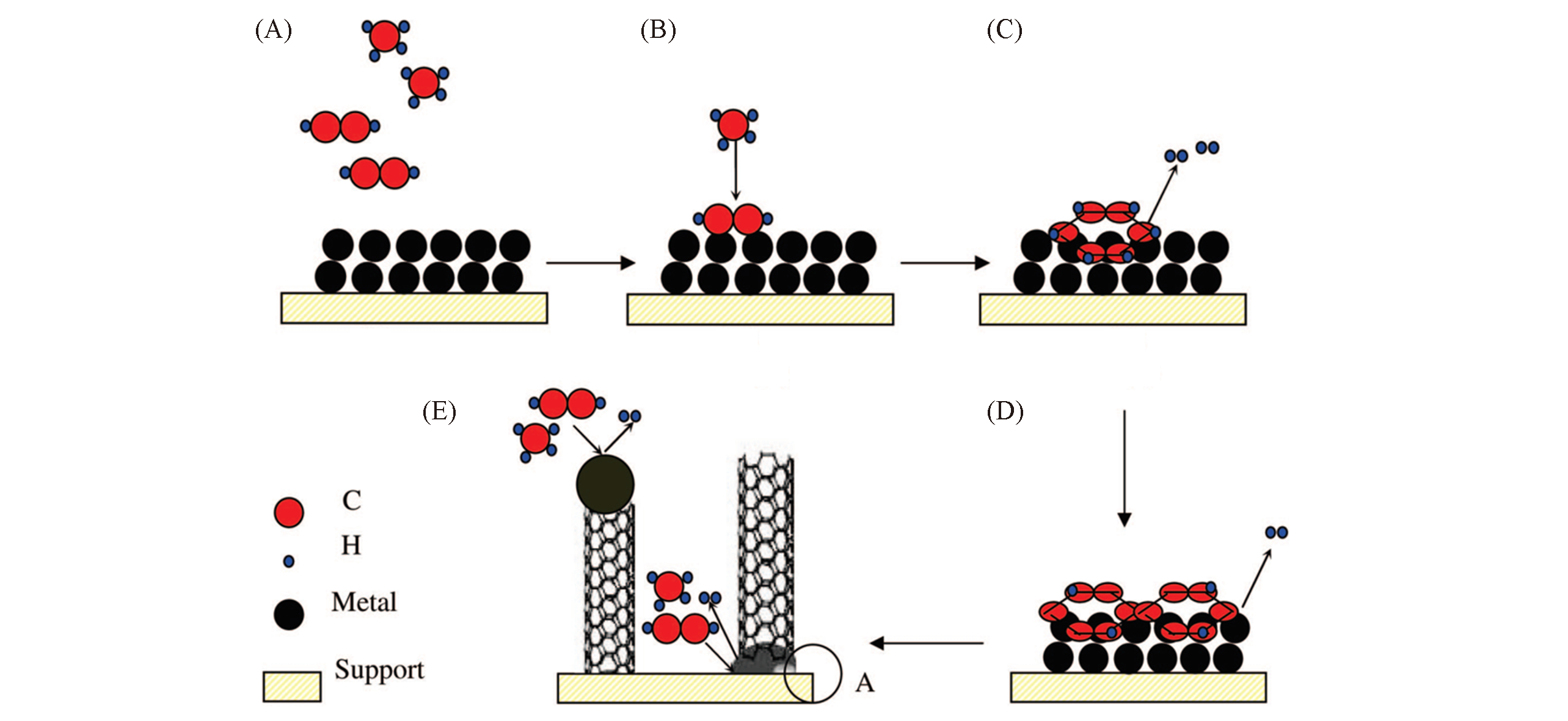
Fig.20 Model of the combined decomposition of CH4 and C2H2 on a catalyst to produce H2 and CNTs[75](A) C2H2 in the bulk gas phase to the surface of the catalyst; (B) CH4 insertion into absorbed C2H2 on the surface of the catalyst; (C) formation of an aromatic intermediate on the surface of the catalyst; (D) formation of a polyaromatic intermediate on the surface of the catalyst; (E) gradual dehydrogenation of intermediates to form the final CNT product. Note: (D) is the local A region of (E). Copyright 2008, American Chemical Society.
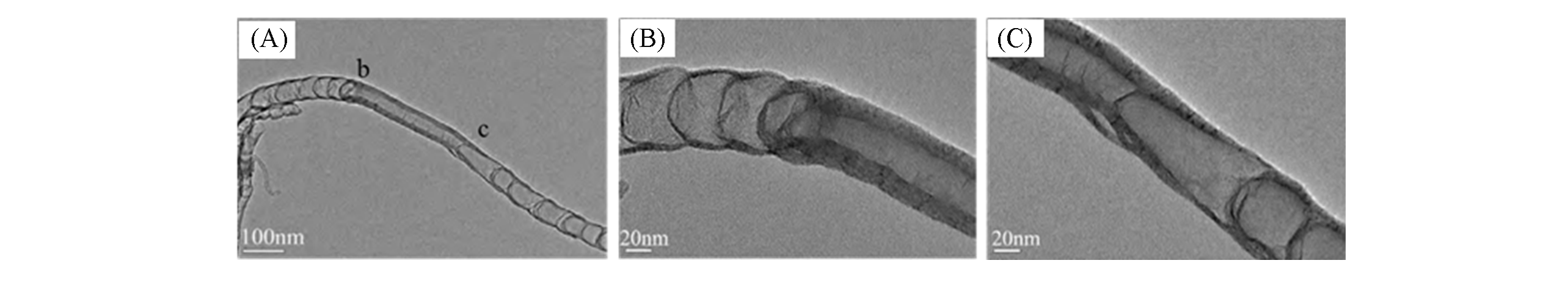
Fig.21 HRTEM images for two junctions within an individual tube[77](A) N-CNTs/MWCNTs/N-CNTs junction;(B, C) high-magnification images of areas b and c in (A), respectively.Copyright 2016, the authors.
