

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (2): 20240455.doi: 10.7503/cjcu20240455
王斓懿1, 王世伟1, 陈心宇1, 于迪2, 张春雷2, 范晓强1, 于学华1( ), 赵震1,2(
), 赵震1,2( )
)
收稿日期:2024-10-08
出版日期:2025-02-10
发布日期:2024-12-07
通讯作者:
于学华,赵震
E-mail:yuxuehua1986@163.com;zhenzhao@cup.edu.cn
基金资助:
WANG Lanyi1, WANG Shiwei1, CHEN Xinyu1, YU Di2, ZHANG Chunlei2, FAN Xiaoqiang1, YU Xuehua1( ), ZHAO Zhen1,2(
), ZHAO Zhen1,2( )
)
Received:2024-10-08
Online:2025-02-10
Published:2024-12-07
Contact:
YU Xuehua, ZHAO Zhen
E-mail:yuxuehua1986@163.com;zhenzhao@cup.edu.cn
Supported by:摘要:
炭烟颗粒和NOx作为柴油机尾气的主要污染物严重危害了人体健康与环境. 因此, 炭烟与NOx的催化净化得到了广泛关注. 本文设计制备了具有独特孔结构的三维有序大孔(3DOM)ZSM-5分子筛催化剂, 并将其作为载体, 制备了一系列3DOM ZSM-5担载MOδ (M=Mn, Fe, Co, Ce, Pr, W)的催化剂. 所制备催化剂均具有独特的多级孔结构, 有利于炭烟与NOx等小分子的捕捉与传质, 可进一步增强催化剂的催化性能. 其中, MnOδ/3DOM ZSM-5催化剂具有最佳的同时消除炭烟与NOx的催化性能, 其炭烟燃烧的峰值温度最低(453 ℃), 80%以上NO转化率的初始温度最低(184 ℃)且温度窗口较宽(184~362 ℃). 该催化剂优异的催化性能与其良好的氧化还原性能、 丰富的酸位点、 充足的活性氧以及丰富的多级孔结构有关. 动力学测试结果表明, MnOδ/3DOM ZSM-5催化剂具有最高的本征活性. 根据催化剂的活性测试和原位漫反射红外光谱(in situ DRIFTS)结果, 推断了该催化剂在不同温度下的反应机理. 在低温(<300 ℃)条件下, 反应以脱硝为主, 主要遵循Eley-Rideal(E-R)机理; 在高温条件下(>300 ℃)主要以炭烟燃烧为主, 包括活性氧机理和NO2辅助机理.
中图分类号:
TrendMD:
王斓懿, 王世伟, 陈心宇, 于迪, 张春雷, 范晓强, 于学华, 赵震. MO δ /3DOM ZSM-5催化剂的制备及同时消除炭烟和NOx的催化性能. 高等学校化学学报, 2025, 46(2): 20240455.
WANG Lanyi, WANG Shiwei, CHEN Xinyu, YU Di, ZHANG Chunlei, FAN Xiaoqiang, YU Xuehua, ZHAO Zhen. Preparation of MO δ /3DOM ZSM-5 Catalysts and Their Catalytic Performance for the Simultaneous Removal of Soot and NOx. Chem. J. Chinese Universities, 2025, 46(2): 20240455.
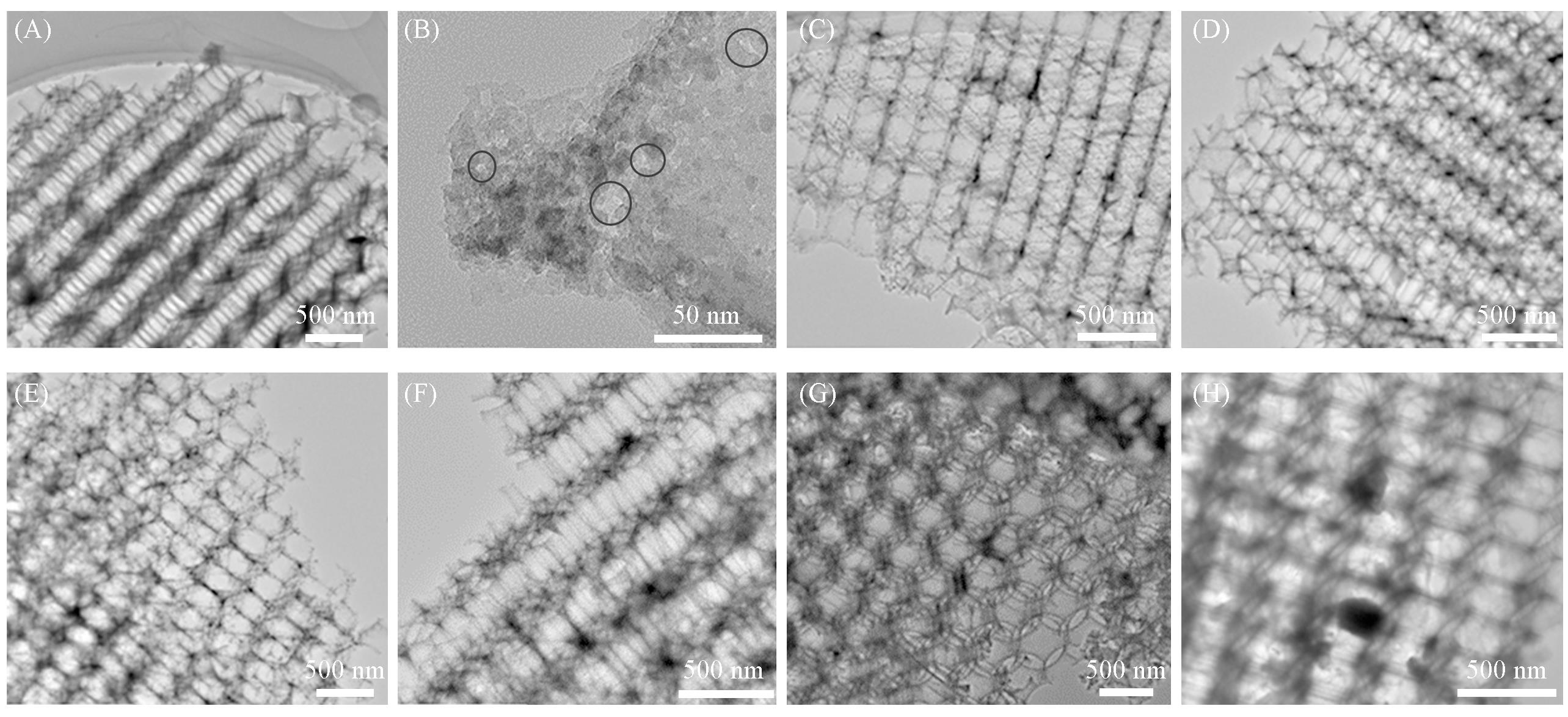
Fig.2 TEM images of MOδ/3DOM ZSM⁃5 catalysts(A, B) 3DOM ZSM-5; (C) MnOδ/3DOM ZSM-5; (D) FeOδ/3DOM ZSM-5; (E) CoOδ/3DOM ZSM-5; (F) CeOδ/3DOM ZSM-5; (G) PrOδ/3DOM ZSM-5; (H) WOδ/3DOM ZSM-5.
