

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (9): 2832.doi: 10.7503/cjcu20210360
收稿日期:2021-05-25
出版日期:2021-09-10
发布日期:2021-09-08
通讯作者:
叶青
E-mail:yeqing@bjut.edu.cn
基金资助:
MENG Fanwei, GAO Qi, YE Qing( ), LI Chenxi
), LI Chenxi
Received:2021-05-25
Online:2021-09-10
Published:2021-09-08
Contact:
YE Qing
E-mail:yeqing@bjut.edu.cn
Supported by:摘要:
研究了不同水热老化温度对钾(K)中毒0.4K-Cu-SAPO-18样品的结构及其NH3-SCR(NH3作为还原剂的选择性还原技术)催化活性的影响. 结果表明, K中毒对样品结构影响较小, 但明显降低了其NH3-SCR性能, 在350 ℃ 时, K中毒样品0.4K-Cu-SAPO-18的NO转化率为65.88%, 明显低于未中毒Cu-SAPO-18样品的90.85%. 水热老化温度明显影响催化剂的结构, 减少了活性位点, 降低了表面酸性. 随着水热老化温度升高, 催化剂的AEI结构被破坏, 活性物种数量降低, 催化活性明显下降. 氢气程序升温还原 (H2-TPR)结果表明, 孤立的Cu2+和Cu+的总量分别从未中毒Cu-SAPO-18样品的66.61和1.32 μmol/g变化到K中毒0.4K-Cu-SAPO-18样品的39.52和101.96 μmol/g, 表明K中毒样品中孤立Cu2+ 容易转化为Cu2O. K中毒后, 样品的弱酸、 中强酸、 强酸性位点的数量降低, 分别从未中毒Cu-SAPO-18样品的0.201, 0.103和0.302 mmol/g降低到中毒0.4K-Cu-SAPO-18样品的0.102, 0.086和0.071 mmol/g. 氨气程序升温脱附(NH3-TPD)和原位红外结果表明, K竞争性地取代了催化剂中孤立的Cu2+和H+, 使K中毒0.4K-Cu-SAPO-18样品的活性位和酸性位减少, 导致催化活性下降. 在低温 NH3-SCR反应中, K中毒和未中毒样品均以Eley-Rideal(E-R)和Langmuir-Hinshelwood(L-H)机理进行, 而L-H机理占主导地位, 但K中毒样品的反应速率明显降低.
中图分类号:
TrendMD:
孟繁伟, 高琦, 叶青, 李晨曦. Cu-SAPO-18催化剂氨选择性催化还原NOx钾中毒机理的研究. 高等学校化学学报, 2021, 42(9): 2832.