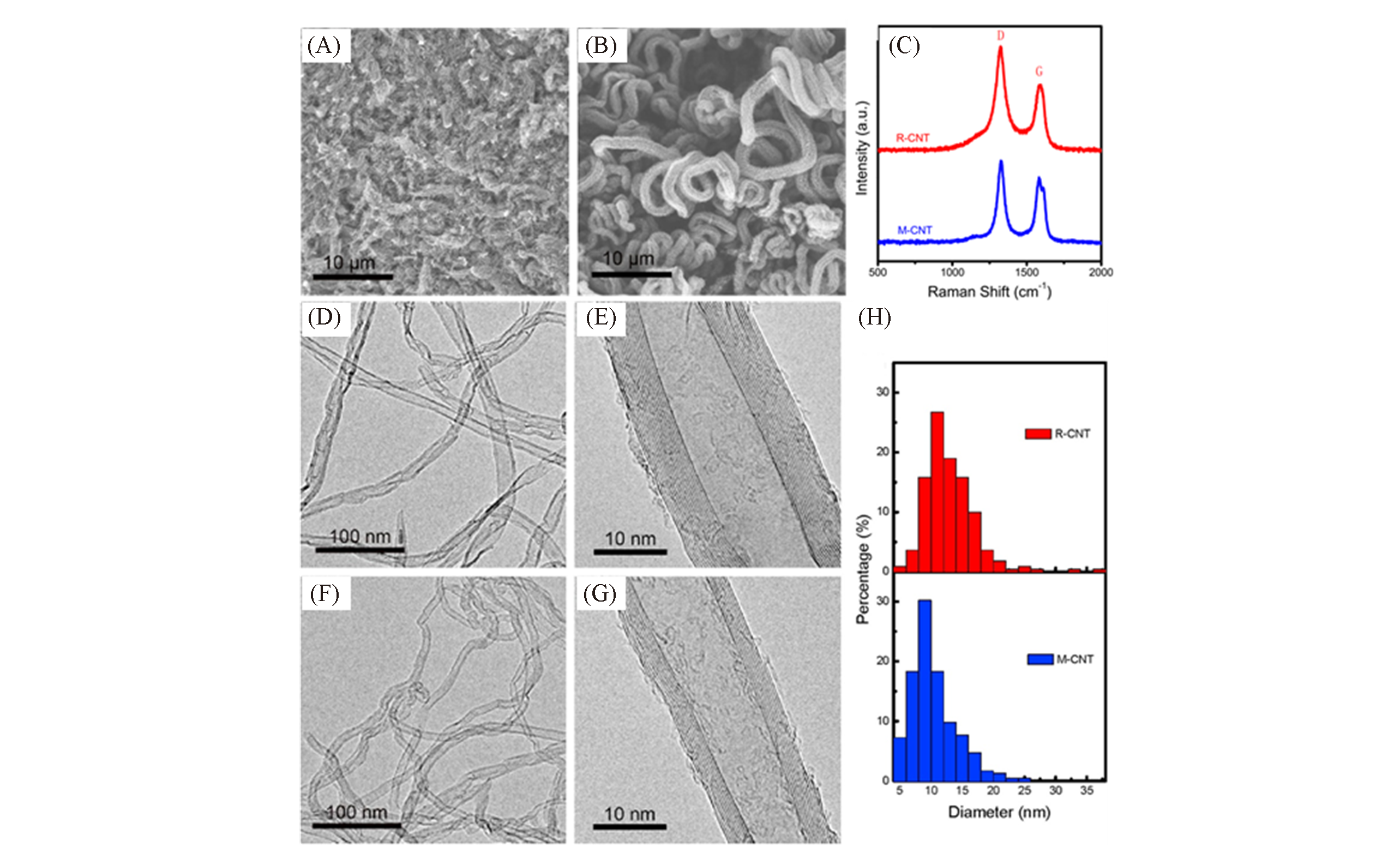
Fig.23 Comparison of R?CNTs(A, D, E) and M?CNTs(B, F, G)[84](A, B) SEM images; (C) Raman spectra; (D, F) TEM images; (E, G) HRTEM images; (H) the corresponding diameter distributions of the CNT products. Copyright 2016, Elsevier.
