

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (5): 20220032.doi: 10.7503/cjcu20220032
收稿日期:2022-01-13
出版日期:2022-05-10
发布日期:2022-02-28
通讯作者:
王宁,孙启明
E-mail:wangning2021@qdu.edu.cn;sunqiming@suda.edu.cn
基金资助:
LI Jiafu1, ZHANG Kai2, WANG Ning1( ), SUN Qiming2(
), SUN Qiming2( )
)
Received:2022-01-13
Online:2022-05-10
Published:2022-02-28
Contact:
WANG Ning,SUN Qiming
E-mail:wangning2021@qdu.edu.cn;sunqiming@suda.edu.cn
Supported by:摘要:
分子筛由于具有规则的微孔孔道结构、 较大的表面积、 优异的(水)热稳定性, 被认为是限域合成超小尺寸金属物种的理想载体. 近年来, 分子筛限域单原子金属催化剂由于超高的金属分散度、 接近100%的金属利用率以及独特的电子结构, 被广泛地应用于重要的催化反应和气体吸附分离过程. 本文系统地总结了近年来分子筛限域不同类型单原子金属催化剂的合成策略, 以及其在多相催化和气体分离等领域的研究进展. 最后, 提出了分子筛限域单原子金属催化剂在合成与表征方面存在的挑战和未来的发展方向.
中图分类号:
TrendMD:
李加富, 张凯, 王宁, 孙启明. 分子筛限域单原子金属催化剂的研究进展. 高等学校化学学报, 2022, 43(5): 20220032.
LI Jiafu, ZHANG Kai, WANG Ning, SUN Qiming. Research Progress of Zeolite-encaged Single-atom Metal Catalysts. Chem. J. Chinese Universities, 2022, 43(5): 20220032.
| Catalyst | Noble or non?noble metal species | Zeolite type | Metal loading/(mass fraction, %) | Synthetic method | Characterization method | Application | Ref. |
|---|---|---|---|---|---|---|---|
| Rh@S?1?H | Rh atoms | MFI | 0.28—0.71 | Insitu hydrothermal synthesis | HAADF?STEM, EXAFS, XANES,CO?DRIFTS | Ammonia borane hydrolysis; Hydrogenation of nitroarenes compounds | [ |
| Rh@MFI | Rh atoms | MFI | 0.95 | Insitu hydrothermal synthesis | CO?DRIFTS, HAADF?STEM | Methanol carbonylation | [ |
| Rh?ZSM?5washed | Rh atoms | MFI | 0.5 | Impregnation method coupled with washing | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Oxidation of methane to acetic acid | [ |
| Rh?ZSM?5 | Rh atoms | MFI | 0.1 | Incipient wetness impregnation | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Oxidation of methane to acetic acid | [ |
| Rh(C2H4)2/SAPO?37 | Rh(C2H4)2 | FAU | 1.0 | Adsorption of organometallic compounds | CO?DRIFTS, EXAFS, XANES | Hydrogenation and dimerization of ethylene | [ |
| Rh(CO)2/HY | Rh(CO)2 | FAU | 0.5 | Adsorption of organometallic compounds | FTIR, EXAFS, XANES | Water gas shift reaction | [ |
| Pt@Y | Pt atoms | FAU | 0.6 | Insitu hydrothermal synthesis | HAADF?STEM, EXAFS, XANES | Selective hydrogenation of α,β?unsaturated aldehydes and nitroarenes | [ |
| Pt?ISAS@Y | Pt atoms | FAU | 0.22 | Insitu hydrothermal synthesis | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Ethane dehydrogenation and n?hexane isomerization | [ |
| Catalyst | Noble or non?noble metal species | Zeolite type | Metal loading/(mass fraction, %) | Synthetic method | Characterization method | Application | Ref. |
| Pt?Zn?DeAlBEA | Pt atoms | BEA | 0.73 | Impregnation method | HAADF?STEM, EXAFS, XANES | Propane dehydrogenation | [ |
| Pt/HZSM?5 | Pt atoms | MFI | 0.5 | Chemical vapor deposition | CO?DRIFTS, HAADF?STEM | CO oxidation Water?gas shift | [ |
| Pt/KLTL | Pt atoms | LTL | 1.0 | Ion exchange | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | CO oxidation | [ |
| Ir(C2H4)2/HY | Ir(C2H4)2 | FAU | 1.0 | Adsorption of organometallic compounds | HAADF?STEM, EXAFS | Cyclohexene hydrogenation | [ |
| Ir(C2H4)2/HSSZ-53 | Ir(C2H4)2 | SFH | 1.0 | Adsorption of organometallic compounds | FTIR, HAADF?STEM, EXAFS | Ethylene hydrogenation | [ |
| Ir@MWW?air | Ir atoms | MWW | 0.24 | Insitu hydrothermal synthesis | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Hydrogenolysis of alkane | [ |
| Au(CH3)2/NaY | Au(CH3)2 | FAU | 1.0 | Adsorption of organometallic compounds | FTIR, HAADF?STEM, EXAFS | CO oxidation | [ |
| Au?K/KLTL | Au atoms | LTL | 0.25 | Impregnation method | HAADF?STEM, EXAFS, XANES | Water?gas shift reaction | [ |
| Pd/ZSM?5 | Pd atoms | MFI | 0.01—0.04 | Incipient wetness impregnation | TEM, EXAFS | Methane oxidation | [ |
| Ru(acac)·(C2H4)2/HY | Ru(acac)(C2H4)2 | FAU | 1.0 | Adsorption of organometallic compounds | FTIR, EXAFS | Ethylene dimerization | [ |
| Ru@S?1 | Ru atoms | MFI | 0.27 | Insitu hydrothermal synthesis | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Ammonia synthesis | [ |
| Fe?BEA | Fe atoms | BEA | 0.3 | Impregnation method | Magnetic circular dichroism, M?ssbauer spectroscopy | Methane hydroxylation | [ |
| FeS?1?EDTA | Fe atoms | MFI | 1.2 | Insitu hydrothermal synthesis | UV Raman spectra, EXAFS, H2?TPR | Ethane dehydrogenation | [ |
| Ni@CHA | Ni atoms | CHA | 3.5 | Insitu hydrothermal synthesis | XANES, EXAFS, UV?Vis?NIR | Acetylene?selective hydrogenation | [ |
| Ni@FAU | Ni atoms | FAU | 4.5 | Insitu hydrothermal synthesis | XANES, in situ neutron powder diffraction, TEM | Chemoselective alkyne/olefin separation | [ |
| Cu?LTA | Cu atoms | LTA | 3.6 | Ion exchange | XANES, synchrotron powder XRD, ESR | NH3?SCR | [ |
| Cu?SSZ?13 | Cu atoms | CHA | 2.1—3.1 | Ion exchange | XANES, EXAFS, UV?Vis?NIR | NH3?SCR | [ |
| Ga/H?MFI | Ga atoms | MFI | 0.3—3.0 | Vapor?phase exchange | XANES, EXAFS | Propane dehydrogenation | [ |
| In?CHA | In atoms | CHA | ca. 6.0 | Solid?state ion?exchange | FTIR, XANES, EXAFS | Ethane dehydrogenation | [ |
| Ti/UCB?4 | calix[ | -SVY | 0.37 | Grafting of Ti complex | XANES | Cyclohexene epoxidation | [ |
