

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (9): 1771.doi: 10.7503/cjcu20150144
• Physical Chemistry • Previous Articles Next Articles
PAN Wenbo, LI Mingxue, SU Yaqiong, WU Deyin*( ), TIAN Zhongqun
), TIAN Zhongqun
Received:2015-02-11
Online:2015-09-10
Published:2015-08-21
Contact:
WU Deyin
E-mail:dywu@xmu.edu.cn
Supported by:CLC Number:
TrendMD:
PAN Wenbo, LI Mingxue, SU Yaqiong, WU Deyin, TIAN Zhongqun. Theoretical Study of Reactions Between Polysulfides and Ethylene Carbonate and Raman Spectra in Lithium-sulfur Battery†[J]. Chem. J. Chinese Universities, 2015, 36(9): 1771.
| Parameter | EC | Li+(EC) | ||||
|---|---|---|---|---|---|---|
| 6-311++G(d,p) | Expt.(crys)[ | Expt.(MW)[ | aug-cc-pVDZ[ | 6-311++G(d,p) | ||
| Bond length/nm | C=O | 0.1188 | 0.1203 | 0.1200 | 0.1203 | 0.1208 |
| C3—O | 0.1361 | 0.1342 | 0.1358 | 0.1370 | 0.1333 | |
| C—O | 0.1437 | 0.1457 | 0.1428 | 0.1440 | 0.1456 | |
| C—C | 0.1529 | 0.1522 | 0.1540 | 0.1520 | 0.1531 | |
| C—H | 0.1089 | 0.1091 | 0.1095 | 0.1090 | 0.1089 | |
| Bending angle/(°) | O=C—O | 124.9 | 124.2 | 124.2 | 124.0 | |
| C—O—C | 109.4 | 108.7 | 109.5 | 108.4 | 109.3 | |
| O—C—O | 110.1 | 111.7 | 110.4 | 110.4 | 112.4 | |
| C—C—O | 102.6 | 102.2 | 102.4 | 102.5 | 102.6 | |
| H—C—H | 109.9 | 110.8 | 114.0 | 110.6 | ||
| Dihedral angle/(°) | O=C—O—C | -171.7 | -171.3 | -171.5 | 170.6 | 179.9 |
| C—O—C—C | -20.0 | -21.3 | -20.2 | 22.9 | -16.3 | |
| O—C—C—O | 23.5 | 24.8 | 23.9 | 27.2 | 19.1 | |
| O—C—O—C | 8.3 | 8.7 | 8.5 | 9.4 | 6.7 | |
Table 1 Comparison of theoretical and experimental geometrical parameters for EC and Li+(EC)
| Parameter | EC | Li+(EC) | ||||
|---|---|---|---|---|---|---|
| 6-311++G(d,p) | Expt.(crys)[ | Expt.(MW)[ | aug-cc-pVDZ[ | 6-311++G(d,p) | ||
| Bond length/nm | C=O | 0.1188 | 0.1203 | 0.1200 | 0.1203 | 0.1208 |
| C3—O | 0.1361 | 0.1342 | 0.1358 | 0.1370 | 0.1333 | |
| C—O | 0.1437 | 0.1457 | 0.1428 | 0.1440 | 0.1456 | |
| C—C | 0.1529 | 0.1522 | 0.1540 | 0.1520 | 0.1531 | |
| C—H | 0.1089 | 0.1091 | 0.1095 | 0.1090 | 0.1089 | |
| Bending angle/(°) | O=C—O | 124.9 | 124.2 | 124.2 | 124.0 | |
| C—O—C | 109.4 | 108.7 | 109.5 | 108.4 | 109.3 | |
| O—C—O | 110.1 | 111.7 | 110.4 | 110.4 | 112.4 | |
| C—C—O | 102.6 | 102.2 | 102.4 | 102.5 | 102.6 | |