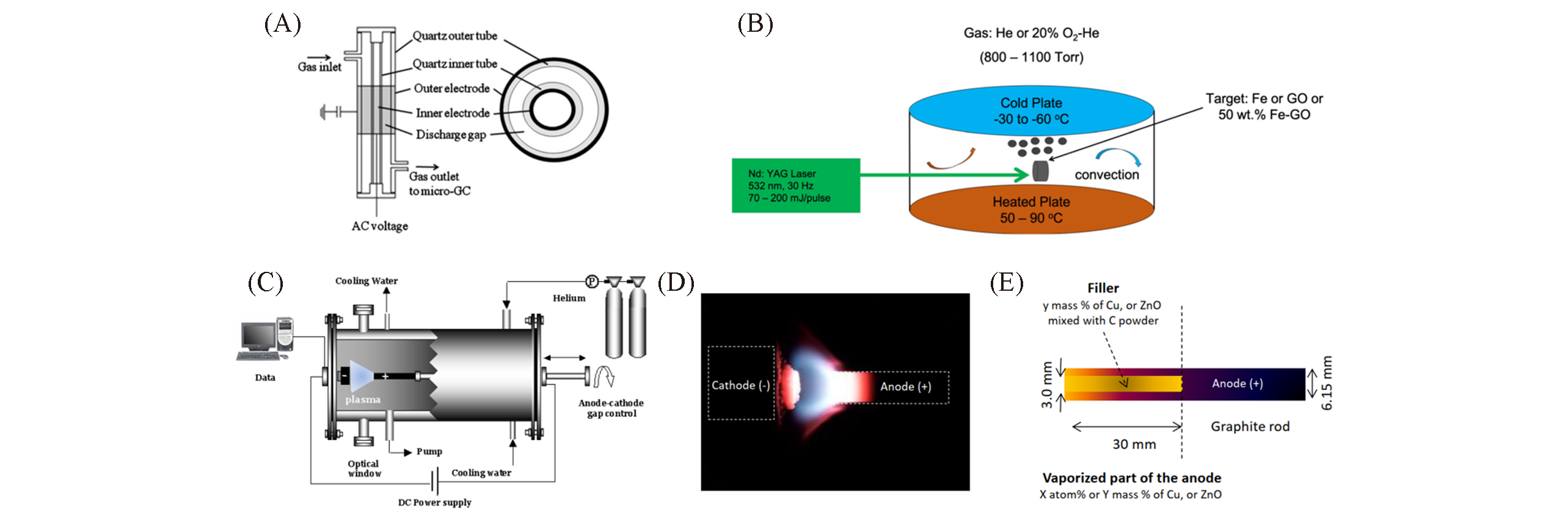
Fig.24 Progress in photo/electric preparation of carbon nanomaterials(A) DBD reaction device[85]; (B) schematic diagram of LVCC[86]; (C―E) arc discharge set-up: (C) schematic; (D) photography of the luminous plasma zone created between the anode and the cathode; (E) anode composition[87].(A) Copyright 2011, Elsevier. (B) Copyright 2020, Springer Nature. (C―E) Copyright 2020, Multidisciplinary Digital Publishing Institute.
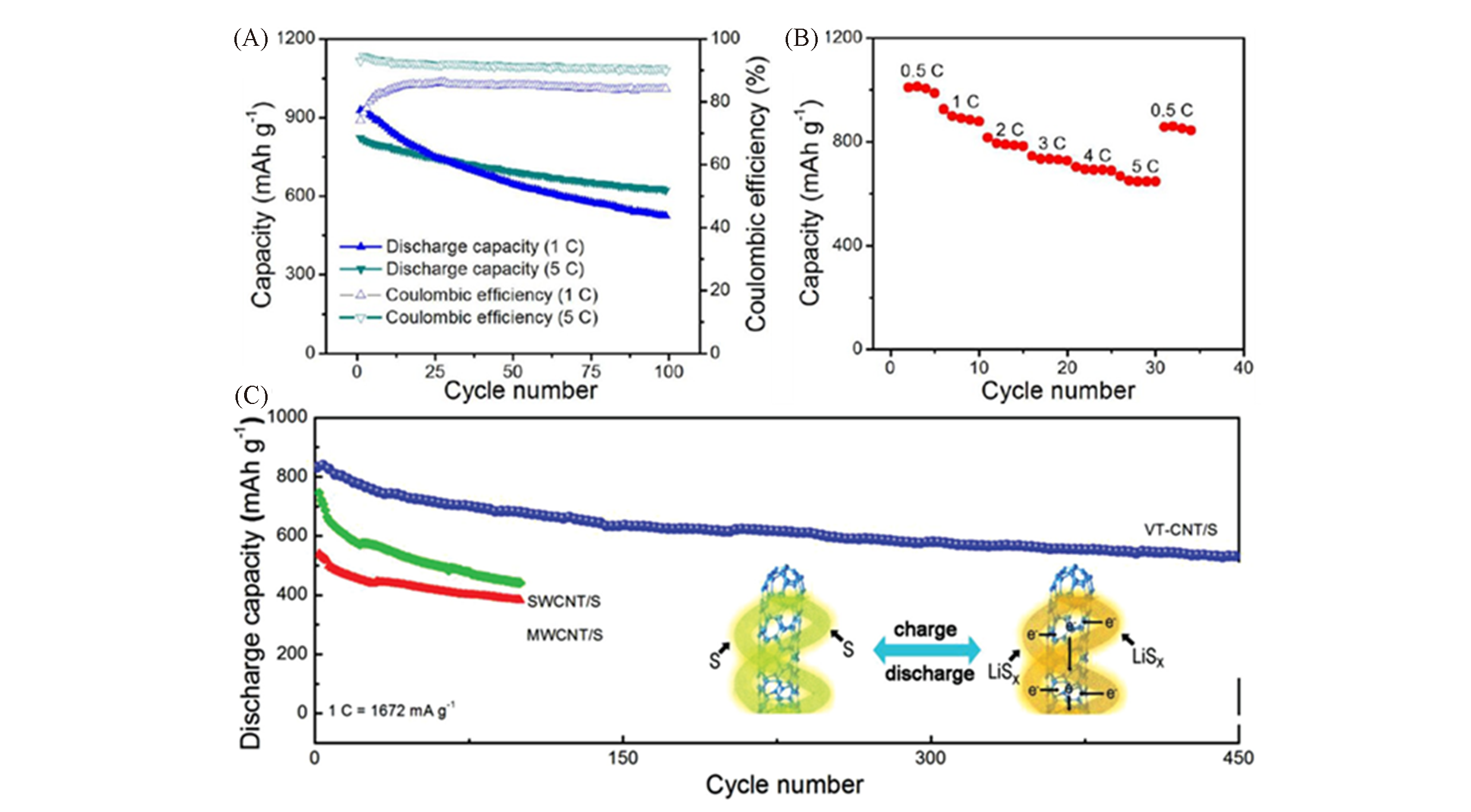
Fig.25 Performance diagrams as electrode materials of carbon nanocomposites grown by LDH?based catalystsElectrode performance of the G/SWCNT/S nanocomposites for Li-S cells: (A) cycling stability, (B) rate performance[90]; (C) cycling profiles of the VT-CNT/S, SWCNT/S, and MWCNT/S cathodes(the inset image show the illustration of VT-CNT/S cathode during discharge/charge cycle)[21]. (A, B) Copyright 2012, American Chemical Society. (C) Copyright 2014, Wiley-VCH.
