

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (1): 20240116.doi: 10.7503/cjcu20240116
• 综合评述 • 上一篇
孙玉洁, 牛振田, 佟昊轩, 胡悦, 俞丙然( ), 徐福建(
), 徐福建( )
)
收稿日期:2024-03-11
出版日期:2025-01-10
发布日期:2024-04-23
通讯作者:
俞丙然,徐福建
E-mail:yubr@mail.buct.edu.cn;xufj@mail.buct.edu.cn
基金资助:
SUN Yujie, NIU Zhentian, TONG Haoxuan, HU Yue, YU Bingran( ), XU Fujian(
), XU Fujian( )
)
Received:2024-03-11
Online:2025-01-10
Published:2024-04-23
Contact:
YU Bingran, XU Fujian
E-mail:yubr@mail.buct.edu.cn;xufj@mail.buct.edu.cn
Supported by:摘要:
二硫键的动态交换与重组使其易于进行开环聚合(ROP)反应得到聚二硫化物. 聚二硫化物因其还原环境敏感性而被广泛应用于药物递送领域. 本文综合评述了二硫化物的ROP策略, 主要分为开环自聚和巯基引发的双硫交换聚合; 讨论了聚二硫化物在药物递送方面的最新研究进展, 主要包括核酸递送、 蛋白质递送和小分子药物递送; 最后, 对聚二硫化物的开环制备策略及其在药物递送方面的应用前景进行了展望.
中图分类号:
TrendMD:
孙玉洁, 牛振田, 佟昊轩, 胡悦, 俞丙然, 徐福建. 开环制备聚二硫化物及其在药物递送方面的应用. 高等学校化学学报, 2025, 46(1): 20240116.
SUN Yujie, NIU Zhentian, TONG Haoxuan, HU Yue, YU Bingran, XU Fujian. Ring-opening Preparation of Poly(disulfide)s for Drug Delivery. Chem. J. Chinese Universities, 2025, 46(1): 20240116.
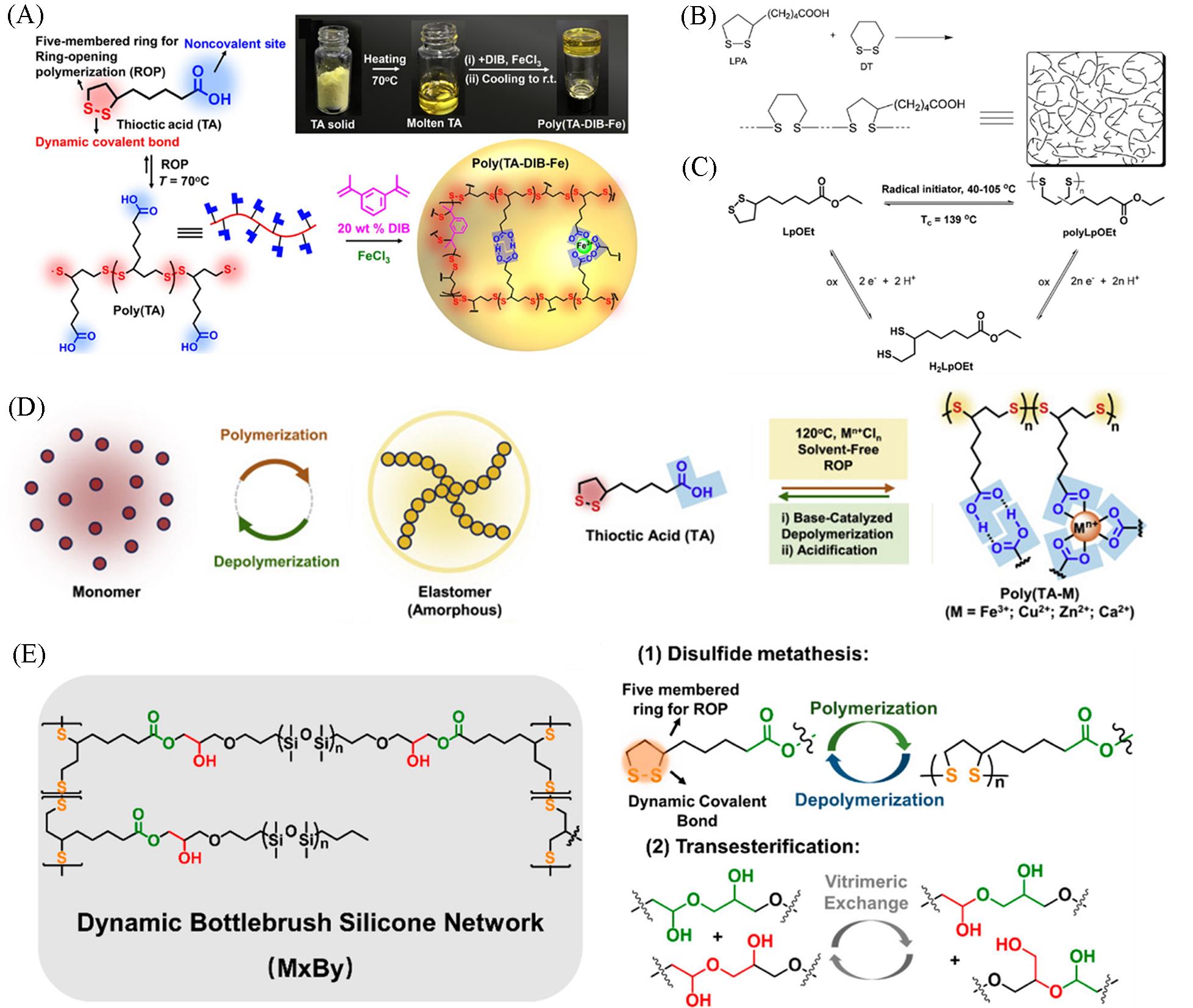
Fig.1 Schematic representation of the synthesis route of the copolymer network and photographs of TA powder, molten TA liquid, and poly(TA⁃DIB⁃Fe) copolymer solid(A)[18], the copolymerization of LPA and DT induced to give high⁃molecular⁃weight polymers(B)[20], synthesis and polymerization of LpOEt, and degradation(thermal and reductive) of the corresponding polymer(C)[21], the schematic representation and molecular structures of the interconversion between two kinds of polymeric products(D)[22], molecular structure of dynamic network prepared using TA and possible dynamic mechanisms(E)[23](A) Copyright 2018, American Association for the Advancement of Science; (B) Copyright 2006, American Chemical Society; (C) Copyright 2021, Wiley-VCH; (D) Copyright 2021, Elsevier; (E) Copyright 2023, American Chemical Society.