Table 1 Summary of the synthesis, characterization and application of zeolite-encaged single-atom catalysts*
| Catalyst | Noble or non?noble metal species | Zeolite type | Metal loading/(mass fraction, %) | Synthetic method | Characterization method | Application | Ref. |
|---|---|---|---|---|---|---|---|
| Rh@S?1?H | Rh atoms | MFI | 0.28—0.71 | Insitu hydrothermal synthesis | HAADF?STEM, EXAFS, XANES,CO?DRIFTS | Ammonia borane hydrolysis; Hydrogenation of nitroarenes compounds | [ |
| Rh@MFI | Rh atoms | MFI | 0.95 | Insitu hydrothermal synthesis | CO?DRIFTS, HAADF?STEM | Methanol carbonylation | [ |
| Rh?ZSM?5washed | Rh atoms | MFI | 0.5 | Impregnation method coupled with washing | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Oxidation of methane to acetic acid | [ |
| Rh?ZSM?5 | Rh atoms | MFI | 0.1 | Incipient wetness impregnation | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Oxidation of methane to acetic acid | [ |
| Rh(C2H4)2/SAPO?37 | Rh(C2H4)2 | FAU | 1.0 | Adsorption of organometallic compounds | CO?DRIFTS, EXAFS, XANES | Hydrogenation and dimerization of ethylene | [ |
| Rh(CO)2/HY | Rh(CO)2 | FAU | 0.5 | Adsorption of organometallic compounds | FTIR, EXAFS, XANES | Water gas shift reaction | [ |
| Pt@Y | Pt atoms | FAU | 0.6 | Insitu hydrothermal synthesis | HAADF?STEM, EXAFS, XANES | Selective hydrogenation of α,β?unsaturated aldehydes and nitroarenes | [ |
| Pt?ISAS@Y | Pt atoms | FAU | 0.22 | Insitu hydrothermal synthesis | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Ethane dehydrogenation and n?hexane isomerization | [ |
| Catalyst | Noble or non?noble metal species | Zeolite type | Metal loading/(mass fraction, %) | Synthetic method | Characterization method | Application | Ref. |
| Pt?Zn?DeAlBEA | Pt atoms | BEA | 0.73 | Impregnation method | HAADF?STEM, EXAFS, XANES | Propane dehydrogenation | [ |
| Pt/HZSM?5 | Pt atoms | MFI | 0.5 | Chemical vapor deposition | CO?DRIFTS, HAADF?STEM | CO oxidation Water?gas shift | [ |
| Pt/KLTL | Pt atoms | LTL | 1.0 | Ion exchange | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | CO oxidation | [ |
| Ir(C2H4)2/HY | Ir(C2H4)2 | FAU | 1.0 | Adsorption of organometallic compounds | HAADF?STEM, EXAFS | Cyclohexene hydrogenation | [ |
| Ir(C2H4)2/HSSZ-53 | Ir(C2H4)2 | SFH | 1.0 | Adsorption of organometallic compounds | FTIR, HAADF?STEM, EXAFS | Ethylene hydrogenation | [ |
| Ir@MWW?air | Ir atoms | MWW | 0.24 | Insitu hydrothermal synthesis | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Hydrogenolysis of alkane | [ |
| Au(CH3)2/NaY | Au(CH3)2 | FAU | 1.0 | Adsorption of organometallic compounds | FTIR, HAADF?STEM, EXAFS | CO oxidation | [ |
| Au?K/KLTL | Au atoms | LTL | 0.25 | Impregnation method | HAADF?STEM, EXAFS, XANES | Water?gas shift reaction | [ |
| Pd/ZSM?5 | Pd atoms | MFI | 0.01—0.04 | Incipient wetness impregnation | TEM, EXAFS | Methane oxidation | [ |
| Ru(acac)·(C2H4)2/HY | Ru(acac)(C2H4)2 | FAU | 1.0 | Adsorption of organometallic compounds | FTIR, EXAFS | Ethylene dimerization | [ |
| Ru@S?1 | Ru atoms | MFI | 0.27 | Insitu hydrothermal synthesis | CO?DRIFTS, HAADF?STEM, EXAFS, XANES | Ammonia synthesis | [ |
| Fe?BEA | Fe atoms | BEA | 0.3 | Impregnation method | Magnetic circular dichroism, M?ssbauer spectroscopy | Methane hydroxylation | [ |
| FeS?1?EDTA | Fe atoms | MFI | 1.2 | Insitu hydrothermal synthesis | UV Raman spectra, EXAFS, H2?TPR | Ethane dehydrogenation | [ |
| Ni@CHA | Ni atoms | CHA | 3.5 | Insitu hydrothermal synthesis | XANES, EXAFS, UV?Vis?NIR | Acetylene?selective hydrogenation | [ |
| Ni@FAU | Ni atoms | FAU | 4.5 | Insitu hydrothermal synthesis | XANES, in situ neutron powder diffraction, TEM | Chemoselective alkyne/olefin separation | [ |
| Cu?LTA | Cu atoms | LTA | 3.6 | Ion exchange | XANES, synchrotron powder XRD, ESR | NH3?SCR | [ |
| Cu?SSZ?13 | Cu atoms | CHA | 2.1—3.1 | Ion exchange | XANES, EXAFS, UV?Vis?NIR | NH3?SCR | [ |
| Ga/H?MFI | Ga atoms | MFI | 0.3—3.0 | Vapor?phase exchange | XANES, EXAFS | Propane dehydrogenation | [ |
| In?CHA | In atoms | CHA | ca. 6.0 | Solid?state ion?exchange | FTIR, XANES, EXAFS | Ethane dehydrogenation | [ |
| Ti/UCB?4 | calix[ | -SVY | 0.37 | Grafting of Ti complex | XANES | Cyclohexene epoxidation | [ |
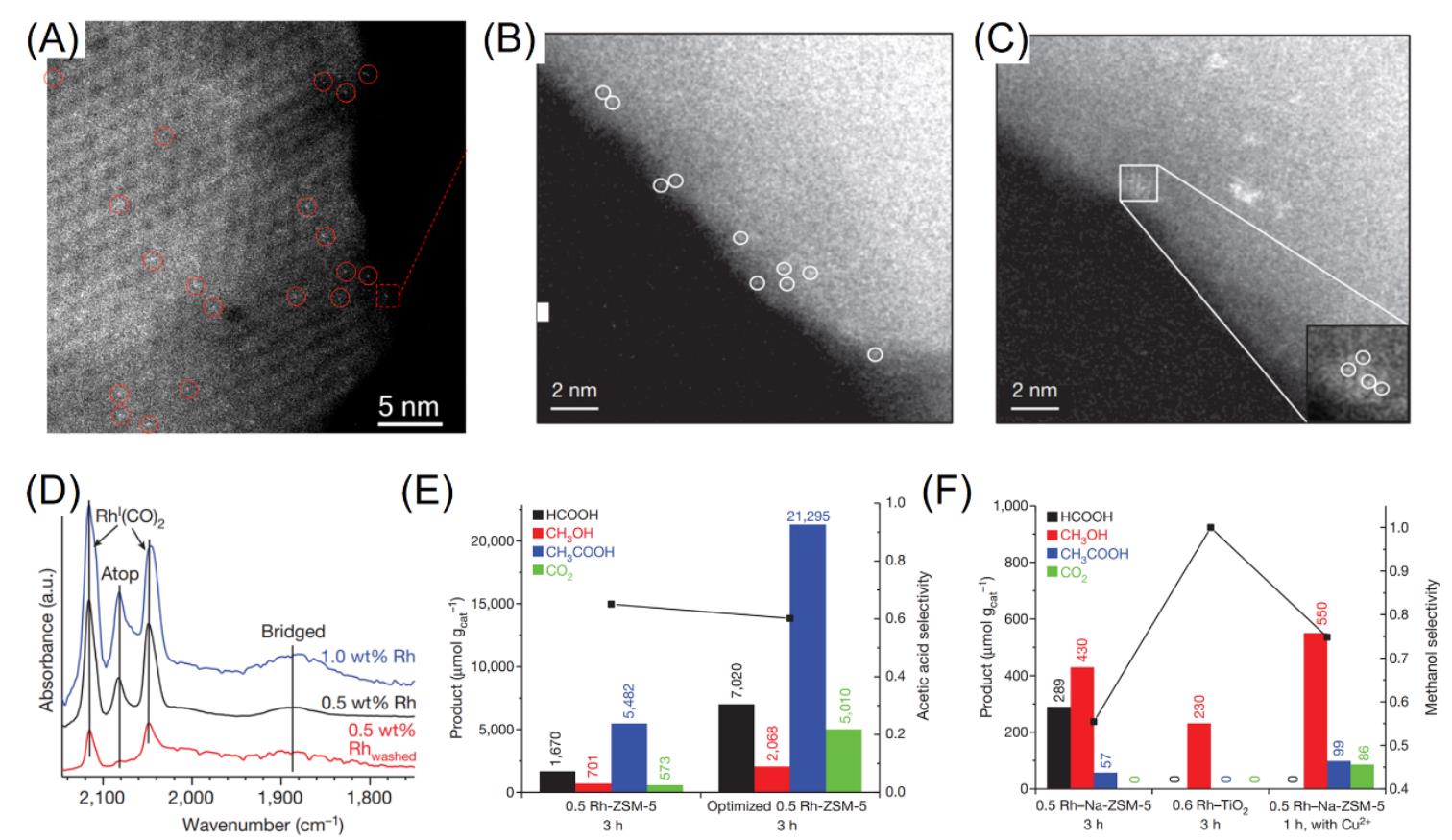
Fig.2 Cs?corrected HAADF?STEM image of zeolite HY?encaged [Rh(C2H4)2]+ catalysts[51](A), Cs?corrected HAADF?STEM image of as?synthesized Rh?ZSM?5 catalysts with(B) and without(C) washing by water, CO?DRIFTS spectra of 1.0% Rh?ZSM?5, 0.5% Rh?ZSM?5, and 0.5% Rh?ZSM?5washed(D), catalytic performance of 0.5% RhZSM?5 and optimized 0.5% Rh?ZSM?5 catalysts in the methane conversion(E), product yields and methanol selectivity for 0.5% Rh?Na?ZSM?5 with and without Cu2+, as well as on 0.6% Rh/TiO2 catalysts(F)[41](A) Bright features encircled are individual Rh atoms, Copyright 2016, American Chemical Society. Reaction conditions: 20 mg catalyst, 2×10-5 Pa O2, 5×10-5 Pa CO, 2×10-4 Pa CH4, 20 mL water, 3 h reaction time, 150 ℃ reaction temperature. (B―F) Copyright 2017, Springer Nature.