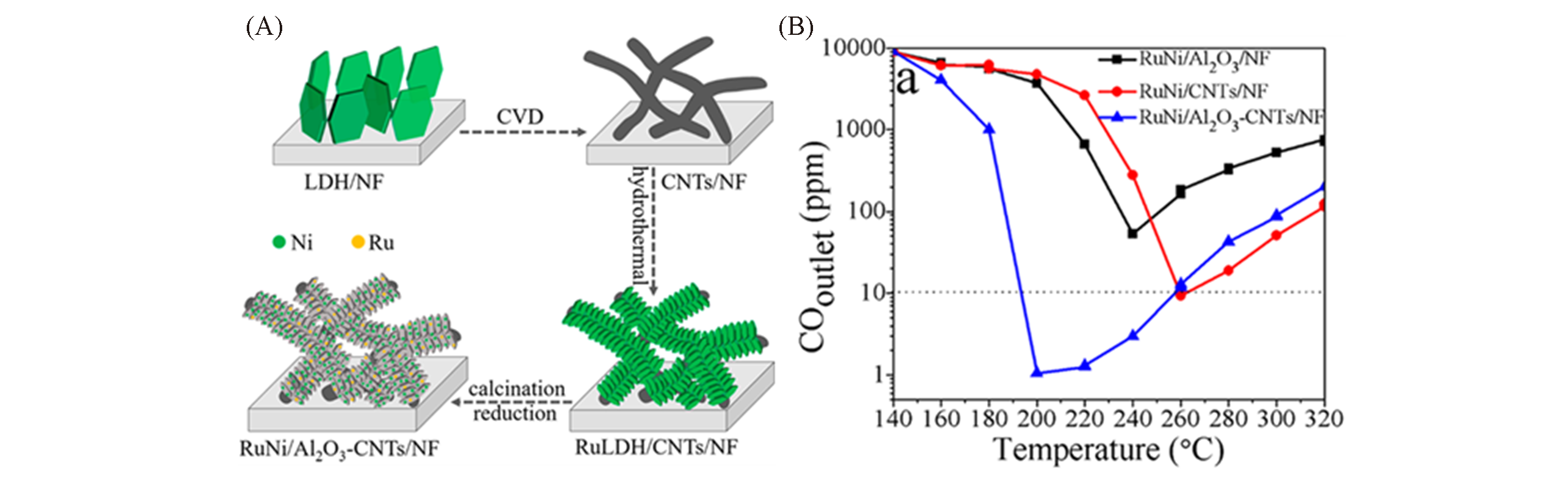
Fig.26 Preparation schematic diagram and electrocatalytic performance diagrams of RuNi/Al2O3?CNTs/NF[100](A) Schematic illustration of the preparation of RuNi/Al2O3-CNTs/NF catalyst from RuLDH/CNTs/NF hierarchical composite; (B) outlet CO concentrations over prepared catalysts for CO-SMET. Copyright 2018, American Chemical Society.