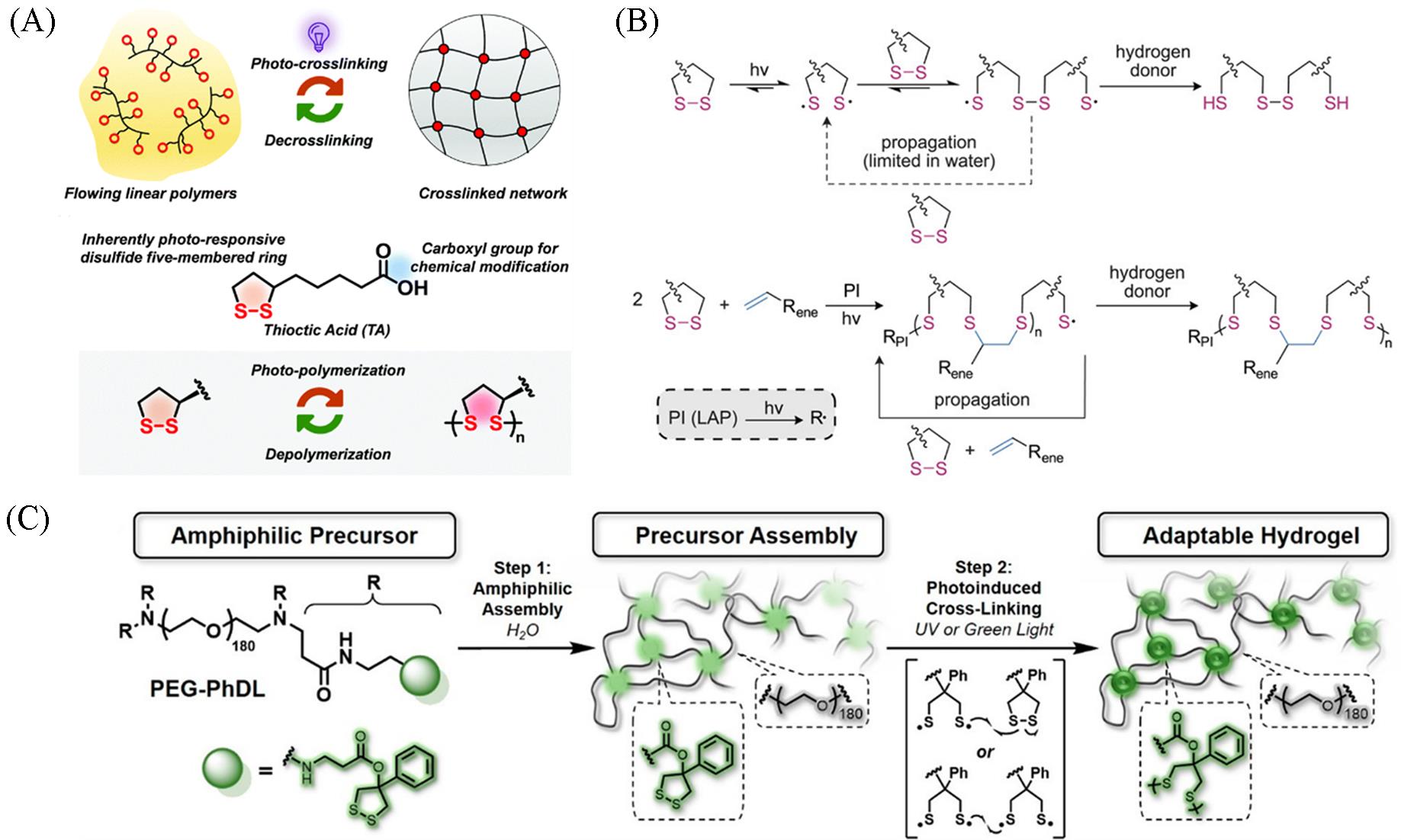
Fig.2 Reversible photo⁃crosslinking strategy mediated by dynamic covalent disulfide bonds of TA(A)[27], dithiolane⁃mediated photo⁃crosslinking mechanisms(B)[25], the 1,2⁃dithiolane photochemistry ring⁃opening for disulfide hydrogels under light irradiation(C)[26](A) Copyright 2021, the Royal Society of Chemistry; (B) Copyright 2023, Wiley-VCH; (C) Copyright 2020, American Chemical Society.
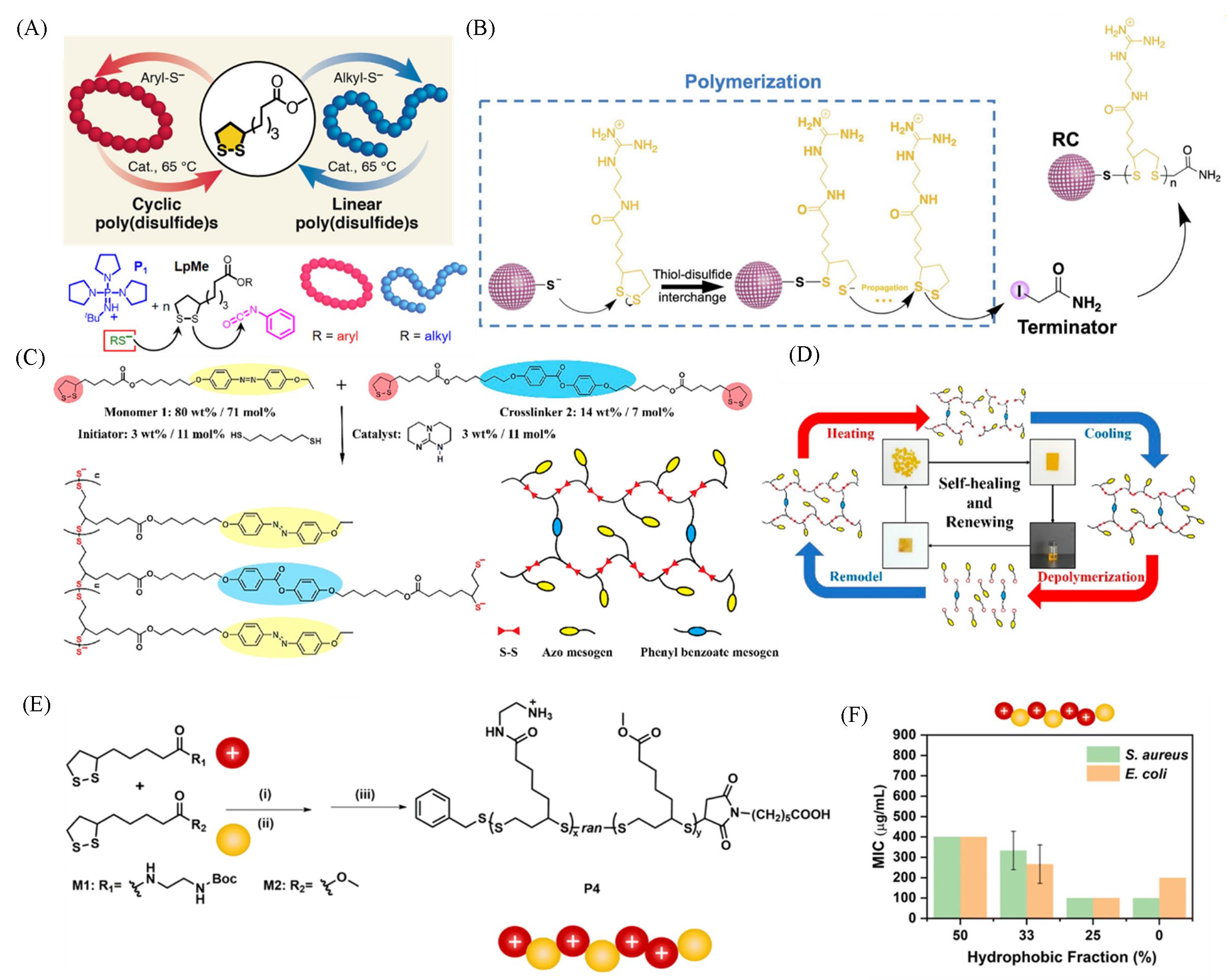
Fig.4 Aryl thiol and alkyl thiols yield poly(disulfide)s by ring⁃opening polymerization(A)[33], schematic illustration of in⁃situ polymerization on the thiol⁃functionalized nanoparticles via thiol⁃disulfide interchange reaction(B)[36], molecular design and chemical structures of the liquid crystal networks film(C), schematic illustration of the reversible polymerization and depolymerization of the liquid crystal networks(LCNs)(D)[37], random copolymerization of cationic amphiphilic poly(disulfide)s(E), minimal inhibitory concentration(MIC) of poly(disulfide)s with a different hydrophobic fraction of hydrophobic groups and similar Mn(F)[43](E) Reaction conditions: (i) benzyl mercaptan(BnSH), phosphazene base P1-t-Bu-tris(tetramethylene), tetrahydrofuran(THF), 0 °C, 2 h; (ii) 6-maleimidohexanoic acid, r.t., 30 min; (iii) hydrogen chloride(HCl), dioxane/ methyl alcohol(MeOH), 0 °C, 4 h.(A) Copyright 2019, American Chemical Society; (B) Copyright 2022, Elsevier; (C, D) Copyright 2021, American Chemical Society; (E, F) Copyright 2022, the Royal Society of Chemistry.
