

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (7): 20220198.doi: 10.7503/cjcu20220198
收稿日期:2022-03-30
出版日期:2022-07-10
发布日期:2022-05-04
通讯作者:
祝艳,杨艳辉
E-mail:zhuyan@nju.edu.cn;yhyang@njtech.edu.cn
基金资助:
YANG Dan1, LIU Xu2, DAI Yihu1, ZHU Yan2( ), YANG Yanhui1(
), YANG Yanhui1( )
)
Received:2022-03-30
Online:2022-07-10
Published:2022-05-04
Contact:
ZHU Yan,YANG Yanhui
E-mail:zhuyan@nju.edu.cn;yhyang@njtech.edu.cn
Supported by:摘要:
二氧化碳电还原反应(CO2RR)在改善能源利用方式、 实现可持续碳循环以及生产高附加值液体燃料和化学品等方面具有广阔的应用前景, 近年来受到广泛关注. 有机配体保护的金团簇具有确定的晶体结构, 其不同的尺寸、 配体及组成可以有效调控氧化还原电位, 作为一种独特的模型催化剂, 为探索原子水平的CO2RR反应机理提供了新机遇. 本文综合评述了纯金团簇和异金属原子掺杂的金团簇催化CO2RR的研究进展, 包括金团簇的电荷、 尺寸、 配体以及掺杂对CO2RR性能的影响, 重点讨论了CO2RR的反应机理, 总结了金团簇在CO2RR中所面临的挑战, 并展望了金团簇在CO2RR中未来的研究方向和发展前景.
中图分类号:
TrendMD:
杨丹, 刘旭, 戴翼虎, 祝艳, 杨艳辉. 金团簇电催化二氧化碳还原反应的研究进展. 高等学校化学学报, 2022, 43(7): 20220198.
YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters. Chem. J. Chinese Universities, 2022, 43(7): 20220198.
| Product | Reaction | Potential/V(vs. RHE*) |
|---|---|---|
| Hydrogen | 0 | |
| -1.90 | ||
| Carbon monoxide | -0.10 | |
| Formic acid | -0.12 | |
| Formaldehyde | -0.07 | |
| Methanol | 0.03 | |
| Methane | 0.17 | |
| Ethylene | 0.08 | |
| Ethane | 0.14 | |
| Ethanol | 0.09 | |
| Acetic acid | 0.06 | |
| Propanol | 0.10 |
Table 1 Major products, reactions and potentials of CO2RR
| Product | Reaction | Potential/V(vs. RHE*) |
|---|---|---|
| Hydrogen | 0 | |
| -1.90 | ||
| Carbon monoxide | -0.10 | |
| Formic acid | -0.12 | |
| Formaldehyde | -0.07 | |
| Methanol | 0.03 | |
| Methane | 0.17 | |
| Ethylene | 0.08 | |
| Ethane | 0.14 | |
| Ethanol | 0.09 | |
| Acetic acid | 0.06 | |
| Propanol | 0.10 |
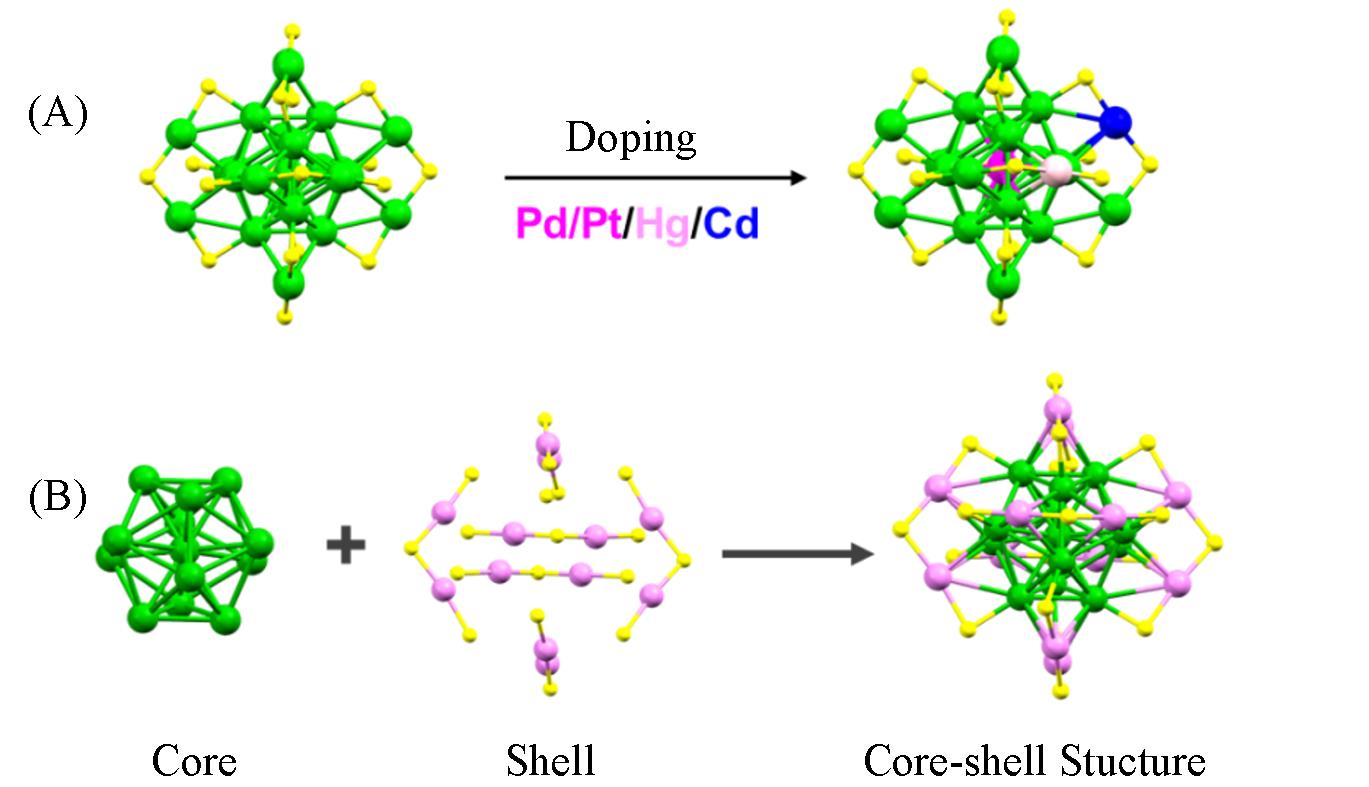
Fig.1 Crystal structures of Au25(PET)18(A) and M1Au24(PET)18(M=Pd, Pt, Hg, Cd) nanoclusters(B)Green/pink: Au, magenta: Pd/Pt, light pink: Hg, blue: Cd, yellow: S.
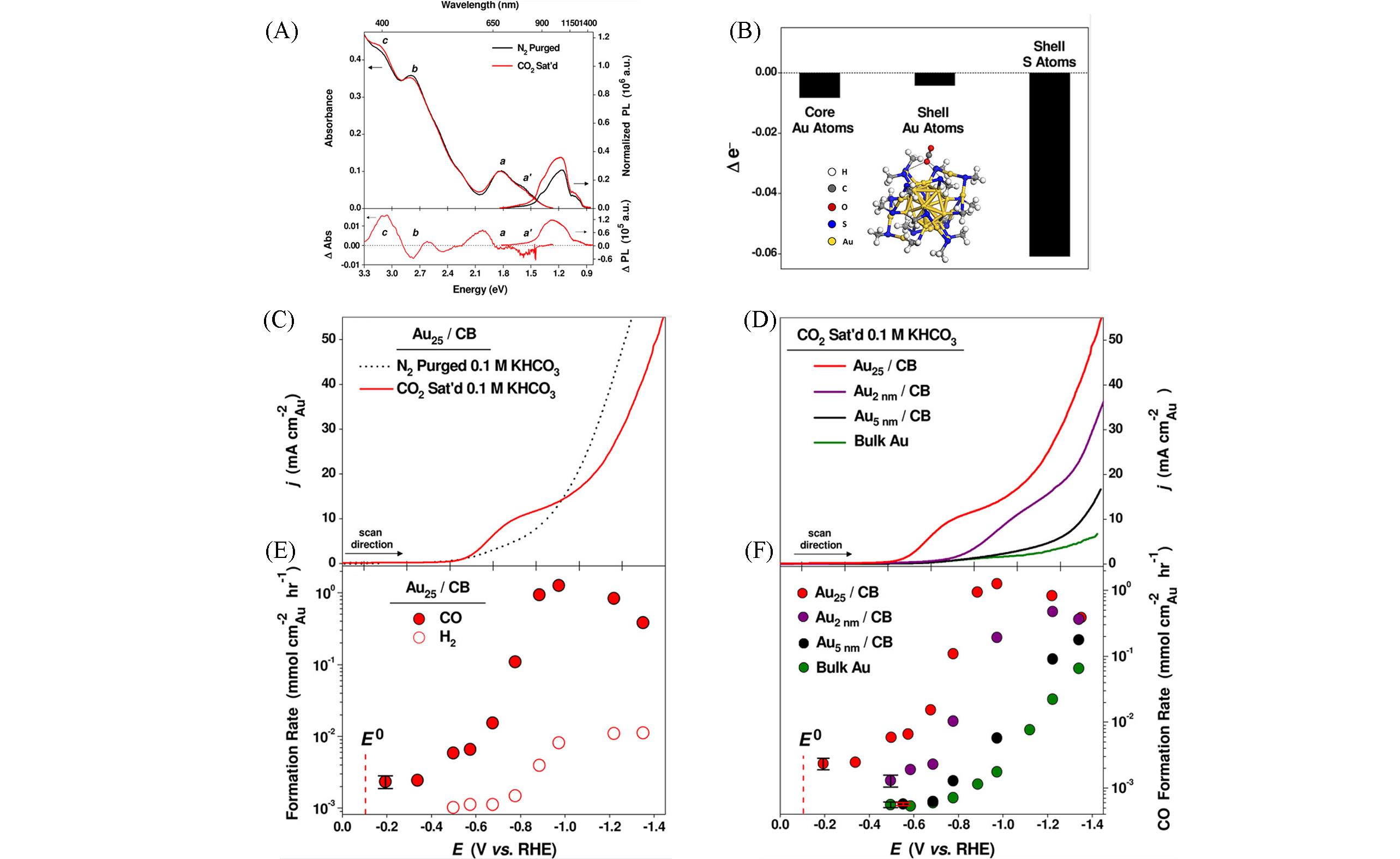
Fig.2 Catalytic performance of metal nanoclusters in CO2RR[56](A) UV-Vis absorption, PL spectra and difference spectra of Au25 in N2 purged and CO2 saturated dimethylformamide; (B) bader charge analysis showing the change in Au25 valence electrons upon CO2 adsorption; negative values indicate electron loss, and the picture of inset is DFT simulation of a stable CO2 adsorption model on the Au25; Linear sweep voltammetric(LSV) curves of carbon black(CB) supported Au25 catalysts(C) and various Au-based catalysts in CO2 saturated 0.1 mol/L KHCO3(D); (E) potential-dependent H2 and CO formation rates for Au25/CB; (F) Potential-dependent CO formation rates for the various Au-based catalysts.Copyright 2012, American Chemical Society.