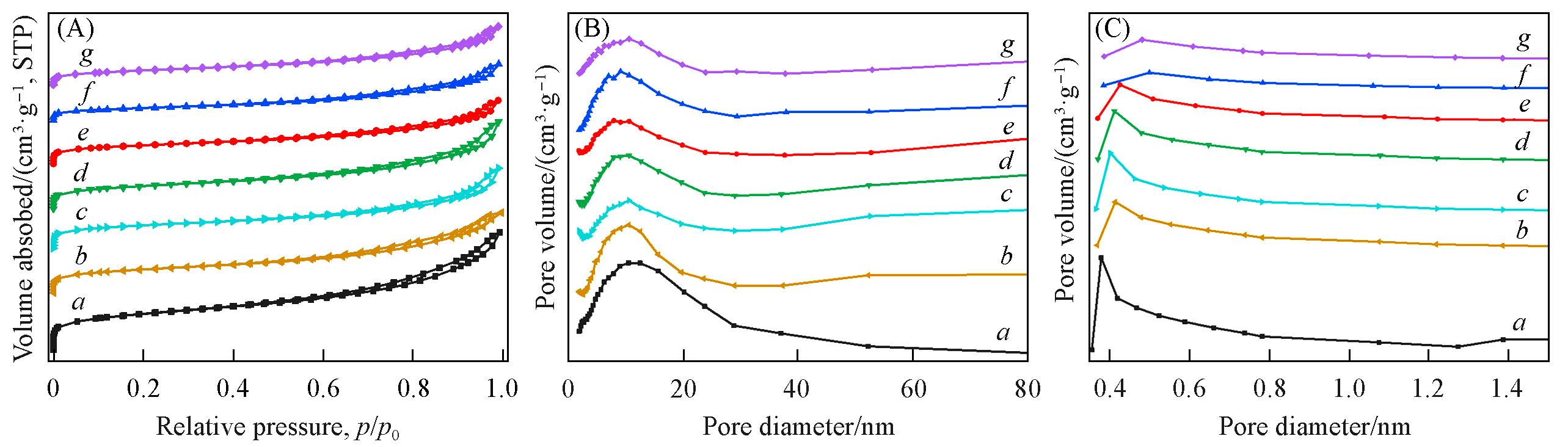
Fig.3 Nitrogen adsorption⁃desorption isotherms(A), mesoporous(B) and microporous(C) distribution curves of MOδ/3DOM ZSM⁃5 catalystsCurves a—g: 3DOM ZSM-5; MnOδ/3DOM ZSM-5; FeOδ/3DOM ZSM-5; CoOδ/3DOM ZSM-5; CeOδ/3DOM ZSM-5; PrOδ/3DOM ZSM-5; WOδ/3DOM ZSM-5.
| Catalyst | SBETa /(m2‧g‒1) | Smicrob /(m2‧g‒1) | Vtotalc /(cm3‧g‒1) | Vmicrod /(cm3‧g‒1) | Dpe /nm |
|---|---|---|---|---|---|
| 3DOM ZSM⁃5 | 641.3 | 177.7 | 0.784 | 0.091 | 4.5 |
| MnOδ/3DOM ZSM⁃5 | 435.4 | 121.4 | 0.624 | 0.062 | 5.7 |
| FeOδ/3DOM ZSM⁃5 | 426.9 | 142.0 | 0.632 | 0.073 | 5.9 |
| CoOδ/3DOM ZSM⁃5 | 432.1 | 116.5 | 0.685 | 0.059 | 6.3 |
| CeOδ/3DOM ZSM⁃5 | 354.1 | 109.9 | 0.505 | 0.056 | 5.7 |
| PrOδ/3DOM ZSM⁃5 | 255.8 | 57.3 | 0.449 | 0.029 | 7.0 |
| WOδ/3DOM ZSM⁃5 | 292.5 | 57.9 | 0.466 | 0.030 | 6.4 |
Table 1 Textural properties of MOδ/3DOM ZSM-5 catalysts
| Catalyst | SBETa /(m2‧g‒1) | Smicrob /(m2‧g‒1) | Vtotalc /(cm3‧g‒1) | Vmicrod /(cm3‧g‒1) | Dpe /nm |
|---|---|---|---|---|---|
| 3DOM ZSM⁃5 | 641.3 | 177.7 | 0.784 | 0.091 | 4.5 |
| MnOδ/3DOM ZSM⁃5 | 435.4 | 121.4 | 0.624 | 0.062 | 5.7 |
| FeOδ/3DOM ZSM⁃5 | 426.9 | 142.0 | 0.632 | 0.073 | 5.9 |
| CoOδ/3DOM ZSM⁃5 | 432.1 | 116.5 | 0.685 | 0.059 | 6.3 |
| CeOδ/3DOM ZSM⁃5 | 354.1 | 109.9 | 0.505 | 0.056 | 5.7 |
| PrOδ/3DOM ZSM⁃5 | 255.8 | 57.3 | 0.449 | 0.029 | 7.0 |
| WOδ/3DOM ZSM⁃5 | 292.5 | 57.9 | 0.466 | 0.030 | 6.4 |
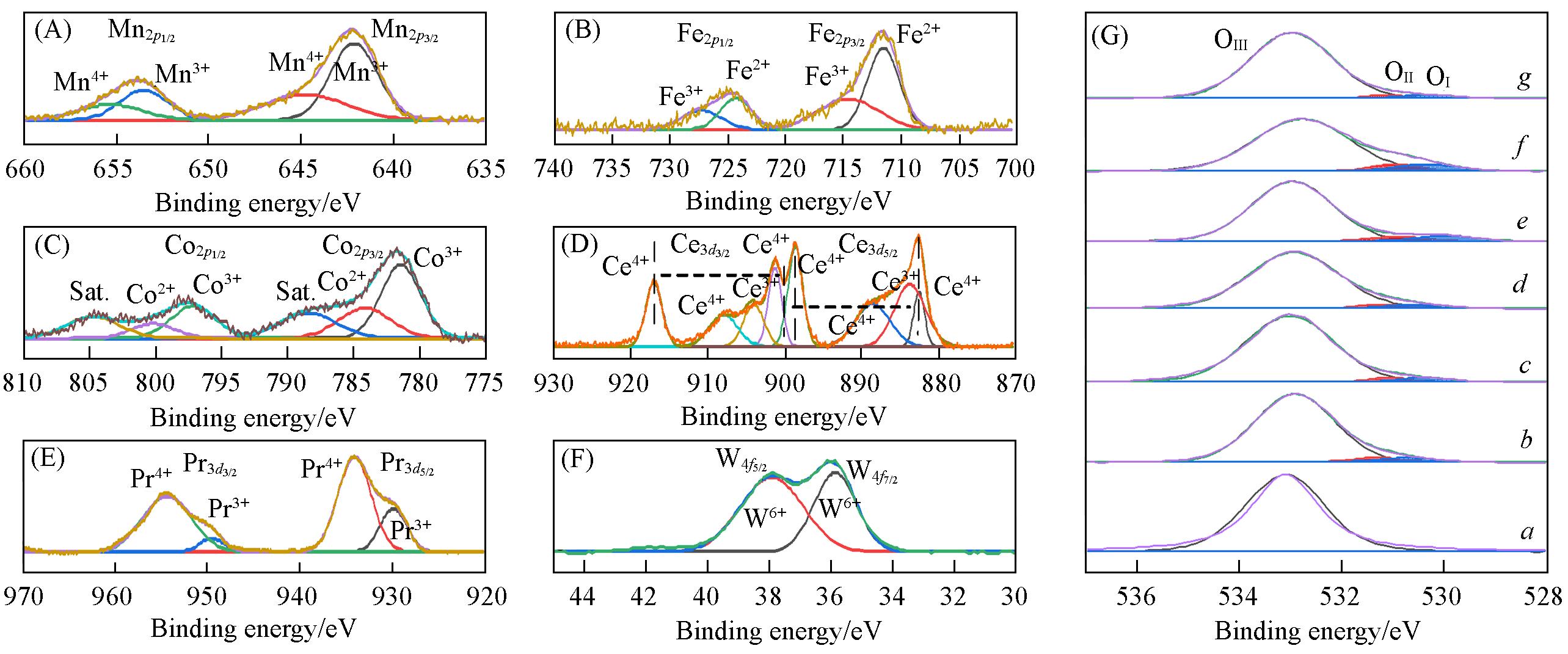
Fig.4 XPS spectra of MOδ/3DOM ZSM⁃5 catalysts(A)—(G) Mn2p, Fe2p, Co2p, Ce3d, Pr3d, W4f, O1s . Different metal elements; (G) a. 3DOM ZSM-5; b. MnOδ/3DOM ZSM-5;c. FeOδ/3DOM ZSM-5; d. CoOδ/3DOM ZSM-5; e. CeOδ/3DOM ZSM-5; f. PrOδ/3DOM ZSM-5; g. WOδ/3DOM ZSM-5.
