

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (9): 2842.doi: 10.7503/cjcu20210354
收稿日期:2021-05-21
出版日期:2021-09-10
发布日期:2021-09-08
通讯作者:
马丽娟
E-mail:malijuan19852223@163.com
基金资助:
MA Lijuan( ), GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun
), GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun
Received:2021-05-21
Online:2021-09-10
Published:2021-09-08
Contact:
MA Lijuan
E-mail:malijuan19852223@163.com
Supported by:摘要:
3d过渡金属修饰是改善石墨烯储氢性能的最有效途径, 但仍存在金属团聚和H2解离导致难以脱附的问题. 提出了B/N掺杂单缺陷石墨烯(BMG/NMG)的策略来避免以上两个问题. 密度泛函理论计算结果表明, N掺杂可以使Sc, Ti, V与石墨烯的结合能提高3~4倍, B掺杂可以将Sc与石墨烯的结合能提高3倍. Sc/BMG和Sc/NMG吸附的第一个H2不会解离. Sc/BMG中Sc吸附5个H2, 平均氢分子结合能为-0.18~-0.43 eV, 并且可以通过在同侧锚定多个Sc原子形成Sc/C3B2五元环增加H2吸附位点. Sc/NMG中每个Sc吸附6个H2, 平均氢分子结合能为-0.17~-0.29 eV, 还可以通过在异侧修饰形成Sc/N3/Sc单元进一步提高储氢能力. 研究结果将为设计基于3d过渡金属修饰碳材料的储氢材料提供理论基础.
中图分类号:
TrendMD:
马丽娟, 高升启, 荣祎斐, 贾建峰, 武海顺. Sc, Ti, V修饰B/N掺杂单缺陷石墨烯的储氢研究. 高等学校化学学报, 2021, 42(9): 2842.
MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene. Chem. J. Chinese Universities, 2021, 42(9): 2842.
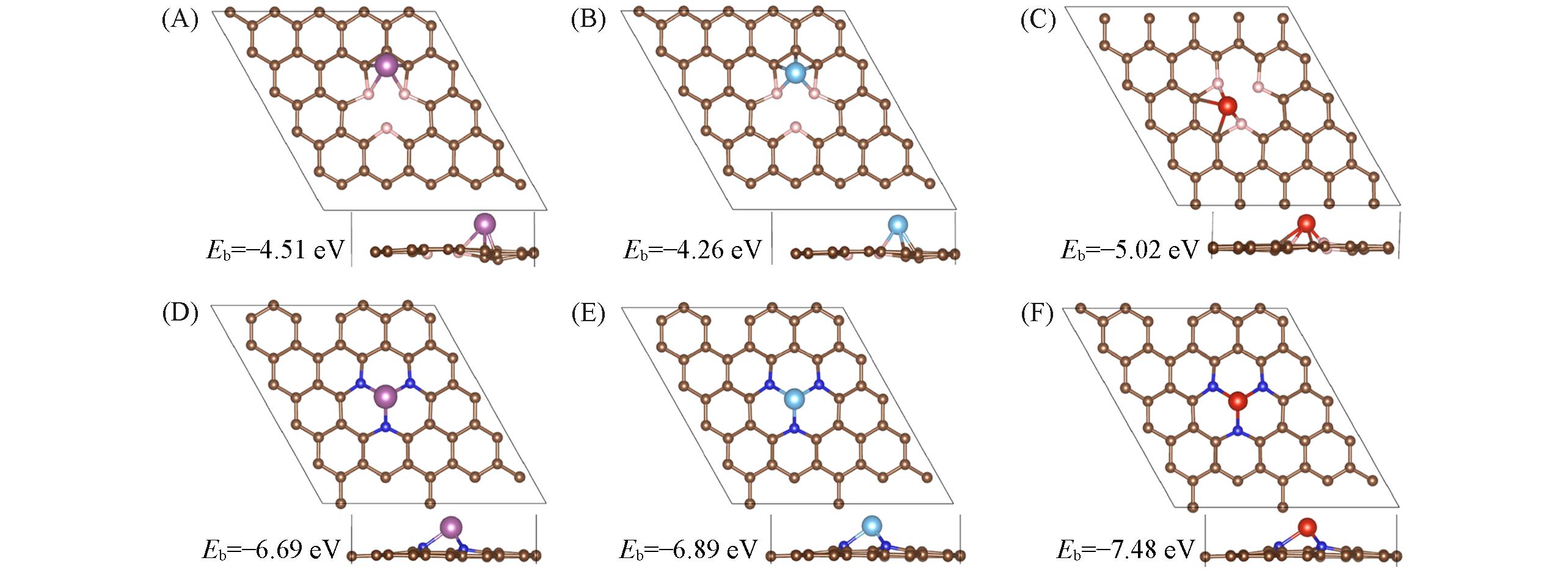
Fig.1 Top and side views of optimized structures of Sc, Ti, V decorated BMG(A—C) and NMG(D—F)The purple, blue and red balls represent Sc, Ti, and V atoms.