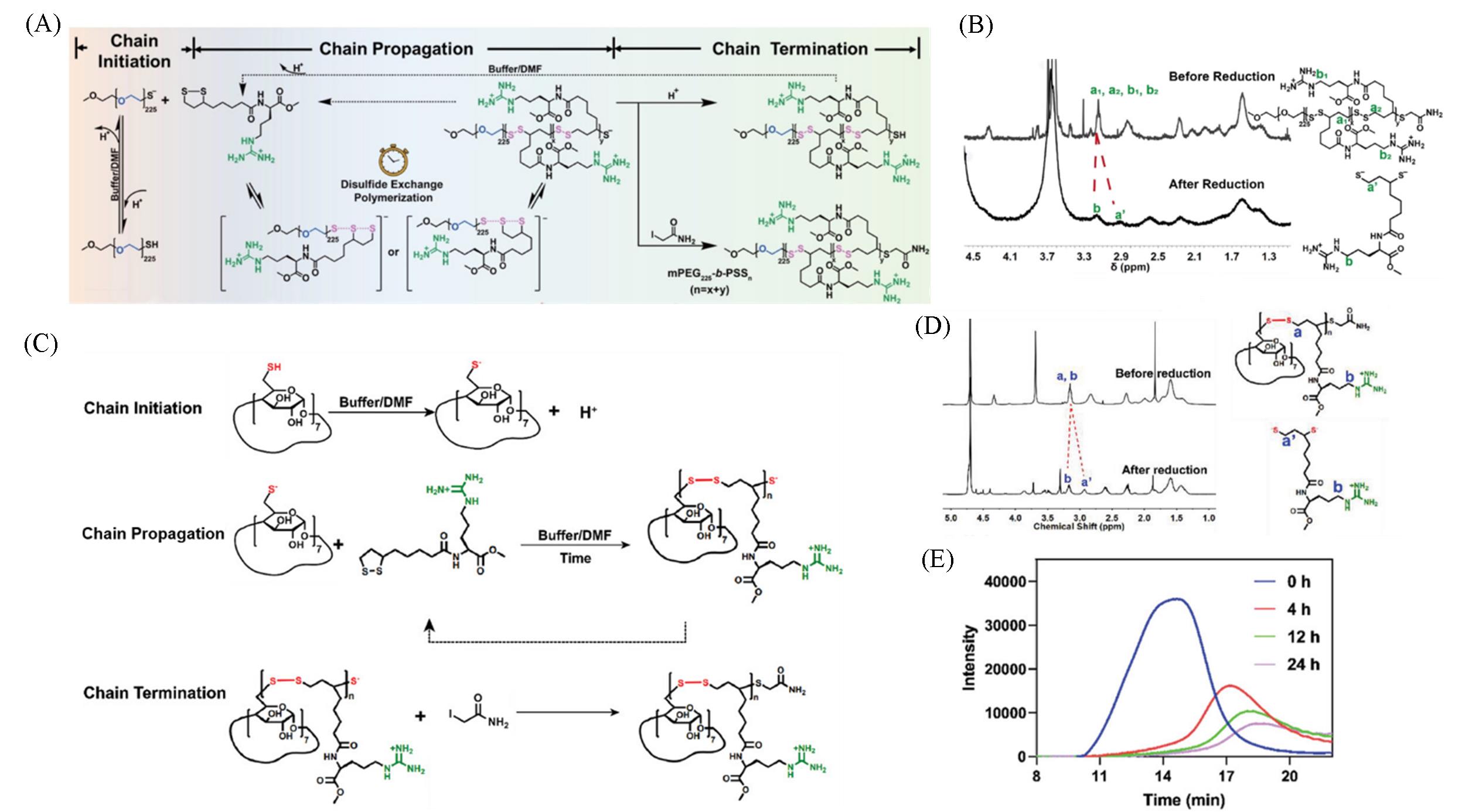
Fig.5 Controllable disulfide exchange polymerization of mPEG225⁃b⁃PSS n (A), typical 1H NMR(400 MHz) spectra of mPEG225⁃b⁃PSS n before and after reduction(B)[30], the reaction mechanism of controllable disulfide exchange polymerization between β⁃CD⁃SH and La⁃Arg monomer(C), typical 1H NMR(400 MHz) spectra of β⁃CD⁃g⁃PSS20 before and after reduction(D), gel permeation chromato⁃ graphy(GPC) curves of β⁃CD⁃g⁃PSS20 in the presence of GSH at different time periods(E)[49](A, B) Copyright 2022, Wiley⁃VCH; (C—E) Copyright 2023, Wiley⁃VCH.