| Catalyst | OⅡ/OⅠ content ratio | NH3 desorption | |
|---|---|---|---|
| Weak acid content/(μmol‧g‒1) | Medium acid content/(μmol‧g‒1) | ||
| 3DOM ZSM⁃5 | — | 37.5 | — |
| MnOδ/3DOM ZSM⁃5 | 0.9 | 19.5 | 8.0 |
| FeOδ/3DOM ZSM⁃5 | 0.8 | 19.9 | 6.8 |
| CoOδ/3DOM ZSM⁃5 | 0.8 | 23.0 | 7.8 |
| CeOδ/3DOM ZSM⁃5 | 0.7 | 21.5 | 6.6 |
| PrOδ/3DOM ZSM⁃5 | 0.8 | 20.4 | 4.3 |
| WOδ/3DOM ZSM⁃5 | 0.7 | 32.2 | 9.1 |
Table 2 Ionic ratios and acid content of MOδ/3DOM ZSM-5 catalysts
| Catalyst | OⅡ/OⅠ content ratio | NH3 desorption | |
|---|---|---|---|
| Weak acid content/(μmol‧g‒1) | Medium acid content/(μmol‧g‒1) | ||
| 3DOM ZSM⁃5 | — | 37.5 | — |
| MnOδ/3DOM ZSM⁃5 | 0.9 | 19.5 | 8.0 |
| FeOδ/3DOM ZSM⁃5 | 0.8 | 19.9 | 6.8 |
| CoOδ/3DOM ZSM⁃5 | 0.8 | 23.0 | 7.8 |
| CeOδ/3DOM ZSM⁃5 | 0.7 | 21.5 | 6.6 |
| PrOδ/3DOM ZSM⁃5 | 0.8 | 20.4 | 4.3 |
| WOδ/3DOM ZSM⁃5 | 0.7 | 32.2 | 9.1 |
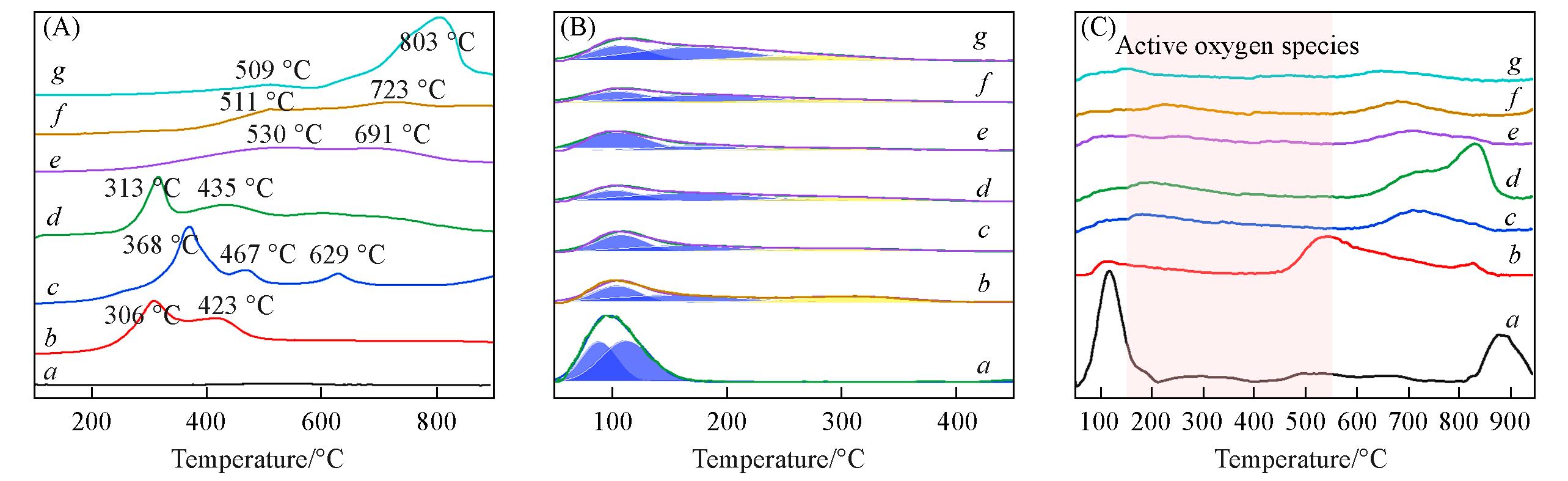
Fig.5 H2⁃TPR (A), NH3⁃TPD(B) and O2⁃TPD(C) curves of MOδ/3DOM ZSM⁃5 catalystsCurves a—g: 3DOM ZSM-5, MnOδ/3DOM ZSM-5, FeOδ/3DOM ZSM-5, CoOδ/3DOM ZSM-5, CeOδ/3DOM ZSM-5, PrOδ/3DOM ZSM-5, WOδ/3DOM ZSM-5.