| H—C—H | 109.9 | 110.8 | 114.0 | 110.6 | ||
| Dihedral angle/(°) | O=C—O—C | -171.7 | -171.3 | -171.5 | 170.6 | 179.9 |
| C—O—C—C | -20.0 | -21.3 | -20.2 | 22.9 | -16.3 | |
| O—C—C—O | 23.5 | 24.8 | 23.9 | 27.2 | 19.1 | |
| O—C—O—C | 8.3 | 8.7 | 8.5 | 9.4 | 6.7 | |
| Modea | 6-311++G(d,p) | Scaled | Expt.[ | aug-cc-pVDZ[ | 1019AR/(nm4·kg-1) | PED(%)b |
|---|---|---|---|---|---|---|
| ν1(b) | 3135 | 3031 | 3011 | 3205 | 46.6 | νCH2(100) |
| ν2(a) | 3123 | 3020 | 3004 | 3194 | 57.6 | νCH2(86) |
| ν3(b) | 3058 | 2957 | 3000 | 3112 | 18.3 | νCH2(100) |
| ν4(a) | 3054 | 2953 | 2990 | 3109 | 132.3 | νCH2(86) |
| ν5(a) | 1895 | 1860 | 1868 | 1898 | 10.4 | ν(C=O) (86) |
| ν6(a) | 1529 | 1500 | 1570 | 1546 | 2.5 | sciCH2(99) |
| ν7(b) | 1521 | 1492 | 1480 | 1539 | 6.3 | sciCH2(98) |
| ν8(b) | 1400 | 1373 | 1421 | 1421 | 0.6 | ωCH2(81) |
| ν9(a) | 1386 | 1360 | 1386 | 1420 | 2.6 | ωCH2(87) |
| ν10(a) | 1245 | 1222 | 1223 | 1271 | 4.4 | τCH2(91) |
| ν11(a) | 1238 | 1214 | 1218 | 1267 | 4.6 | τCH2(79) |
| ν12(a) | 1154 | 1132 | 1157 | 1175 | 0.2 | rockCH2(95) |
| ν13(b) | 1115 | 1070 | 1125 | 1138 | 0.4 | ν(C—O)(59) |
| β(C=O)(12) | ||||||
| ν14(a) | 1090 | 1094 | 1090 | 1123 | 1.4 | ν(C—O)(76) |
| ν(C—C)(14) | ||||||
| ν15(b) | 1051 | 1031 | 1087 | 1079 | 0.1 | ν(C—O)(72) |
| ν16(a) | 964 | 946 | 960 | 991 | 4.5 | ν(C—C)(62) |
| ν17(a) | 891 | 874 | 891 | 895 | 0.9 | R breathing |
| ν18(b) | 889 | 872 | 881 | 919 | 8.3 | rockCH2(52) |
| ν19(b) | 772 | 758 | 768 | 779 | 0.3 | β(C=O)(97) |
| ν20(a) | 718 | 704 | 715 | 719 | 3.7 | νR(68) |
| ν21(b) | 691 | 678 | 673 | 673 | 1.6 | τR(62) |
| ν22(b) | 523 | 513 | 527 | 527 | 0.3 | γ(C=O)(70) |
| ν23(a) | 187 | 184 | 227 | 227 | 0.2 | τR(97) |
| ν24(b) | 178 | 175 | 184 | 184 | 0.2 | τR(97.3) |
Table 2 Calculated frequencies, Raman activity(AR) and assignment of EC along with the reference dataa
| Modea | 6-311++G(d,p) | Scaled | Expt.[ | aug-cc-pVDZ[ | 1019AR/(nm4·kg-1) | PED(%)b |
|---|---|---|---|---|---|---|
| ν1(b) | 3135 | 3031 | 3011 | 3205 | 46.6 | νCH2(100) |
| ν2(a) | 3123 | 3020 | 3004 | 3194 | 57.6 | νCH2(86) |
| ν3(b) | 3058 | 2957 | 3000 | 3112 | 18.3 | νCH2(100) |
| ν4(a) | 3054 | 2953 | 2990 | 3109 | 132.3 | νCH2(86) |
| ν5(a) | 1895 | 1860 | 1868 | 1898 | 10.4 | ν(C=O) (86) |