| System | TM cohesive energy/(eV·atom-1) | ETM/eV | dB—B or dN—N/nm | dTM—B or dTM—N/nm | Δz/nm | Charge/|e| |
|---|---|---|---|---|---|---|
| BMG | — | — | 0.220—0.221 | — | — | — |
| Sc?BMG | -3.90[ | -4.51 | 0.199—0.208 | 0.245—0.378 | 0.007 | 1.44 |
| Ti?BMG | -4.85[ | -4.26 | 0.182—0.232 | 0.211—0.317 | 0.003 | 1.16 |
| V?BMG | -5.31[ | -5.02 | 0.220—0.249 | 0.180—0.255 | 0.023 | 0.87 |
| NMG | — | — | 0.260—0.261 | — | — | — |
| Sc/NMG | -3.90[ | -6.69 | 0.272—0.273 | 0.198—0.199 | 0.168 | 1.66 |
| Ti/NMG | -4.85[ | -6.89 | 0.238—0.308 | 0.180—0.212 | 0.165 | 1.40 |
| V/NMG | -5.31[ | -7.48 | 0.238—0.293 | 0.172—0.205 | 0.158 | 1.25 |
Table 1 Relevant parameters of Sc, Ti and V decorated BMG and NMG*
| System | TM cohesive energy/(eV·atom-1) | ETM/eV | dB—B or dN—N/nm | dTM—B or dTM—N/nm | Δz/nm | Charge/|e| |
|---|---|---|---|---|---|---|
| BMG | — | — | 0.220—0.221 | — | — | — |
| Sc?BMG | -3.90[ | -4.51 | 0.199—0.208 | 0.245—0.378 | 0.007 | 1.44 |