| 1 | Georgakilas V., Perman J. A., Tucek J., Zboril R., Chem. Rev., 2015, 115(11), 4744—4822 |
| 2 | Lin X., Liu C., Wang J. B., Yang S., Shi J. Y., Hong Y. Z., Separation and Purification Technology, 2019, 226, 117—127 |
| 3 | Pan M. J., Wang J. N., Fu W. Z., Chen B. X., Lei J. Q., Chen W. Y., Duan X. Z., Chen D., Qian G., Zhou X. G., Green Energy Environ., 2020, 5(1), 76—82 |
| 4 | Wu Y. Y., Deng P. H., Tian Y. L., Ding Z. Y., Li G. L., Liu J., Zuberi Z., He Q. G., Bioelectrochemistry, 2020, 131, 107393—107401 |
| 5 | Kim S., Ou R. W., Hu Y. X., Li X. Y., Zhang H. C., Simon G. P., Wang H. T., J. Membrane Sci., 2018, 562, 47—55 |
| 6 | Zhang S. C., Wang X., Yao F. R., He M. S., Lin D. W., Ma H., Sun Y. Y., Zhao Q. C., Liu K. H., Ding F., Zhang J., Chem, 2019, 5(5), 1182—1193 |
| 7 | Kumar R., Sahoo S., Joanni E., Singh R. K., Tan W. K., Kar K. K., Matsuda A., Prog. Energ. Combust., 2019, 75, 100786—100841 |
| 8 | Chen K. Y., Li G. J., Wang Y. J., Chen W. H., Mi L. W., Green Energy Environ., 2020, 5(1), 50—58 |
| 9 | Ma R. Z., Wei B. Q., Xu C. L., Liang J., Wu D. H., Sci. China Technol. Sci., 2000, 30(2), 112—116(马仁志, 魏秉庆, 徐才录, 梁吉, 吴德海. 中国科学E辑: 技术科学, 2000, 30(2), 112—116) |
| 10 | Bai Y. X., Zhang R. F., Ye X., Zhu Z., Xie H., Shen B., Cai D., Liu B., Zhang C., Jia Z., Zhang S., Li X., Wei F., Nat. Nanotechnol., 2018, 13(7), 589—595 |
| 11 | Shen Z. M., Zhao D. L., New Carbon Mater., 2001, 16(1), 1—4(沈曾民, 赵东林, 新型炭材料, 2001, 16(1), 1—4) |
| 12 | Du F. L., Wu B. X., Liu J., Xu C. C.,Li G. F., Wang X., Chem. J. Chinese Universities, 2021, 42(4), 1177—1187(杜芳林, 吴冰昕, 刘娇, 徐聪聪, 李国锋, 王兴. 高等学校化学学报, 2021, 42(4), 1177—1187) |
| 13 | Gong P. W., Sun L., Wang F., Liu X. C., Yan Z. Q., Wang M. Z., Zhang L., Tian Z. Z., Liu Z., You J. M., Chem. Eng. J., 2019, 356, 994—1002 |
| 14 | Zhang Q., Huang J. Q., Qian W. Z., Zhang Y. Y., Wei F., Small, 2013, 9(8), 1237—1265 |
| 15 | Zhang S. C., Qian L., Zhao Q. C., Wang Z. Q., Lin D. W., Liu W. M., Chen Y. B., Zhang J., Sci. China Mater., 2019, 63(1), 16—34 |
| 16 | Xiong H., Xie X. W., Wang M., Hou Y. Q., Hou X., Acta Phys.⁃Chim. Sin., 2020, 36(9), 1912008(熊辉, 谢歆雯, 王苗, 侯雅琦, 侯旭. 物理化学学报, 2020, 36(9), 1912008) |
| 17 | Fan G., Li F., Evans D. G., Duan X., Chem. Soc. Rev., 2014, 43(20), 7040—7066 |
| 18 | Li T., Hao X. J., Bai S., Zhao Y. F., Song Y. F., Acta Phys.⁃Chim. Sin., 2020, 36(9), 1912005(李天, 郝晓杰, 白莎, 赵宇飞, 宋宇飞. 物理化学学报, 2020, 36(9), 1912005) |
| 19 | Sideris P. J., Blanc F., Gan Z. H., Grey C. P., Chem. Mater., 2012, 24(13), 2449—2461 |
| 20 | Xu M., He S., Chen H., Cui G. Q., Zheng L. R., Wang B., Wei M., ACS Catal., 2017, 7(11), 7600—7609 |
| 21 | Zhao M. Q., Peng H. J., Tian G. L., Zhang Q., Huang J. Q., Cheng X. B., Tang C., Wei F., Adv. Mater., 2014, 26(41), 7051—7058 |
| 22 | Zhang Q., Zhao M. Q., Tang D. M., Li F., Huang J. Q., Liu B., Zhu W. C., Zhang Y. H., Wei F., Angew. Chem. Int. Ed., 2010, 49(21), 3642—3645 |
| 23 | Kroto H. W., Heath J. R., O’brien S. C., Curl R. F., Smalley R. E., Nature, 1985, 318(6042), 162—163 |
| 24 | Iijima S., Nature, 1991, 354(6348), 56—58 |
| 25 | Peng B., Locascio M., Zapol P., Li S., Mielke S. L., Schatz G. C., Espinosa H. D., Nat. Nanotechnol., 2008, 3(10), 626—631 |
| 26 | Li J., Song K., Zhang H. T., Guo Y., He F., Zhao N. Q., Shi C. S., Transactions of Tianjin University, 2020, 26(5), 399—408 |
| 27 | Zhao M. Q., Zhang Q., Huang J. Q., Wei F., Adv. Funct. Mater., 2012, 22(4), 675—694 |