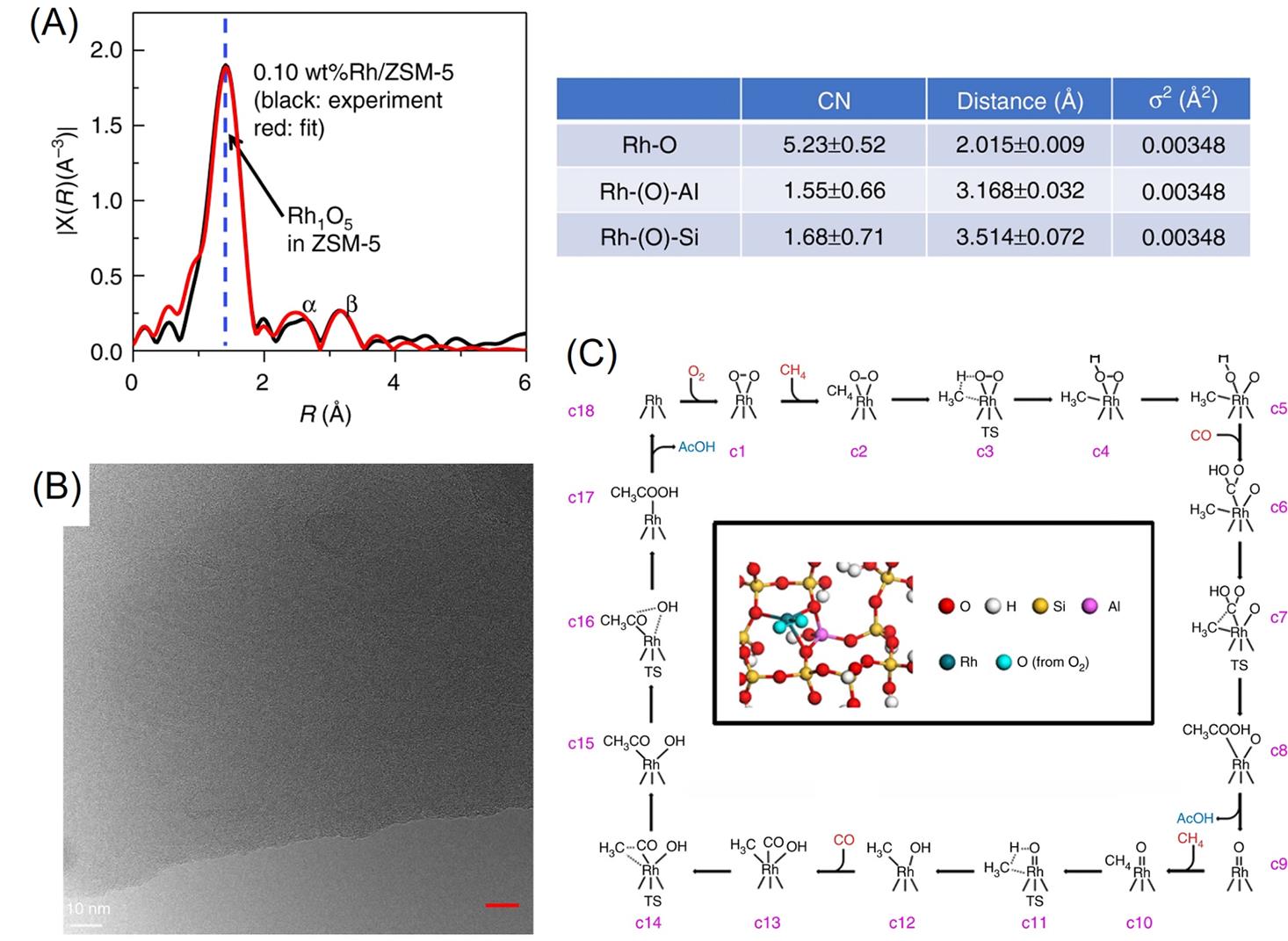
Fig.3 R?space of Rh K?edge of experimental(black) and calculated(red) data of the k2?weighted Rh K?edge EXAFS spectra of spent 0.10% Rh/ZSM?5, and the coordination number and bond length of Rh—O, Rh—(O)—Al, and Rh—(O)—Si(A), TEM image of 0.10%Rh/ZSM?5(scale bar: 10?nm)(B), computational studies of reaction pathway of methane oxidation reactions on Rh1O5/ZSM?5(C)[54]Copyright 2018, Springer Nature.
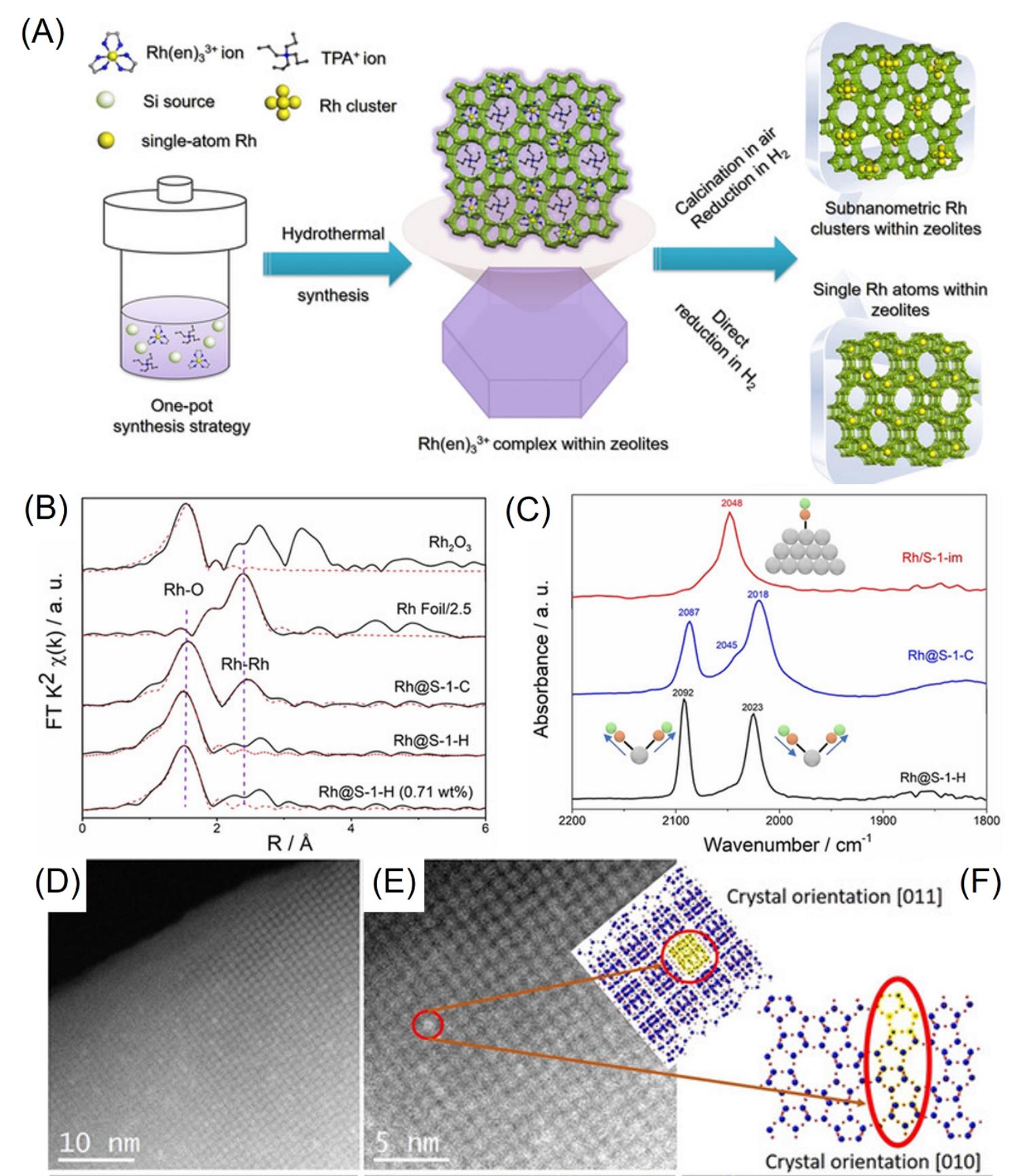
Fig.4 Schematic of the synthetic procedure of the Rh@S?1 catalyst(A), Fourier transform of k2?weighted EXAFS spectra of Rh foil and various zeolite?encaged Rh catalysts at Rh K?edge(B), in situ CO?DRIFTS spectra of Rh@S?1?H(Rh single atoms), Rh@S?1?C(Rh clusters), and Rh@S?1?H (Rh nanoparticles)(C), Cs?corrected STEM images(D,E) of Rh@S?1?H viewed different orientation as well as the schematic models(F)[16]Copyright 2019, Wiley?VCH.