| Catalyst | Tm/℃ | TNO,>80/℃ | XNO,m(%) |
|---|---|---|---|
| Soot | 641 | — | — |
| 3DOM ZSM⁃5 | 601 | — | 31.4 |
| MnOδ/3DOM ZSM⁃5 | 453 | 184—362 | 96.3 |
| FeOδ/3DOM ZSM⁃5 | 494 | 319—411 | 87.8 |
| CoOδ/3DOM ZSM⁃5 | 462 | — | 66.1 |
| CeOδ/3DOM ZSM⁃5 | 538 | 257—448 | 96.0 |
| PrOδ/3DOM ZSM⁃5 | 573 | — | 30.3 |
| WOδ/3DOM ZSM⁃5 | 563 | — | 30.7 |
| MnOδ/3DOM ZSM⁃5⁃cycle1 | 457 | 180—352 | 95.0 |
| MnOδ/3DOM ZSM⁃5⁃cycle2 | 465 | 187—356 | 96.3 |
| MnOδ/3DOM ZSM⁃5⁃cycle3 | 462 | 185—361 | 96.2 |
| MnOδ/ZSM⁃5 | 470 | 199—380 | 96.0 |
| MnOδ⁃powder | 451 | — | 51.5 |
| MnOδ/3DOM ZSM⁃5⁃100 mg/m3 SO2+10%H2O | 498 | — | 67.3 |
Table 3 Peak temperature of maximum CO2 concentration(Tm), temperature window with over 80% conversion(TNO,>80) and maximum NO conversion(XNO, m) of MOδ/3DOM ZSM-5 catalysts
| Catalyst | Tm/℃ | TNO,>80/℃ | XNO,m(%) |
|---|---|---|---|
| Soot | 641 | — | — |
| 3DOM ZSM⁃5 | 601 | — | 31.4 |
| MnOδ/3DOM ZSM⁃5 | 453 | 184—362 | 96.3 |
| FeOδ/3DOM ZSM⁃5 | 494 | 319—411 | 87.8 |
| CoOδ/3DOM ZSM⁃5 | 462 | — | 66.1 |
| CeOδ/3DOM ZSM⁃5 | 538 | 257—448 | 96.0 |
| PrOδ/3DOM ZSM⁃5 | 573 | — | 30.3 |
| WOδ/3DOM ZSM⁃5 | 563 | — | 30.7 |
| MnOδ/3DOM ZSM⁃5⁃cycle1 | 457 | 180—352 | 95.0 |
| MnOδ/3DOM ZSM⁃5⁃cycle2 | 465 | 187—356 | 96.3 |