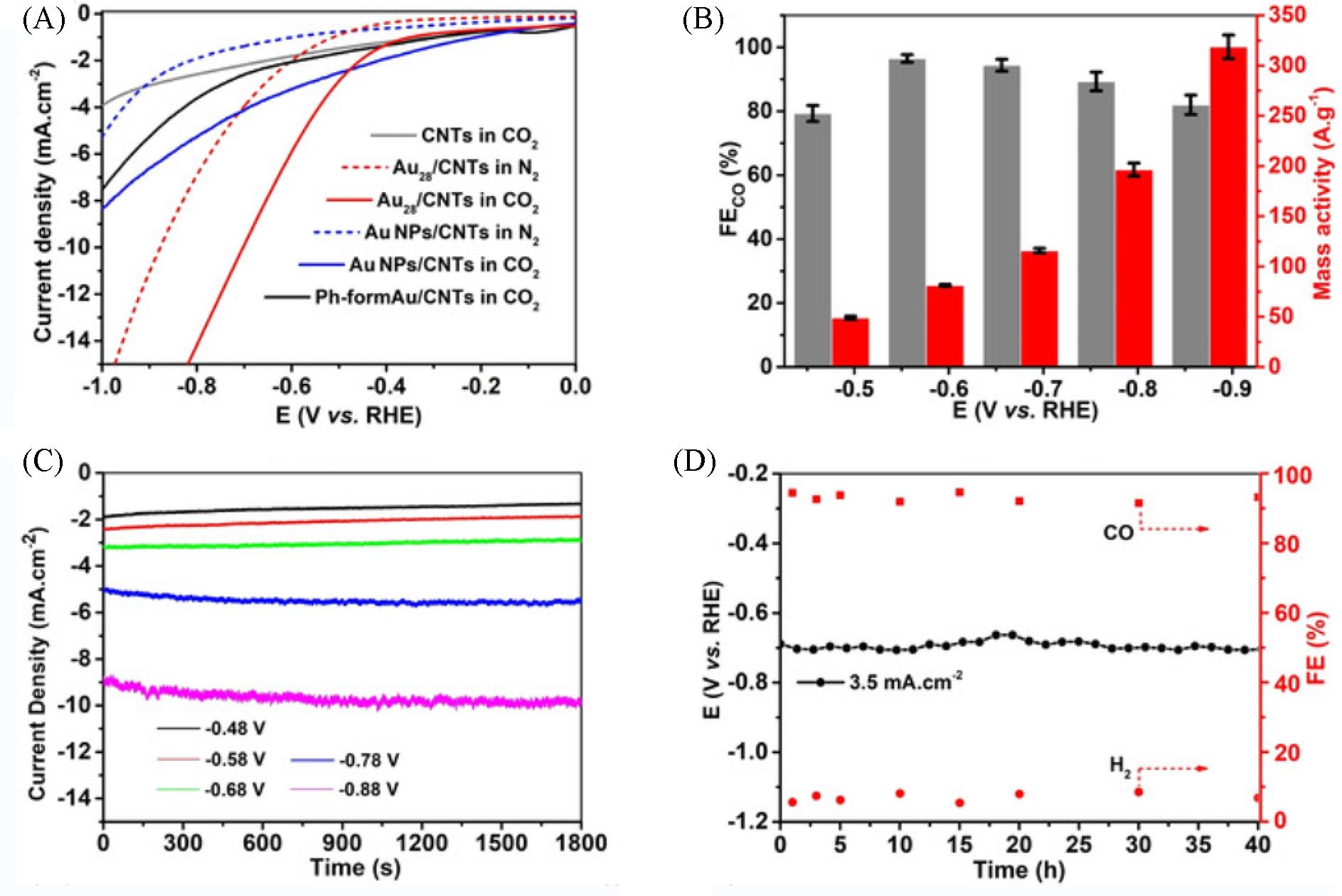
Fig.5 Catalytic performance of metal nanoclusters in CO2RR[62](A) LSV of supported nanoclusters in quiescent N2 purged and CO2 saturated 0.5 mol/L KHCO3; (B) FE and mass activity of CO in CO2RR on Au28/CNTs in 0.5 mol/L KHCO3; (C) I?t test of Au28/CNTs at different fixed voltage; (D) long-term operation of Au28/CNTs.Copyright 2021, John Wiley & Sons, Inc.
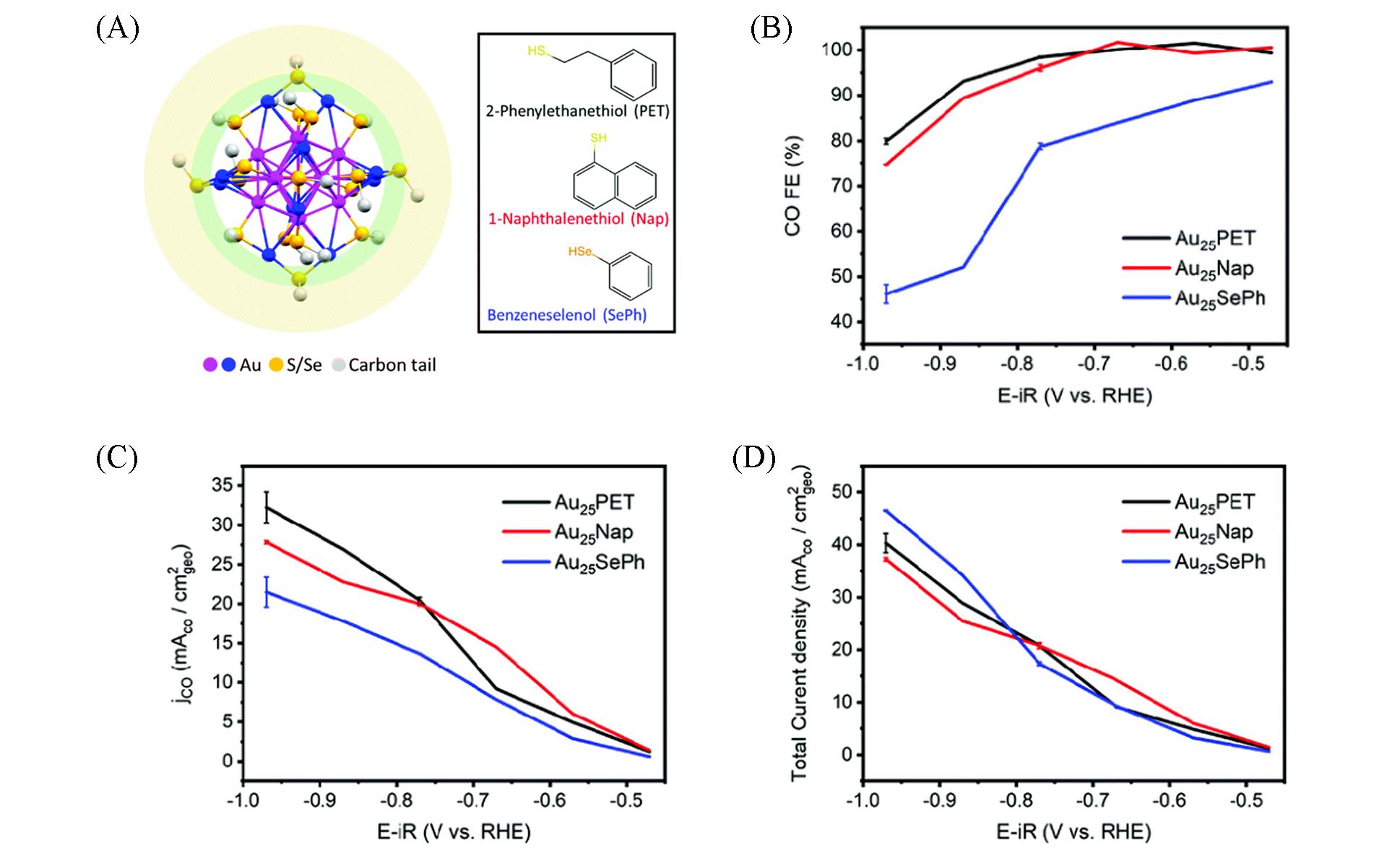
Fig.6 Catalytic performance of metal nanoclusters in CO2RR[63](A) Crystal structure of Au25 nanoclusters with different ligands. Electrocatalytic CO2 reduction performance of various Au25 nanoclusters:(B) FECO; (C) CO mass activity; (D) CO partial current density; (E) total current density.Copyright 2021, the Royal Society of Chemistry.