MENG Fanwei, GAO Qi, YE Qing, LI Chenxi. Potassium Poisoning Mechanism of Cu-SAPO-18 Catalyst for Selective Catalytic Reduction of NOx by Ammonia. Chem. J. Chinese Universities, 2021, 42(9): 2832.
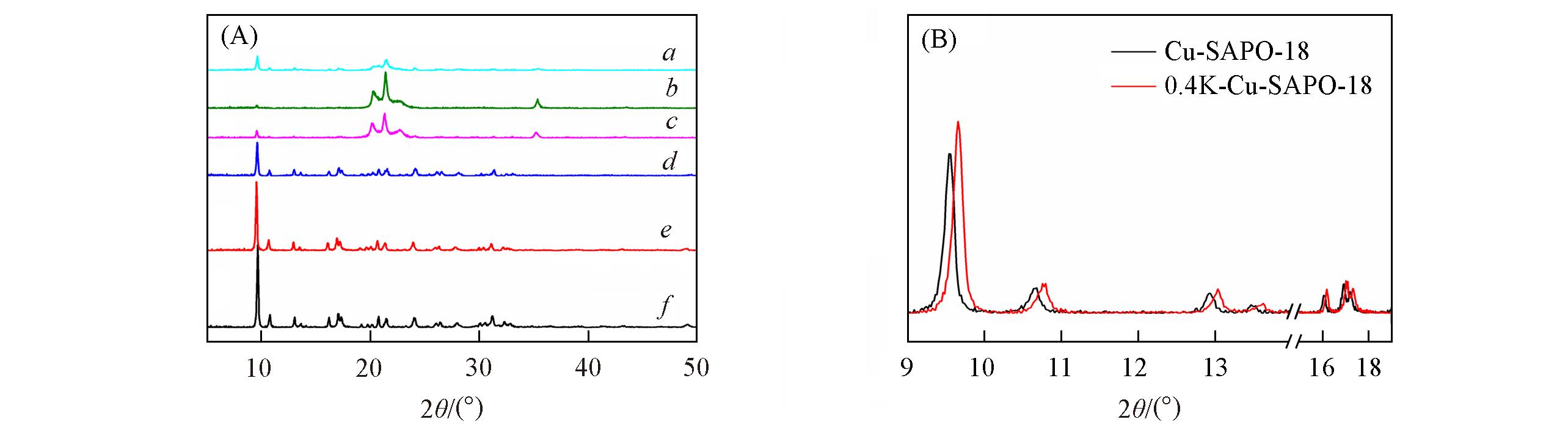
Fig.3 XRD patterns of the samples(A) a. Cu?SAPO?18?850; b. 0.4K?Cu?SAPO?18?850; c. 0.4K?Cu?SAPO?18?750; d. 0.4K?Cu?SAPO?18?650; e. 0.4K?Cu?SAPO?18; f. Cu?SAPO?18; (B) unpoisoned Cu?SAPO?8 and 0.4K?Cu?SAPO?18.
| Sample | SBET/(m2?g?1) | Vmic/(cm3?g?1) | Sample | SBET/(m2?g?1) | Vmic/(cm3?g?1) |
|---|---|---|---|---|---|
| Cu?SAPO?18 | 597.33 | 0.24 | 0.4K?Cu?SAPO?18?750 | 271.05 | 0.12 |
| 0.4K?Cu?SAPO?18 | 556.73 | 0.22 | 0.4K?Cu?SAPO?18?850 | 254.49 | 0.11 |
| 0.4K?Cu?SAPO?18?650 | 543.80 | 0.21 | Cu?SAPO?18?50 | 267.40 | 0.11 |
Table 1 BET results of the samples
| Sample | SBET/(m2?g?1) | Vmic/(cm3?g?1) | Sample | SBET/(m2?g?1) | Vmic/(cm3?g?1) |
|---|---|---|---|---|---|
| Cu?SAPO?18 | 597.33 | 0.24 | 0.4K?Cu?SAPO?18?750 | 271.05 | 0.12 |
| 0.4K?Cu?SAPO?18 | 556.73 | 0.22 | 0.4K?Cu?SAPO?18?850 | 254.49 | 0.11 |
| 0.4K?Cu?SAPO?18?650 | 543.80 | 0.21 | Cu?SAPO?18?50 | 267.40 | 0.11 |
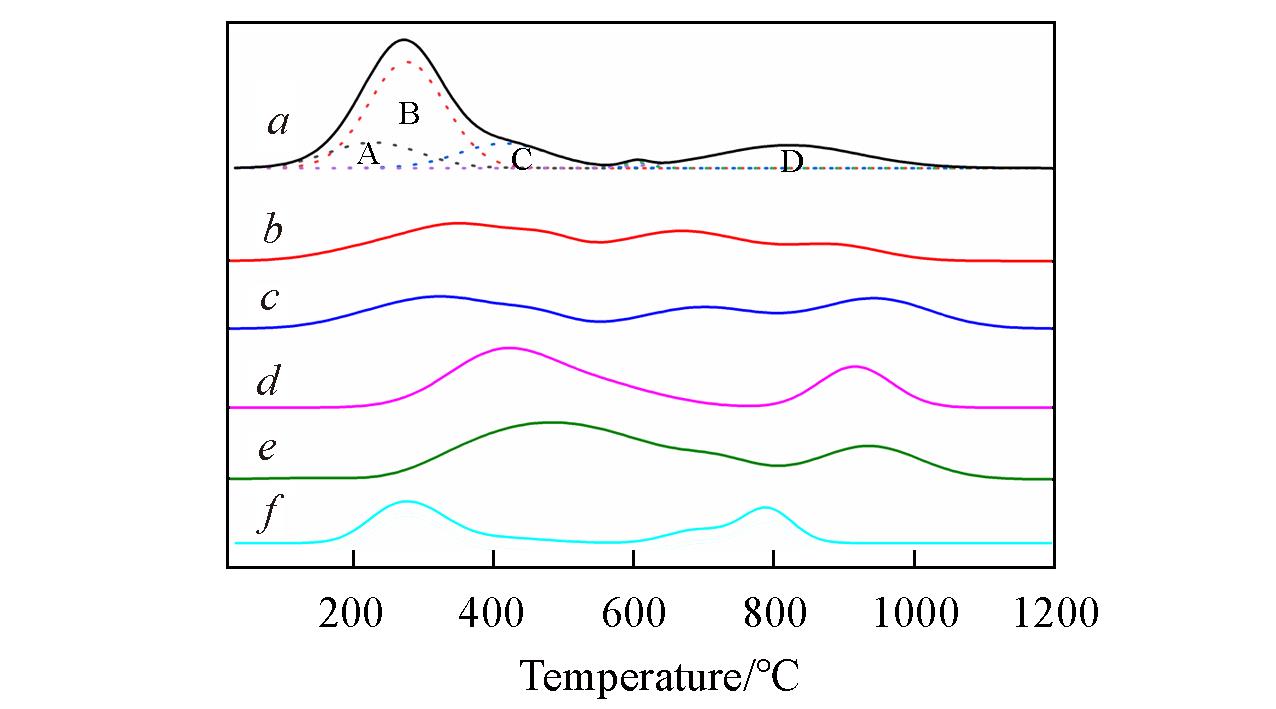
Fig.4 H2?TPR profiles of the samplesa. Cu?SAPO?18; b. 0.4K?Cu?SAPO?18; c. 0.4K?Cu?SAPO?18?650;d. 0.4K?Cu?SAPO?18?750; e. 0.4K?Cu?SAPO?18?850; f. Cu?SAPO?18?850.
