

高等学校化学学报 ›› 2014, Vol. 35 ›› Issue (6): 1267.doi: 10.7503/cjcu20140046
宫健1, 曹洪玉2, 李慎敏1,2, 唐乾2, 杨彦杰2, 郑学仿1,2( )
)
收稿日期:2014-01-15
出版日期:2014-06-10
发布日期:2014-04-21
作者简介:联系人简介: 郑学仿, 男, 博士, 教授, 主要从事生物无机化学研究. E-mail:基金资助:
GONG Jian1, CAO Hongyu2, LI Shenmin1,2, TANG Qian2, YANG Yanjie2, ZHENG Xuefang1,2,*( )
)
Received:2014-01-15
Online:2014-06-10
Published:2014-04-21
Contact:
ZHENG Xuefang
E-mail:dlxfzheng@126.com
Supported by:摘要:
采用密度泛函理论在B3LYP/6-31+G(d)水平上研究了4种金属Mg, Ni, Cu, Zn配位的自由卟啉(FBP)及氮混杂卟啉(NECP)的几何结构及分子轨道能级. 采用含时密度泛函理论(TD-DFT)方法计算了金属与2种卟啉配位后在气体条件下的电子吸收光谱, 包括激发能、 吸收波长、 跃迁组成和振子强度.计算结果表明, 与金属配位的FBP(M-FBP)具有D4h对称性, 分子轨道能级HOMO/HOMO-1和LUMO/LUMO+1因能级相近发生简并, HOMO-LUMO轨道能级差大约3.0 eV, 在Soret带出现较强吸收峰.由于C/N原子位置的改变, 非对称性结构的M-NECP前线轨道组成发生改变, 轨道能级差(HOMO-LUMO)减小至2.6 eV左右, 且能级发生分裂, Soret带出现多个电子吸收谱峰, Q带也出现吸收峰. 本文研究了水、 氯仿和苯3种不同极性溶剂对M-FBP和M-NECP的分子轨道及电子吸收光谱的影响, 结果表明, 随溶剂极性减弱金属配合物的电子吸收光谱发生红移, 并且吸收峰强度增强.
中图分类号:
TrendMD:
宫健, 曹洪玉, 李慎敏, 唐乾, 杨彦杰, 郑学仿. 氮混杂金属卟啉吸收光谱性质的理论研究. 高等学校化学学报, 2014, 35(6): 1267.
GONG Jian, CAO Hongyu, LI Shenmin, TANG Qian, YANG Yanjie, ZHENG Xuefang. Theoritical Studies on the Structure and Absorption Spectra of Neo-Confused Metal Porphyrin†. Chem. J. Chinese Universities, 2014, 35(6): 1267.
| Species | Mg-FBP | Ni-FBP | Cu-FBP | Zn-FBP |
|---|---|---|---|---|
| M—N21/nm | 0.2066(0.2052[ | 0.1975(0.1972[ | 0.2023(0.2007[ | 0.2056(0.2058[ |
| N21—C4/nm | 0.1374(0.1366[ | 0.1378(0.1380[ | 0.1375(0.1376[ | 0.1373(0.1372[ |
| C4—C3/nm | 0.1448(0.1443[ | 0.1441(0.1438[ | 0.1445(0.1443[ | 0.1447(0.1446[ |
| C3—C2/nm | 0.1367(0.1354[ | 0.1360(0.1360[ | 0.1363(0.1361[ | 0.1366(0.1366[ |
| C4—C5/nm | 0.1402(0.1386[ | 0.1383(0.1380[ | 0.1391(0.1388[ | 0.1398(0.1398[ |
| N24—M—N21/(°) | 90.00 | 90.00(90.00[ | 90.00 | 90.00(90.00[ |
| N21—C1—C2/(°) | 109.50(108.9[ | 111.02(110.93[ | 110.13 | 109.58(109.52[ |
| C2—C3—C4/(°) | 106.93(107.2[ | 106.61(106.68[ | 106.79 | 106.91(106.92[ |
| C1—N21—C4/(°) | 107.14(107.8[ | 104.74(104.79[ | 106.17 | 107.02(107.13[ |
| C20—C1—N21/(°) | 124.95(125.6[ | 125.45(125.53[ | 125.31 | 125.05(125.07[ |
| C4—C5—C6/(°) | 127.24(126.6[ | 123.85(123.72[ | 125.55 | 126.92(126.99[ |
Table 1 Geometric parameters of M-FBP(M: Mg, Ni, Cu and Zn) at B3LYP/6-31+G(d) level