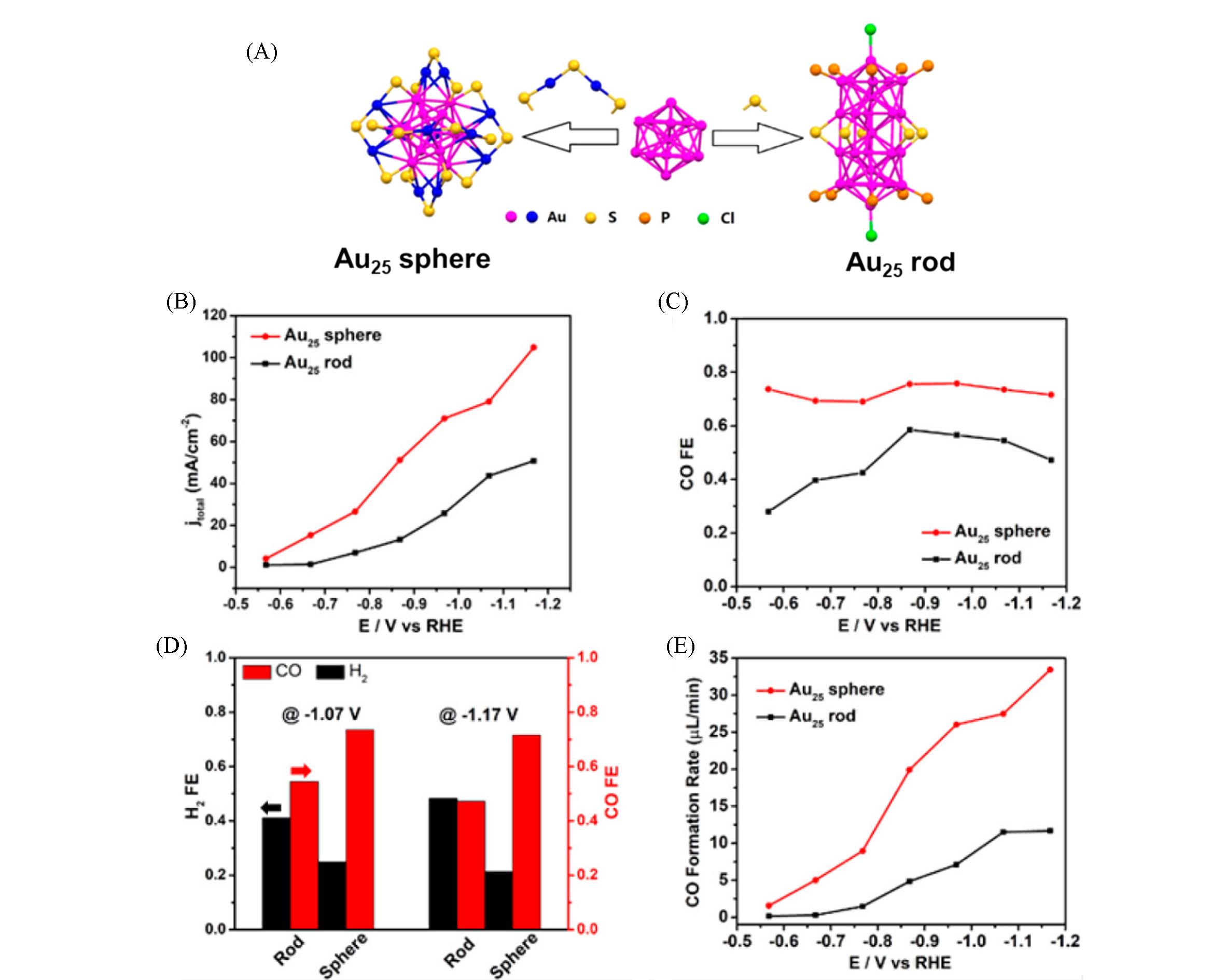
Fig.7 Structures and catalytic CO2RR performances of Au25 nanosphere and nanorod nanoclusters[64](A) Atom structures; (B) total current density of CO2 reduction; (C) FEs for CO production; (D) FEs for CO and H2 production at -1.07 and -1.17 V; (E) CO formation rates.Copyright 2018, American Chemical Society.
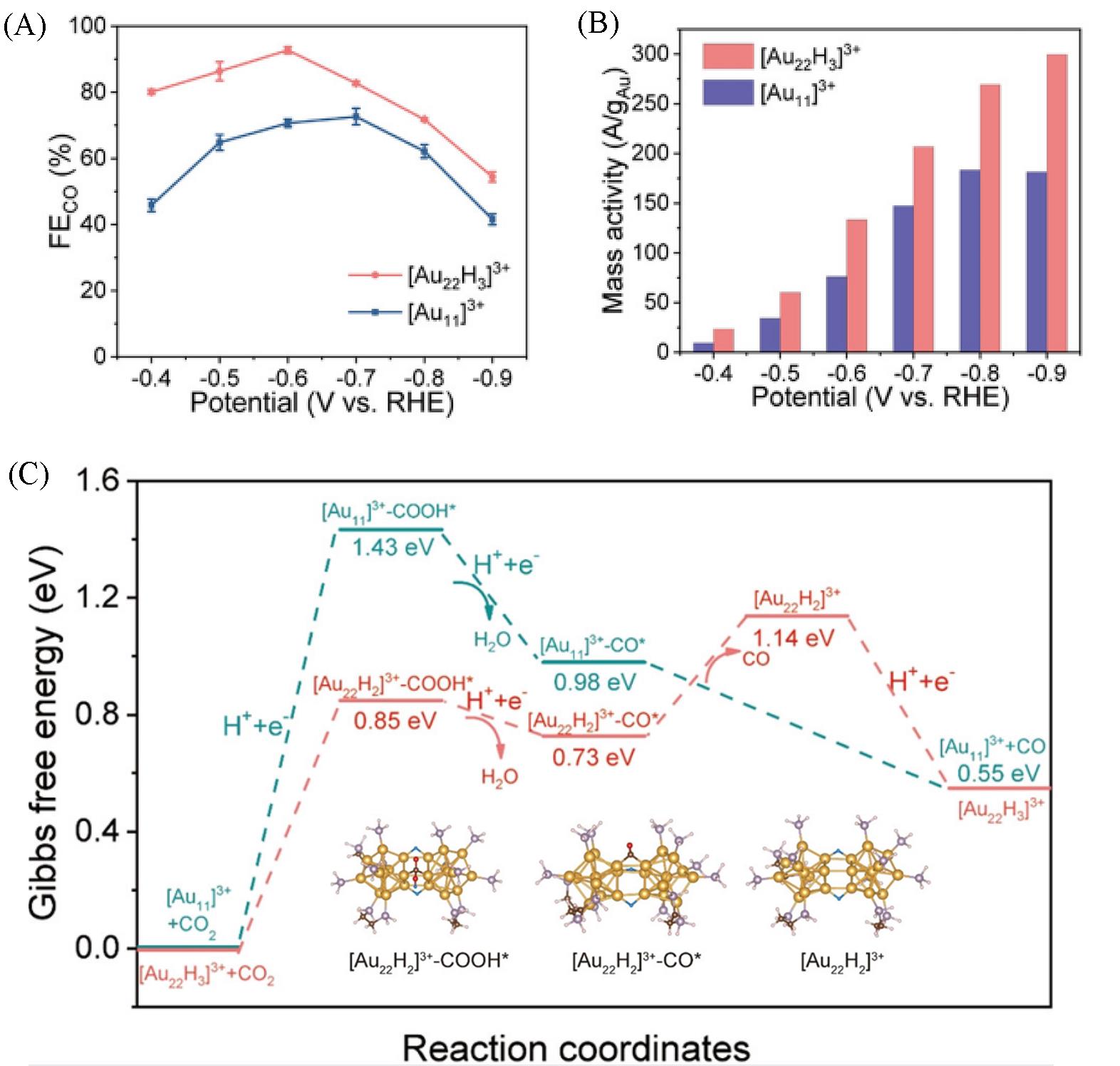
Fig.8 Selectivity and activity of [Au22H3]3+ and [Au11]3+ in CO2RR to CO[66]FECO(A) and potential?dependent mass activity(B) of the two nanoclusters; (C) DFT structures and energetics of CO2RR on the[Au11]3+(cyan lines) and [Au22H3]3+(red lines) nanoclusters at 0 V(vs. RHE).Copyright 2022, American Chemical Society.