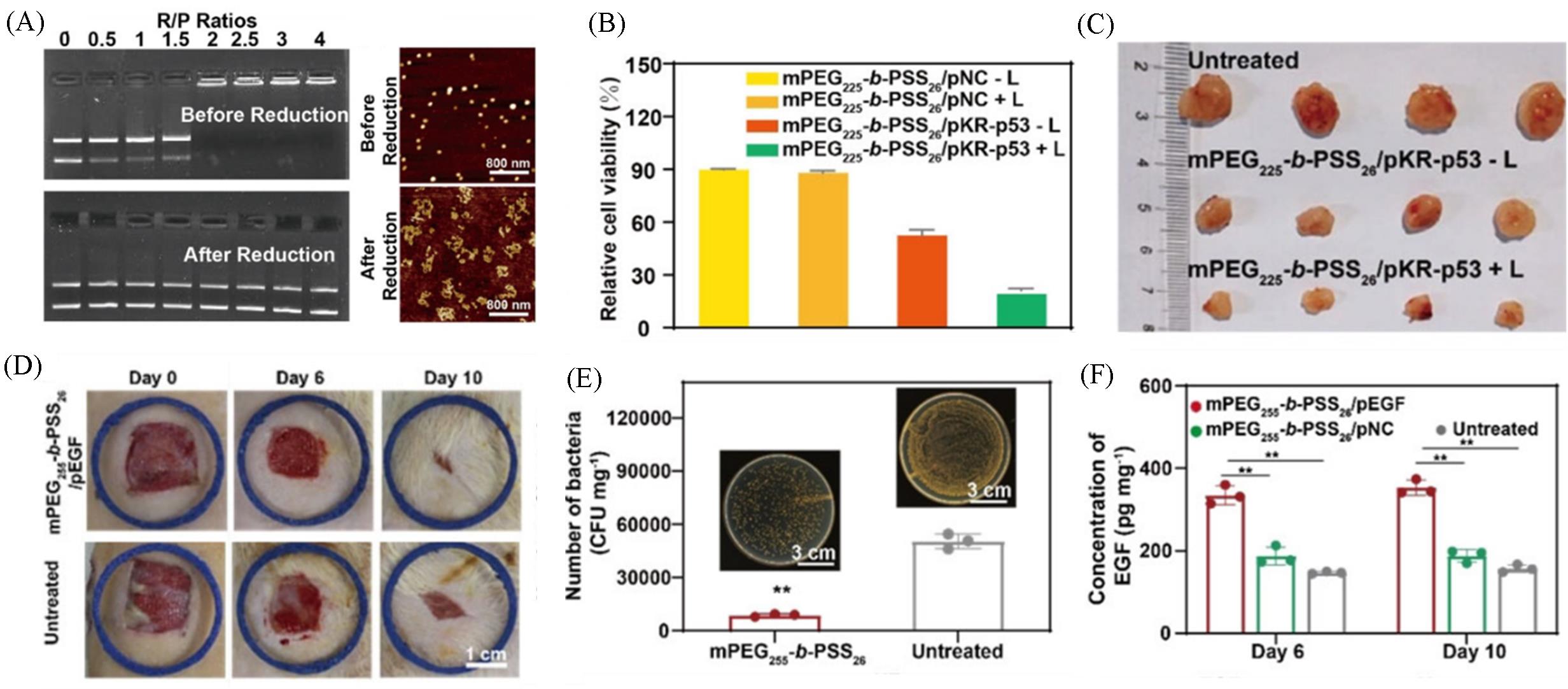
Fig.6 Electrophoretic mobility and atomic force microscope(AFM) images of mPEG225⁃b⁃PSS26/pDNA before and after degradation(A), relative cell viabilities of mPEG225⁃b⁃PSS26/pDNA complexes in 4T1 cells with or without irradiation(B), images of 4T1 tumors after different treatments on day 14(C), photographs of wound healing in the rat model on day 0, day 6 and day 10(D), statistical analysis of bacterial colonies cultured from infected tissues on day 2(E), concentration of epidermal growth factor(EGF) in the harvested tissues with different treatments on day 6 and day 10(F)[30]Copyright 2022, Wiley⁃VCH.
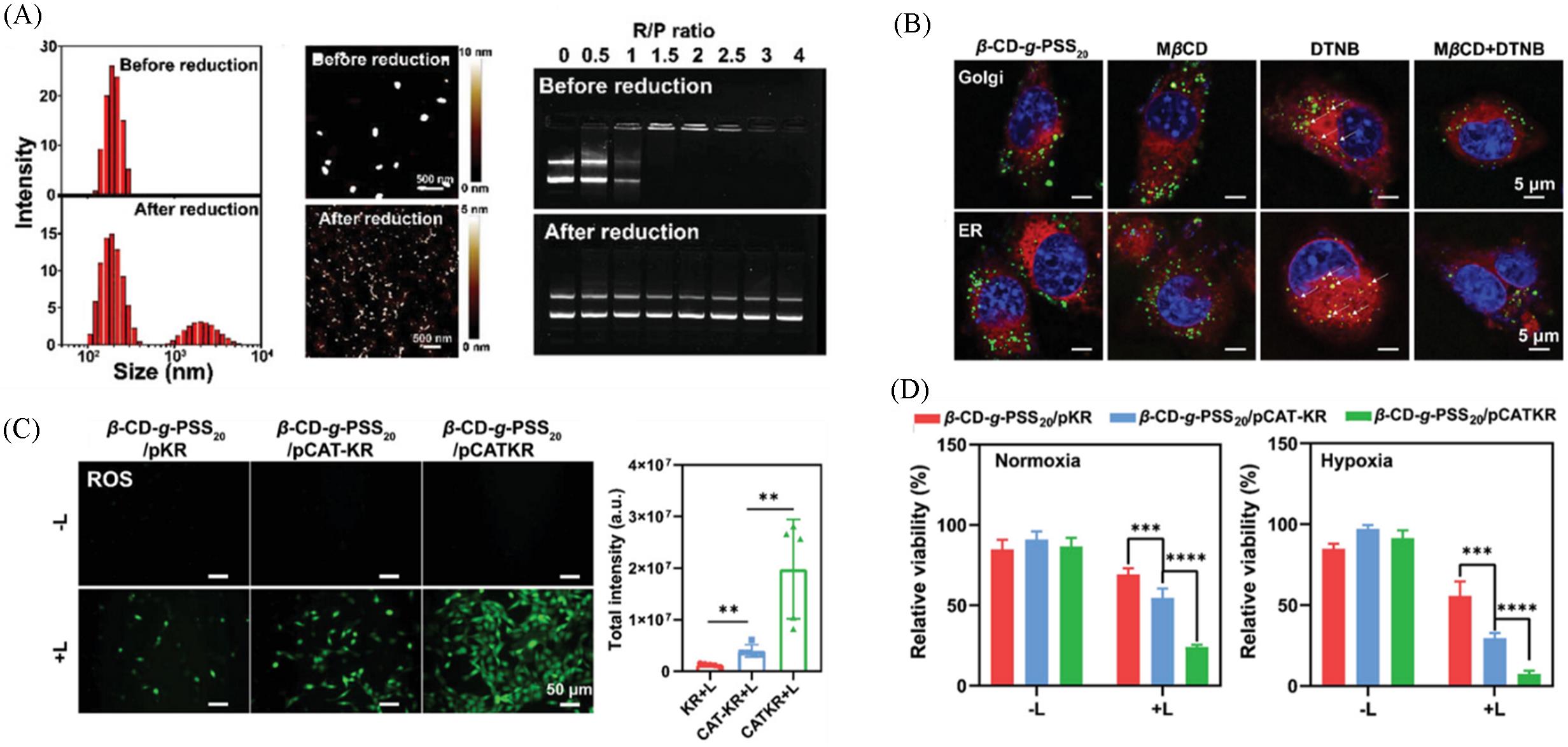
Fig.7 Size distribution, representative AFM images and electrophoretic mobility retardation assays of β⁃CD⁃g⁃PSS20/pDNA complexes before and after reduction(A), co⁃localization of β⁃CD⁃g⁃PSS20/pDNA complexes with Golgi or endoplasmic reticulum compartments(B), representative images of intracellular ROS accumulation after different treatment(C), in vitro relative SCC⁃7 cell viabi⁃lity treated with complexes in normoxic or hypoxic atmospheres, with or without irradiation(D)[49]
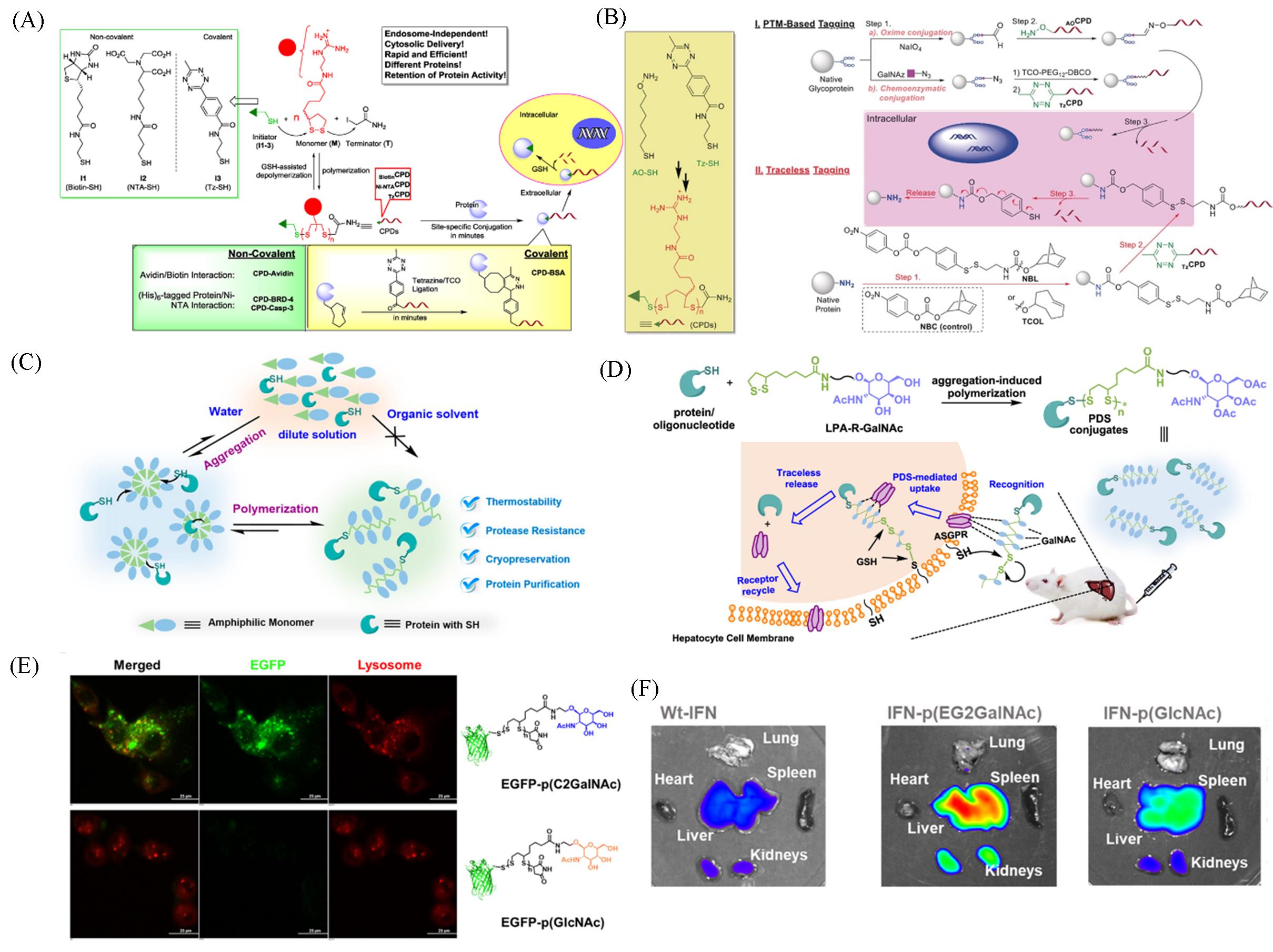