| Ti?BMG | -4.85[ | -4.26 | 0.182—0.232 | 0.211—0.317 | 0.003 | 1.16 |
| V?BMG | -5.31[ | -5.02 | 0.220—0.249 | 0.180—0.255 | 0.023 | 0.87 |
| NMG | — | — | 0.260—0.261 | — | — | — |
| Sc/NMG | -3.90[ | -6.69 | 0.272—0.273 | 0.198—0.199 | 0.168 | 1.66 |
| Ti/NMG | -4.85[ | -6.89 | 0.238—0.308 | 0.180—0.212 | 0.165 | 1.40 |
| V/NMG | -5.31[ | -7.48 | 0.238—0.293 | 0.172—0.205 | 0.158 | 1.25 |
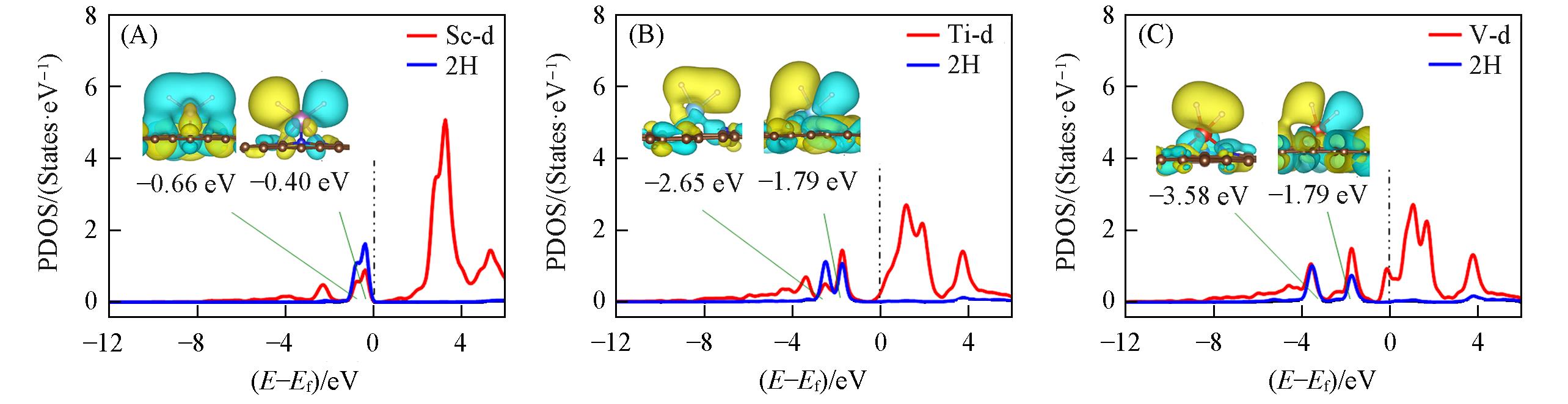
Fig.3 PDOS of 2H?Sc/NMG(A), 2H?Ti/NMG(B), 2H?V/NMG(C), and the main orbitals involved in the Kubas interaction(inset)Yellow and blue indicate the positive and negative phase, respectively. The isosurface is 1.0×10-7. Ef: Fermi energy.
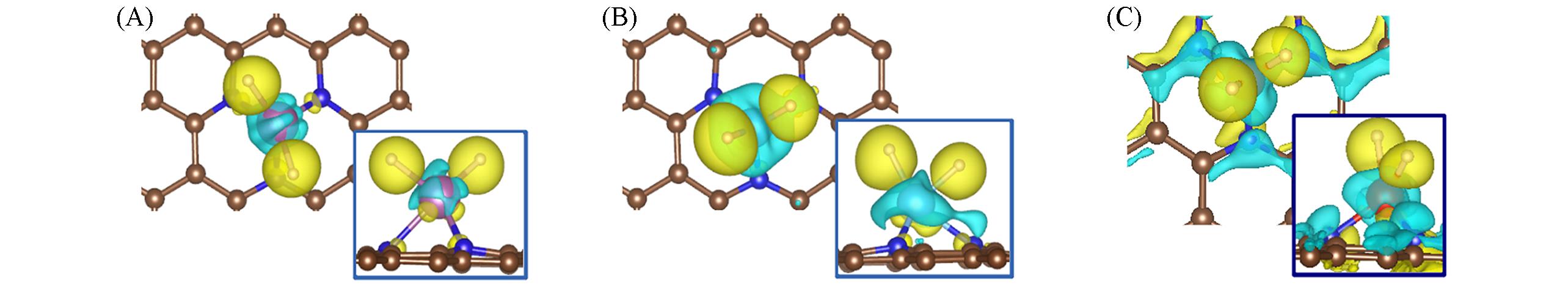
Fig.4 Charge density difference of 2H?Sc/NMG(A), 2H?Ti/NMG(B) and 2H?V/NMG(C)Yellow and blue indicate the charge accumulation and depletion regions, respectively. The isosurface value is 8 e/nm3.