| 28 | Zhang S. C., Zhang N., Zhang J., Acta Phys.⁃Chim. Sin., 2020, 36(1), 1907021(张树辰, 张娜, 张锦. 物理化学学报, 2020, 36(1), 1907021) |
| 29 | Zhu Y. Q., Zhao X. J., Zhong Y., Chen Z. R., Yan H., Duan X., Chem. J. Chinese Universities, 2020, 41(11), 2287—2305(朱玉荃, 赵晓婕, 钟嫄, 陈子茹, 鄢红, 段雪. 高等学校化学学报, 2020, 41(11), 2287—2305) |
| 30 | Zhao Y. F., Zhang X., Jia X. D., Waterhouse G. I. N., Shi R., Zhang X. R., Zhan F., Tao Y., Wu L. Z., Tung C. H., O’hare D., Zhang T. R., Adv. Energy Mater., 2018, 8(18), 1703585—1703592 |
| 31 | Hao X. J., Tan L., Xu Y. Q., Wang Z. L., Wang X., Bai S., Ning C. J., Zhao J. W., Zhao Y. F., Song Y. F., Ind. Eng. Chem. Res., 2020, 59(7), 3008—3015 |
| 32 | Qiu C. H., Hao X. J., Tan L., Wang X., Cao W., Liu J., Zhao Y., Song Y. F., Chem. Commun., 2020, 56(40), 5354—5357 |
| 33 | Ma X. D., Xu Y. Q., Tan L., Zhao Y. F., Song Y. F., Ind. Eng. Chem. Res., 2020, 59(32), 14315—14322 |
| 34 | Qiu C. H., Bai S., Cao W. J., Tan L., Liu J. Y., Zhao Y. F., Song Y. F., Transactions of Tianjin University, 2020, 26(5), 352—361 |
| 35 | Wang Z. L., Xu S. M., Tan L., Liu G. H., Shen T. Y., Yu C., Wang H., Tao Y., Cao X. Z., Zhao Y. F., Song Y. F., Appl. Catal. B: Environ., 2020, 270, 118884—118893 |
| 36 | He S., An Z., Wei M., Evans D. G., Duan X., Chem. Commun., 2013, 49(53), 5912—5920 |
| 37 | Zhao Y. F., Zhao B., Liu J. J., Chen G. G., Gao R., Yao S. Y., Li M. Z., Zhang Q. H., Gu L., Xie J. L., Wen X. D., Wu L. Z., Tung C. H., Ma D., Zhang T. R., Angew. Chem. Int. Ed., 2016, 55(13), 4215—4219 |
| 38 | Li Z. H., Liu J. J., Zhao Y. F., Waterhouse G. I. N., Chen G. B., Shi R., Zhang X., Liu X. W., Wei Y. M., Wen X. D., Wu L. Z., Tung C. H., Zhang T. R., Adv. Mater., 2018, 30(31), 1800527—1800534 |
| 39 | Zhao Y. F., Li Z. H., Li M. Z., Liu J. J., Liu X. W., Waterhouse G. I. N., Wang Y. S., Zhao J. Q., Gao W., Zhang Z. S., Long R., Zhang Q. H., Gu L., Liu X., Wen X. D., Ma D., Wu L. Z., Tung C. H., Zhang T. R., Adv. Mater., 2018, 30, 1803127—1803134 |
| 40 | Chen G. B., Gao R., Zhao Y. F., Li Z. H., Waterhouse G. I. N., Shi R., Zhao J. Q., Zhang M. T., Shang L., Sheng G. Y., Zhang X. P., Wen X. D., Wu L. Z., Tung C. H., Zhang T. R., Adv. Mater., 2018, 30(3), 1704663—1704670 |
| 41 | Zhao M. Q., Zhang Q., Huang J. Q., Nie J. Q., Wei F., Carbon, 2010, 48(11), 3260—3270 |
| 42 | Zhao M. Q., Zhang Q., Jia X. L., Huang J. Q., Zhang Y. H., Wei F., Adv. Funct. Mater., 2010, 20(4), 677—685 |
| 43 | Zhao M. Q., Zhang Q., Zhang W., Huang J. Q., Zhang Y., Su D. S., Wei F., J. Am. Chem. Soc., 2010, 132(42), 14739—14741 |
| 44 | Li Y. W., Gao W., Peng M., Zhang J. B., Sun J. L., Xu Y., Hong S., Liu X., Liu X. W., Wei M., Zhang B. S., Ma D., Nat. Commun., 2020, 11(1), 61—69 |
| 45 | Xu M., Yao S. Y., Rao D. M., Niu Y. M., Liu N., Peng M., Zhai P., Man Y., Zheng L. R., Wang B., Zhang B. S., Ma D., Wei M., J. Am. Chem. Soc., 2018, 140(36), 11241—11251 |
| 46 | Song J. Q., Xu X. Y., Lin Y. J., Li D. Q., Duan X., CN 101905861A. 2010⁃12⁃08(宋家庆, 徐向宇, 林彦军, 李殿卿, 段雪. CN 101905861A, 2010⁃12⁃08) |
| 47 | Li F., Tan Q., Evans D. G., Duan X., Catal. Lett., 2005, 99(3/4), 151—156 |
| 48 | Zhang X. X., Li Z. Q., Wen G. H., Fung K. K., Chen J. L., Li Y. D., Chem. Phys. Lett., 2001, 333(6), 509—514 |
| 49 | Li Y. D., Chen J. L., Liu C., Zhao J. S., Studies in Surface Science & Catalysis, 1998, 118, 321—329 |
| 50 | Hima H. I., Xiang X., Zhang L., Li F., Evans D. G.. Chinses J. Inorg. Chem., 2008, 24(6), 886—891(Hima H. I., 项顼, 张璐, 李峰, Evans D. G.. 无机化学学报, 2008, 24(6), 886—891) |