Fig.8 Polymerization/depolymerization process of CPDs, and the two⁃step approach for conjugation of protein cargos with CPDs in non⁃covalent and covalent approaches(A)[63], overview of CPD⁃facilitated intracellular delivery of native proteins(B)[64], the protein⁃mediated, aggregation⁃induced polymerization(AIP) of amphiphilic LPA⁃derived monomers at room temperature(C)[66], schematic illustration of the grafting⁃from synthesis and targeted intracellular delivery of protein/oligonucleotide⁃cell⁃penetrating polydisulfides(PDS) conjugates into liver cells(D), representative images of HepG2 cells incubated with EGFP⁃p(C2GalNAc) or EGFP⁃p(GlcNAc)(E), fluorescent images of the extracted organs at 24 h(F)[67](A) Copyright 2015, American Chemical Society; (B) Copyright 2018, Wiley-VCH; (C) Copyright 2022, American Chemical Society; (D—F) Copyright 2024, American Chemical Society.
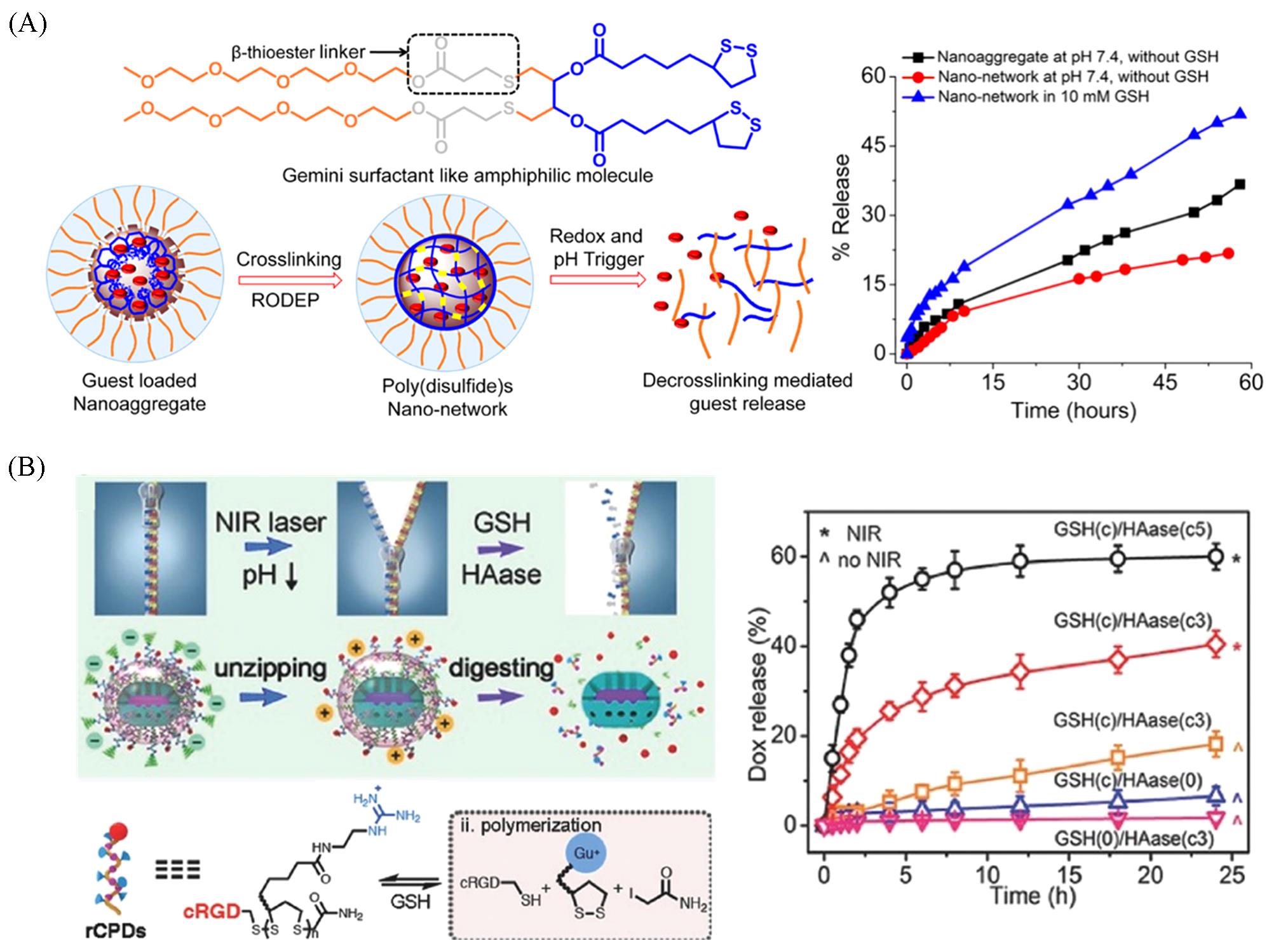
Fig.9 Chemical structure of the Gemini surfactant⁃like molecule and redox⁃responsive release profile of the doxorubicin⁃loaded nanonetwork(A)[70], the surface state variations during drug delivery(the polymer zipper decoding and the sandwich protective shell degradation) and the drug release profiles of pPTP@DMSGRs(B)[71](A) Copyright 2023, American Chemical Society; (B) Copyright 2017, Wiley-VCH.