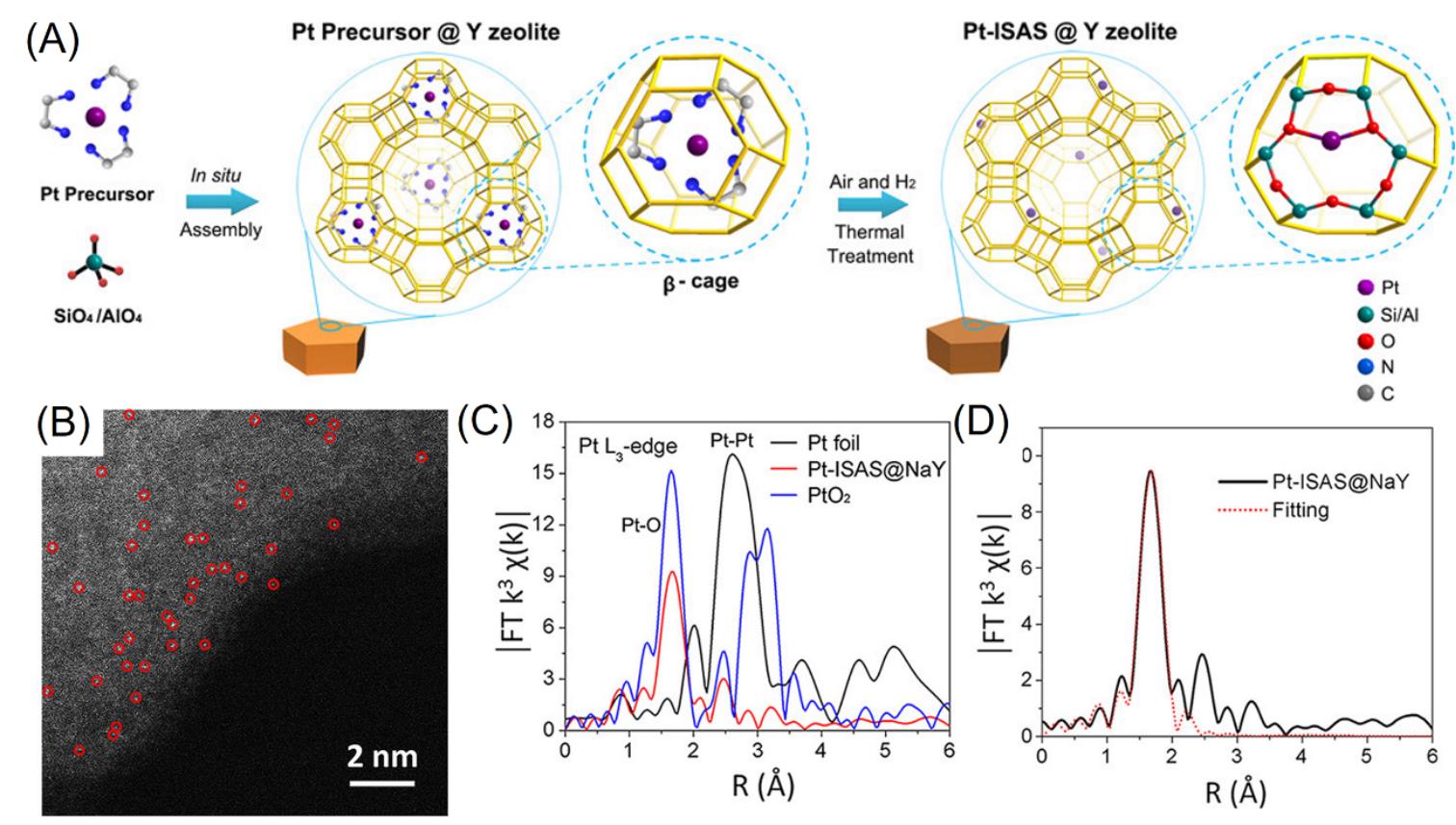
Fig.5 Schematic of the zeolite?encaged Pt single?atom catalyst(A), Cs?corrected STEM image of Pt?ISAS@NaY(B), Fourier transforms of k3?weighted Pt L?edge EXAFS experimental data for Pt?ISAS@NaY as compared with Pt foil and PtO2(C), EXAFS fitting curves of Pt?ISAS@NaY at the R space(D)[17]Copyright 2019, American Chemical Society.
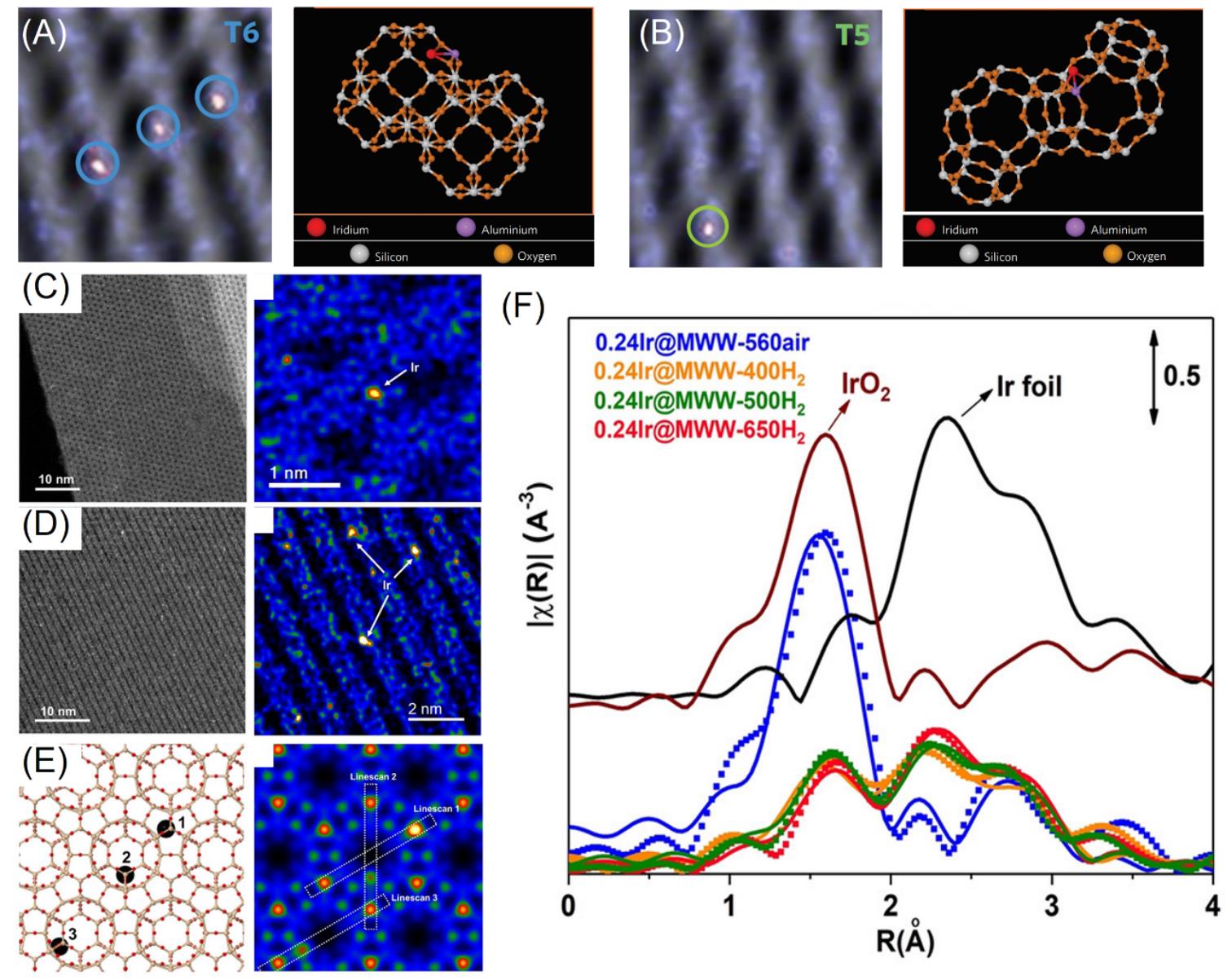
Fig.6 Cs?corrected HAADF?STEM images and the corresponding models illustrating the positions of the Ir+ ions for T6 site and the T5 site(A,B)[44], HAADF?STEM images of Ir@MWW?560?air sample along different orientations(C,D),?model of MWW zeolite with isolated Ir atoms located at different positions and?simulated HAADF?STEM image(E), in situ EXAFS spectra of 0.24Ir@MWW samples reduced at given temperature by H2(F)[48](A, B) Copyright 2010, Springer Nature; (C―F) Copyright 2020, Wiley?VCH.