| Species | Mg-FBP | Ni-FBP | Cu-FBP | Zn-FBP |
|---|---|---|---|---|
| M—N21/nm | 0.2066(0.2052[ | 0.1975(0.1972[ | 0.2023(0.2007[ | 0.2056(0.2058[ |
| N21—C4/nm | 0.1374(0.1366[ | 0.1378(0.1380[ | 0.1375(0.1376[ | 0.1373(0.1372[ |
| C4—C3/nm | 0.1448(0.1443[ | 0.1441(0.1438[ | 0.1445(0.1443[ | 0.1447(0.1446[ |
| C3—C2/nm | 0.1367(0.1354[ | 0.1360(0.1360[ | 0.1363(0.1361[ | 0.1366(0.1366[ |
| C4—C5/nm | 0.1402(0.1386[ | 0.1383(0.1380[ | 0.1391(0.1388[ | 0.1398(0.1398[ |
| N24—M—N21/(°) | 90.00 | 90.00(90.00[ | 90.00 | 90.00(90.00[ |
| N21—C1—C2/(°) | 109.50(108.9[ | 111.02(110.93[ | 110.13 | 109.58(109.52[ |
| C2—C3—C4/(°) | 106.93(107.2[ | 106.61(106.68[ | 106.79 | 106.91(106.92[ |
| C1—N21—C4/(°) | 107.14(107.8[ | 104.74(104.79[ | 106.17 | 107.02(107.13[ |
| C20—C1—N21/(°) | 124.95(125.6[ | 125.45(125.53[ | 125.31 | 125.05(125.07[ |
| C4—C5—C6/(°) | 127.24(126.6[ | 123.85(123.72[ | 125.55 | 126.92(126.99[ |
| Species | Mg-NECP | Ni-NECP | Cu-NECP | Zn-NECP |
|---|---|---|---|---|
| M—C21/nm | 0.2085 | 0.1923(0.1907[ | 0.1975 | 0.1990 |
| M—N22/nm | 0.2074 | 0.1984(0.1951[ | 0.2050 | 0.2106 |
| M—N23/nm | 0.2036 | 0.1991(0.1970[ | 0.2014 | 0.2017 |
| M—N24/nm | 0.2070 | 0.1954(0.1929[ | 0.2031 | 0.2105 |
| C21—N1/nm | 0.1373 | 0.1385(0.1399[ | 0.1379 | 0.1379 |
| C21—C4/nm | 0.1411 | 0.1419(0.1402[ | 0.1413 | 0.1412 |
| N23—C1/nm | 0.1376 | 0.1377(0.1367[ | 0.1375 | 0.1379 |
| N23—C14/nm | 0.1371 | 0.1375(0.1384[ | 0.1373 | 0.1378 |
| N24—M—C21/(°) | 88.67 | 90.15 | 89.97 | 90.13 |
| C21—N1—C2/(°) | 110.95 | 112.32 | 111.36 | 110.69 |
| C2—C3—C4/(°) | 107.05 | 106.70 | 106.97 | 107.18 |
| N1—C21—C4/(°) | 105.85 | 103.15 | 104.81 | 105.79 |
| C20—N1—C21/(°) | 125.22 | 126.86 | 126.73 | 126.68 |
| C4—C5—C6/(°) | 127.29 | 122.73 | 124.93 | 126.65 |
Table 2 Geometric parameters of M-NECP(M: Mg, Ni, Cu, Zn) at B3LYP/6-31+G(d) level
| Species | Mg-NECP | Ni-NECP | Cu-NECP | Zn-NECP |
|---|---|---|---|---|
| M—C21/nm | 0.2085 | 0.1923(0.1907[ | 0.1975 | 0.1990 |
| M—N22/nm | 0.2074 | 0.1984(0.1951[ | 0.2050 | 0.2106 |
| M—N23/nm | 0.2036 | 0.1991(0.1970[ | 0.2014 | 0.2017 |
| M—N24/nm | 0.2070 | 0.1954(0.1929[ | 0.2031 | 0.2105 |