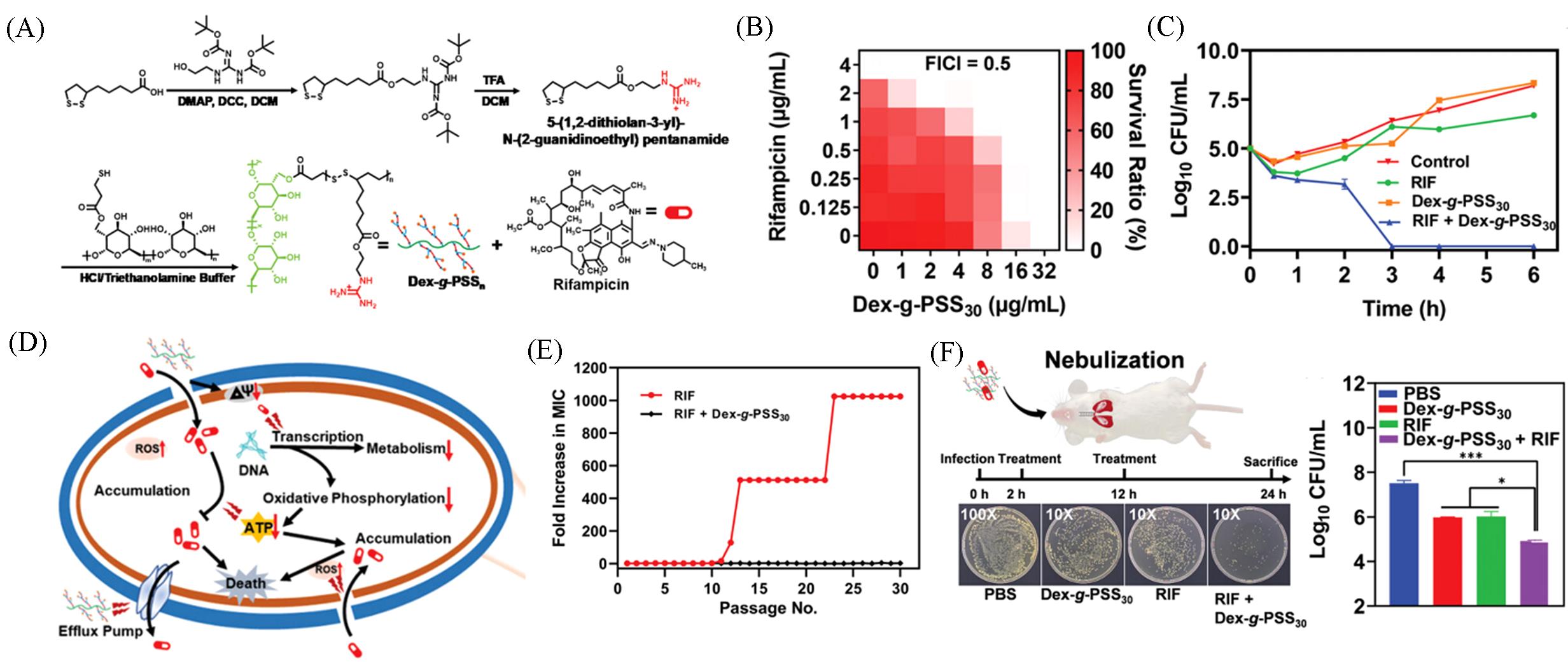
Fig.10 Schematic illustration of cationic polysaccharide conjugates Dex⁃g⁃PSS n (A), synergism between rifampicin(RIF) and Dex⁃g⁃PSS30 against MDR⁃AB(B), time⁃dependent killing against MDR⁃AB after different treatment(C), schematic illustration of associated preventative mechanisms toward bacterial resistance(D), dex⁃g⁃PSS30 addition preventing rifampicin(RIF) resistance in MDR⁃AB(E), representative Luria⁃Bertani agar plates and statistical analysis of bacterial colonies cultured from MDR⁃AB infected lungs(F)[72](A—F) Copyright 2022, Wiley⁃VCH
| 1 | Arun Y., Ghosh R., Domb A. J., Adv. Funct. Mater., 2021, 31(44), 2010284 |
| 2 | Kamaly N., Yameen B., Wu J., Farokhzad O. C., Chem. Rev., 2016, 116(4), 2602—2663 |
| 3 | Yang P., Zhu F., Zhang Z. B., Cheng Y. Y., Wang Z., Li Y. W., Chem. Soc. Rev., 2021, 50(14), 8319—8343 |
| 4 | Jin Y., Yu C., Denmana R. J, Zhang W., Chem. Soc. Rev., 2013, 42(16), 6634—6654 |
| 5 | Komáromy D., Stuart M. C. A., Santiago G. M., Tezcan M., Krasnikov V. V., Otto S., J. Am. Chem. Soc., 2017, 139(17), 6234—6241 |
| 6 | Fan F. Q., Ji S. B., Sun C. X., Liu C., Yu Y., Fu Y., Xu H. P., Angew. Chem. Int. Ed., 2018, 57(50), 16426—16430 |
| 7 | Li D. D., Zhang R. H., Liu G. T., Kang Y., Wu J., Adv. Healthcare Mater., 2020, 9(20), 2000605 |
| 8 | Karandish F., Mamnoon B., Feng L., Haldar M. K., Xia L., Gange K. N., You S., Choi Y., Sarkar K., Mallik S., Biomacromolecules, 2018, 19(10), 4122—4132 |
| 9 | Bechtel T. J., Weerapana E., Proteomics, 2017, 17(6), 1600391 |
| 10 | Yan C. M., Yang F., Wu M. J., Yuan Y., Chen F. Y., Chen Y. L., Macromolecules, 2019, 52(23), 9376—9382 |
| 11 | Goethals E. J., Sillis C., Die Makromolekulare Chemie, 1968, 119, 249—251 |
| 12 | Lin C., Zhong Z. Y., Lok M. C., Jiang X. L., Hennink W. E., Feijen J., Engbersen J. F. J., Bioconjugate Chem., 2007, 18(1), 138—145 |
| 13 | Wang B. S., Zhang Q., Wang Z. Q., Shi C. Y., Gong X. Q., Tian H., Qu D. H., Angew. Chem. Int. Ed., 2023, 62(11), e202215329 |
| 14 | Felber J. G., Poczka L., Scholzen K. C., Zeisel L., Maier M. S., Busker S., Theisen U., Brandstädter C., Becker K., Arnér E. S. J., Thorn⁃Seshold J., Thorn⁃Seshold O., Nat. Commun., 2022, 13, 1754 |
| 15 | Maczurek A., Hager K., Kenklies M., Sharman M., Martins R., Engel J., Carlson D. A., Münch G., Adv. Drug Deliver. Rev., 2008, 60(13/14), 1463—1470 |
| 16 | Barcan G. A., Zhang X. Y., Waymouth R. M., J. Am. Chem. Soc., 2015, 137(17), 5650—5653 |
| 17 | Zhang Q., Qu D. H., Feringa B. L., Tian H., J. Am. Chem. Soc., 2022, 144(5), 2022—2033 |
| 18 | Zhang Q., Shi C. Y., Qu D. H., Long Y. T., Feringa B. L., Tian H., Sci. Adv., 2018, 4(7), eaat8192 |
| 19 | Deng Y. X., Zhang Q., Feringa B. L., Tian H., Qu D. H., Angew. Chem. Int. Ed., 2020, 59(13), 5278—5283 |
| 20 | Endo K., Yamanaka T., Macromolecules, 2006, 39(12), 4038—4043 |
| 21 | Raeisi M., Tsarevsky N. V., J. Polym. Sci., 2021, 59(8), 675—684 |
| 22 | Zhang Q., Deng Y. X., Shi C. Y., Feringa B. L., Tian H., Qu D. H., Matter, 2021, 4(7), 1352—1364 |
| 23 | Zheng S. J., Xue H. Y., Yao J., Chen Y., Brook M. A., Noman M. E., Cao Z. H., ACS Appl. Mater. Interfaces, 2023, 15(34), 41043—41054 |
| 24 | Fan F. Q., Ji S. B., Sun C. X., Liu C., Yu Y., Fu Y., Xu H. P., Angew. Chem. Int. Ed., 2018, 57(50), 16426—16430 |
| 25 | Nelson B. R., Kirkpatrick B. E., Miksch C. E., Davidson M. D., Skillin N. P., Hach G. K., Khang A., Hummel S. N., Fairbanks B. D., Burdick J. A., Bowman C. N., Anseth K. S., Adv. Mater., 2024, 36, 2211209 |