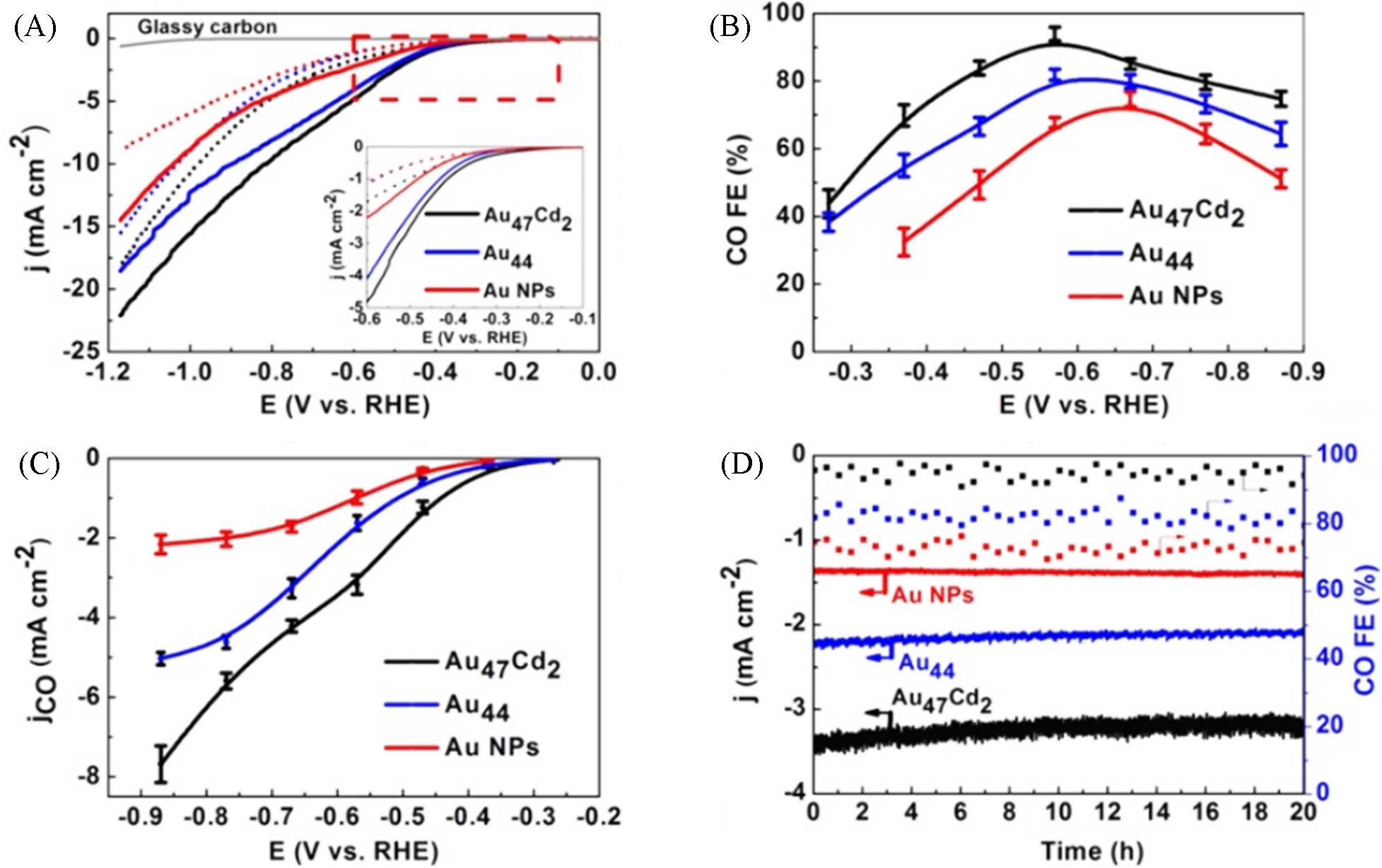
Fig.9 Selectivity and activity of metal nanoclusters in CO2RR to CO[42](A) LSV curves of Au47Cd2(TBBT)31, Au44(TBBT)28 and the ca. 1.5 nm Au NPs in an Ar-saturated(dotted line) and a CO2-saturated(full line) 0.5 mol/L KHCO3 solution; (B) FEs of CO for the catalysts performed with different applied potentials; (C) The corresponding CO partial current density; (D) Stability test conducted at -0.57 V for gold nanoclusters and at -0.67 V for the Au NPs.Copyright 2019, John Wiley & Sons, Inc.
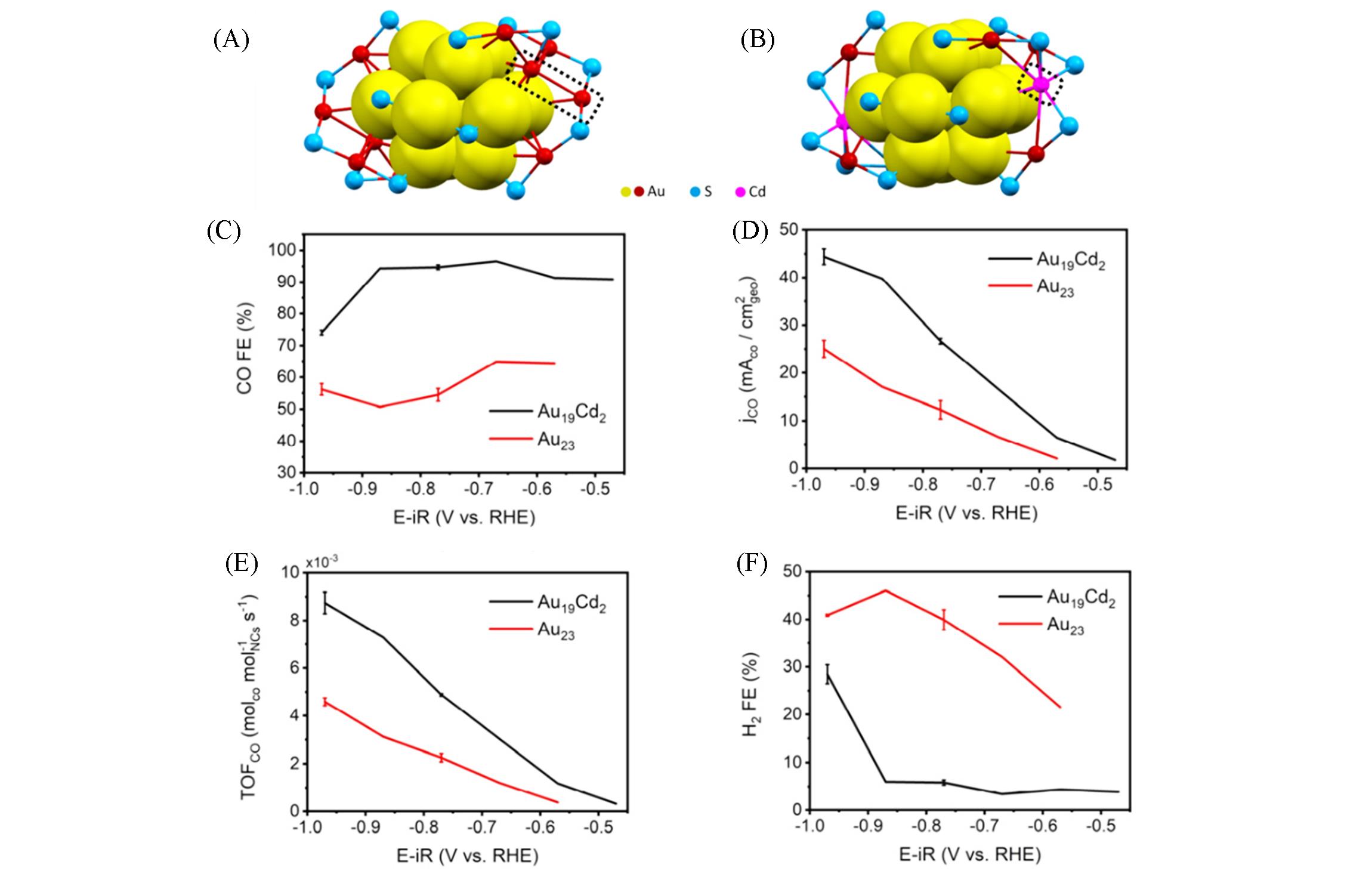
Fig.10 Catalytic performance of metal nanoclusters in CO2RR[41]Crystal structures of Au23(S?c?C6H11)16(A) and Au19Cd2(S?c?C6H11)16(B)(C); FECO for the Au23(S?c?C6H11)16 and Au19Cd2 (S?c?C6H11)16 with different potentials in a CO2?saturated 0.5 mol/L KHCO3 solution; (D) the corresponding CO partial current density; (E) TOF of CO production for CO2 reduction at different applied potentials; (F) FEH2 for the catalysts performed with different potentials.Copyright 2020, John Wiley & Sons, Inc.