| MnOδ/3DOM ZSM⁃5⁃cycle3 | 462 | 185—361 | 96.2 |
| MnOδ/ZSM⁃5 | 470 | 199—380 | 96.0 |
| MnOδ⁃powder | 451 | — | 51.5 |
| MnOδ/3DOM ZSM⁃5⁃100 mg/m3 SO2+10%H2O | 498 | — | 67.3 |
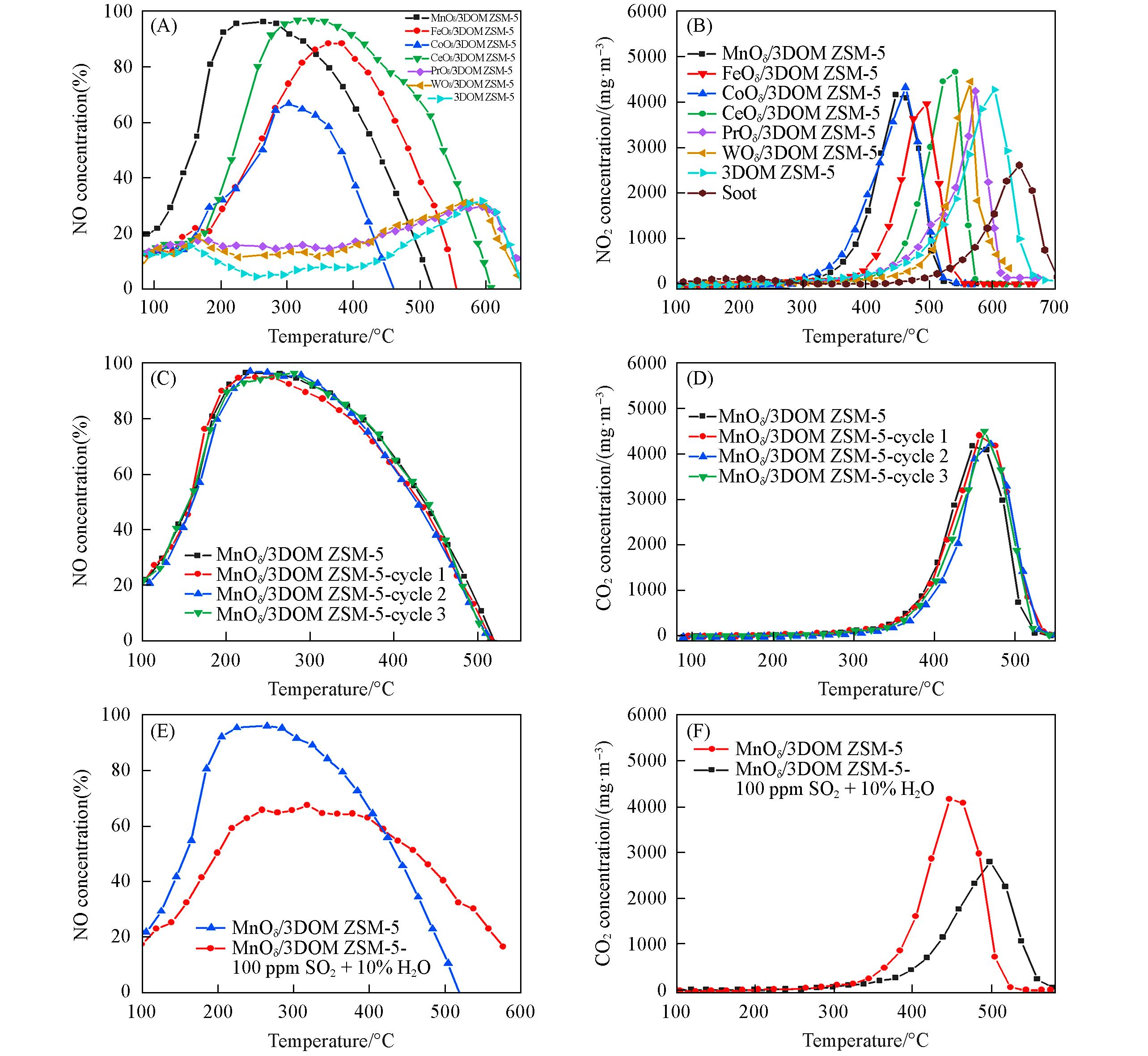
Fig.7 NO conversion curves(A) and CO2 concentration cures(B) for the simultaneous removal of soot and NOx over MOδ/3DOM ZSM⁃5 catalysts, NO conversion curves(C) and CO2 concentration cures(D) of MnOδ/3DOM ZSM⁃5 catalysts with three cycle testing, NO conversion curves(E) and CO2 concentration cures(F) of MnOδ/3DOM ZSM⁃5 catalysts with 100 mg/m3 SO2 and 10%H2O
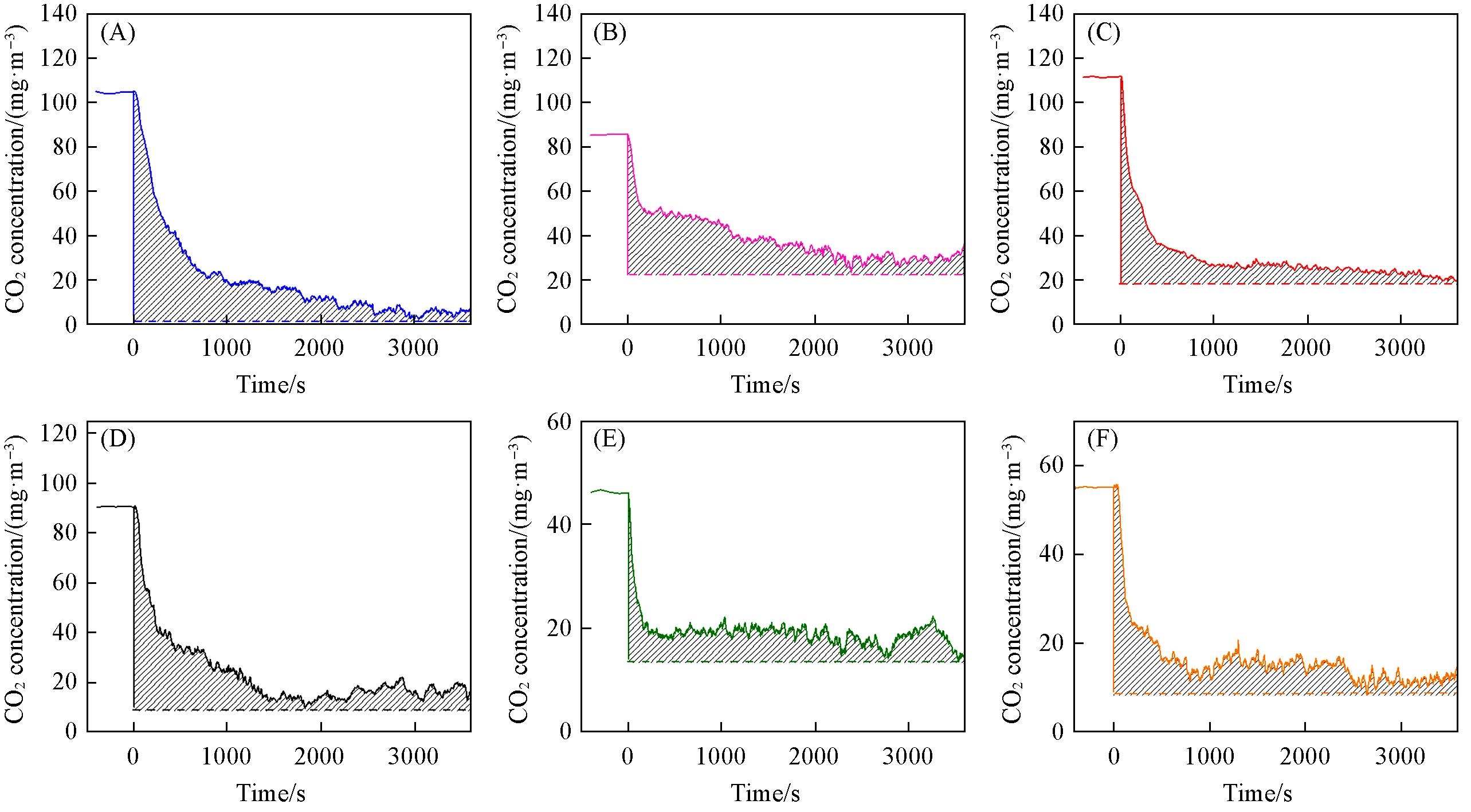
Fig.8 CO2 concentrations at 350 ℃ as a function of time over catalysts before and after O2 removed from the reactant feed(A) MnOδ/3DOM ZSM-5; (B) FeOδ/3DOM ZSM-5; (C) CoOδ/3DOM ZSM-5; (D) CeOδ/3DOM ZSM-5; (E) PrOδ/3DOM ZSM-5; (F) WOδ/3DOM ZSM-5. Reaction conditions: 1000 mg/m3 NO, 1000 mg/m3 NH3, 5% O2, balance N2, flow rate=150 mL/min
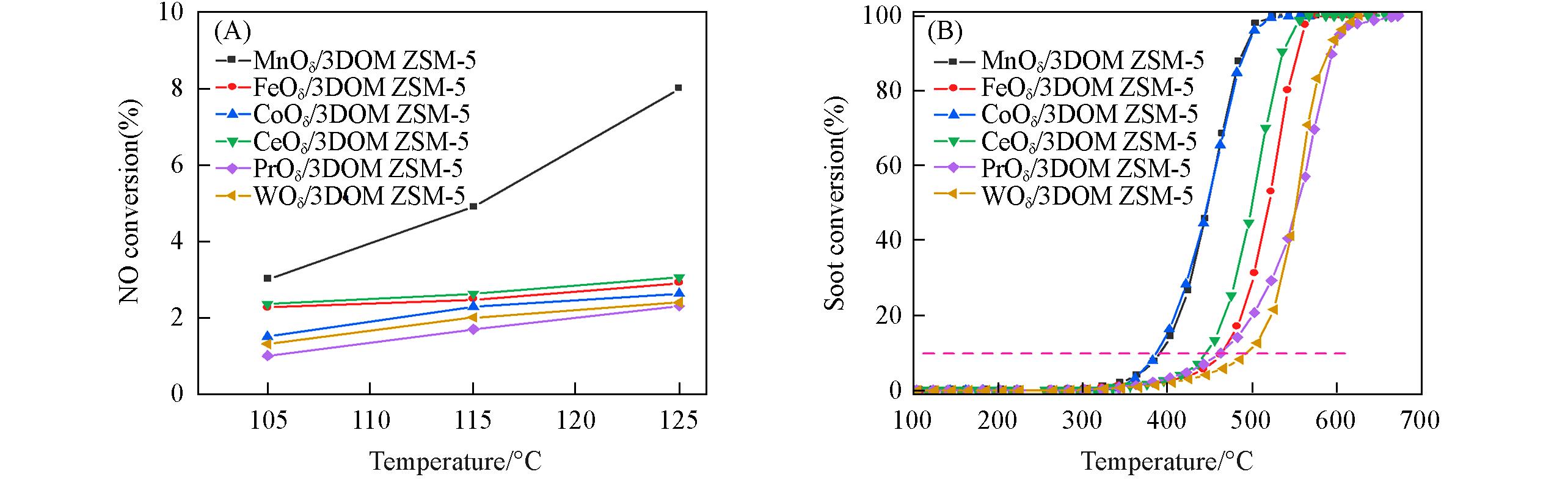
Fig.9 NO conversion(A) and soot conversion(B) of the catalystsReaction conditions: (A) 1000 mg/m3 NO, 1000 mg/m3 NH3, 5% O2 and balance with N2, flow rate=300 mL/min;(B) 1000 mg/m3 NO, 1000 mg/m3 NH3, 5% O2 and balance with N2, flow rate=100 mL/min.