| 51 | Xiang X., Zhang L., Halidou I. H., Li F., Evans D., Appl. Clay Sci., 2009, 42(3/4), 405—409 |
| 52 | Li M. Z., Fan G. L., Qin H., Li F., Ind. Eng. Chem. Res., 2012, 51(37), 11892—11900 |
| 53 | Lan M., Fan G. L., Chen Q. L., Li F., J. Ind. Eng. Chem., 2014, 20(4), 1523—1531 |
| 54 | Li C. H., Zhao Y., Yao K. F., Liang J., Carbon, 2003, 41(12), 2443—2446 |
| 55 | Chen J. L., Li Y. D., Ma Y. M., Qin Y. N., Chang L., Carbon, 2001, 39(10), 1467—1475 |
| 56 | Yu Z. X., Chen D., Tøtdal B., Holmen A., Mater. Chem. Phys., 2005, 92(1), 71—81 |
| 57 | Chen Q. L., Wang J., Li F., Ind. Eng. Chem. Res., 2011, 50(15), 9034—9042 |
| 58 | Sun T. T., Fan G. L., Li F., Ind. Eng. Chem. Res., 2013, 52(16), 5538—5547 |
| 59 | Cao Y., Zhao Y., Li Q. X., Jiao Q. Z., J. Chem. Sci., 2009, 121(2), 225—229 |
| 60 | Cao Y., Zhao Y., Jiao Q. Z., Mater. Chem. Phys., 2010, 122(2/3), 612—616 |
| 61 | Cheng X. B., Tian G. L., Liu X. F., Nie J. Q., Zhao M. Q., Huang J. Q., Zhu W. C., Hu L., Zhang Q., Wei F., Carbon, 2013, 62, 393—404 |
| 62 | Dussault L., Dupin J. C., Latorre N., Ubieto T., Noé L., Monthioux M., Romeo E., Royo C., Monzón A., Guimon C., J. Phys. Chem. Solids, 2006, 67(5/6), 1162—1167 |
| 63 | Zhao M. Q., Tian G. L., Zhang Q., Huang J. Q., Nie J. Q., Wei F., Nanoscale, 2012, 4(7), 2470—2477 |
| 64 | Tian G. L., Zhao M. Q., Zhang B. S., Zhang Q., Zhang W., Huang J. Q., Chen T. C., Qian W. Z., Su D. S., Wei F., J. Mater. Chem. A, 2014, 2(6), 1686—1696 |
| 65 | Zhao M. Q., Zhang Q., Tian G. L., Huang J. Q., Wei F., ACS Nano, 2012, 6(5), 4520—4529 |
| 66 | Zhao M. Q., Huang J. Q., Zhang Q., Nie J. Q., Wei F., Carbon, 2011, 49(6), 2148—2152 |
| 67 | Lee K. N., Park D. Y., Choi G., Nguyen D. A., Choi Y. C., Jeong M. S., ACS Appl. Mater. Inter., 2020, 12(31), 35716—35724 |
| 68 | He M. S., Wang X., Zhang S. C., Jiang H., Cavalca F., Cui H. Z., Wagner J. B., Hansen T. W., Kauppinen E., Zhang J., Ding F., Sci. Adv., 2019, 5(12), 1—8 |
| 69 | Zhang S. C., Kang L. X., Wang X., Tong L. M., Yang L. W., Wang Z. Q., Qi K., Deng S. B., Li Q. W., Bai X. D., Ding F., Zhang J., Nature, 2017, 543(7644), 234—238 |
| 70 | Wang J. T., Jin X., Liu Z. B., Yu G., Ji Q. Q., Wei H. M., Zhang J., Zhang K., Li D. Q., Yuan Z., Li J. C., Liu P., Wu Y., Wei Y., Wang J. P., Li Q. Q., Zhang L. N., Kong J., Fan S. S., Jiang K. L., Nat. Catal., 2018, 1(5), 326—331 |
| 71 | Bai Y. X., Yue H. J., Wang J., Shen B. Y., Sun S. L., Wang S. J., Wang H. D., Li X. D., Xu Z. P., Zhang R. F., Wei F., Science, 2020, 369(6507), 1104—1106 |
| 72 | Wang B. W., Jiang S., Zhu Q. B., Sun Y., Luan J., Hou P. X., Qiu S., Li Q. W., Liu C., Sun D. M., Cheng H. M., Adv. Mater., 2018, 30(32), 1802057—1802064 |
| 73 | Zhang D. Y., Lei L. Y., Shang Y. H., Chem. Ind. Eng. Prog., 2016, 35(3), 831—836(张德懿, 雷龙艳, 尚永花. 化工进展, 2016, 35(3), 831—836) |
| 74 | Li Y. D., Chen J. L., Chang L., Appl. Catal. A: Gen., 1997, 163(1/2), 45—57 |
| 75 | Qian W. Z., Tian T., Guo C. Y., Wen Q., Li K. J., Zhang H. B., Shi H. B., Wang D. Z., Liu Y., Zhang Q., Zhang Y. X., Wei F., Wang Z. W., Li X. D., Li Y. D., J. Phys. Chem. C, 2008, 112(20), 7588—7593 |
| 76 | Xue R. L., Sun Z. P., Su L. H., Zhang X., Catalysis Letters, 2010, 135(3/4), 312—320 |
| 77 | Cao Y., Liu B. T., Jiao Q. Z., Zhao Y., S. Afr. J. Chem., 2011, 64, 67—70 |
| 78 | Kvande I., Chen D., Yu Z., Ronning M., Holmen A., J. Catal., 2008, 256(2), 204—214 |