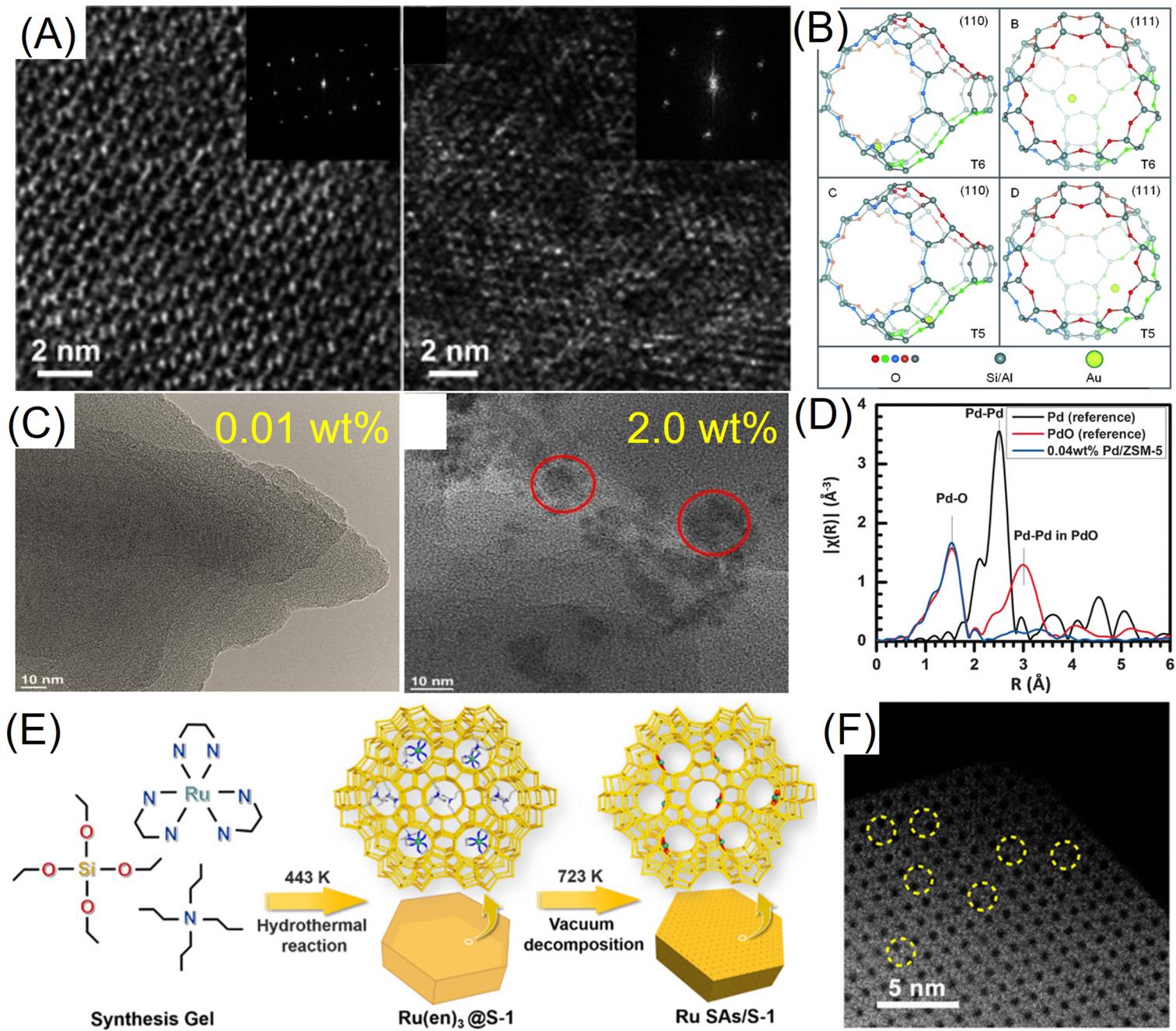
Fig.7 Cs?corrected HAADF?STEM images of the sample prepared by adsorption of Au(CH3)2(acac) in zeolite?NaY(A), perspective views of gold atoms at T6 and T5?positions in an isolated FAU supercage, viewed from the [110] and [111]?projections(B)[45], TEM images(C) of 0.01% Pd/ZSM?5 and 2.0% Pd/ZSM?5 catalysts, Fourier transform magnitudes of k2?weighted EXAFS data of 0.04% Pd/ZSM?5, Pd foil, and PdO nanoparticles(D)[65] schematic of synthesis procedure of Ru SAs/S?1(E), Cs?corrected HAADF?STEM image of Ru SAs/S?1(F)[42](A, B) Copyright 2012, Wiley?VCH; (C, D) Copyright 2016, Wiley?VCH; (E, F) Copyright 2019, American Chemical Society.
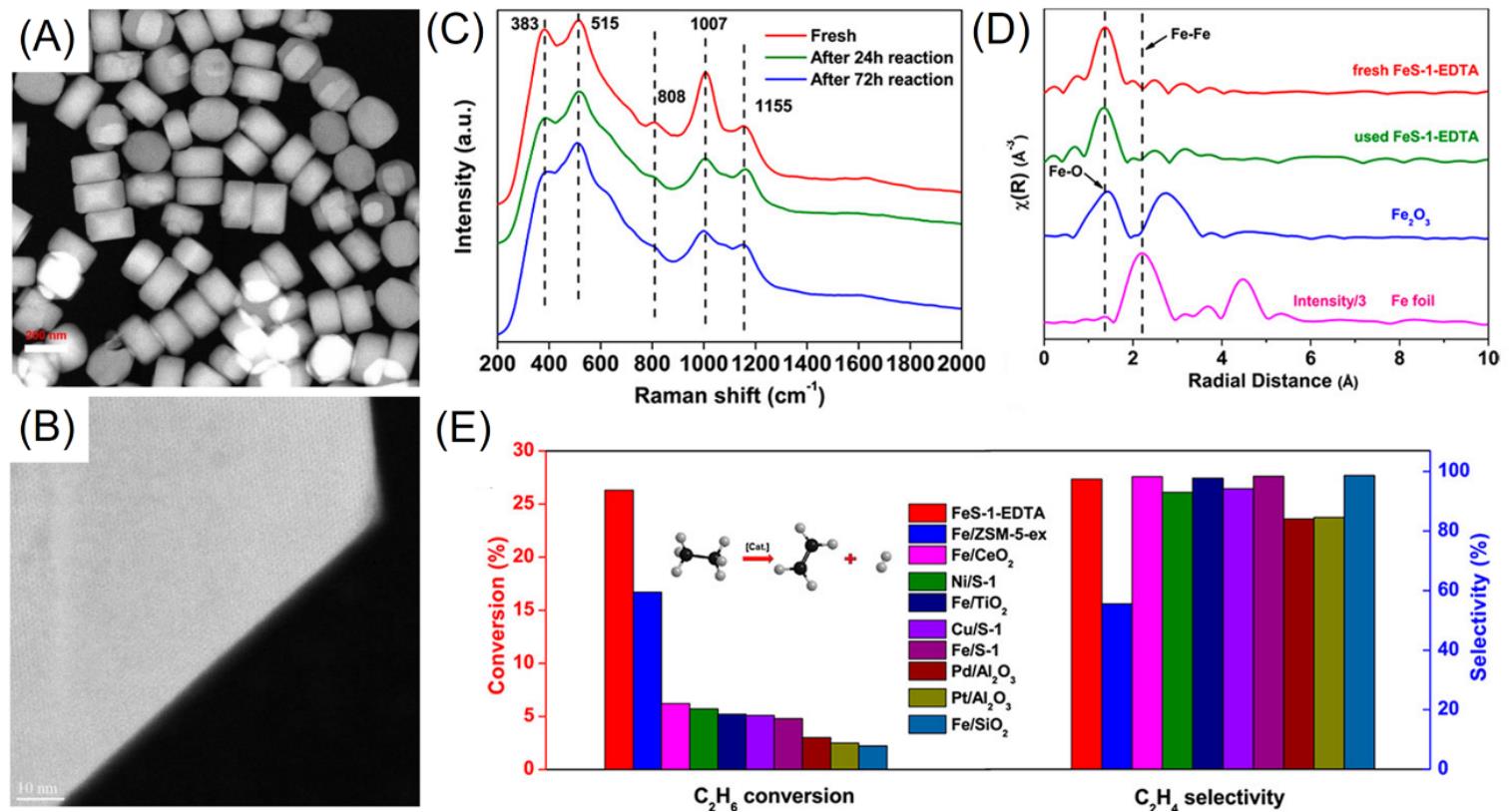
Fig.8 Cs?corrected HAADF?STEM images of FeS?1?EDTA with different amplifications(A, B), UV resonance Raman(λex=325 nm)(C) and EXAFS spectra of fresh and spent FeS?1?EDTA catalysts after the EDH reaction(D), ethane conversion and ethylene selectivity of various catalysts in EDH reactions(E)[43]Reaction conditions: 0.2 g of catalyst, 873 K, gas flowing rate at 2 L?gcat-1?h-1(30% ethane balanced with Ar), and pressure at 1.01×10-5 Pa. Copyright 2020, American Chemical Society.