| Catalyst | 10‒5 rNO/ (mol‧g‒1‧s‒1) | 10‒8 rsoot/ (mol‧g‒1‧s‒1) | 10‒5 mO/ (mol‧g‒1) | 103 TOFSCR,115 ℃/s‒1 | 103 TOFSCR,125 ℃/s‒1 | 103 TOFsoot,350 ℃/s‒1 |
|---|---|---|---|---|---|---|
| MnOδ/3DOM ZSM⁃5 | 0.7(105 ℃) | 5.1 | 3.8 | 1.0 | 1.6 | 1.4 |
| 1.1(115 ℃) | ||||||
| 1.9(125 ℃) | ||||||
| FeOδ/3DOM ZSM⁃5 | 0.5(105 ℃) | 4.2 | 3.6 | 0.4 | 0.5 | 1.2 |
| 0.6(115 ℃) | ||||||
| 0.7(125 ℃) | ||||||
| CoOδ/3DOM ZSM⁃5 | 0.3(105 ℃) | 5.3 | 2.9 | 0.4 | 0.5 | 1.3 |
| 0.5(115 ℃) | ||||||
| 0.6(125 ℃) | ||||||
| CeOδ/3DOM ZSM⁃5 | 0.5(105 ℃) | 4.2 | 3.2 | 0.9 | 1.2 | 1.3 |
| 0.6(115 ℃) | ||||||
| 0.7(125 ℃) | ||||||
| PrOδ/3DOM ZSM⁃5 | 0.2(105 ℃) | 2.5 | 2.3 | 0.6 | 0.7 | 1.1 |
| 0.4(115 ℃) | ||||||
| 0.5(125 ℃) | ||||||
| WOδ/3DOM ZSM⁃5 | 0.3(105 ℃) | 2.7 | 2.6 | 0.6 | 0.8 | 1.1 |
| 0.5(115 ℃) | ||||||
| 0.5(125 ℃) |
Table 4 Reaction rate(r) for NH3-SCR/soot combustion and the amount of active oxygen species(mO) for soot combustion and the TOF and Ea values of catalysts
| Catalyst | 10‒5 rNO/ (mol‧g‒1‧s‒1) | 10‒8 rsoot/ (mol‧g‒1‧s‒1) | 10‒5 mO/ (mol‧g‒1) | 103 TOFSCR,115 ℃/s‒1 | 103 TOFSCR,125 ℃/s‒1 | 103 TOFsoot,350 ℃/s‒1 |
|---|---|---|---|---|---|---|
| MnOδ/3DOM ZSM⁃5 | 0.7(105 ℃) | 5.1 | 3.8 | 1.0 | 1.6 | 1.4 |
| 1.1(115 ℃) | ||||||
| 1.9(125 ℃) | ||||||
| FeOδ/3DOM ZSM⁃5 | 0.5(105 ℃) | 4.2 | 3.6 | 0.4 | 0.5 | 1.2 |
| 0.6(115 ℃) | ||||||
| 0.7(125 ℃) | ||||||
| CoOδ/3DOM ZSM⁃5 | 0.3(105 ℃) | 5.3 | 2.9 | 0.4 | 0.5 | 1.3 |
| 0.5(115 ℃) | ||||||
| 0.6(125 ℃) | ||||||
| CeOδ/3DOM ZSM⁃5 | 0.5(105 ℃) | 4.2 | 3.2 | 0.9 | 1.2 | 1.3 |
| 0.6(115 ℃) | ||||||
| 0.7(125 ℃) | ||||||
| PrOδ/3DOM ZSM⁃5 | 0.2(105 ℃) | 2.5 | 2.3 | 0.6 | 0.7 | 1.1 |
| 0.4(115 ℃) | ||||||
| 0.5(125 ℃) | ||||||
| WOδ/3DOM ZSM⁃5 | 0.3(105 ℃) | 2.7 | 2.6 | 0.6 | 0.8 | 1.1 |
| 0.5(115 ℃) | ||||||
| 0.5(125 ℃) |
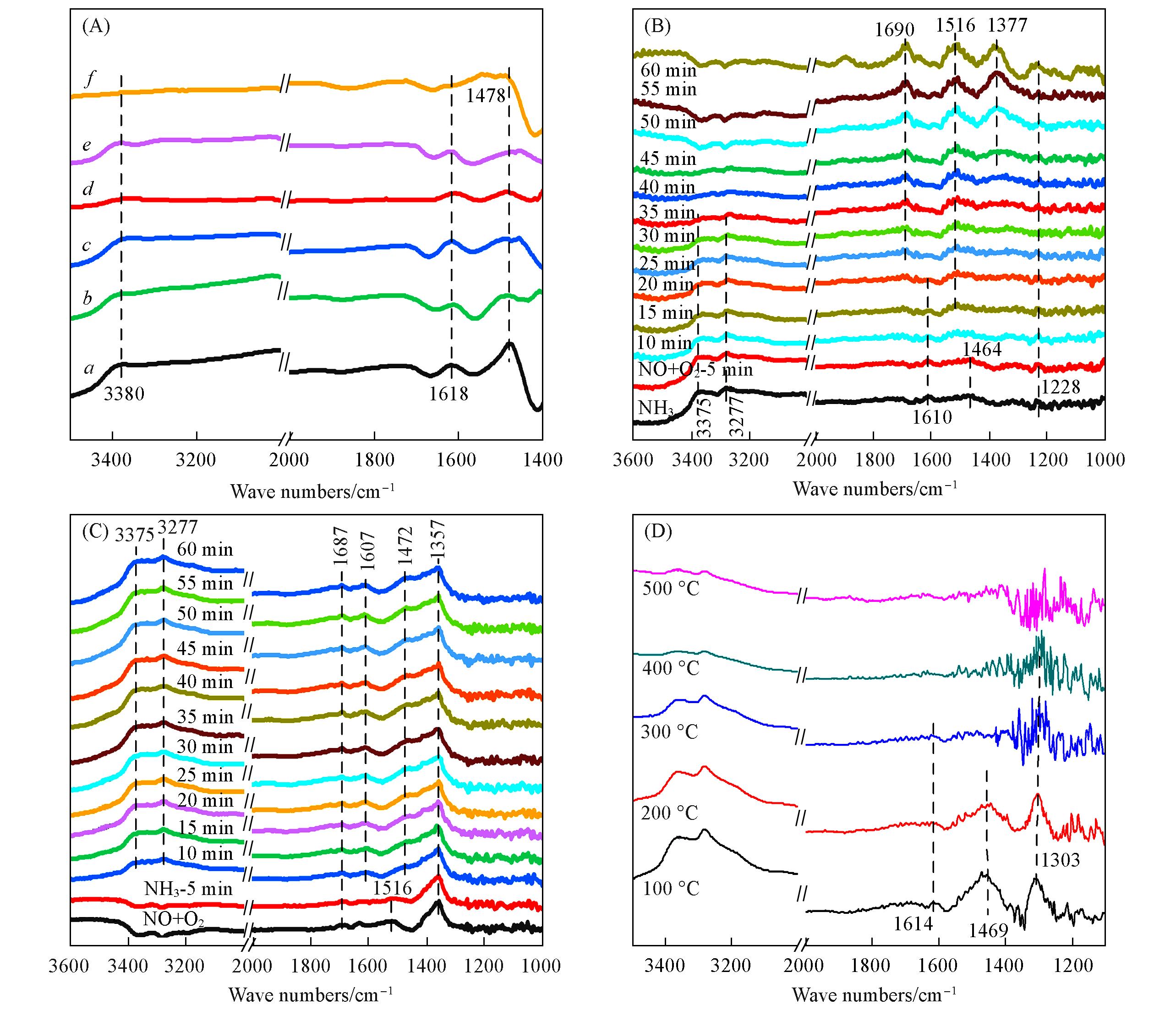
Fig.10 In situ DRIFT spectra of MOδ/3DOM ZSM⁃5 catalyst(A) The reaction pre-adsorbed NH3, a—f: MnOδ/3DOM ZSM-5, FeOδ/3DOM ZSM-5, CoOδ/3DOM ZSM-5, CeOδ/3DOM ZSM-5, PrOδ/3DOM ZSM-5, WOδ/3DOM ZSM-5; (B) the reaction pre-adsorbed NH3 and NO+O2 of the MnOδ/3DOM ZSM-5 catalyst at 150 ℃; (C) the reaction pre-adsorbed NO+O2 and NH3 of the MnOδ/3DOM ZSM-5 catalyst at 150 ℃; (D) the co-adsorption of NH3+NO+O2 over MnOδ/3DOM ZSM-5 catalyst.