| nH2?Sc/BMV | Ec/eV | Ea/eV | dH—H/nm | dSc—H/nm |
|---|---|---|---|---|
| n=1 | -0.23 | -0.23 | 0.077 | 0.208—0.234 |
| n=2 | -0.63 | -0.43 | 0.080—0.083 | 0.202—0.214 |
| n=3 | -0.50 | -0.24 | 0.079—0.081 | 0.206—0.215 |
| n=4 | -0.22 | -0.22 | 0.078—0.082 | 0.205—0.226 |
| n=5 | -0.17 | -0.18 | 0.077—0.082 | 0.205—0.225 |
Table 2 Continuous adsorption energies of hydrogen(Ec), average adsorption energies of hydrogen(Ea), and structural parameters of nH2-Sc/BMV
| nH2?Sc/BMV | Ec/eV | Ea/eV | dH—H/nm | dSc—H/nm |
|---|---|---|---|---|
| n=1 | -0.23 | -0.23 | 0.077 | 0.208—0.234 |
| n=2 | -0.63 | -0.43 | 0.080—0.083 | 0.202—0.214 |
| n=3 | -0.50 | -0.24 | 0.079—0.081 | 0.206—0.215 |
| n=4 | -0.22 | -0.22 | 0.078—0.082 | 0.205—0.226 |
| n=5 | -0.17 | -0.18 | 0.077—0.082 | 0.205—0.225 |
| System | n | Ec/eV | Ea/eV | dH—H/nm | dSc—H/nm | dTi—H/nm | dV—H/nm |
|---|---|---|---|---|---|---|---|
| nH2?Sc/NMV | n=1 | -0.29 | -0.29 | 0.078 | 0.223—0.232 | ||
| n=2 | -0.27 | -0.28 | 0.077—0.078 | 0.222—0.238 | |||
| n=3 | -0.17 | -0.24 | 0.076—0.078 | 0.222—0.238 | |||
| n=4 | -0.13 | -0.22 | 0.077—0.078 | 0.231—0.243 | |||
| n=5 | -0.06 | -0.18 | 0.075—0.078 | 0.228—0.370 | |||
| n=6 | -0.08 | -0.17 | 0.075—0.076 | 0.264—0.375 | |||
| nH2?Ti/NMV | n=1 | -0.055(-0.083) | -0.055(-0.083) | 0.085(0.173) | 0.182—0.187 | ||
| n=2 | -0.035(-0.010) | -0.045(-0.046) | 0.083—0.161 | 0.162—0.186 | |||
| nH2?V/NMV | n=1 | -0.70(-0.72) | -0.70(-0.72) | 0.086(0.197) | 0.169—0.178 | ||
| n=2 | -0.40(-0.23) | -0.55(-0.47) | 0.070—0.165 | 0.153—0.209 | |||
| n=3 | -0.37(-0.12) | -0.49(-0.36) | 0.084—0.190 | 0.163—0.203 | |||
| n=4 | 0.39(-0.49) | -0.27(-0.39) | 0.086—0.156 | 0.161—0.198 |
Table 3 Continuous adsorption energies of hydrogen(Ec), average adsorption energies of hydrogen(Ea), and structural parameters of nH2-TM/NMG(TM = Sc, Ti, V)*
| System | n | Ec/eV | Ea/eV | dH—H/nm | dSc—H/nm | dTi—H/nm | dV—H/nm |
|---|---|---|---|---|---|---|---|
| nH2?Sc/NMV | n=1 | -0.29 | -0.29 | 0.078 | 0.223—0.232 | ||
| n=2 | -0.27 | -0.28 | 0.077—0.078 | 0.222—0.238 | |||
| n=3 | -0.17 | -0.24 | 0.076—0.078 | 0.222—0.238 | |||
| n=4 | -0.13 | -0.22 | 0.077—0.078 | 0.231—0.243 | |||
| n=5 | -0.06 | -0.18 | 0.075—0.078 | 0.228—0.370 | |||
| n=6 | -0.08 | -0.17 | 0.075—0.076 | 0.264—0.375 | |||
| nH2?Ti/NMV | n=1 | -0.055(-0.083) | -0.055(-0.083) | 0.085(0.173) | 0.182—0.187 | ||