| C21—N1/nm | 0.1373 | 0.1385(0.1399[ | 0.1379 | 0.1379 |
| C21—C4/nm | 0.1411 | 0.1419(0.1402[ | 0.1413 | 0.1412 |
| N23—C1/nm | 0.1376 | 0.1377(0.1367[ | 0.1375 | 0.1379 |
| N23—C14/nm | 0.1371 | 0.1375(0.1384[ | 0.1373 | 0.1378 |
| N24—M—C21/(°) | 88.67 | 90.15 | 89.97 | 90.13 |
| C21—N1—C2/(°) | 110.95 | 112.32 | 111.36 | 110.69 |
| C2—C3—C4/(°) | 107.05 | 106.70 | 106.97 | 107.18 |
| N1—C21—C4/(°) | 105.85 | 103.15 | 104.81 | 105.79 |
| C20—N1—C21/(°) | 125.22 | 126.86 | 126.73 | 126.68 |
| C4—C5—C6/(°) | 127.29 | 122.73 | 124.93 | 126.65 |
| Molecular | State | Excitation energy/eV | λ/nm | f | Main configuration(%) |
|---|---|---|---|---|---|
| Mg-FBP | Eu | 2.348 | 528 | 0.0007 | H-1→L(47), H→L+1(52) |
| Eu | 2.348 | 528 | 0.0007 | H-1→L+1(47), H→L(52) | |
| Eu | 3.451 | 359 | 0.9685 | H-1→L(49), H→L+1(43) | |
| Eu | 3.451 | 359 | 0.9685 | H-1→L+1(49), H→L(43) | |
| Eu | 3.738 | 332 | 0.0474 | H-2→L(87) | |
| Eu | 3.738 | 332 | 0.0474 | H-2→L+1(87) | |
| Mg-NECP | A | 2.088 | 594 | 0.0578 | H-1→L+1(10), H→L(84) |
| A | 2.342 | 529 | 0.0026 | H-1→L(39), H→L+1(56) | |
| A | 2.980 | 416 | 0.0465 | H-2→L(51), H-1→L+1(41) | |
| A | 3.249 | 382 | 0.4637 | H-2→L+1(17), H-1→L(34), H→L+1(25) | |
| A | 3.504 | 354 | 0.3420 | H-4→L(17), H-2→L(33), H→L+2(17) | |
| A | 3.814 | 325 | 0.2335 | H-6→L(17), H-5→L(23), H→L+2(35) |
Table 3 Excitation energy levels, oscillators strength(f) and transition configurations for Mg-FBP and Mg-NECP molecules in the gas state
| Molecular | State | Excitation energy/eV | λ/nm | f | Main configuration(%) |
|---|---|---|---|---|---|
| Mg-FBP | Eu | 2.348 | 528 | 0.0007 | H-1→L(47), H→L+1(52) |
| Eu | 2.348 | 528 | 0.0007 | H-1→L+1(47), H→L(52) | |
| Eu | 3.451 | 359 | 0.9685 | H-1→L(49), H→L+1(43) | |
| Eu | 3.451 | 359 | 0.9685 | H-1→L+1(49), H→L(43) | |
| Eu | 3.738 | 332 | 0.0474 | H-2→L(87) | |
| Eu | 3.738 | 332 | 0.0474 | H-2→L+1(87) | |
| Mg-NECP | A | 2.088 | 594 | 0.0578 | H-1→L+1(10), H→L(84) |
| A | 2.342 | 529 | 0.0026 | H-1→L(39), H→L+1(56) | |
| A | 2.980 | 416 | 0.0465 | H-2→L(51), H-1→L+1(41) | |
| A | 3.249 | 382 | 0.4637 | H-2→L+1(17), H-1→L(34), H→L+1(25) | |
| A | 3.504 | 354 | 0.3420 | H-4→L(17), H-2→L(33), H→L+2(17) | |