| 1 | Frank B., Schuster M. E., Schlögl R., Su D., Angew. Chem., 2013, 52, 2673—2677 |
| 2 | Niessner R., Angew. Chem., 2014, 53, 12366—12379 |
| 3 | Choi B., Lee K. S., Chem. Eng. J., 2014, 240, 476—486 |
| 4 | Wei Y. C., Zhang Y. L., Zhang P., Xiong J., Mei X. L., Yu Q., Zhao Z., Liu J., Environ. Sci. Technol., 2020, 54, 2002—2011 |
| 5 | Meng D., Xu Q., Jiao Y., Guo Y., Guo Y., Wang L., Lu G., Zhan W., Appl. Catal. B, 2018, 221, 652—663 |
| 6 | Mihai O., Stenfeldt M., Olsson L., Catal. Today, 2018, 306, 243—250 |
| 7 | Cheng Y., Song W. Y., Liu J., Zhao Z., Wei Y. C., RSC Adv., 2017, 7, 56509—56518 |
| 8 | Cheng Y., Song W. Y., Liu J., Zheng H., Zhao Z., Xu C. M., Wei Y. C., Hensen E. J. M., ACS Catal., 2017, 7, 3883—3892 |
| 9 | Xiong J., Mei X. L., Liu J., Wei Y. C., Zhao Z., Xie Z. A., Li, J., Appl. Catal. B, 2019, 251, 247—260 |
| 10 | Wei Y. C., Zhang P., Xiong J., Yu Q., Wu Q., Zhao Z., Liu J., Environ. Sci. Technol., 2020, 54, 6947—6956 |
| 11 | Sellers⁃Antón B., Bailón⁃García E., Cardenas⁃Arenas A., Davó⁃Quiñonero A., Lozano⁃Castelló D., Bueno⁃López A., Environ. Sci. Technol., 2020, 54, 2439—2447 |
| 12 | Cheng Y., Liu J., Zhao Z., Song W. Y., Wei Y. C., Chem. Eng. Sci., 2017, 167, 219—228 |
| 13 | Cheng Y., Liu J., Zhao Z., Song W. Y., Wei Y. C., J. Hazard. Mater., 2018, 342, 317—325 |
| 14 | Li R. J., Li D., Wang L. Y., Zhou Q., Li J. M., Yu X. H., Liu J., Zhao Z., Chem. Phys. Impact, 2023, 6, 100135 |
| 15 | Zhong C. M., Ren Y., Yin C. Y., Wang R. D., Hou J., Wang L. Y., Zhao Z., Mozgawa B., Pietrzyk P., Sojka Z., Song Y. Y., ACS Catal., 2023, 13, 10927—10944 |
| 16 | Guo X. N., Zhang R. D., Di Z. Y., Kang B., Shen H. X., Wei Y., Jia J. B., Zheng L. R., Appl. Catal. B, 2024, 343, 123519 |
| 17 | Weissenberger T., Machoke A., Bauer J., Dotzel R., Casci J., Hartmann M., Schwieger W., ChemCatChem, 2020, 12, 2461—2468 |
| 18 | Machoke A., Beltrán A., Inayat A., Winter B., Weissenberger T., Kruse N., Güttel R., Spiecker E., Schwieger W., Adv. Mater., 2014, 27, 1066—1070 |
| 19 | Wang L. Y., Yu D., Zhao Z., Yu X. H., Li D., Zhou S. R., Zhong C. M., Hou J., Yin C. Y., Fan X. Q., Ind. Eng. Chem. Res., 2023, 62, 21950—21966 |
| 20 | Wang L. Y., Ren Y., Yu X. H., Peng C., Yu D., Zhong C. M., Hou J., Yin C. Y., Fan X. Q., Zhao Z., Liu J., Wei Y. C., J. Catal., 2023, 417, 226—247 |
| 21 | Xu J. F., Liu, J., Zhao Z., Xu C. M., Zheng J. X., Duan A. J., Jiang G. Y., J. Catal., 2011, 282, 1—12 |
| 22 | Zhang Z., Han D., Wei S., Zhang Y., J. Catal., 2010, 276, 16—23 |
| 23 | Li Y. L., Yang S. U., Peng H. G., Liu W. M., Mi Y. Y., Wang Z., Tang C. J., Wu D. S., An T. C., J. Catal., 2021, 395, 195—209 |
| 24 | Wang L. Y., Chen M. Z., Yu X. H., Zhao Z., Fan X. Q., Wei Y. C., Liu J., Catal. Today, 2021, 364, 21—34 |
| 25 | Zhao T., Zhao B. B., Niu Y. C., Liang Y., Liu L., Dong J. X., Tang M. X., Li X. K., J. Fuel Chem. Technol., 2021, 49, 1181—1189 |
| 26 | Muraza O., Bakare I. A., Tago T., Konno H., Taniguchi T., Al-amer A. M., Yamani Z. H., Nakasaka Y., Masuda T., Fuel, 2014, 135, 105—111 |
| 27 | Bleken F. L., Barbera K., Bonino F., Lsbye U., Lillerud K. P., Bordiga S., Beato P., Janssens T. V. W., Svelle S., J. Catal., 2013, 307, 62—73 |
| 28 | Liu S., Wu X. D., Weng D., Li M., Ran R., ACS Catal., 2015, 5, 909—919 |
| 29 | Chen J. P., SI X. L., Yu J. S., Zhang X. H., Appl. Surf. Sci., 2015, 330, 191—199 |
| 30 | Liu F. D, Shan W. P., Lian Z. H., Liu J. J., He H., Appl. Catal. B, 2018, 230, 165—176 |
| 31 | Yu X. H., Zhao Z., Wei Y. C., Liu J., Li J. M., Duan A. J., Jiang G. Y., RSC Adv., 2015, 5, 49780—49790 |
| 32 | Sullivan J. A., Doherty J. A., Appl. Catal. B, 2005, 3, 185—194 |
| 33 | Xue H. Y., Guo X. M., Meng T., Guo Q. S., Mao D. S., Wang S., ACS Catal., 2021, 11, 7702—7718 |