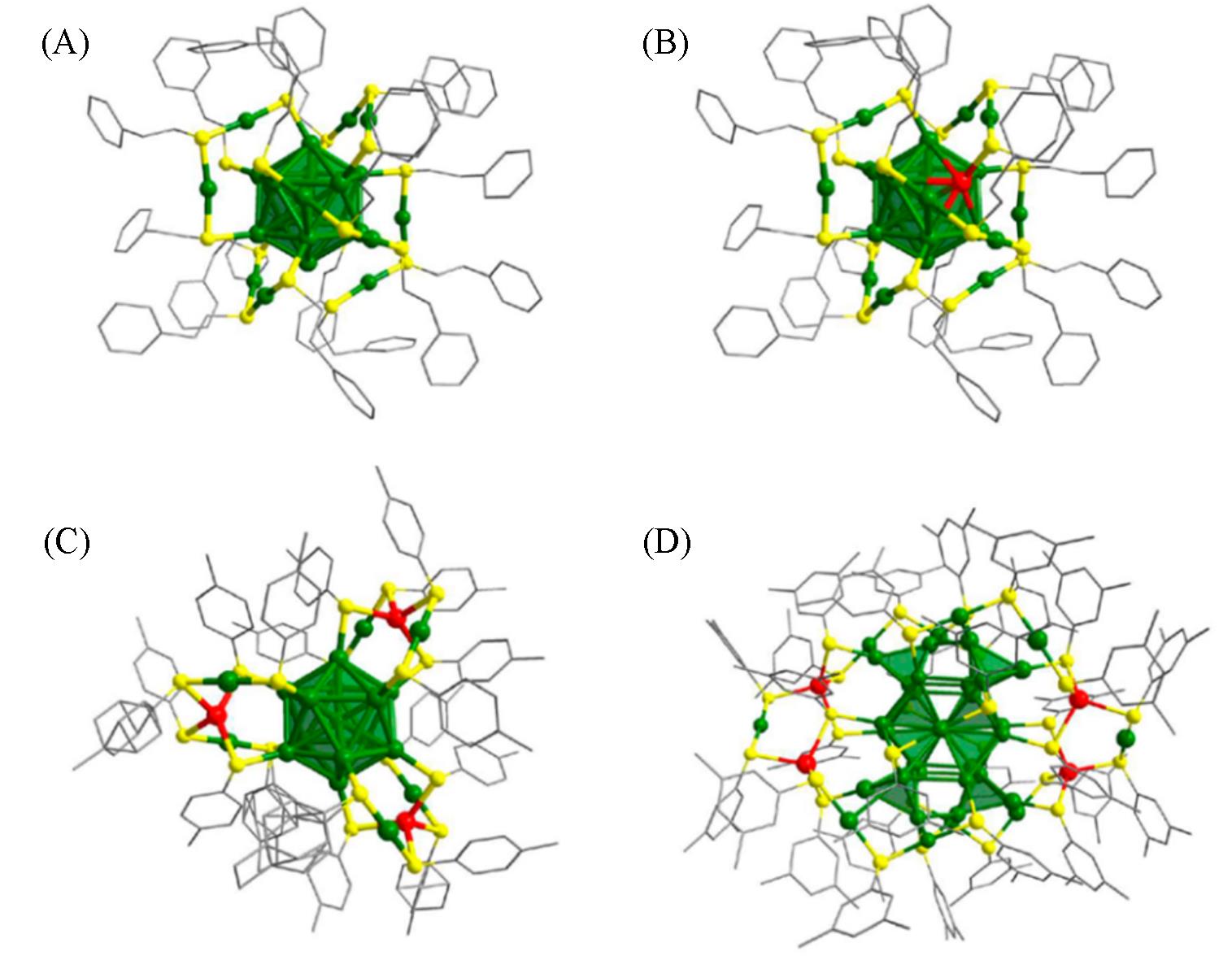
Fig.11 Crystal structures of Au25(PET)18(A), Au24Cd1(PET)18(B), Au19Cd3(S?tol)18(C) and Au38Cd4(d?MBT)30 nanoclusters(D)[68]Green: Au, red: Cd, yellow: S, gray: C.Copyright 2021, American Chemical Society.
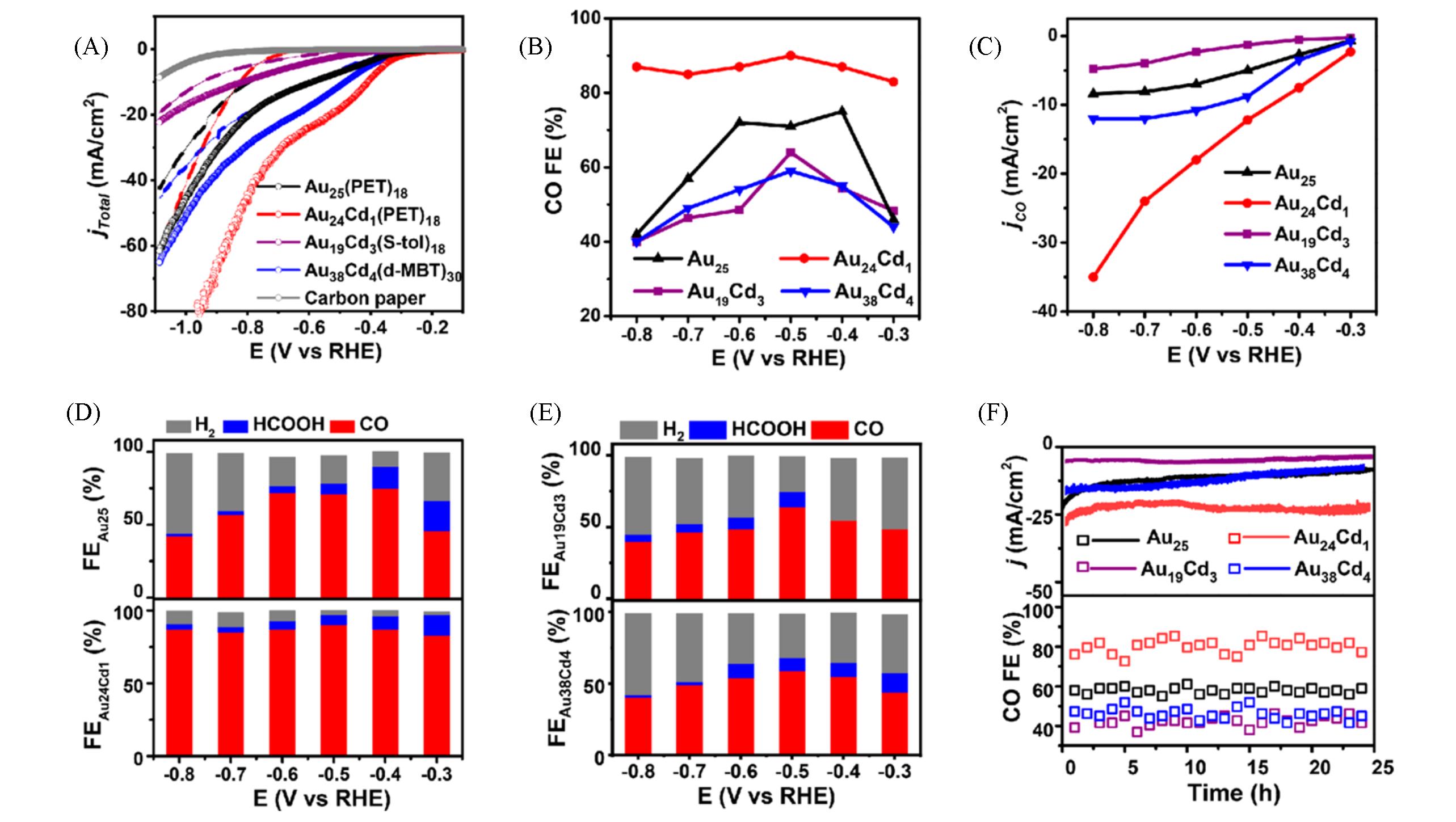
Fig.12 Catalytic performance of metal nanoclusters in CO2RR[68](A) LSV curves of Au25(PET)18, Au24Cd1(PET)18, Au19Cd3(S-tol)18, and Au38Cd4(d-MBT)30 nanoclusters in an Ar-saturated(dotted line) and a CO2-saturated(full line) 1 mol/L KHCO3 solution; (B) FECO for the catalysts examined with different applied potentials; (C) the corresponding CO partial current density; FEs for various CO2RR products obtained on Au25(PET)18 and Au24Cd1(PET)18(D), Au19Cd3(S-tol)18 and Au38Cd4(d-MBT)30(E); (F) the stability test conducted at -0.7 V for gold nanocluster.Copyright 2021, American Chemical Society.