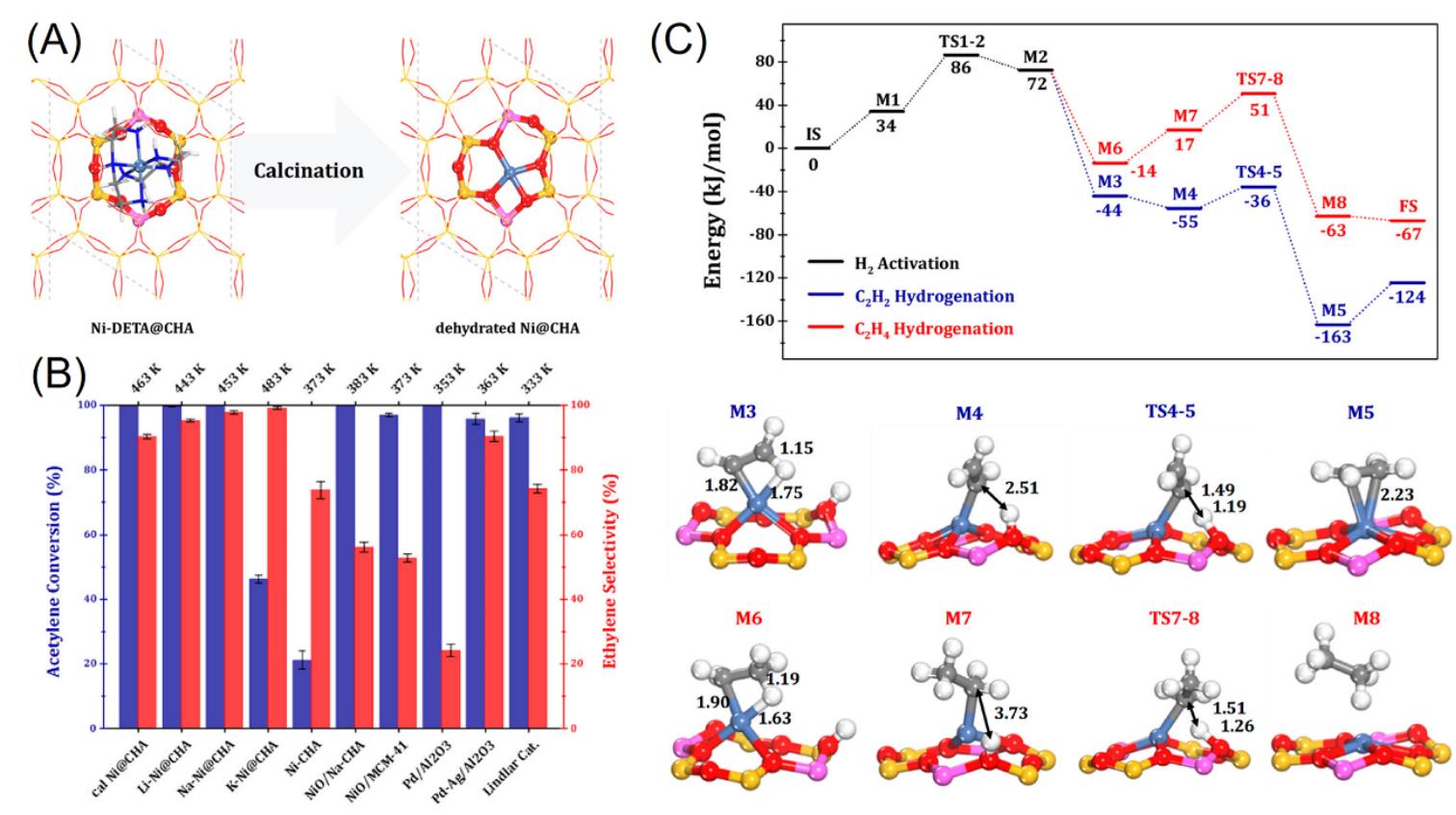
Fig.9 Schematic structure evolution of nickel species confined in CHA zeolite(A), comparison of different catalysts in acetylene?selective hydrogenation(B) and calculated Gibbs free energy profile of Ni@CHA?catalyzed acetylene?selective hydrogenation at 453 K(C)[46]Reaction conditions: 0.2 g of catalyst, 1% C2H2, 16% H2 in He, GHSV=15000 h-1.Copyright 2019, American Chemical Society.
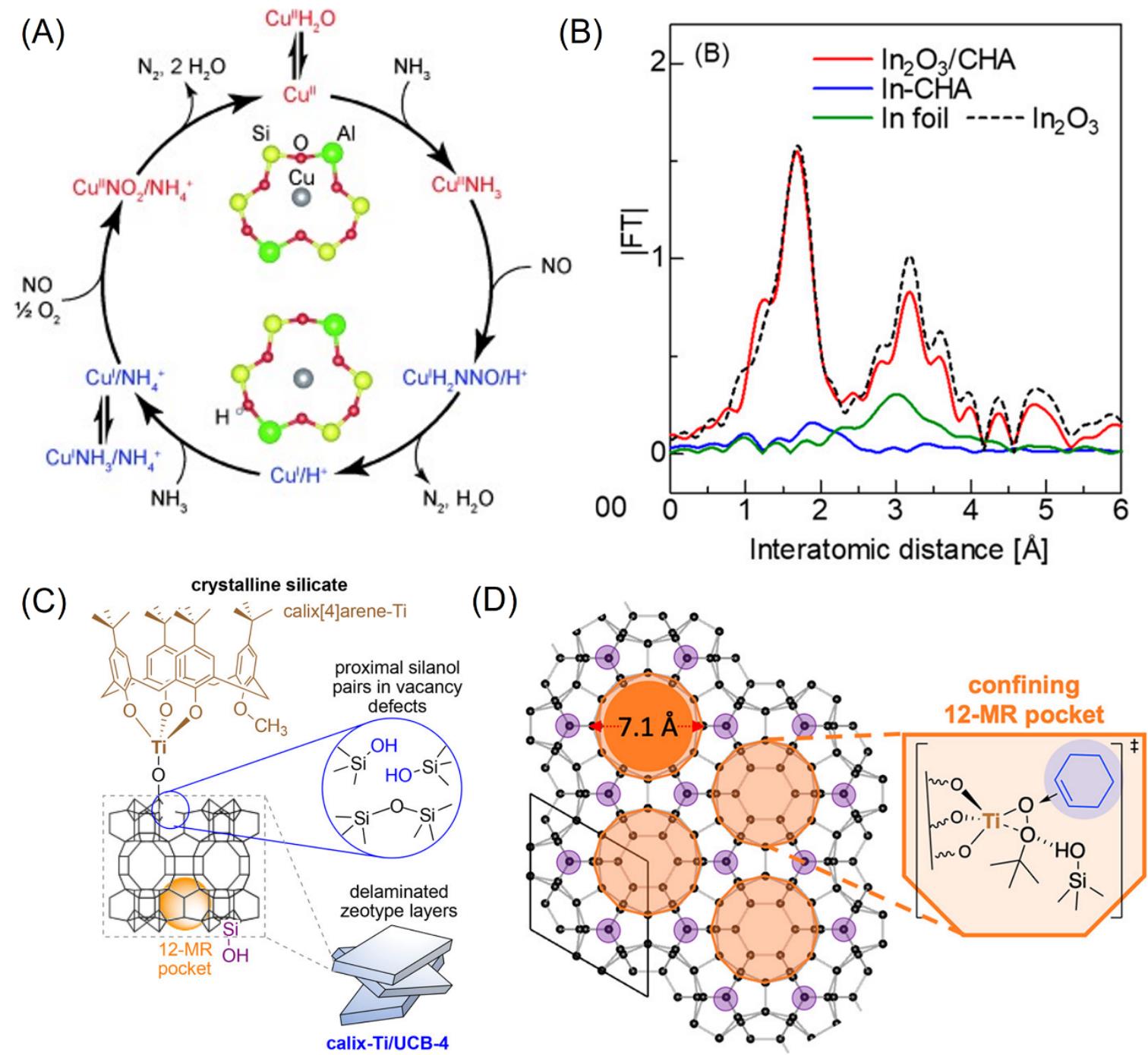
Fig.10 Proposed NH3?SCR cycle over Cu?SSZ?13 catalyst[71](A), EXAFS spectra of In?CHA after reductive solid?state ion?exchange under H2 atmosphere at room temperature(B)[76], schematic representation of zeolite supported calix[4]arene?TiIV(C)[77], grafting model of calix[4]arene?TiIV in the confining 12?MR ring(D)[78](A) Copyright 2014, Wiley?VCH; (B) Copyright 2020, American Chemical Society; (C) Copyright 2018, American Chemical Society; (D) Copyright 2019, American Chemical Society.