| 79 | Chen J. L., Li Y. D., Li Z. Q., Zhang X. X., Appl. Catal. A: Gen., 2004, 269(1/2), 179—186 |
| 80 | Pacuła A., Uosaki K., Socha R. P., Bielańska E., Pietrzyk P., Zimowska M., Electrochim. Acta, 2016, 212, 47—58 |
| 81 | Jia X. L., Wei F., Top. Curr. Chem., 2017, 375(18), 1—35 |
| 82 | Liu S. W., Li Y. D., J. Chem. Ind. Eng., 2007, 58(1), 102—107(刘少文, 李永丹. 化工学报, 2007, 58(1), 102—107) |
| 83 | Qian W. Z., Liu T., Wang Z. W., Wei F., Li Z. F., Luo G. H., Li Y. D., Appl. Catal. A: Gen., 2004, 260(2), 223—228 |
| 84 | Tian G. L., Huang J. Q., Li J., Zhang Q., Wei F., Carbon, 2016, 108, 404—411 |
| 85 | Gallon H. J., Tu X., Twigg M. V., Whitehead J. C., Appl. Catal. B: Environ., 2011, 106, 616—620 |
| 86 | Bobb J. A., Awad F. S., Moussa S., El⁃Shall M. S., J. Mater. Sci., 2020, 55(13), 5351—5363 |
| 87 | Kane A., Hinkov I., Brinza O., Hosni M., Barry A. H., Cherif S. M., Farhat S., Coatings, 2020, 10(4), 1—24 |
| 88 | Gao W., Gao R., Zhao Y. F., Peng M., Song C. Q., Li M. Z., Li S. W., Liu J. J., Li W. Z., Deng Y. C., Zhang M. T., Xie J. L., Hu G., Zhang Z. S., Long R., Wen X. D., Ma D., Chem., 2018, 4(12), 2917—2928 |
| 89 | Zhao D. M. Li Z. W., Liu L. D., Zhang Y. H., Ren D. C., Li J., Acta Chim. Sinica, 2014, 72(2), 185—200(赵冬梅, 李振伟, 刘领弟, 张艳红, 任德财, 李坚. 化学学报, 2014, 72(2), 185—200) |
| 90 | Zhao M. Q., Liu X. F., Zhang Q., Tian G. L., Huang J. Q., Zhu W., Wei F., ACS Nano, 2012, 6(12), 10759—10769 |
| 91 | Li Z. H., Shao M. F., Yang Q. H., Tang Y., Wei M., Evans D. G., Duan X., Nano Energy, 2017, 37, 98—107 |
| 92 | Zhang L., Zhang C. F., Xiang X., Li F., Chem. Eng. Technol., 2010, 33(1), 44—51 |
| 93 | Zhang L., Li F., Electrochim. Acta, 2010, 55(22), 6695—6702 |
| 94 | Zhang L., Yang L., Li F., Mater. Lett., 2011, 65(1), 38—40 |
| 95 | Zhang L., Li F., Appl. Clay Sci., 2010, 50(1), 64—72 |
| 96 | Zhang S. Y., Fan G., Zhang C. F., Li F., Mater. Chem. Phys., 2012, 135(1), 137—143 |
| 97 | Zhang S. L., Zhang Y., Jiang W. J., Liu X., Xu S. L., Huo R. J., Zhang F. Z., Hu J. S., Carbon, 2016, 107, 162—170 |
| 98 | Li W., Sun T. T., Li F., Ind. Eng. Chem. Res., 2014, 53(47), 18095—18103 |
| 99 | Kang J., Han R. R., Wang J., Yang L., Fan G. L., Li F., Chem. Eng. J., 2015, 275, 36—44 |
| 100 | Ping D., Dong C. J., Zhao H., Dong X. F., Ind. Eng. Chem. Res., 2018, 57(16), 5558—5567 |
| [1] | 曹舒杰, 李泓君, 管文丽, 任梦田, 周传政. 硫代磷酸酯寡聚核苷酸的立体控制合成研究进展[J]. 高等学校化学学报, 2022, 43(Album-4): 20220304. |
| [2] | 唐全骏, 刘颖馨, 孟蓉炜, 张若天, 凌国维, 张辰. 单原子催化在海洋能源领域的应用[J]. 高等学校化学学报, 2022, 43(9): 20220324. |
| [3] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [4] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [5] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [6] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [7] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [8] | 程前, 杨博龙, 吴文依, 向中华. S掺杂Fe-N-C高活性氧还原反应催化剂[J]. 高等学校化学学报, 2022, 43(9): 20220341. |
| [9] | 杨静怡, 李庆贺, 乔波涛. 铱单原子和纳米粒子在N2O分解反应中的协同催化[J]. 高等学校化学学报, 2022, 43(9): 20220388. |
| [10] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [11] | 任诗杰, 谯思聪, 刘崇静, 张文华, 宋礼. 铂单原子催化剂同步辐射X射线吸收谱的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220466. |
| [12] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [13] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [14] | 杨静怡, 施思齐, 彭怀涛, 杨其浩, 陈亮. Ga-C3N4单原子催化剂高效光驱动CO2环加成[J]. 高等学校化学学报, 2022, 43(9): 20220349. |
| [15] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||