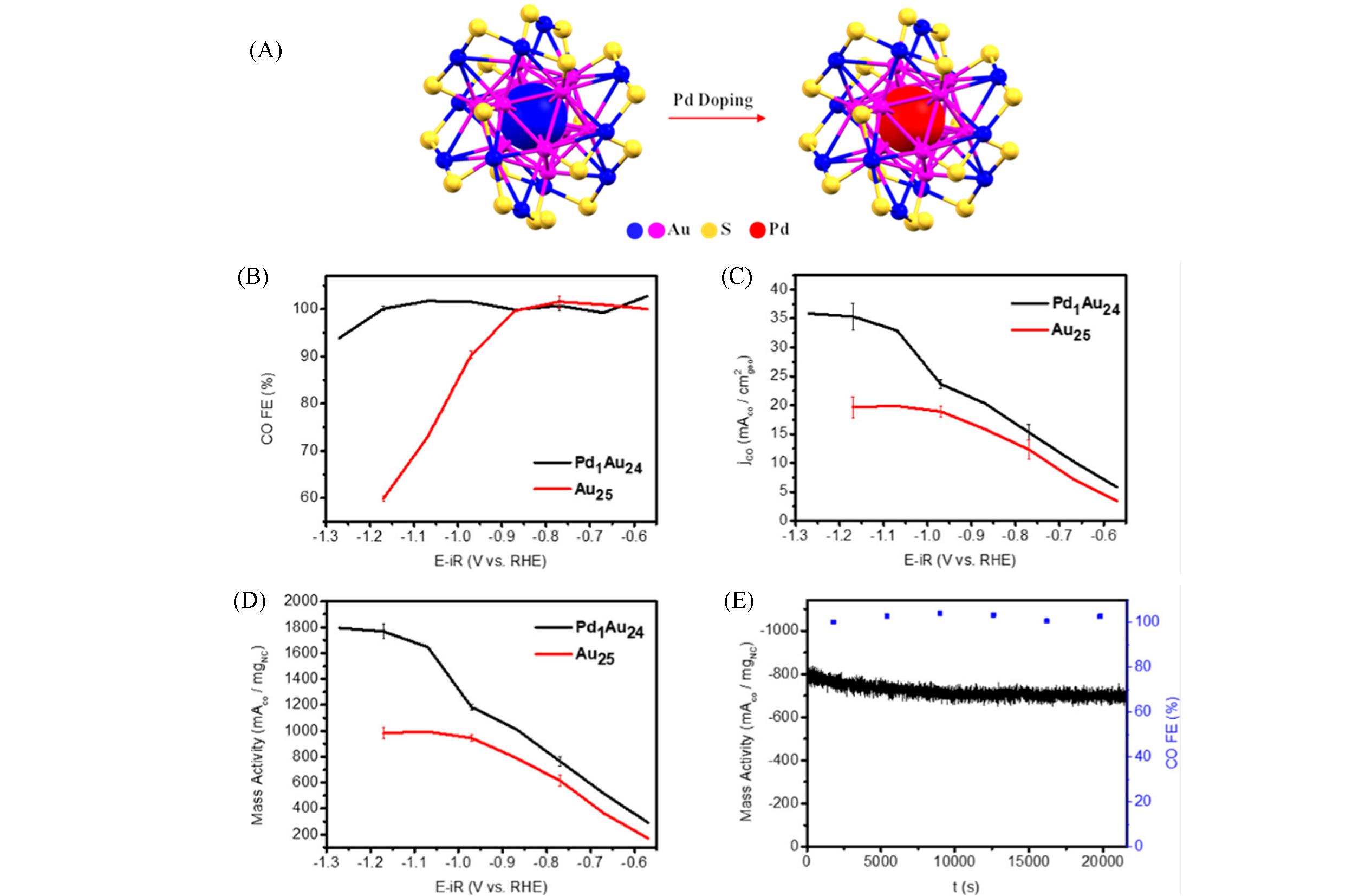
Fig.13 Catalytic performances of different metal nanoclusters in CO2RR[69](A) Crystal structures of Au25(PET)18 and Pd1Au24(PET)18 nanoclusters; (B) the performance of Au nanoclusters in CO2RR: FECO; (C) CO partial current density; (D) CO mass activity; (E) stability of Pd1Au24(PET)18 at -0. 8 V(vs. RHE) for 6 h.Copyright 2020, American Chemical Society.
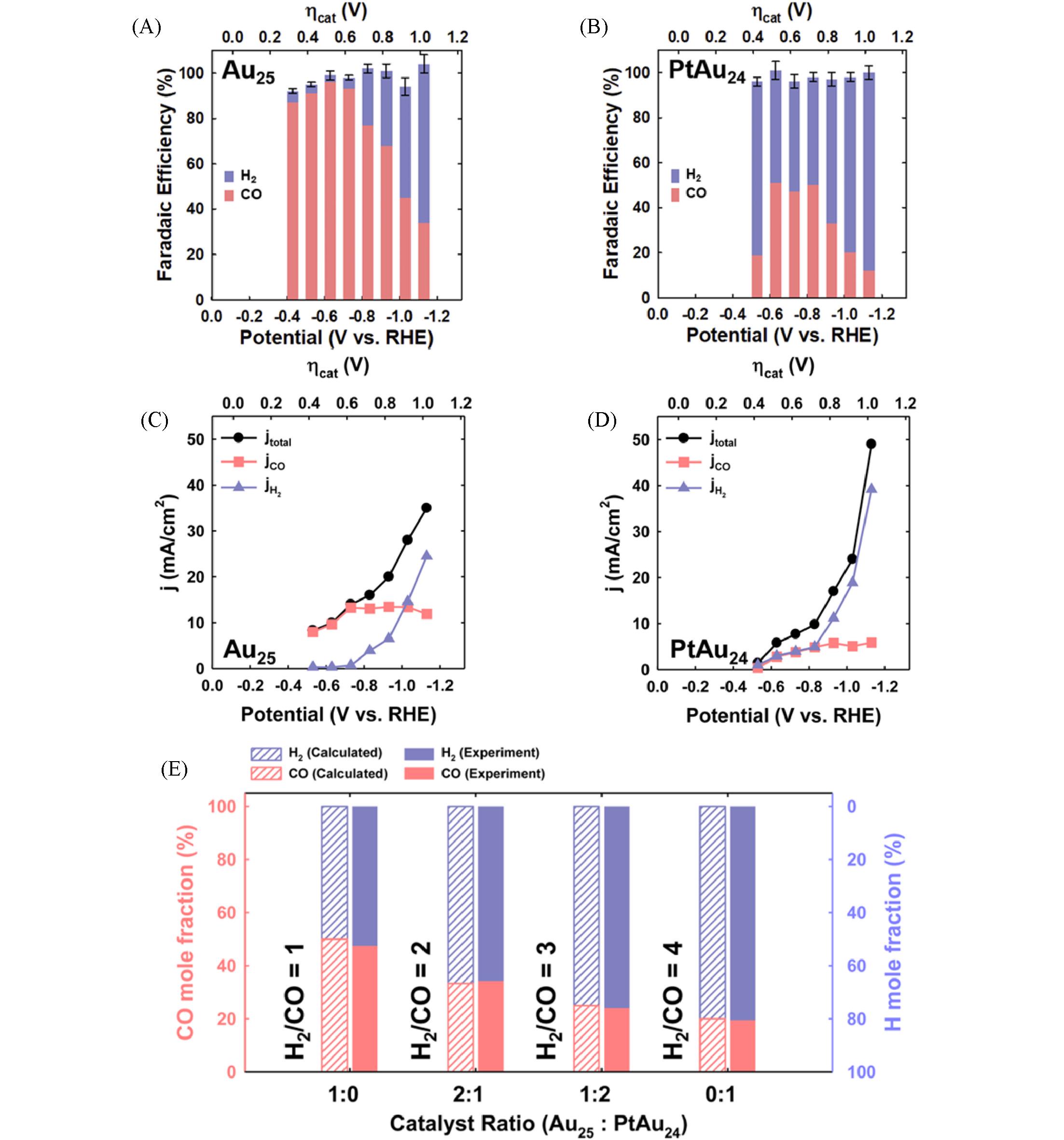
Fig.14 Catalytic performances of different metal nanoclusters in CO2RR[70]FEs for CO and H2 production measured on the Au25/C/GDE(A) and PtAu24/C/GDE(B) in the CO2?saturated solution of 0.1 mol/L KHCO3 and 0.4 mol/L KCl at various applied potentials; the corresponding electric current density obtained on the Au25/C/GDE(C) and PtAu24/C/GDE(D) at various potentials; (E) calculated(shaded) and experimentally determined(filled) H2/CO mole ratio on formulated Au25 and PtAu24.Copyright 2021, AIP Publishing LLC.