| 26 | Scheutz G. M., Rowell J. L., Ellison S. T., Garrison J. B., Angelini T. E., Sumerlin B. S., Macromolecules, 2020, 53(10), 4038—4046 |
| 27 | Sieredzinska B., Zhang Q., Berg K. J., Flapperc J., Feringa B. L., Chem. Commun., 2021, 57(77), 9838—9841 |
| 28 | Shi C. Y., Zhang Q., Wang B. S., Chen M., Qu D. H., ACS Appl. Mater. Interfaces, 2021, 13(37), 44860—44867 |
| 29 | Singh R., Whiteside G. M., J. Am. Chem. Soc., 1990, 112(3), 1190—1197 |
| 30 | Zhu Y.W., Lin M. Y., Hu W. T., Wang J. K., Zhang Z. G., Zhang K., Yu B. R., Xu F. J., Angew. Chem. Int. Ed., 2022, 61(23), e202200535 |
| 31 | An S. Y., Noh S. M., Oh J. K., Macromol. Rapid Commun., 2017, 38(8), 1600777 |
| 32 | Yu H. S., Wang Y. N., Yang H. Y., Peng K., Zhang X. Y., J. Mater. Chem. B, 2017, 5(22), 4121—4127 |
| 33 | Liu Y., Jia Y., Wu Q., Moore J. S., J. Am. Chem. Soc., 2019, 141(43), 17075—17080 |
| 34 | Gasparini G., Bang E. K., Molinard G., Tulumello D. V., Ward S., Kelley S. O., Roux A., Sakai N., Matile S., J. Am. Chem. Soc., 2014, 136(16), 6069—6074 |
| 35 | Bang E. K., Gasparini G., Molinard G., Roux A., Sakai N., Matile S., J. Am. Chem. Soc., 2013, 135(6), 2088—2091 |
| 36 | Li X., Wang C., Wang L. L., Huang R., Li W. C., Wang X. N., Wong S. S. W., Cai Z. W., Leung K. C. F., Jin L. J., J. Colloid Interface Sci., 2022, 614, 322—336 |
| 37 | Huang S., Shen Y. K., Bisoyi H. K., Tao Y., Liu Z. C., Wang M., Yang H., Li Q., J. Am. Chem. Soc., 2021, 143(32), 12543—12551 |
| 38 | Li Y. L., Zhu L., Liu Z. Z., Cheng R., Meng F. H., Cui J. H., Ji S. J., Zhong Z. Y., Angew. Chem. Int. Ed., 2009, 48(52), 9914—9918 |
| 39 | Houk J., Whitesides G. M., J. Am. Chem. Soc., 1987, 109(22), 6825—6836 |
| 40 | Singh R., Whitesides G. M., J. Am. Chem. Soc., 1990, 112(17), 6304—6309 |
| 41 | Kim S., Wittek K. I., Lee Y., Chem. Sci., 2020, 11(19), 4882—4886 |
| 42 | Behrendt F. N., Schlaad H., Macromol. Rapid Commun., 2018, 39(6), 1700735 |
| 43 | Guo J., Zhang S. Q., Tao Y. Q., Fan B. E., Tang W., Polym. Chem., 2022, 13(48), 6637—6649 |
| 44 | Pięta M., Purohit V. B., Pietrasik J., Plummer C. M., Polym. Chem., 2023, 14(1), 7—31 |
| 45 | Zhang X. Y., Waymouth R. M., J. Am. Chem. Soc., 2017, 139(10), 3822—3833 |
| 46 | Margulis K., Zhang X. Y., Joubert L. M., Bruening K., Tassone C. J., Zare R. N., Waymouth R. M., Angew. Chem. Int. Ed., 2017, 56(51), 16357—16362 |
| 47 | Carmine A., Domoto Y., Sakai N., Matile S., Chem. Eur. J., 2013, 19(35), 11558—11563 |
| 48 | Du T. Y., Shen B. M., Dai J. Y., Zhang M. M., Chen X. J., Yu P. Y., Liu Y., J. Am. Chem. Soc., 2023, 145(50), 27788—27799 |
| 49 | Yu D., Wang Y. C., Qu S., Zhang N., Nie K. L., Wang J. K., Huang Y. C., Sui D. D., Yu B. R., Qin M., Xu F. J., Adv. Mater., 2023, 35(52), 2307190 |
| 50 | Zhang Y. M., Røise J. J., Lee K., Li J., Murthy N., Curr. Opin. Biotechnol., 2018, 52, 25—31 |
| 51 | Hasan M., Khatun A., Kogure K., Pharmaceutics, 2022, 14(3), 525 |
| 52 | Zhao Y., Cao W. Q., Liu Y., Chem. J. Chinese Universities, 2020, 41(5), 909—923 |
| 赵宇, 曹琬晴, 刘阳. 高等学校化学学报, 2020, 41(5), 909—923 | |
| 53 | Bauhuber S., Hozsa C., Breunig M., Göpferich A., Adv. Mater., 2009, 21(32/33), 3286—3306 |
| 54 | Wang F., Gao L., Meng L. Y., Xie J. M., Xiong J. W., Luo Y., ACS Appl. Mater. Interfaces, 2016, 8(49), 33529—33538 |
| 55 | Won Y. W., Yoon S. M., Lee K. M., Kim Y. H., Mol. Ther., 2011, 19(2), 372—380 |