| A | 3.814 | 325 | 0.2335 | H-6→L(17), H-5→L(23), H→L+2(35) |
| Molecular | State | Excitation energy/eV | λ/nm | f | Main configuration(%) |
|---|---|---|---|---|---|
| Ni-FBP | Eu | 2.478 | 500 | 0.0059 | H-1→L(46), H→L+1(54) |
| Eu | 2.478 | 500 | 0.0059 | H-1→L+1(46), H→L(54) | |
| Eu | 3.456 | 359 | 0.7851 | H-1→L+1(51), H→L(45) | |
| Eu | 3.456 | 359 | 0.7852 | H-1→L(51), H→L+1(45) | |
| Eu | 3.919 | 316 | 0.006 | H-5→L(96) | |
| Eu | 3.919 | 316 | 0.006 | H-5→L+1(96) | |
| Ni-NECP | A | 2.133 | 581 | 0.0161 | H-3→L+2(48), H→L(40) |
| A | 2.188 | 567 | 0.0222 | H-3→L+2(45), H→L(42) | |
| A | 3.150 | 394 | 0.145 | H-4→L+1(34), H-1→L(10), H-1→L+1(24) | |
| A | 3.529 | 351 | 0.3046 | H-4→L+1(34), H-2→L+1(25), H-1→L(13) | |
| A | 3.655 | 339 | 0.2265 | H-5→L(56), H-1→L+1(13) |
Table 4 Excitation energy levels, oscillators strength(f) and transition configurations for Ni-FBP and Ni-NECP molecules in the gas state
| Molecular | State | Excitation energy/eV | λ/nm | f | Main configuration(%) |
|---|---|---|---|---|---|
| Ni-FBP | Eu | 2.478 | 500 | 0.0059 | H-1→L(46), H→L+1(54) |
| Eu | 2.478 | 500 | 0.0059 | H-1→L+1(46), H→L(54) | |
| Eu | 3.456 | 359 | 0.7851 | H-1→L+1(51), H→L(45) | |
| Eu | 3.456 | 359 | 0.7852 | H-1→L(51), H→L+1(45) | |
| Eu | 3.919 | 316 | 0.006 | H-5→L(96) | |
| Eu | 3.919 | 316 | 0.006 | H-5→L+1(96) | |
| Ni-NECP | A | 2.133 | 581 | 0.0161 | H-3→L+2(48), H→L(40) |
| A | 2.188 | 567 | 0.0222 | H-3→L+2(45), H→L(42) | |
| A | 3.150 | 394 | 0.145 | H-4→L+1(34), H-1→L(10), H-1→L+1(24) | |
| A | 3.529 | 351 | 0.3046 | H-4→L+1(34), H-2→L+1(25), H-1→L(13) | |
| A | 3.655 | 339 | 0.2265 | H-5→L(56), H-1→L+1(13) |
| Molecular | State | Excitation energy/eV | λ/nm | f | Main configuration(%) |
|---|---|---|---|---|---|
| Cu-FBP | Eu | 2.424 | 511 | 0.0030 | H-1(α)→L+1(α)(25), H(α)→L(α)(23), H-1(β)→L+1(β)(28), |
| H(β)→L+2(β)(23) | |||||
| Eu | 2.424 | 511 | 0.0030 | H-1(α)→L(α)(25), H(α)→L+1(α)(23), H-1(β)→L+2(β)(28), | |
| H(β)→L+1(β)(23) | |||||
| Eu | 3.285 | 377 | 0.0055 | H-5(α)→L+1(α)(35), H-4(β)→L+2(β)(40) | |
| Eu | 3.285 | 377 | 0.0055 | H-5(α)→L(α)(35), H-4(β)→L+1(β)(40) | |
| Eu | 3.409 | 364 | 0.7721 | H-1(α)→L(α)(24), H(α)→L+1(α)(22), H-1(β)→L+2(β)(24), | |