| n=2 | -0.035(-0.010) | -0.045(-0.046) | 0.083—0.161 | 0.162—0.186 | |||
| nH2?V/NMV | n=1 | -0.70(-0.72) | -0.70(-0.72) | 0.086(0.197) | 0.169—0.178 | ||
| n=2 | -0.40(-0.23) | -0.55(-0.47) | 0.070—0.165 | 0.153—0.209 | |||
| n=3 | -0.37(-0.12) | -0.49(-0.36) | 0.084—0.190 | 0.163—0.203 | |||
| n=4 | 0.39(-0.49) | -0.27(-0.39) | 0.086—0.156 | 0.161—0.198 |
| System | ESc/eV | Energy of structure with H2/eV | Energy of structure with 2H/eV | ΔE/eV |
|---|---|---|---|---|
| 1B | -5.95 | -459.08 | -458.11 | 0.97 |
| 2B | -4.75 | -456.58 | -455.91 | 0.67 |
| 3B | -4.42 | -454.51 | -454.52 | -0.01 |
| 1N | -7.03 | -462.58 | -460.78 | 1.80 |
| 2N | -7.39 | -462.78 | -461.31 | 1.47 |
| 3N | -6.69 | -462.74 | -462.60 | 0.14 |
| 1B1N1C | -5.14 | -459.17 | -459.03 | 0.16 |
| 1B2N | -5.14 | -459.59 | -458.86 | 0.74 |
| 2B1N | -6.19 | -457.14 | -456.52 | 0.62 |
Table 4 Binding energies of Sc(Eb), the energies of configurations with H2 and 2H, and their energy differences of Sc decorated various B/N doped graphenes
| System | ESc/eV | Energy of structure with H2/eV | Energy of structure with 2H/eV | ΔE/eV |
|---|---|---|---|---|
| 1B | -5.95 | -459.08 | -458.11 | 0.97 |
| 2B | -4.75 | -456.58 | -455.91 | 0.67 |
| 3B | -4.42 | -454.51 | -454.52 | -0.01 |
| 1N | -7.03 | -462.58 | -460.78 | 1.80 |
| 2N | -7.39 | -462.78 | -461.31 | 1.47 |
| 3N | -6.69 | -462.74 | -462.60 | 0.14 |
| 1B1N1C | -5.14 | -459.17 | -459.03 | 0.16 |
| 1B2N | -5.14 | -459.59 | -458.86 | 0.74 |
| 2B1N | -6.19 | -457.14 | -456.52 | 0.62 |
| 1 | Gomes I. L. R., Pousinho H. M. I., Melício R., Mendes V. M. F., Energy, 2017, 124, 310—320 |
| 2 | Niaz S., Manzoor T., Pandith A. H., Renewable Sustainable Energy Rev., 2015, 50, 457—469 |
| 3 | Møller K. T., Jensen T. R., Akiba E., Li H. W., Prog. Nat. Sci.: Mater. Int., 2017, 27(1), 34—40 |
| 4 | Hirscher M., Yartys V. A., Baricco M., Bellosta V. C. J., Blanchard D., Bowman R. C., Broom D. P., Buckley C. E., Chang F., Chen P., Cho Y. W., Crivello J. C., Cuevas F., David W. I. F., Jongh P. E. D., Denys R. V., Dornheim M., Felderhoff M., Filinchuk Y., Froudakis G. E., Grant D. M., Gray E. M., Hauback B. C., He T., Humphries T. D., Jensen T. R., Kim S., Kojima Y., Latroche M., Li H. W., Lototskyy M. V., Makepeace J. W., Møller K. T., Naheed L., Ngene P., Noréus D., Nygård M. M., Orimo S. I., Paskevicius M., Pasquini L., Ravnsbæk D. B., Veronica S. M., Udovic T. J., Vegge T., Walker G. S., Webb C. J., Weidenthaler C., Zlotea C., J. Alloys Compd., 2020, 827, 153548 |