| 1 | Yang X., Wang A., Qiao B., Li J., Liu J., Zhang T., Acc. Chem. Res., 2013, 46, 1740—1748 |
| 2 | Chen Y., Ji S., Chen C., Peng Q., Wang D., Li Y., Joule, 2018, 2, 1242—1264 |
| 3 | Wang A., Li J., Zhang T., Nat. Rev. Chem., 2018, 2, 65—81 |
| 4 | Su X., Yang X., Huang Y., Liu B., Zhang T., Acc. Chem. Res., 2019, 52, 656—664 |
| 5 | Wang X., Li Z., Qu Y., Yuan T., Wang W., Wu Y., Li Y., Chem, 2019, 5, 1486—1511 |
| 6 | Ji S., Chen Y., Wang X., Zhang Z., Wang D., Li Y., Chem. Rev., 2020, 120, 11900—11955 |
| 7 | Qin R., Liu K., Wu Q., Zheng N., Chem. Rev., 2020, 120, 11810—11899 |
| 8 | Wei Y., Zhang M., Zou R., Xu Q., Chem. Rev., 2020, 120, 12089—12174 |
| 9 | Chen Y., Ji S., Wang Y., Dong J., Chen W., Li Z., Shen R., Zheng L., Zhuang Z., Wang D., Li Y., Angew. Chem. Int. Ed., 2017, 56, 6937—6941 |
| 10 | Xiong Y., Dong J., Huang Z., Xin P., Chen W., Wang Y., Li Z., Jin Z., Xing W., Zhuang Z., Ye J., Wei X., Cao R., Gu L., Sun S., Zhuang L., Chen X., Yang H., Chen C., Peng Q., Chang C., Wang D., Li Y., Nat. Nanotech., 2020, 15, 390—397 |
| 11 | Qiao B., Wang A., Yang X., Allard F., Jiang Z., Cui Y., Liu J., Li J., Zhang T., Nat. Chem., 2011, 3, 634-641 |
| 12 | Bai S., Liu F., Huang B., Li F., Lin H., Wu T., Sun M., Wu J., Shao Q., Xu Y., Huang X., Nat. Commun., 2020, 11, 954 |
| 13 | Zhang L., Cong M., Ding X., Jin Y., Xu F., Wang Y., Chen L., Zhang L., Angew. Chem. Int. Ed., 2020, 59, 10888—10893 |
| 14 | Ji S., Chen Y., Zhao S., Chen W., Shi L., Wang Y., Dong J., Li Z., Li F., Chen C., Peng Q., Li J., Wang D., Li Y., Angew. Chem. Int. Ed., 2019, 58, 4271—4275 |
| 15 | Zhong W., Sa R., Li L., He Y., Li L., Bi J., Zhuang Z., Yu Y., Zou Z., J. Am. Chem. Soc., 2019, 141, 7615—7621 |
| 16 | Sun Q., Wang N., Zhang T., Bai R., Mayoral A., Zhang P., Zhang Q., Terasaki O., Yu J., Angew. Chem. Int. Ed., 2019, 58, 18570—18576 |
| 17 | Liu Y., Li Z., Yu Q., Chen Y., Chai Z., Zhao G., Liu S., Cheong W., Pan Y., Zhang Q., Gu L., Zheng L., Wang Y., Lu Y., Wang D., Chen C., Peng Q., Liu Y., Liu L., Chen J., Li Y., J. Am. Chem. Soc., 2019, 141, 9305—9311 |
| 18 | Li Y., Li L., Yu J., Chem., 2017, 3, 928—949 |
| 19 | Sun Q., Xie Z., Yu J., Natl. Sci. Rev., 2018, 5, 542—558 |
| 20 | Chen L., Sun M., Wang Z., Yang W., Xie Z., Su B., Chem. Rev., 2020, 120, 11194—11294 |
| 21 | Wen J. L., Zhang J. H., Jiang J. X., Chem. J. Chinese Universities, 2021, 42(1), 101—116 |
| 闻嘉丽, 张钧豪, 姜久兴. 高等学校化学学报, 2021, 42(1), 101—116 | |
| 22 | Chai Y. C., Guan N. J., Li L D.., Liu S. S., Chem. J. Chinese Universities, 2021, 42(1), 268—288 |
| 柴玉超, 关乃佳, 李兰冬, 刘珊珊. 高等学校化学学报, 2021, 42(1), 268—288 | |
| 23 | Chen S. Q., Li L., Li Y., Yu J. H., Chem. J. Chinese Universities, 2021, 42(1), 179—187 |
| 陈思琦, 李莉, 李乙, 于吉红. 高等学校化学学报, 2021, 42(1), 179—187 | |
| 24 | Jin S. Q., Sun H. M., Yang W. M., Chem. J. Chinese Universities, 2021, 42(1), 217—226 |
| 金少青, 孙洪敏, 杨为民. 高等学校化学学报, 2021, 42(1), 217—226 | |
| 25 | Jin K. Y., Li J., Li L. Wang B. Y., Yan W. F., Zhang J. N., Zhang S. Q., Chem. J. Chinese Universities, 2021, 42(1), 40—59 |
| 靳科研, 李菁, 李莉, 王彬宇, 闫文付, 张佳楠, 张少卿. 高等学校化学学报, 2021, 42(1), 40—59 | |
| 26 | Meng X. J., Wang Y. Q., Wu Q. M., Xiao F. S., Chem. J. Chinese Universities, 2021, 42(1), 21—28 |
| 孟祥举, 王叶青, 吴勤明, 肖丰收. 高等学校化学学报, 2021, 42(1), 21—28 | |
| 27 | Sun Q., Wang N., Yu J., Adv. Mater., 2021, 33, 2104442 |
| 28 | Wang N., Sun Q., Yu J., Adv. Mater., 2019, 31, 1803966 |
| 29 | Sun Q., Wang N., Xu Q., Yu J., Adv. Mater., 2020, 32, 2001818 |
| 30 | Wang H., Wang L., Xiao F., ACS Cent. Sci., 2020, 6, 1685—1697 |
| 31 | Liu L., Corma A., Nat. Rev. Mater., 2021, 6, 244—263 |
| 32 | Liu L., Corma A., Chem. Rev., 2018, 118, 4981—5079 |
| 33 | Dai C., Zhang S., Zhang A., Song C., Shi C., Guo X., J. Mater. Chem. A, 2015, 3, 16461—16468 |
| 34 | Liu L., Lopez⁃Haro M., Lopes W., Li C., Concepcion P., Simonelli L., Calvino J., Corma A., Nat. Mater., 2019, 18, 866—873 |
| 35 | Wang N., Sun Q., Bai R., Li X., Guo G., Yu J., J. Am. Chem. Soc., 2016, 138, 7484—7487 |
| 36 | Sun Q., Wang N., Bing Q., Si R., Liu J. , Bai R., Zhang P., Jia M., Yu J., Chem, 2017, 3, 477—493 |
| 37 | Sun Q., Wang N., Bai R., Hui Y., Zhang T., Do A., Zhang P., Song L., Miao S., Yu J., Adv. Sci., 2019, 6, 1802350 |
| 38 | Sun Q., Chen J., Wang N., He Q., Chang A., Yang C., Asakura H., Tanaka T., Hulsey J., Wang C., Yu J., Yan N., Angew. Chem. Int. Ed., 2020, 59, 20183—20191 |
| 39 | Sun Q., Wang N., Fan Q., Zeng L., Mayoral A., Miao S., Yang R., Jiang Z., Zhou W., Zhang J., Zhang T., Xu J., Zhang P., Cheng J., Yang D., Jia R., Li L., Zhang Q., Wang Y., Terasaki O., Yu J., Angew. Chem. Int. Ed., 2020, 59, 19450 |
| 40 | Wang N., Sun Q., Zhang T., Mayoral A., Li L., Zhou X., Xu J., Zhang P., Yu J., J. Am. Chem. Soc., 2021, 143, 6905—6914 |
| 41 | Shan J., Li M., Allard L., Lee S., Flytzani⁃Stephanopoulos M., Nature, 2017, 551, 605—608 |
| 42 | Qiu J., Hu J., Lan J., Wang L., Fu G., Xiao R., Ge B., Jiang J., Chem. Mater., 2019, 31, 9413—9421 |
| 43 | Yang Z., Li H., Zhou H., Wang L., Wang L., Zhu Q., Xiao J., Meng X., Chen J., Xiao F., J. Am. Chem. Soc., 2020, 142, 16429—16436 |
| 44 | Ortalan V., Uzun A., Gates B., Browning N., Nat. Nanotech., 2010, 5, 506—510 |
| 45 | Lu J., Aydin C., Browning N., Gates B., Angew. Chem. Int. Ed., 2012, 51, 5842—5846 |
| 46 | Chai Y., Wu G., Liu X., Ren Y., Dai W., Wang C., Xie Z., Guan N., Li L., J. Am. Chem. Soc., 2019, 141, 9920—9927 |
| 47 | Qi L., Babucci M., Zhang Y., Lund A., Liu L., Li J., Chen Y., Hoffman A., Bare S., Han Y., Gates B., Bell A., J. Am. Chem. Soc., 2021, 143, 21364—21378 |
| 48 | Liu L., Lopez‐Haro M., Meira D., Concepcion P., Calvino J., Corma A., Angew. Chem. Int. Ed., 2020, 59, 15695—15702 |
| 49 | Ryu T., Ahn N. , Seo S., Cho J., Kim H., Jo D., Park G., Kim P., Kim C., Bruce E., Wright P., Nam I., Hong S., Angew. Chem. Int. Ed., 2017, 56, 3256—3260 |
| 50 | Kistler J., Chotigkrai N., Xu P., Enderle B., Praserthdam P., Chen C., Browning N., Gates B., Angew. Chem. Int. Ed., 2014, 53, 8904—8907 |
| 51 | Yang D., Xu P., Browning N., Gates B., J. Phys. Chem. Lett., 2016, 7, 2537—2543 |
| 52 | Bernales V., Yang D., Yu J., Gümüşlü G., Cramer C., Gates B., Gagliardi L., ACS Appl. Mater. Interfaces, 2017, 9, 33511—33520 |
| 53 | Hou Y., Nagamatsu S., Asakura K., Fukuoka A., Kobayashi H., Commun. Chem., 2018, 1, 41 |
| 54 | Tang Y., Li Y., Fung V., Jiang D., Huang W., Zhang S., Iwasawa Y., Sakata T., Nguyen L., Zhang X., Frenkel A., Tao F., Nat. Commun., 2018, 9, 1231 |