| 1 | Liu Y. T., Deng D. H., Bao X. H., Chem, 2020, 6(10), 2497―2514 |
| 2 | Tao Z. X., Wang H. L., Chem. Res. Chinese Universities, 2020, 36(6), 1145―1146 |
| 3 | Fu X. L., Zhu A. N., Chen X. J., Zhang S. F., Wang M., Yuan M. J., Chem. Res. Chinese Universities, 2021, 37(6), 1328―1333 |
| 4 | Bai X. F., Chen W., Wang B. Y., Feng G. H., Wei W., Jiao Z., Sun Y. H., Acta Phys. Chim. Sin., 2017, 33(12), 2388—2403 |
| 白晓芳, 陈为, 王白银, 冯光辉, 魏伟, 焦正, 孙予罕. 物理化学学报, 2017, 33(12), 2388—2403 | |
| 5 | Jin X. Y., Zhang L. B., Sun X. F., Han B. X., Chem. J. Chinese Universities, 2022, 43(5), 20220035 |
| 金湘元, 张礼兵, 孙晓甫, 韩布兴. 高等学校化学学报, 2022, 43(5), 20220035 | |
| 6 | Zhu M. Z, Aikens C. M., Hollander F. J., Schatz G. C., Jin R. C., J. Am. Chem. Soc., 2008, 130(18), 5883—5885 |
| 7 | Tian S. B., Liao L. W., Yuan J. Y., Yao C. H., Chen J. S., Yang J. L., Wu Z. K., Chem. Commun., 2016, 52(64), 9873—9875 |
| 8 | Yao C. H., Lin Y. J., Yuan J. Y., Liao L. W., Zhu M., Weng L. H., Yang J. L., Wu Z. K., J. Am. Chem. Soc., 2015, 137(49), 15350—15353 |
| 9 | Liao L. W., Zhou S. M., Dai Y. F., Liu L. R., Yao C. H., Fu C. F., Yang J. L., Wu Z. K., J. Am. Chem. Soc., 2015, 137(30), 9511—9514 |
| 10 | Yamazoe S., Matsuo S., Muramatsu S., Takano S., Nitta K., Tsukuda T., Inorg. Chem., 2017, 56(14), 8319—8325 |
| 11 | McKenzie L. C., Zaikova T. O., Hutchison J. E., J. Am. Chem. Soc., 2014, 136(38), 13426—13435 |
| 12 | Narouz M. R., Takano S., Lummis P. A., Levchenko T. I., Nazemi A., Kaappa S., Malola S., Yousefalizadeh G., Calhoun L. A., Stamplecoskie K. G., Häkkinen H., Tsukuda T., Crudden C. M., J. Am. Chem. Soc., 2019, 141(38), 14997—15002 |
| 13 | Yang S., Chen S., Xiong L., Liu C., Yu H. Z., Wang S. X., Rosi N. L., Pei Y., Zhu M. Z., J. Am. Chem. Soc., 2018, 140(35), 10988—10994 |
| 14 | Itteboina R., Madhuri U. D., Ghosal P., Kannan M., Sau T. K., Tsukuda T., Bhardwaj S., J. Phys. Chem. A, 2018, 122(5), 1228—1234 |
| 15 | Zhu M. Z., Qian H. F., Jin R. C., J. Am. Chem. Soc., 2009, 131(21), 7220—7221 |
| 16 | Chen S., Xiong L., Wang S. X., Ma Z. Y., Jin S., Sheng H. T., Pei Y., Zhu M. Z., J. Am. Chem. Soc., 2016, 138(34), 10754—10757 |
| 17 | Hesari M., Workentin M. S., J. Mater. Chem. C, 2014, 2(18), 3631—3638 |
| 18 | Das A., Li T., Li G., Nobusada K., Zeng C. J., Rosi N. L., Jin R. C., Nanoscale, 2014, 6(12), 6458—6462 |
| 19 | Zeng C. J., Li T., Das A., Rosi N. L., Jin R. C., J. Am. Chem. Soc., 2013, 135(27), 10011—10013 |
| 20 | Higaki T., Liu C., Zeng C. J., Jin R. X., Chen Y. X., Rosi N. L., Jin R. C., Angew. Chem. Int. Ed., 2016, 55(23), 6694—6697 |
| 21 | Yuan S. F., Xu C. Q., Li J., Wang Q. M., Angew. Chem. Int. Ed., 2019, 58(18), 5906—5909 |
| 22 | Zeng C. J., Qian H. F., Li T., Li G., Rosi N. L., Yoon B., Barnett R. N., Whetten R. L., Landman U., Jin R. C., Angew. Chem. Int. Ed., 2012, 51(52), 13114—13118 |
| 23 | Donkers R. L., Lee D., Murray R. W., Langmuir, 2008, 24(11), 5976—5976 |
| 24 | Zeng C. J., Chen Y. X., Liu C., Nobusada K., Rosi N. L., Jin R. C., Sci. Adv., 2015, 1(9), e1500425 |
| 25 | Dong H. W., Liao L. W., Zhuang S. L., Yao C. H., Chen J. S., Tian S. B., Zhu M., Liu X., Li L. L., Wu Z. K., Nanoscale, 2017, 9(11), 3742—3746 |
| 26 | Zhuang S. L., Liao L. W., Zhao Y., Yuan J. Y., Yao C. H., Liu X., Li J., Deng H. T., Yang J. L., Wu Z. K., Chem. Sci., 2018, 9(9), 2437—2442 |
| 27 | Zeng C. J., Chen Y. X., Iida K., Nobusada K., Kirschbaum K., Lambright K. J., Jin R. C., J. Am. Chem. Soc., 2016, 138(12), 3950—3953 |
| 28 | Liao L. W., Zhuang S. L., Wang P., Xu Y. N., Yan N., Dong H. W., Wang C. M., Zhao Y., Xia N., Li J., Deng H. T., Pei Y., Tian S. K., Wu Z. K., Angew. Chem. Int. Ed., 2017, 56(41), 12644—12648 |
| 29 | Zhuang S. L., Liao L. W., Li M. B., Yao C. H., Zhao Y., Dong H. W., Li J., Deng H. T., Li L. L., Wu Z. K., Nanoscale, 2017, 9(39), 14809—14813 |
| 30 | Gan Z. B., Chen J. S., Wang J., Wang C. M., Li M. B., Yao C. H., Zhuang S. L., Xu A., Li L. L., Wu Z. K., Nat. Commun., 2017, 8, 14739 |
| 31 | Zeng C. J., Liu C., Chen Y. X., Rosi N. L., Jin R. C., J. Am. Chem. Soc., 2016, 138(28), 8710—8713 |
| 32 | Jadzinsky P. D., Calero G., Ackerson C. J., Bushnell D. A., Kornberg R. D., Science, 2007, 318(5849), 430—433 |
| 33 | Chen Y. X., Zeng C. J., Liu C., Kirschbaum K., Gayathri C., Gil R. R., Rosi N. L., Jin R. C., J. Am. Chem. Soc., 2015, 137(32), 10076—10079 |
| 34 | Qian H. F., Jin R. C., Nano Lett., 2009, 9(12), 4083—4087 |
| 35 | Sakthivel N. A., Shabaninezhad M., Sementa L., Yoon B., Stener M., Whetten R. L, Ramakrishna G., Fortunelli A., Landman U., Dass A., J. Am. Chem. Soc., 2020, 142(37), 15799—15814 |
| 36 | Zeng C. J., Chen Y. X., Kirschbaum K., Lambright K. J., Jin R. C., Science, 2016, 354(6319), 1580—1584 |
| 37 | Sakthivel N. A., Theivendran S., Ganeshraj V., Oliver A. G., Dass A., J. Am. Chem. Soc., 2017, 139(43), 15450—15459 |
| 38 | Liu X., Saranya G., Huang X. Y., Cheng X. L., Wang R., Chen M. Y., Zhang C. F., Li T., Zhu Y., Angew. Chem. Int. Ed., 2020, 59(33), 13941—13946 |
| 39 | Xu J. Y., Xiong L., Cai X., Tang S. S., Tang A. C., Liu X., Pei Y., Zhu Y., Chem. Sci., 2022, 13(9), 2778—2782 |
| 40 | Ito E., Takano S., Nakamura T., Tsukuda T., Angew. Chem. Int. Ed., 2020, 60(2), 645—649 |