| 5 | Hanley E. S., Deane J. P., Gallachóir B. P. Ó., Renewable Sustainable Energy Rev., 2018, 82, 3027—3045 |
| 6 | Wei T. Y., Lim K. L., Tseng Y. S., Chan S. L. I., Renewable Sustainable Energy Rev., 2017, 79, 1122—1133 |
| 7 | Salehabadi A., Umar M. F., Ahmad A., Ahmad M. I., Ismail N., Rafatullah M., Int. J. Energy Res., 2020, 44(14), 11044—11058 |
| 8 | Alekseeva O. K., Pushkareva I. V., Pushkarev A. S., Fateev V. N., Nanotechnol. Russ., 2020, 15, 273—300 |
| 9 | García⁃Holley P., Schweitzer B., Islamoglu T., Liu Y., Lin L., Rodriguez S., Rodriguez S., Weston M. H., Hupp J. T., Gómez⁃ Gualdrón D. A.,Yildirim T., Farha O. K., ACS Energy Lett., 2018, 3(3), 748—754 |
| 10 | Jose B. V. C., Ares J.R., Barale J., Baricco M., Buckley C., Capurso G., Gallandat N., Grant D. M., Guzik M. N., Jacob I., Jensen E. H., Jensen T., Jepsen J., Klassen T., Lototskyy M. V., Manickam K., Montone A., Puszkiel J., Sartori S., Sheppard D. A., Stuart A., Walker G., Webb C. J., Yang H., Yartys V., Züttel A., Dornheim M., Int. J. Hydrogen Energy, 2019, 44(15), 7780—7808 |
| 11 | Moradi R., Groth K. M., Int. J. Hydrogen Energy, 2019, 44(23), 12254—12269 |
| 12 | Shiraz H. G., Tavakoli O., Renewable Sustainable Energy Rev., 2017, 74, 104—109 |
| 13 | Nachimuthu S., He L., Cheng H., Tiono R. D., Jiang J., Sustainable Energy & Fuels, 2021, 5(7), 2159—2168 |
| 14 | Kubas G. J., Chem Rev., 2007, 107, 4152—4205 |
| 15 | Jena P., J. Phys. Chem. Lett., 2011, 2, 206—211 |
| 16 | Niu J., Rao B. K., Jena P., Phys. Rev. Lett., 1992, 68(15), 2277 |
| 17 | Guo Y., Lan X., Cao J., Xu B., Xia Y., Yin J., Liu Z., Int. J. Hydrogen Energy, 2013, 38(10), 3987—3993 |
| 18 | Satyapal S., Petrovic J., Read C., Thomas G., Ordaz G., Catal. Today, 2007, 120, 246—256 |
| 19 | Sun Q., Wang Q., Jena P., Kawazoe Y., J. Am. Chem. Soc., 2005, 127(42), 14582—14583 |
| 20 | Valencia H., Gil A., Frapper G., J. Phys. Chem. C, 2015, 119(10), 5506—5522 |
| 21 | Ma L. J., Wang J., Han M., Jia J., Wu H. S., Zhang X., Energy, 2019, 171(15), 315—325 |
| 22 | Dixit M., Adit M. T., Ghatak K., Ahuja R., Pal S., J. Phys. Chem. C, 2012, 116(33), 17336—17342 |
| 23 | Manade M., Vines F., Gil A., Illas F., Phys. Chem. Chem. Phys., 2018, 20, 3819—3830 |