| 55 | Perez⁃Aguilar J., Chen C., Hughes J., Fang C., Gates B., J. Am. Chem. Soc., 2020, 142, 11474—11485 |
| 56 | Shang W., Gao M., Chai Y., Wu G., Guan N., Li L., ACS Catal., 2021, 11, 7249—7256 |
| 57 | Moliner M., Gabay J., Kliewer C., Carr R., Guzman J., Casty G., Serna P., Corma A., J. Am. Chem. Soc., 2016, 138, 15743—15750 |
| 58 | Deng X., Qin B., Liu R., Qin X., Dai W., Wu G., Guan N., Ma D., Li L., J. Am. Chem. Soc., 2021, 143, 20898—20906 |
| 59 | Aydin C., Lu J., Liang A., Chen C., Browning N., Gates B., Nano Lett., 2011, 11, 5537—5541 |
| 60 | Lu J., Aydin C., Liang A., Chen C., Browning N., Gates B., ACS Catal., 2012, 2, 1002—1012 |
| 61 | Bayram E., Lu J., Aydin C., Browning N., Özkar S., Finney E., Gates B., Finke R., ACS Catal., 2015, 5, 3514—3527 |
| 62 | Hoffman A., Fang C., Gates B., J. Phys. Chem. Lett., 2016, 7, 3854—3860 |
| 63 | Fierro⁃Gonzalez J., Gates B., J. Phys. Chem. B, 2004, 108, 16999—17002 |
| 64 | Yang M., Li S., Wang Y., Herron J., Xu Y., Allard L., Lee S., Huang J., Mavrikakis M., Flytzani⁃Stephanopoulos M., Science, 2014, 346, 1498—1501 |
| 65 | Huang W., Zhang S., Tang Y., Li Y., Nguyen L., Li Y., Shan J., Xiao D., Gagne R., Frenkel A., Tao F., Angew. Chem. Int. Ed., 2016, 55, 13441—13445 |
| 66 | Ogino I., Gates B., J. Am. Chem. Soc., 2008, 130, 13338—13346 |
| 67 | Ogino I., Chen M., Dyer J., Kletnieks P., Haw J., Dixon D., Gates B., Chem. Eur. J., 2010, 16, 7427—7436 |
| 68 | Snyder B., Vanelderen P., Bols M., Hallaert S., Böttger L., Ungur L., Pierloot K., Schoonheydt R., Sels B., Solomon E., Nature, 2016, 536, 317—321 |
| 69 | Deng X., Bai R., Chai Y., Hu Z., Guan N., Li L., CCS Chem., 2021, 3, 1101—1114 |
| 70 | Chai Y., Han X., Li W., Liu S., Yao S., Wang C., Shi W., Da⁃Silva I., Manuel P., Cheng Y., Daemen L., Ramirez⁃Cuesta A., Tang C., Jiang L., Yang S., Guan N., Li L., Science, 2020, 368, 1002—1006 |
| 71 | Paolucci C., Verma A., Bates S., Kispersky V., Miller J., Gounder R., Delgass W., Ribeiro F., Schneider W., Angew. Chem. Int. Ed., 2014, 53, 11828—11833 |
| 72 | Gao F., Mei D., Wang Y., Szanyi J., Peden C., J. Am. Chem. Soc., 2017, 139, 4935—4942 |
| 73 | Li Y., Shen J., Peng S., Zhang J., Wu J., Liu X., Sun L., Nat. Commun., 2020, 11, 3206 |
| 74 | Phadke N., Van der Mynsbrugge J., Mansoor E., Getsoian A., Head⁃Gordon M., Bell A., ACS Catal., 2018, 8, 6106—6126 |
| 75 | Phadke N., Mansoor E., Bondil M., Head⁃Gordon M., Bell A., J. Am. Chem. Soc., 2019, 141, 1614—1627 |
| 76 | Maeno Z., Yasumura S., Wu X., Huang M., Liu C., Toyao T., Shimizu K., J. Am. Chem. Soc., 2020, 142, 4820—4832 |
| 77 | Grosso⁃Giordano N., Schroeder C., Okrut A., Solovyov A., Schöttle C., Chassé W., Marinković N., Koller H., Zones S., Katz A., J. Am. Chem. Soc., 2018, 140, 4956—4960 |
| 78 | Grosso⁃Giordano N., Hoffman A., Boubnov A., Small D., Bare S., Zones S., Katz A., J. Am. Chem. Soc., 2019, 141, 7090—7106 |
| 79 | Fang C., Zhang S., Hu Y., Vasiliu M., Perez⁃Aguilar J., Conley, E. , Dixon D., Chen C., Gates B., ACS Catal., 2019, 9, 3311—3321 |
| 80 | Ding K., Gulec A., Johnson A., Schweitzer N., Stucky G., Marks L., Stair P., Science, 2015, 350, 189—192 |
| 81 | Vaska L., Rhodes R., J. Am. Chem. Soc., 1965, 87, 4970—4971 |
| 82 | Hayashi T., Yamasaki K., Chem. Rev., 2003, 103, 2829—2844 |
| 83 | Colby D., Bergman R., Ellman J., Chem. Rev., 2010, 110, 624—655 |
| 84 | Goellner J., Gates B., Vayssilov G., Rösch N., J. Am. Chem. Soc., 2000, 122, 8056—8066 |
| 85 | Ehresmann J., Kletnieks P., Liang A., Bhirud V., Bagatchenko O., Lee E., Klaric M., Gates B., Haw J., Angew. Chem. Int. Ed., 2006, 45, 574—576 |
| 86 | Kletnieks P., Liang A., Craciun R., Ehresmann J., Marcus D., Bhirud V., Klaric M., Hayman M., Guenther D., Bagatchenko O., Dixon D., Gates, B., Haw, J., Chem. Eur. J., 2007, 13, 7294—7304 |
| 87 | Ogino I., Gates B., J. Phys. Chem. C, 2010, 114, 2685—2693 |
| 88 | Serna P., Gates B., Angew. Chem. Int. Ed., 2011, 50, 5528—5531 |
| 89 | Martinez⁃Macias C., Serna P., Gates B., ACS Catal., 2015, 5, 5647—5656 |
| 90 | Uzun A., Bhirud V., Kletnieks P., Haw J., Gates B., J. Phys. Chem. C, 2007, 111, 15064—15073 |
| 91 | Bayram E., Lu J., Aydin C., Uzun A., Browning N., Gates B., Finke R., ACS Catal., 2012, 2, 1947—1957 |
| 92 | Martinez⁃Macias C., Xu P., Hwang S., Lu J., Chen C., Browning N., Gates B., ACS Catal., 2014, 4, 2662—2666 |
| 93 | Sazama P., Moravkova J., Sklenak S., Vondrov A., Tabor E., Sadovska G., Pilar R., ACS Catal., 2020, 10, 3984—4002 |
| 94 | Zhu Y., Chen B., Zhao R., Zhao Q., Gies H., Xiao F., De Vos D., Yokoi T., Bao X., Kolb U., Feyen M., Maurer S., Moini A., Mueller U., Shi C., Zhang, W., Catal. Sci. Technol., 2016, 6, 6581—6592 |
| 95 | Zhu N., Lian Z., Zhang Y., Shan W., He H., Chinese Chem. Lett., 2019, 30, 867—870 |
| 96 | Tsunoji N., Opanasenko M., Kubů M., Čejka J., Nishida H., Hayakawa S., Ide Y., Sadakane M., Sano T., ChemCatChem, 2018, 10, 2536—2540 |
| 97 | Bai R., Navarro M., Song Y., Zhang T., Zou Y., Feng Z., Zhang P., Corma A., Yu J., Chem. Sci., 2020, 11, 12341—12349 |
| 98 | Bates S., Verma A., Paolucci C., Parekh A., Anggara T., Yezerets A., Schneider W., Miller J., Delgass W., Ribeiro F., J. Catal., 2014, 312, 87—97 |
| 99 | Shen B., Chen X., Wang H., Xiong H., Bosch E., Lazić I., Cai D., Qian W., Jin S., Liu X., Han Y., Wei F., Nature, 2021, 592, 541—544 |
| [1] | 杨静怡, 李庆贺, 乔波涛. 铱单原子和纳米粒子在N2O分解反应中的协同催化[J]. 高等学校化学学报, 2022, 43(9): 20220388. |
| [2] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [3] | 任诗杰, 谯思聪, 刘崇静, 张文华, 宋礼. 铂单原子催化剂同步辐射X射线吸收谱的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220466. |
| [4] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [5] | 唐全骏, 刘颖馨, 孟蓉炜, 张若天, 凌国维, 张辰. 单原子催化在海洋能源领域的应用[J]. 高等学校化学学报, 2022, 43(9): 20220324. |
| [6] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [7] | 吴玉, 李轩, 杨恒攀, 何传新. 钴单原子的双重限域制备策略及高效CO2电还原性能[J]. 高等学校化学学报, 2022, 43(9): 20220343. |
| [8] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [9] | 杨静怡, 施思齐, 彭怀涛, 杨其浩, 陈亮. Ga-C3N4单原子催化剂高效光驱动CO2环加成[J]. 高等学校化学学报, 2022, 43(9): 20220349. |
| [10] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| [11] | 王新天, 李攀, 曹越, 洪文浩, 耿忠璇, 安志洋, 王昊宇, 王桦, 孙斌, 朱文磊, 周旸. 单原子材料在二氧化碳催化中的技术经济分析与产业化应用前景[J]. 高等学校化学学报, 2022, 43(9): 20220347. |
| [12] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [13] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [14] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [15] | 江博文, 陈敬轩, 成永华, 桑微, 寇宗魁. 单原子材料在电化学生物传感中的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220334. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||