| ν6(a) | 1529 | 1500 | 1570 | 1546 | 2.5 | sciCH2(99) |
| ν7(b) | 1521 | 1492 | 1480 | 1539 | 6.3 | sciCH2(98) |
| ν8(b) | 1400 | 1373 | 1421 | 1421 | 0.6 | ωCH2(81) |
| ν9(a) | 1386 | 1360 | 1386 | 1420 | 2.6 | ωCH2(87) |
| ν10(a) | 1245 | 1222 | 1223 | 1271 | 4.4 | τCH2(91) |
| ν11(a) | 1238 | 1214 | 1218 | 1267 | 4.6 | τCH2(79) |
| ν12(a) | 1154 | 1132 | 1157 | 1175 | 0.2 | rockCH2(95) |
| ν13(b) | 1115 | 1070 | 1125 | 1138 | 0.4 | ν(C—O)(59) |
| β(C=O)(12) | ||||||
| ν14(a) | 1090 | 1094 | 1090 | 1123 | 1.4 | ν(C—O)(76) |
| ν(C—C)(14) | ||||||
| ν15(b) | 1051 | 1031 | 1087 | 1079 | 0.1 | ν(C—O)(72) |
| ν16(a) | 964 | 946 | 960 | 991 | 4.5 | ν(C—C)(62) |
| ν17(a) | 891 | 874 | 891 | 895 | 0.9 | R breathing |
| ν18(b) | 889 | 872 | 881 | 919 | 8.3 | rockCH2(52) |
| ν19(b) | 772 | 758 | 768 | 779 | 0.3 | β(C=O)(97) |
| ν20(a) | 718 | 704 | 715 | 719 | 3.7 | νR(68) |
| ν21(b) | 691 | 678 | 673 | 673 | 1.6 | τR(62) |
| ν22(b) | 523 | 513 | 527 | 527 | 0.3 | γ(C=O)(70) |
| ν23(a) | 187 | 184 | 227 | 227 | 0.2 | τR(97) |
| ν24(b) | 178 | 175 | 184 | 184 | 0.2 | τR(97.3) |
| Species | Structure | Charge distribution | ||||
|---|---|---|---|---|---|---|
| Bond length/nm | Bending angle/(°) | S1 | S2 | S3 | S4 | |
| 0.2220 | -1.00 | -1.00 | ||||
| 0.2151 | 110.5 | -1.05 | -0.34 | -0.61 | ||
| 0.2127 | 111.4 | -0.61 | -0.39 | -0.39 | -0.61 | |
Table 3 Structure parameters and NBO charge distribution of polysulfide anions at the PCM-B3LYP/6-311++G(d,p) level
| Species | Structure | Charge distribution | ||||
|---|---|---|---|---|---|---|
| Bond length/nm | Bending angle/(°) | S1 | S2 | S3 | S4 | |
| 0.2220 | -1.00 | -1.00 | ||||
| 0.2151 | 110.5 | -1.05 | -0.34 | -0.61 | ||
| 0.2127 | 111.4 | -0.61 | -0.39 | -0.39 | -0.61 | |
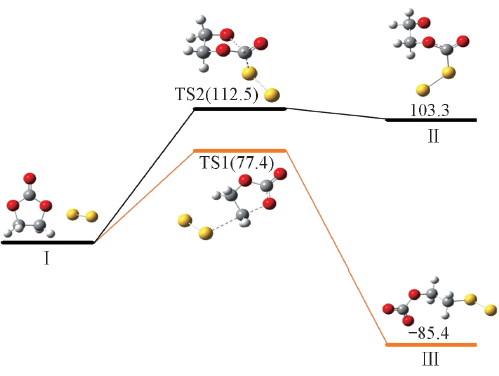
Fig.3 Reaction activation energies and Gibbs free energies(kJ/mol) for nucleophilic attack of S22- at the carbonyl and ethyl carbon atoms of EC calculated at the PCM-B3LYP/6-311++G(d,p) level
| Species | Attacking ethyl carbon | Attacking carbonyl carbon | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔHact,1 | ΔGact,1 | ΔHr,1 | ΔGr,1 | ΔHact,2 | ΔGact,2 | ΔHr,2 | ΔGr,2 | |
| 40.9 | 77.4 | -116.0 | -85.4 | 71.3 | 112.5 | 68.8 | 103.3 | |