| 24 | Long J., Li J., Nan F., Yin S., Li J., Cen W., Appl. Surf. Sci., 2018, 435(30), 1065—1071 |
| 25 | Banerjee P., Thapa R., Rajkamal A., Chandrakumar K. R. S., Das G. P., Int. J. Hydrogen Energy, 2019, 44(41), 23196—23209 |
| 26 | Faye O., Hussain T., Karton A., Szpunar J., Nanotechnology, 2019, 30(7), 075404 |
| 27 | Granja⁃DelRío A., Alonso J. A., López M. J., Comput. Theor. Chem., 2017, 1107(1), 23—29 |
| 28 | Nachimuthu S., Lai P. J., Jiang J. C., Carbon, 2014, 73, 132—140 |
| 29 | Alhameedi K., Hussain T., Bae H., Jayatilaka D., Lee H., Karton A., Carbon, 2019, 152, 344—353 |
| 30 | Gao H., Song L., Guo W., Huang L., Yang D., Wang F., Carbon, 2012, 50(12), 4476—4482 |
| 31 | Lin Y. C., Teng P. Y., Yeh C. H., Koshino M., Chiu P. W., Suenaga K., Nano Lett., 2015, 15(11), 7408—7413 |
| 32 | Wang L., Yang F., Yang R., AIChE J., 2009, 55(7), 1823—1833 |
| 33 | Liu P., Liang J., Xue R., Int. J. Hydrogen Energy, 2019, 44(51), 27853—27861 |
| 34 | Luo Z., Fan X., Pan R., An Y., Int. J. Hydrogen Energy, 2017, 42(5), 3106—3113 |
| 35 | Mananghaya M., Rodulfo E., Santos G. N., Villagracia A. R., Ladines A. N., J. Nanomater., 2012, 2012, 104891 |
| 36 | Mananghaya M., Yu D., Santos G. N., Rodulfo E., Sci. Rep., 2016, 6, 27370 |
| 37 | Liang X., Ng S. P., Ding N., Wu C. M. L., Appl. Surf. Sci., 2019, 473(15), 174—181 |
| 38 | Song N., Wang Y., Sun Q., Jia Y., Appl. Surf. Sci., 2012, 263(15), 182—186 |
| 39 | Valencia H., Gil A., Frapper G., J. Phys. Chem. C, 2015, 119(10), 5506—5522 |
| 40 | Wang J., Chen Y., Yuan L., Zhang M., Zhang C., Molecules, 2019, 24(13), 2382 |
| 41 | Kresse G., Furthmüller J., Phys. Rev. B, 1996, 54(16), 11169—11186 |
| 42 | Perdew J. P., Burke Kieron., Ernzerhof M., Phys. Rev. Lett., 1996, 77(18), 3865—3868 |
| 43 | Blöchl P. E., Phys. Rev. B, 1994, 50(24), 17953—17979 |
| 44 | Chadi D. J., Phys. Rev. B, 1977, 16(4), 1746—1747 |
| 45 | Grimme S., Antony J., Ehrlich S., Krieg S., J. Chem. Phys., 2010, 132, 154104 |
| 46 | Grimme S., Ehrlich S., Goerigk L., J. Comput. Chem., 2011, 32(7), 1456—1465 |
| 47 | Zhou X., Qiao J., Yang L., Zhang J., Adv. Energy Mater.,2014, 4, 1301523 |
| 48 | Gao H., Song L., Guo W., Huang L., Yang D., Wang F., Carbon, 2012, 50, 4476-4482 |
| 49 | Parambhath V. B., Nagar R., Ramaprabhu S., Langmuir,2012, 28, 7826-7833 |