| 56 | Bang E. K., Lista M., Sforazzini G., Sakaia N., Matile S., Chem. Sci., 2012, 3(6), 1752—1763 |
| 57 | Yuan P. Y., Mao X., Chong K. C., Fu J. Q., Pan S. J., Wu S. Z., Yu C. M., Yao S. Q., Small, 2017, 13(27), 1700569 |
| 58 | Hei M. W., Zhan Y. R., Chen P., Zhao R. M., Tian X. L., Yu X. Q., Zhang J., Mol. Pharmaceutics, 2023, 20(6), 3210—3222 |
| 59 | Yang W. H., Yu C. M., Wu C. X., Yao S. Q., Wu S. Z., Polym. Chem., 2017, 8(27), 4043—4051 |
| 60 | Cheng L., Yang L., Meng F. H., Zhong Z. Y., Adv. Healthcare Mater., 2018, 7(20), 1800685 |
| 61 | Chen C. Y., Gao P., Wang H., Cheng Y. Y., Lv J., Biomater. Sci., 2023, 11(5), 1765—1775 |
| 62 | Yang Y. X., Zuo S. Y., Zhang J. X., Liu T., Li X. M., Zhang H. T., Cheng M. S., Wang S. J., He Z. G., Sun B. J., Sun J., Nano Today, 2022, 44, 101480 |
| 63 | Fu J. Q., Yu C. M., Li L., Yao S. Q., J. Am. Chem. Soc., 2015, 137(37), 12153—12160 |
| 64 | Qian L. H., Fu J. Q., Yuan P. Y., Du S. B., Huang W., Li L., Yao S. Q., Angew. Chem. Int. Ed., 2018, 57(6), 1532—1536 |
| 65 | Lu J. H., Wang H., Tian Z. Y., Hou Y. Q., Lu H., J. Am. Chem. Soc., 2020, 142(3), 1217—1221 |
| 66 | Lu J. H., Xu Z., Fu H. L., Lin Y., Wang H., Lu H., J. Am. Chem. Soc., 2022, 144(34), 15709—15717 |
| 67 | Lu J. H., Dai Y. H., He Y. H., Zhang T., Zhang J., Chen X. M., Jiang C. T., Lu H., J. Am. Chem. Soc., 2024, 146(6), 3974—3983 |
| 68 | Zhang R. H., Nie T. Q., Fang Y. F., Huang H., Wu J., Biomacromolecules, 2022, 23(1), 1—19 |
| 69 | Chen Z., Trends Mol. Med., 2010, 16(12), 594—602 |
| 70 | Mondal A., Das S., Ali S. M., Kolay S., Sengupta A., Molla M. R., Bioconjugate Chem., 2023, 34(3), 489—500 |
| 71 | Zhang P. H., Wang Y., Lian J., Shen Q., Wang C., Ma B. H., Zhang Y. C., Xu T. T., Li J. X., Shao Y. P., Xu F., Zhu J. J., Adv. Mater., 2017, 29(36), 1702311 |
| 72 | Mu S. W., Zhu Y. W., Wang Y., Qu S., Huang Y. C., Zheng L., Duan S., Yu B. R., Qin M., Xu F. J., Adv. Mater., 2022, 34(41), 2204065 |
| 73 | Son S., Namgung R., Kim J., Singha K., Kim W. J., Acc. Chem. Res., 2012, 45(7), 1100—1112 |
| [1] | 张荡, 孙小敏, 杨海跃, 宋勃翰, 丛萌, 王宇新, 丁锋, 徐珊珊, 毕赛, 王磊. 基于纳米酶的微纳米马达在智能药物递送中的应用[J]. 高等学校化学学报, 2025, 46(1): 76. |
| [2] | 严子谅, 李蓓, 代云路. 基于多酚的超分子纳米药物递送系统的研究进展[J]. 高等学校化学学报, 2025, 46(1): 14. |
| [3] | 梁惠闲, 王淼, 张雨璨, 白丽, 李雪妹, 于法标, 程子译, 赵琳璐. 基于Ag2S量子点的光热治疗协同药物治疗在动脉粥样硬化中的应用[J]. 高等学校化学学报, 2025, 46(1): 196. |
| [4] | 陈俊年, 张海峰, 王海兵, 杨火诚, 罗忠. 基于超分子纳米递送系统的精准医疗研究进展[J]. 高等学校化学学报, 2025, 46(1): 50. |
| [5] | 盛劲菡, 郑琪臻, 汪铭. CRISPR/Cas9基因编辑非病毒递送系统[J]. 高等学校化学学报, 2023, 44(3): 20220344. |
| [6] | 杨霁野, 孙大吟, 王妍, 谷安祺, 叶一兰, 丁书江, 杨振忠. 若干典型中空结构材料的模板合成与应用进展[J]. 高等学校化学学报, 2023, 44(1): 20220665. |
| [7] | 王慧, 赵德偲, 杨乃亮, 王丹. 智能中空药物载体的门控设计[J]. 高等学校化学学报, 2023, 44(1): 20220237. |
| [8] | 仵宇帅, 尚颖旭, 蒋乔, 丁宝全. 可控自组装DNA折纸结构作为药物载体的研究进展[J]. 高等学校化学学报, 2022, 43(8): 20220179. |
| [9] | 储彬彬, 何耀. 硅基纳米探针用于眼部疾病的成像检测与治疗[J]. 高等学校化学学报, 2022, 43(12): 20220546. |
| [10] | 汪诗琪, 罗博文, 俞计成, 顾臻. 近红外二区活体荧光成像在肿瘤诊疗中的应用[J]. 高等学校化学学报, 2022, 43(12): 20220577. |
| [11] | 张万斌, 王艳蒙, 王少武, 童欣, 韩小倩, 张策, 张光华, 朱秀忠. 烷基功能化聚烯丙基缩水甘油醚的制备及对PVC的增塑及抗静电作用[J]. 高等学校化学学报, 2021, 42(9): 2961. |
| [12] | 马钰琨, 金慧, 任传利, 李志波. 聚苯乙烯微球负载脲催化剂与碱协同催化环内酯的开环聚合[J]. 高等学校化学学报, 2021, 42(9): 2968. |
| [13] | 李荣烨, 倪云霞, 刘丹丹, 李志, 程玉新, 夏明欣, 付小会. 一种新型温度响应性聚氨基酸/聚类肽嵌段共聚物的合成与表征[J]. 高等学校化学学报, 2021, 42(3): 850. |
| [14] | 李琛, 李悦生. 吡啶基有机碱催化O-羧基酐的活性开环聚合[J]. 高等学校化学学报, 2021, 42(10): 3203. |
| [15] | 任玉双, 郭园园, 刘学怡, 宋杰, 张川. 顺铂前药接枝修饰硫代DNA及其自组装靶向纳米药物研究[J]. 高等学校化学学报, 2020, 41(8): 1721. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||