| 53.7 | 93.3 | -80.0 | -43.5 | 116.6 | 163.5 | 116.2 | 156.6 | |
| 62.4 | 100.9 | -60.4 | -24.9 | 135.6 | 187.3 | 141.0 | 178.2 | |
Table 4 Reaction activation energies, enthalpies and Gibbs free energies(kJ/mol) for nucleophilic attack of polysulfide anions S2-n(n=2—4) at the carbonyl and ethyl carbon atoms of EC calculated at the PCM-B3LYP/6-311++G(d,p) level
| Species | Attacking ethyl carbon | Attacking carbonyl carbon | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔHact,1 | ΔGact,1 | ΔHr,1 | ΔGr,1 | ΔHact,2 | ΔGact,2 | ΔHr,2 | ΔGr,2 | |
| 40.9 | 77.4 | -116.0 | -85.4 | 71.3 | 112.5 | 68.8 | 103.3 | |
| 53.7 | 93.3 | -80.0 | -43.5 | 116.6 | 163.5 | 116.2 | 156.6 | |
| 62.4 | 100.9 | -60.4 | -24.9 | 135.6 | 187.3 | 141.0 | 178.2 | |
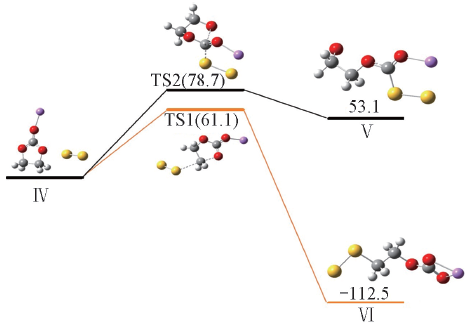
Fig.5 Reaction activation energies and Gibbs free energies(kJ/mol) for nucleophilic attack of S22- at the carbonyl and ethyl carbon atoms of Li+EC calculated at the PCM-B3LYP/6-311++G(d,p) level
| Species | Attacking ethyl carbon | Attacking carbonyl carbon | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔHact,1 | ΔGact,1 | ΔHr,1 | ΔGr,1 | ΔHact,2 | ΔHr,2 | ΔGact,2 | ΔGr,2 | |
| 21.8 | 61.1 | -168.0 | -112.5 | 28.9 | -41.2 | 78.7 | 53.1 | |
| 34.3 | 78.5 | -131.6 | -81.7 | 77.7 | 203.1 | 132.6 | 62.5 | |
| 42.8 | 86.7 | -256.1 | -57.5 | 114.2 | 41.2 | 165.8 | 91.5 | |
Table 5 Reaction activation energies, enthalpies and Gibbs free energies(kJ/mol) for nucleophilic attack of polysulfides anions S2-n(n=2—4) at the carbonyl and ethyl carbon atoms of Li+(EC) calculated at the PCM-B3LYP/6-311++G(d,p) level
| Species | Attacking ethyl carbon | Attacking carbonyl carbon | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔHact,1 | ΔGact,1 | ΔHr,1 | ΔGr,1 | ΔHact,2 | ΔHr,2 | ΔGact,2 | ΔGr,2 | |
| 21.8 | 61.1 | -168.0 | -112.5 | 28.9 | -41.2 | 78.7 | 53.1 | |
| 34.3 | 78.5 | -131.6 | -81.7 | 77.7 | 203.1 | 132.6 | 62.5 | |
| 42.8 | 86.7 | -256.1 | -57.5 | 114.2 | 41.2 | 165.8 | 91.5 | |
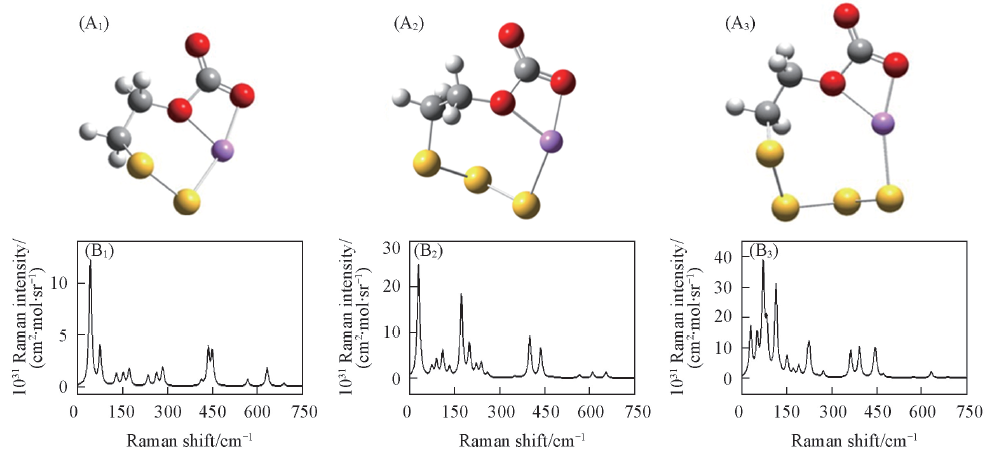
Fig.7 Optimized structures(A1—A3) and simulated Raman spectra(B1—B3) of isomerization products calculated at the PCM-B3LYP/6-311++G(d,p) level(A1), (B1) Ring-Li+(EC)S22-; (A2), (B2) ring-Li+(EC)S32-; (A3), (B3) ring-Li+(EC)S42-.