| 50 | Gu J., Du Q., Han Y., He Z., Li W., Zhang J., Phys. Chem. Chem. Phys., 2014, 16(46), 25498—25507 |
| 51 | Lazar P., Zboril R., Pumera M., Otyepka M., Phys. Chem. Chem. Phys., 2014, 16(27), 14231—14235 |
| 52 | Hou M., Cen W., Nan F., Li J., Chu Y., Yin H., RSC Adv., 2015, 6(9), 7015—7021 |
| 53 | Charles K., Introduction To Solid State Physics, 8th Ed., John Wiley & Sons, In., Hoboken, 2005 |
| 54 | Ernst O. K., Bartol T., Sejnowski T., Mjolsness E., J. Chem. Phys., 2018, 149(3), 034107 |
| 55 | Luo Z., Fan X., Pan R., An Y., Int. J. Hydrogen Energy, 2017, 42(5), 3106—3113 |
| 56 | Hussain T., Mortazavi B., Bae H., Rabczuk T., Lee H., Karton A., Carbon, 2019, 147, 199—205 |
| 57 | Liu P., Liang J., Xue R., Du Q., Jiang M., Int. J. Hydrogen Energy, 2019, 44(51), 27853—27861 |
| 58 | Jia J., Li X., Ma L., Wu H. S., Comput. Theor. Chem., 2014, 1027(1), 128—134 |
| [1] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [2] | 杨丽君, 于洋, 张蕾. 双功能2D/3D杂化结构Co2P-CeO x 异质结一体化电极的构筑及电催化尿素氧化辅助制氢性能[J]. 高等学校化学学报, 2022, 43(6): 20220082. |
| [3] | 张义超, 赵付来, 王宇, 王亚玲, 沈永涛, 冯奕钰, 封伟. 基于多层二硒化钨的高性能场效应晶体管的实验优化和理论模拟[J]. 高等学校化学学报, 2022, 43(6): 20220113. |
| [4] | 黄汉浩, 卢湫阳, 孙明子, 黄勃龙. 石墨炔原子催化剂的崭新道路:基于自验证机器学习方法的筛选策略[J]. 高等学校化学学报, 2022, 43(5): 20220042. |
| [5] | 胡慧敏, 崔静, 刘丹丹, 宋佳欣, 张宁, 范晓强, 赵震, 孔莲, 肖霞, 解则安. 过渡金属修饰对Pt/M-DMSN催化剂丙烷脱氢性能的影响[J]. 高等学校化学学报, 2022, 43(4): 20210815. |
| [6] | 刘坤, 尹远, 耿文强, 夏昊天, 李华. 不同组分过渡金属氧化物催化剂对介质阻挡放电固氮的影响机制[J]. 高等学校化学学报, 2022, 43(11): 20220278. |
| [7] | 丁钦, 张梓轩, 徐培程, 李晓宇, 段莉梅, 王寅, 刘景海. Cu, Ni, Co掺杂对Fe碳纳米管的结构及电催化性能的影响[J]. 高等学校化学学报, 2022, 43(11): 20220421. |
| [8] | 刘洋, 李旺昌, 张竹霞, 王芳, 杨文静, 郭臻, 崔鹏. Sc3C2@C80与[12]CPP纳米环之间非共价相互作用的理论研究[J]. 高等学校化学学报, 2022, 43(11): 20220457. |
| [9] | 王祖民, 孟程, 于然波. 过渡金属磷化物析氢催化剂的掺杂调控[J]. 高等学校化学学报, 2022, 43(11): 20220544. |
| [10] | 周成思, 赵远进, 韩美晨, 杨霞, 刘晨光, 贺爱华. 硅烷类外给电子体对丙烯-丁烯序贯聚合的调控作用[J]. 高等学校化学学报, 2022, 43(10): 20220290. |
| [11] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [12] | 王园月, 安梭梭, 郑旭明, 赵彦英. 5-巯基-1, 3, 4-噻二唑-2-硫酮微溶剂团簇的光谱和理论计算研究[J]. 高等学校化学学报, 2022, 43(10): 20220354. |
| [13] | 黄罗仪, 翁约约, 黄旭慧, 王朝杰. 车前草中黄酮类成分结构和性质的理论研究[J]. 高等学校化学学报, 2021, 42(9): 2752. |
| [14] | 钟声广, 夏文生, 张庆红, 万惠霖. 电中性团簇MCu2Ox(M=Cu2+, Ce4+, Zr4+)上甲烷和二氧化碳直接合成乙酸的理论研究[J]. 高等学校化学学报, 2021, 42(9): 2878. |
| [15] | 郑若昕, 张颖, 徐昕. 低标度XYG3双杂化密度泛函的开发与测评[J]. 高等学校化学学报, 2021, 42(7): 2210. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||