| H(β)→L+1(β)(23) | |||||
| Eu | 3.409 | 364 | 0.7721 | H-1(α)→L+1(α)(24), H(α)→L(α)(22), H-1(β)→L+1(β)(24), | |
| H(β)→L+2(β)(23) | |||||
| Cu-NECP | A | 2.196 | 565 | 0.0506 | H(α)→L(α)(41), H(β)→L(β)(41) |
| A | 2.353 | 527 | 0.0001 | H-1(α)→L+1(α)(27), H(β)→L+2(β)(68) | |
| A | 2.993 | 414 | 0.0392 | H-3(α)→L(α)(27), H-2(α)→L+1(α)(14), H-2(β)→L(β)(19), | |
| H-1(β)→L+1(β)(15) | |||||
| A | 3.204 | 387 | 0.3423 | H(α)→L+1(α)(12), H-1(β)→L(β)(14) | |
| A | 3.493 | 355 | 0.3301 | H-4(α)→L(α)(12), H-3(α)→L(α)(18), H-2(β)→L(β)(18) | |
| A | 3.887 | 319 | 0.1371 | H(α)→L+2(α)(38), H(β)→L+3(β)(33) |
Table 5 Excitation energy levels, oscillators strength(f) and transition configurations for Cu-FBP and Cu-NECP molecules in the gas state
| Molecular | State | Excitation energy/eV | λ/nm | f | Main configuration(%) |
|---|---|---|---|---|---|
| Cu-FBP | Eu | 2.424 | 511 | 0.0030 | H-1(α)→L+1(α)(25), H(α)→L(α)(23), H-1(β)→L+1(β)(28), |
| H(β)→L+2(β)(23) | |||||
| Eu | 2.424 | 511 | 0.0030 | H-1(α)→L(α)(25), H(α)→L+1(α)(23), H-1(β)→L+2(β)(28), | |
| H(β)→L+1(β)(23) | |||||
| Eu | 3.285 | 377 | 0.0055 | H-5(α)→L+1(α)(35), H-4(β)→L+2(β)(40) | |
| Eu | 3.285 | 377 | 0.0055 | H-5(α)→L(α)(35), H-4(β)→L+1(β)(40) | |
| Eu | 3.409 | 364 | 0.7721 | H-1(α)→L(α)(24), H(α)→L+1(α)(22), H-1(β)→L+2(β)(24), | |
| H(β)→L+1(β)(23) | |||||
| Eu | 3.409 | 364 | 0.7721 | H-1(α)→L+1(α)(24), H(α)→L(α)(22), H-1(β)→L+1(β)(24), | |
| H(β)→L+2(β)(23) | |||||
| Cu-NECP | A | 2.196 | 565 | 0.0506 | H(α)→L(α)(41), H(β)→L(β)(41) |
| A | 2.353 | 527 | 0.0001 | H-1(α)→L+1(α)(27), H(β)→L+2(β)(68) | |
| A | 2.993 | 414 | 0.0392 | H-3(α)→L(α)(27), H-2(α)→L+1(α)(14), H-2(β)→L(β)(19), | |
| H-1(β)→L+1(β)(15) | |||||
| A | 3.204 | 387 | 0.3423 | H(α)→L+1(α)(12), H-1(β)→L(β)(14) | |
| A | 3.493 | 355 | 0.3301 | H-4(α)→L(α)(12), H-3(α)→L(α)(18), H-2(β)→L(β)(18) | |
| A | 3.887 | 319 | 0.1371 | H(α)→L+2(α)(38), H(β)→L+3(β)(33) |
| Molecular | State | Excitation energy/eV | λ/nm | f | Main configuration(%) |
|---|---|---|---|---|---|
| Zn-FBP | Eu | 2.397 | 517 | 0.0021 | H-1→L(49), H→L+1(49) |
| Eu | 2.397 | 517 | 0.0021 | H-1→L+1(49), H→L(49) | |
| Eu | 3.472 | 357 | 0.9494 | H-1→LO(13), H-1→L+1(36), H→L(35), H→L+1(12) | |