| 34 | Cao C. M., Yang H., Xiao J. Y., Yang X. C., Ren B. Z., Xu L., Liu G. J., Li X. G., Fuel, 2021, 305, 121446—121456 |
| 35 | Wei Y. C., Zhao Z., Li T., Liu J., Duan A. J., Jiang G. Y., Appl. Catal. B, 2014, 146, 57—70 |
| 36 | Xie S. Z., Li L. L., Jin L. J., Wu Y. H., Liu H., Qin Q. J., Wei X. L., Liu J. X., Dong L. H., Li B., Appl. Surf. Sci., 2020, 15, 146014—146026 |
| 37 | Jiang L. J., Liang Y., Liu W. Z., Wu H. L., Aldahri T., Carrero D. S., Liu Q. C., J. Environ. Chem. Eng., 2021, 9, 106360—106373 |
| 38 | Wang F. M., Shen B. X., Zhu S. W., Wang Z., Fuel, 2019, 249, 54—60 |
| 39 | Tan J. B., Wei Y. C., Sun Y. Q., Liu J., Zhao Z., Song W. Y., Li J. M., Zhang X., J. Ind. Eng. Chem., 2018, 63, 84—94 |
| 40 | Chen Z., Liu Q., Guo L., Zhang S., Pang L., Guo Y. B., Li T., Appl. Catal. B, 2021, 286, 119816—119828 |
| 41 | Chen Y. J., Shen G. R., Lang Y., Chen R., Jia L. W., Yue J., Shen M. Q., Du C., Shan B., J. Catal., 2020, 384, 96—105 |
| 42 | Liu J., Li X. Y., Zhao Q. D., Ke J., Xiao H. N., Lv X. J., Liu S. M., Tadé M., Wang S. B., Appl. Catal. B, 2017, 200, 297—308 |
| 43 | Wang F., Xie Z. B., Liang Z. S., Fang B. Z., Piao Y. A., Hao M., Wang Z. S., Environ. Sci. Technol., 2019, 53, 6989—6996 |
| 44 | Wang B., Wang M. X., Han L. N., Hou Y. Q., Bao W. R., Zhang C. M., Feng G., Chang L. P., Huang Z. G., Wang J. C., ACS Catal., 2020, 10, 9034—9045 |
| 45 | Liu B., Liu J., Xin L., Zhang T., Xu Y. B., Jiang F., Liu X. H., ACS Catal., 2021, 11, 7613—7636 |
| 46 | Liu X. S., Jiang P., Chen Y., Wang Y. G., Ding Q. L., Sui Z. M., Chen H. F., Shen Z. Y., Wu X. D., Chem. Eng. J., 2021, 421, 127833—127845 |
| 47 | Meng D. M., Zhan W. C., Guo Y., Guo Y. L., Wang L., Lu G. Z., ACS Catal., 2015, 5, 5973—5983 |
| 48 | Chen T., Guan B., Lin H., Zhu L., Chin. J. Catal., 2014, 35, 294—301 |
| 49 | Liu Z. M., Liu Y. X., Li Y., Su H., Ma L. L., Chem. Eng. J., 2016, 283, 1044—1050 |
| 50 | Wang L. Y., Ren Y., Yu X. H., Yu D., Peng C., Zhou Q., Hou J., Zhong C. M., Yin C. Y., Fan X. Q., Zhao Z., Cheng K., Chen Y. S., Sojka Z., Kotarba A., Wei Y. C., Liu J., Catal. Sci. Technol., 2022, 12, 1950—1967 |
| 51 | Gao C., Shi J. W., Fan Z. Y., Yu Y. K., Chen J. S., Li Z. H., Niu C. M., Fuel Process. Technol., 2017, 167, 322—333 |
| 52 | Yu D., Peng C., Yu X. H., Wang L. Y., Li K. X., Zhao Z., Li Z. G., Fuel, 2022, 307, 121803—121817 |
| [1] | 孔雪, 张海平, 夏文生, 张庆红, 万惠霖. 石墨烯负载单原子Mo上合成气催化转化机理: 碳组分和Mo-C作用的影响[J]. 高等学校化学学报, 2024, 45(7): 20240038. |
| [2] | 曹华文, 唐秋凡, 屈蓓, 霍欢, 郑启龙, 曹意林, 李吉祯. 硝酸酯键断裂触发的含能增塑剂DNTN多阶热分解机理[J]. 高等学校化学学报, 2024, 45(2): 20230398. |
| [3] | 徐佳宁, 白文静, 楼雨寒, 于海鹏, 窦烁. 电催化氧化木质素解聚: 温和高效的生物质增值策略[J]. 高等学校化学学报, 2023, 44(5): 20220749. |
| [4] | 高凤雨, 陈都, 罗宁, 姚小龙, 段二红, 易红宏, 赵顺征, 唐晓龙. MnO x -CeO2催化剂的氯苯氧化性能及反应机理[J]. 高等学校化学学报, 2023, 44(4): 20220690. |
| [5] | 夏文文, 于洪晶, 王时野, 姚丽, 李象远. 用于燃烧反应机理构建的极小反应网络方法—芳香烃燃烧[J]. 高等学校化学学报, 2023, 44(4): 20220616. |
| [6] | 廖爱雪, 李宜蔚, 毛业兵, 李象远. 基于极小反应网络方法构建燃烧反应机理: JP-10燃料燃烧[J]. 高等学校化学学报, 2023, 44(12): 20230322. |
| [7] | 周子豪, 王思皓, 黄玳川, 刘波, 甯红波. 正丙苯高温氧化机理的分子动力学模拟研究[J]. 高等学校化学学报, 2023, 44(11): 20230276. |
| [8] | 周紫璇, 杨海艳, 孙予罕, 高鹏. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220235. |
| [9] | 杨丹, 刘旭, 戴翼虎, 祝艳, 杨艳辉. 金团簇电催化二氧化碳还原反应的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220198. |
| [10] | 任娜娜, 薛洁, 王治钒, 姚晓霞, 王繁. 热力学数据对1, 3-丁二烯燃烧特性的影响[J]. 高等学校化学学报, 2022, 43(6): 20220151. |
| [11] | 孙翠红, 吕立强, 刘迎, 王妍, 杨静, 张绍文. 硝酸异丙酯与Cl原子、 OH和NO3自由基反应的机理及动力学[J]. 高等学校化学学报, 2022, 43(2): 20210591. |
| [12] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [13] | 孟繁伟, 高琦, 叶青, 李晨曦. Cu-SAPO-18催化剂氨选择性催化还原NOx钾中毒机理的研究[J]. 高等学校化学学报, 2021, 42(9): 2832. |
| [14] | 杨一莹, 朱荣秀, 张冬菊, 刘成卜. 金催化炔基苯并二𫫇英环化合成8-羟基异香豆素的理论研究[J]. 高等学校化学学报, 2021, 42(7): 2299. |
| [15] | 李心怡, 刘永军. 人工设计逆醛缩酶RA95.5-8F催化β-羟基酮C—C裂解的理论研究[J]. 高等学校化学学报, 2021, 42(7): 2306. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||