| Reaction | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|
| Li+(EC) | -18.1 | -17.8 | -12.8 |
| Li+(EC) | -18.6 | -26.5 | -10.7 |
| Li+(EC) | -14.3 | -37.9 | -3.0 |
Table 6 Enthalpies and Gibbs free energies for isomerization products calculated at the PCM-B3LYP/6-311++G(d,p) level
| Reaction | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|
| Li+(EC) | -18.1 | -17.8 | -12.8 |
| Li+(EC) | -18.6 | -26.5 | -10.7 |
| Li+(EC) | -14.3 | -37.9 | -3.0 |
| [1] | Yuan K. G., Wang A. B., Yu Z. B., Wang W. K., Yang Y. S., Chem. J. Chinese Universities, 2006, 27(9), 1738—1741 |
| (苑克国, 王安邦, 余仲宝, 王维坤, 杨裕生. 高等学校化学学报, 2006, 27(9), 1738—1741) | |
| [2] | Ji X., Nazar L. F., J. Mater. Chem., 2010, 20(44), 9821—9826 |
| [3] | Yin Y. X., Xin S., Guo Y. G., Wan L. J., Angew. Chem.Int. Ed., 2013, 52(50), 13186—13200 |
| [4] | Chen R. J., Zhao T., Li L., Chen J. Z., Wu F., Sci.China Chem., 2014, 8, 8 |
| (陈人杰, 赵腾, 李丽, 陈君政, 吴锋. 中国科学: 化学, 2014, 8, 8) | |
| [5] | Manthiram A., Fu Y., Su Y. S., Accounts. Chem. Res., 2012, 46(5), 1125—1134 |
| [6] | Manthiram A., Fu Y., Chung S. H., Zu C., Su Y. S., Chem. Rev., 2014, 114(23), 11751—11787 |
| [7] | Zhang S. S., J. Power Sources, 2013, 231, 153—162 |
| [8] | Scheers J., Fantini S., Johansson P., J. Power Sources, 2014, 255, 204—218 |
| [9] | Yamin H., Peled E., J. Power Sources, 1983, 9(3), 281—287 |
| [10] | Peled E., Gorenshtein A., Segal M., Sternberg Y., J. Power Sources, 1989, 26, 269—271 |
| [11] | Nelson J., Misra S., Yang Y., Jackson A., Liu Y., Wang H., Dai H., Andrews J. C., Cui Y., Toney M. F., J. Am. Chem. Soc., 2012, 134(14), 6337—6343 |
| [12] | Hagen M., Schiffels P., Hammer M., Dörfler S., Tübke J., Hoffmann M., Althues H., Kaskel S., J. Electrochem. Soc., 2013, 160(8), A1205—A1214 |
| [13] | Kim S., Jung Y., Park S. J., J. Power Sources, 2005, 152, 272—277 |
| [14] | Kim S., Jung Y., Park S. J., Electrochim. Acta, 2007, 52(5), 2116—2122 |
| [15] | Dong Q. F., Wang C., Zheng M. S., Process Chem., 2011, 23(2), 533—539 |
| (董全峰, 王翀, 郑明森. 化学进展, 2011, 23(2), 533—539) | |
| [16] | Wang L., Zhang T., Yang S., Cheng F., Liang J., Chen J., J. Energ. Chem., 2013, 22(1), 72—77 |
| [17] | Assary R. S., Curtiss L. A., Moore J. S., J. Phys. Chem. C, 2014, 118(22), 11545—11558 |
| [18] | Gao J., Lowe M.a H. C. D., J. Phys. Chem. C, 2011, 115(50), 25132—25137 |
| [19] | Yim T., Park M. S., Yu J. S., Kim K. J., Im K. Y., Kim J. H., Jeong G., Jo Y. N., Woo S. G., Kang K. S., Electrochim. Acta, 2013, 107, 454—460 |
| [20] | Wang J., Chew S. Y., Zhao Z. W., Ashraf S., Wexler D., Chen J., Ng S. H., Chou S. L., Liu H. K., Carbon, 2008, 46(2), 229—235 |
| [21] | Liang X., Wen Z., Liu Y., Zhang H., Jin J., Wu M., Wu X., J. Power Sources, 2012, 206(0), 409—413 |
| [22] | Li T., Balbuena P. B., J. Electrochem. Soc., 1999, 146(10), 3613—3622 |
| [23] | Allen J. L., Borodin O., Seo D. M., Henderson W. A., J. Power Sources, 2014, 267, 821—830 |
| [24] | Masia M., Probst M., Rey R., J. Phys. Chem. B, 2004, 108(6), 2016—2027 |
| [25] | Wang Y., Nakamura S., Ue M., Balbuena P. B., J. Am. Chem. Soc., 2001, 123(47), 11708—11718 |
| [26] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., et al., Gaussian 09, Revision A. 01; Gaussian Inc., Wallingford, CT, 2009 |
| [27] | Krishnan R., Binkley J. S., Seeger R., Pople J. A., J. Chem. Phys., 1980, 72(1), 650—654 |