| Eu | 3.472 | 357 | 0.9494 | H-1→L(36), H-1→L+1(13), H→L+1(35) | |
| Eu | 3.786 | 327 | 0.0376 | H-3→L+1(90) | |
| Eu | 3.786 | 327 | 0.0376 | H-3→L(90) | |
| Zn-NECP | A | 2.193 | 565 | 0.0502 | H-1→L+1(11), H→L(79) |
| A | 2.406 | 515 | 0.0036 | H-1→L(40), H→L+1(52) | |
| A | 2.998 | 414 | 0.0518 | H-3→LUMO(52), H-1→L+1(37) | |
| A | 3.258 | 381 | 0.4238 | H-3→L+1(17), H-1→L(30), H-1→L+1(17), H→L+1(25) | |
| A | 3.562 | 348 | 0.2447 | H-5→L(21), H-4→L(38) | |
| A | 3.875 | 320 | 0.2171 | H-4→L+1(15), H→L+2(46) |
Table 6 Excitation energy levels, oscillators strength(f) and transition configurations for Zn-FBP and Zn-NECP molecules in the gas state
| Molecular | State | Excitation energy/eV | λ/nm | f | Main configuration(%) |
|---|---|---|---|---|---|
| Zn-FBP | Eu | 2.397 | 517 | 0.0021 | H-1→L(49), H→L+1(49) |
| Eu | 2.397 | 517 | 0.0021 | H-1→L+1(49), H→L(49) | |
| Eu | 3.472 | 357 | 0.9494 | H-1→LO(13), H-1→L+1(36), H→L(35), H→L+1(12) | |
| Eu | 3.472 | 357 | 0.9494 | H-1→L(36), H-1→L+1(13), H→L+1(35) | |
| Eu | 3.786 | 327 | 0.0376 | H-3→L+1(90) | |
| Eu | 3.786 | 327 | 0.0376 | H-3→L(90) | |
| Zn-NECP | A | 2.193 | 565 | 0.0502 | H-1→L+1(11), H→L(79) |
| A | 2.406 | 515 | 0.0036 | H-1→L(40), H→L+1(52) | |
| A | 2.998 | 414 | 0.0518 | H-3→LUMO(52), H-1→L+1(37) | |
| A | 3.258 | 381 | 0.4238 | H-3→L+1(17), H-1→L(30), H-1→L+1(17), H→L+1(25) | |
| A | 3.562 | 348 | 0.2447 | H-5→L(21), H-4→L(38) | |
| A | 3.875 | 320 | 0.2171 | H-4→L+1(15), H→L+2(46) |
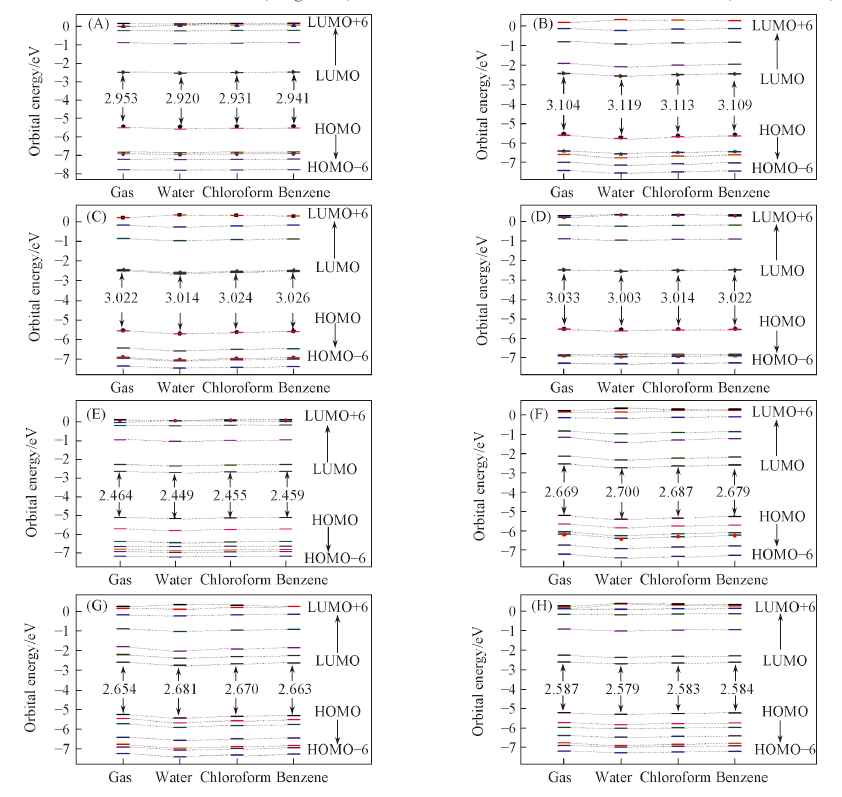
Fig.6 Orbital energy levels and the ΔE(HOMO-LUMO)/eV of Mg-FBP(A), Ni-FBP(B), Cu-FBP(C), Zn-FBP(D), Mg-NECP(E), Ni-NECP(F), Cu-NECP(G) and Zn-NECP(H) in gas and solvents(water, chloroform and benzene)
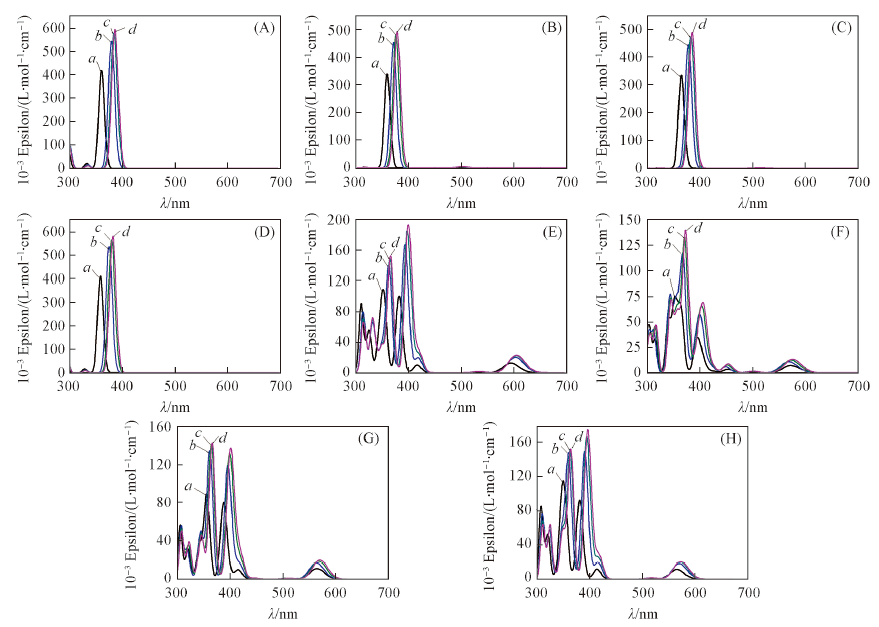
Fig.7 Simulated absorption spectrum of Mg-FBP(A), Ni-FBP(B), Cu-FBP(C), Zn-FBP(D), Mg-NECP(E), Ni-NECP(F), Cu-NECP(G)and Zn-NECP(H) in gas(a), water(b), chloroform(c), benzene(d)
| [1] | Smith K.M., Porphyrins and Metalloporphyrins, Elsevier, Amsterdam, 1975 |
| [2] | Battersby A. R., Fookes C. J., Matcham G. W., McDonald E., Nature, 1980, 285(5759), 17—21 |
| [3] | Kräutler B., Chimia, 1987, 41(9), 277—292 |