| 41 | Li S. T., Nagarajan A. V., Alfonso D. R., Sun M. K., Kauffman D. R., Mpourmpakis G., Jin R. C., Angew. Chem. Int. Ed., 2021, 60(12), 6351—6356 |
| 42 | Zhuang S. L., Chen D., Liao L. W., Zhao Y., Xia N., Zhang W. H., Wang C. M., Yang J., Wu Z. K., Angew. Chem. Int. Ed., 2020, 59(8), 3073—3077 |
| 43 | Liu X., Yao G., Cheng X. L., Xu J. Y., Cai X., Hu W. G., Xu W. W., Zhang C. F., Zhu Y., Chem. Sci., 2021, 12(9), 3290—3294 |
| 44 | Wan X. K., Cheng X. L., Tang Q., Han Y. Z., Hu G. X., Jiang D. E., Wang Q. M., J. Am. Chem. Soc., 2017, 139(28) 9451—9454 |
| 45 | Guan Z. J., Zeng J. L., Yuan S. F., Hu F., Lin Y. M., Wang Q. M., Angew. Chem. Int. Ed., 2018, 57(20), 5703—5707 |
| 46 | Yang D., Pei W., Zhou S., Zhao J. J., Ding W. P., Zhu Y., Angew. Chem. Int. Ed., 2020, 59(5), 1919—1924 |
| 47 | Yang D., Pei W., Zhang Y. Y., Hu W. G., Cai X., Sun Y. N., Li S. H., Cheng X. L., Zhou S., Zhao J. J., Zhu Y., Ding W. P., Liu X., Nano Res., 2020, 14(3), 807—813 |
| 48 | Cai X., Hu W. G., Xu S., Yang D., Chen M. Y., Shu M., Si R., Ding W. P., Zhu Y., J. Am. Chem. Soc., 2020, 142(9), 4141—4153 |
| 49 | Sun Y. N., Yang D., Zhang Y. Y., Hu W. G., Cheng X. L., Liu X., Chen M. Y., Zhu Y., Chem. Commun., 2020, 56(84), 12833—12836 |
| 50 | Yang D., Song Y., Yang F., Sun Y. N., Li S. H., Liu X., Zhu Y., Yang Y. H., J. Chem. Phys., 2021, 155(5), 054305 |
| 51 | Li G. J., Sui X., Cai X., Hu W. G., Liu X., Chen M. Y., Zhu Y., Angew. Chem. Int. Ed., 2021, 60(19), 10573—10576 |
| 52 | Liu Y. Y., Chai X. Q., Cai X., Chen M. Y., Jin R. C., Ding W. P., Zhu Y., Angew. Chem. Int. Ed., 2018, 57(31), 9775—9779 |
| 53 | Lei Z., Wan X. K., Yuan S. F., Guan Z. J., Wang Q. M., Acc. Chem. Res., 2018, 51(10), 2465—2474 |
| 54 | Du Y. X., Sheng H. T., Astruc D., Zhu M. Z., Chem. Rev., 2020, 120(2), 526—622 |
| 55 | Jin R. C., Li G., Sharma S., Li Y. W., Du X. S., Chem. Rev., 2021, 121(2), 567—648 |
| 56 | Kauffman D. R., Alfonso D., Matranga C., Qian H. F., Jin R. C., J. Am. Chem. Soc., 2012, 134(24), 10237—10243 |
| 57 | Hashmi A. S. K., Hutchings G. J., Angew. Chem. Int. Ed., 2006, 45(47), 7896—7936 |
| 58 | Min B. K., Friend C. M., Chem. Rev., 2007, 107(6), 2709—2724 |
| 59 | Li G. J., Hu W. G., Sun Y. N., Xu J.Y., Cai X., Cheng X. L., Zhang Y. Y., Tang A. C., Liu X., Chen M. Y., Ding W. P., Zhu Y., Angew. Chem. Int. Ed., 2020, 59(47), 21135—21142 |
| 60 | Kauffman D. R., Alfonso D., Matranga C., Ohodnicki P., Deng X. Y., Siva R. C., Zeng C. J., Jin R. C., Chem. Sci., 2014, 5(8), 3151—3157 |
| 61 | Narouz M. R., Osten K. M., Unsworth P. J., Man R. W. Y., Salorinne K., Takano S., Tomihara R., Kaappa S., Malola S., Dinh C. T., Padmos J. D., Ayoo K., Garrett P. J., Nambo M., Horton J. H., Sargent E. H., Häkkinen H., Tsukuda T., Crudden C. M., Nat. Chem., 2019, 11(5), 419—425 |
| 62 | Yuan S. F., He R. L., Han X. S., Wang J. Q., Guan Z. J., Wang Q. M., Angew. Chem. Int. Ed., 2021, 60(26), 14345—14349 |
| 63 | Li S. T., Nagarajan A. V., Li Y. W., Kauffman D. R., Mpourmpakis G., Jin R. C., Nanoscale, 2021, 13(4), 2333—2337 |
| 64 | Zhao S., Austin N., Li M., Song Y. B., House S. D., Bernhard S., Yang J. C., Mpourmpakis G., Jin R. C., ACS Catal., 2018, 8(6), 4996—5001 |
| 65 | Wan X. K., Wang J. Q., Wang Q. M., Angew. Chem. Int. Ed., 2021, 60(38), 20748—20753 |
| 66 | Gao Z. H., Wei K. C., Wu T., Dong J., Jiang D. E., Sun S. H., Wang L. S., J. Am. Chem. Soc., 2022, 144(12), 5258—5262 |
| 67 | Tang Q., Lee Y. J., Li D. Y., Choi W., Liu C. W., Lee D., Jiang D. E., J. Am. Chem. Soc., 2017, 139(28), 9728—9736 |
| 68 | Sun Y. N., Liu X., Xiao K., Zhu Y., Chen M. Y., ACS Catal., 2021, 11(18), 11551—11560 |
| 69 | Li S. T., Alfonso D., Nagarajan A. V., House S. D., Yang J. C., Kauffman D. R., Mpourmpakis G., Jin R. C., ACS Catal., 2020, 10(20), 12011—12016 |
| 70 | Choi W., Seong H., Efremov V., Lee Y., Im S., Lim D. H., Yoo J. S., Lee D., J. Chem. Phys., 2021, 155(1), 014305 |
| [1] | 赵盈喆, 张建玲. 金属-有机框架基材料在二氧化碳光催化转化中的应用[J]. 高等学校化学学报, 2022, 43(7): 20220223. |
| [2] | 周紫璇, 杨海艳, 孙予罕, 高鹏. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220235. |
| [3] | 任娜娜, 薛洁, 王治钒, 姚晓霞, 王繁. 热力学数据对1, 3-丁二烯燃烧特性的影响[J]. 高等学校化学学报, 2022, 43(6): 20220151. |
| [4] | 孙翠红, 吕立强, 刘迎, 王妍, 杨静, 张绍文. 硝酸异丙酯与Cl原子、 OH和NO3自由基反应的机理及动力学[J]. 高等学校化学学报, 2022, 43(2): 20210591. |
| [5] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [6] | 孟繁伟, 高琦, 叶青, 李晨曦. Cu-SAPO-18催化剂氨选择性催化还原NOx钾中毒机理的研究[J]. 高等学校化学学报, 2021, 42(9): 2832. |
| [7] | 杨一莹, 朱荣秀, 张冬菊, 刘成卜. 金催化炔基苯并二𫫇英环化合成8-羟基异香豆素的理论研究[J]. 高等学校化学学报, 2021, 42(7): 2299. |
| [8] | 李心怡, 刘永军. 人工设计逆醛缩酶RA95.5-8F催化β-羟基酮C—C裂解的理论研究[J]. 高等学校化学学报, 2021, 42(7): 2306. |
| [9] | 李宜蔚, 申屠江涛, 王静波, 李象远. 燃烧反应机理构建的极小反应网络方法: C1燃料燃烧[J]. 高等学校化学学报, 2021, 42(6): 1871. |
| [10] | 田胜侨, 韦美菊. Rh(Ⅱ)催化吲哚衍生物[3+3]环化机理及产物性质分析[J]. 高等学校化学学报, 2021, 42(6): 1899. |
| [11] | 任颖, 李昌华, 王涛, 薛珊珊, 张婷婷, 贾建峰, 武海顺. 钯催化氧化N—H键羰基化反应合成1,3,4⁃噁二唑⁃2(3H)⁃酮杂环化合物机理的理论研究[J]. 高等学校化学学报, 2021, 42(6): 1793. |
| [12] | 孙海珠, 杨国夺, 杨柏. 碳点的设计合成、 结构调控及应用[J]. 高等学校化学学报, 2021, 42(2): 349. |
| [13] | 侯华, 王宝山. 六氟化硫替代气体绝缘强度的官能团加和理论方法[J]. 高等学校化学学报, 2021, 42(12): 3709. |
| [14] | 齐国栋, 叶晓栋, 徐君, 邓风. 分子筛上糖类催化转化的核磁共振研究[J]. 高等学校化学学报, 2021, 42(1): 148. |
| [15] | 毛庆,赵健,刘松,郭唱,李冰玉,徐可一,曹自强,黄延强. Ni单原子催化剂表面CO2电还原动力学的电化学谱学解析[J]. 高等学校化学学报, 2020, 41(5): 1058. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||