| [28] | McLean A., Chandler G., J. Chem. Phys., 1980, 72(10), 5639—5648 |
| [29] | Pulay P., Fogarasi G., Pang F., Boggs J. E., J. Am. Chem. Soc., 1979, 101(10), 2550—2560 |
| [30] | Wu D. Y., Liu X. M., Huang Y. F., Ren B., Xu X., Tian Z. Q., J. Phys. Chem. C, 2009, 113(42), 18212—18222 |
| [31] | Pulay P., Fogarasi G., Pongor G., Boggs J. E., Vargha A., J. Am. Chem. Soc., 1983, 105(24), 7037—7047 |
| [32] | Angell C., Trans. Faraday Soc., 1956, 52, 1178—1183 |
| [33] | Wang I., Britt C. O., Boggs J. E., J. Am. Chem. Soc., 1965, 87(21), 4950—4951 |
| [34] | Fortunato B., Mirone P., Fini G., Spectrochim. Acta Part A: Mol. Spectro., 1971, 27(9), 1917—1927 |
| [35] | Alonso J. L., Cervellati R., Degli Esposti A., Lister D. G., Palmieri P., J. Chem. Soc. Faraday Transactions 2: Molecular and Chemical Physics, 1986, 82(3), 337—356 |
| [36] | Matias P. M., Jeffrey G., Wingert L. M., Ruble J. R., J. Mol. Struct: Theochem., 1989, 184(3), 247—260 |
| [37] | Cremer D., Pople J. A., J. Am. Chem. Soc., 1975, 97(6), 1354—1358 |
| [38] | Blint R. J., J. Electrochem. Soc., 1995, 142(3), 696—702 |
| [39] | Klassen B., Aroca R., Nazri M., Nazri G. A., J. Phys. Chem. B, 1998, 102(24), 4795—4801 |
| [40] | Brown C., Acta Crystallographica, 1954, 7(1), 92—96 |
| [41] | Berghof V., Sommerfeld T., Cederbaum L., J. Phys. Chem. A, 1998, 102(26), 5100—5105 |
| [42] | Meyer B., Chem. Rev., 1976, 76(3), 367—388 |
| [43] | Yu H. J., Liu Z. J., Yin Y. F., Fu J., Ding L., Mo Y. J., Spectrosc.Spect.Anal., 2009, 29(11), 2975—2979 |
| (余红静, 刘照军, 尹延峰, 符娟, 丁丽, 莫育俊. 光谱学与光谱分析, 2009, 29(11), 2975—2979) |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | CHEN Jiamin, QU Xiaozhang, QI Guohua, XU Weiqing, JIN Yongdong, XU Shuping. SERS Nanoprobe for the Detection of Reactive Oxygen Species in Cells Produced by Electrostimulus [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220033. |
| [3] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [4] | YIN Xiaoju, SUN Xun, ZHAO Chenghao, JIANG Bo, ZHAO Chenyang, ZHANG Naiqing. Research Progress of Single Atomic Catalysts in Lithium-sulfur Batteries [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220076. |
| [5] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [6] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [7] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [8] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [9] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [10] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [11] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [12] | XUE Jin, CAO Xiaowei, LIU Yifan, WANG Min. Preparation of Paper Hollow Gold Nanocage SERS Sensor and Its Rapid and Highly Sensitive Detection for miRNAs in Sputum of Patients with Non-small Cell Lung Cancer [J]. Chem. J. Chinese Universities, 2021, 42(8): 2393. |
| [13] | ZHENG Ruoxin, ZHANG Igor Ying, XU Xin. Development and Benchmark of Lower Scaling Doubly Hybrid Density Functional XYG3 [J]. Chem. J. Chinese Universities, 2021, 42(7): 2210. |
| [14] | YING Fuming, JI Chenru, SU Peifeng, WU Wei. λ-DFCAS: A Hybrid Density Functional Complete Active Space Self Consistent Field Method [J]. Chem. J. Chinese Universities, 2021, 42(7): 2218. |
| [15] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||