| [4] | Kay A., Graetzel M., J. Phys. Chem., 1993, 97(23), 6272—6277 |
| [5] | Kay A., Humphry-Baker R., Graetzel M., J. Phys. Chem., 1994, 98(3), 952—959 |
| [6] | Beljonne D., O'Keefe G. E., Hamer P. J., Friend R. H., Anderson H. L., Brédas J. L., J. Chem. Phys., 1997, 106(23), 9439—9460 |
| [7] | Henari F. Z., Blau W. J., Milgrom L. R., Yahioglu G., Phillips D., Lacey J. A., Chem. Phys. Lett., 1997, 267(3/4), 229—233 |
| [8] | Wang Z. Q., Day P. N., Pachter R ., J. Chem. Phys., 1998, 108(6), 2504—2510 |
| [9] | Merchán M., Ortí E., Roos B. O., Chem. Phys. Lett., 1994, 226(1/2), 27—36 |
| [10] | Nakatsuji H., Hasegawa J. Y., Hada M., J. Chem. Phys., 1996, 104(6), 2321—2329 |
| [11] | Kitao O., Ushiyama H., Miura N., J. Chem. Phys., 1999, 110(6), 2936—2946 |
| [12] | van Gisbergen S. J. A., Rosa A., Ricciardi G., Baerends E. J., J. Chem. Phys., 1999, 111(6), 2499—2506 |
| [13] | Sundholm D., Phys. Chem. Chem. Phys., 2000, 2(10), 2275—2281 |
| [14] | Šeda J., Burda J. V., Brázdová V., Int. J. Mol. Sci., 2004, 5(4), 196—213 |
| [15] | Šeda J., Burda J. V., Leszczynski J., J. Comput. Chem., 2005, 26(3), 294—303 |
| [16] | Zhang Y. H., Ruan W. J., Li Z. Y., Wu Y., Zheng J. Y., Chem. Phys., 2005, 315(1/2), 201—213 |
| [17] | Gouterman M., J. Mol. Spectrosc., 1961, 6(0), 138—163 |
| [18] | Petke J. D., Maggiora G. M., Shipman L. L., Christoffersen R. E., J. Mol. Spectrosc., 1978, 71(1—3), 64—84 |
| [19] | Balanay M. P., Kim D. H., J. Mol. Struct.(Theochem.), 2009, 910(1—3), 20—26 |
| [20] | Lash T. D., Lammer A. D., Ferrence G. M., Angew. Chem. Int. Ed., 2011, 50(41), 9718—9721 |
| [21] | Fujino K., Hirata Y., Kawabe Y., Morimoto T., Srinivasan A., Toganoh M., Miseki Y., Kudo A., Furuta H., Angew. Chem. Int. Ed., 2011, 50(30), 6855—6859 |
| [22] | Bauernschmitt R., Ahlrichs R., Chem. Phys. Lett., 1996, 256(4/5), 454—464 |
| [23] | Stratmann R. E., Scuseria G. E., Frisch M. J., J. Chem. Phys., 1998, 109(19), 8218—8224 |
| [24] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A., Vreven T., Kudin K. N., Burant J. C., Millam J. M., Iyengar S. S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cui Q., Baboul A. G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Gonzalez C., Pople J. A., Gaussian 03, Revision B.04, Wallingford CT, Gaussian Inc., 2004 |
| [25] | Becke A. D., Phys. Rev. A, 1988, 38(6), 3098—3100 |
| [26] | Lee C., Yang W., Parr R. G., Phys. Rev. B, 1988, 37(2), 785—789 |
| [27] | O’Boyle N. M., Tenderholt A. L., Langner K. M., J. Comput. Chem., 2008, 29(5), 839—845 |
| [28] | Wei L., She Y. B., Yu Y. M., Yao X. Q., Zhang S. J., J. Mol .Model., 2012, 18(6), 2483—2491 |
| [29] | Liu X., Yeow E. K. L., Velate S., Steer R. P., Phys. Chem. Chem. Phys., 2006, 8(11), 1298—1309 |
| [30] | Hashimoto T., Choe Y. K., Nakano H., Hirao K., J. Phys. Chem. A, 1999, 103(12), 1894—1904 |
| [31] | Ghosh A., Vangberg T., Inorg. Chem., 1998, 37(24), 6276—6280 |
| [32] | Hirao H., Shaik S., Kozlowski P. M., J. Phys. Chem. A, 2006, 110(18), 6091—6099 |
| [1] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [2] | 邱丽琪, 姚向阳, 何良年. 可见光驱动丰产金属卟啉类配合物催化的二氧化碳选择性还原反应[J]. 高等学校化学学报, 2022, 43(7): 20220064. |
| [3] | 黄汉浩, 卢湫阳, 孙明子, 黄勃龙. 石墨炔原子催化剂的崭新道路:基于自验证机器学习方法的筛选策略[J]. 高等学校化学学报, 2022, 43(5): 20220042. |
| [4] | 刘洋, 李旺昌, 张竹霞, 王芳, 杨文静, 郭臻, 崔鹏. Sc3C2@C80与[12]CPP纳米环之间非共价相互作用的理论研究[J]. 高等学校化学学报, 2022, 43(11): 20220457. |
| [5] | 王园月, 安梭梭, 郑旭明, 赵彦英. 5-巯基-1, 3, 4-噻二唑-2-硫酮微溶剂团簇的光谱和理论计算研究[J]. 高等学校化学学报, 2022, 43(10): 20220354. |
| [6] | 周成思, 赵远进, 韩美晨, 杨霞, 刘晨光, 贺爱华. 硅烷类外给电子体对丙烯-丁烯序贯聚合的调控作用[J]. 高等学校化学学报, 2022, 43(10): 20220290. |
| [7] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [8] | 马丽娟, 高升启, 荣祎斐, 贾建峰, 武海顺. Sc, Ti, V修饰B/N掺杂单缺陷石墨烯的储氢研究[J]. 高等学校化学学报, 2021, 42(9): 2842. |
| [9] | 钟声广, 夏文生, 张庆红, 万惠霖. 电中性团簇MCu2Ox(M=Cu2+, Ce4+, Zr4+)上甲烷和二氧化碳直接合成乙酸的理论研究[J]. 高等学校化学学报, 2021, 42(9): 2878. |
| [10] | 黄罗仪, 翁约约, 黄旭慧, 王朝杰. 车前草中黄酮类成分结构和性质的理论研究[J]. 高等学校化学学报, 2021, 42(9): 2752. |
| [11] | 王建, 张红星. 四配位铂磷光发射体结构与光物理性质关系的理论研究[J]. 高等学校化学学报, 2021, 42(7): 2245. |
| [12] | 胡伟, 刘小峰, 李震宇, 杨金龙. 金刚石纳米线氮空位色心的表面与尺寸效应[J]. 高等学校化学学报, 2021, 42(7): 2178. |
| [13] | 杨一莹, 朱荣秀, 张冬菊, 刘成卜. 金催化炔基苯并二𫫇英环化合成8-羟基异香豆素的理论研究[J]. 高等学校化学学报, 2021, 42(7): 2299. |
| [14] | 柳扬, 李清波, 孙杰, 赵显. Ga对在AlN衬底上直接生长石墨烯的远程催化[J]. 高等学校化学学报, 2021, 42(7): 2271. |
| [15] | 应富鸣, 计辰儒, 苏培峰, 吴玮. 基于完全活性空间自洽场的杂化多组态密度泛函方法λ-DFCAS[J]. 高等学校化学学报, 2021, 42(7): 2218. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||