| Sample | Content of Cu species/(μmol?g?1) | ||||
|---|---|---|---|---|---|
| Cu2+(8MR) | CuO | Cu2+(D6R) | Cu+ | Cutotal | |
| Cu?SAPO?18 | 32.23 | 59.98 | 34.38 | 1.32 | 127.91 |
| 0.4K?Cu?SAPO?18 | 18.47 | 56.76 | 21.05 | 101.96 | 198.23 |
| 0.4K?Cu?SAPO?18?650 | 14.60 | 49.45 | 18.47 | 122.17 | 204.69 |
| 0.4K?Cu?SAPO?18?750 | 5.14 | 43.86 | 5.57 | 209.05 | 263.61 |
| 0.4K?Cu?SAPO?18?850 | 3.85 | 41.49 | 4.28 | 317.86 | 367.47 |
| Cu?SAPO?18?850 | 29.18 | 32.19 | 15.69 | 189.17 | 266.23 |
Table 2 H2-TPR results for all samples
| Sample | Content of Cu species/(μmol?g?1) | ||||
|---|---|---|---|---|---|
| Cu2+(8MR) | CuO | Cu2+(D6R) | Cu+ | Cutotal | |
| Cu?SAPO?18 | 32.23 | 59.98 | 34.38 | 1.32 | 127.91 |
| 0.4K?Cu?SAPO?18 | 18.47 | 56.76 | 21.05 | 101.96 | 198.23 |
| 0.4K?Cu?SAPO?18?650 | 14.60 | 49.45 | 18.47 | 122.17 | 204.69 |
| 0.4K?Cu?SAPO?18?750 | 5.14 | 43.86 | 5.57 | 209.05 | 263.61 |
| 0.4K?Cu?SAPO?18?850 | 3.85 | 41.49 | 4.28 | 317.86 | 367.47 |
| Cu?SAPO?18?850 | 29.18 | 32.19 | 15.69 | 189.17 | 266.23 |
| Sample | Acidity site/(mmol?g?1) | Amount/(mmol?g?1) | ||
|---|---|---|---|---|
| Weak | Moderate | Strong | ||
| Cu?SAPO?18 | 0.201 | 0.103 | 0.302 | 0.636 |
| 0.4K?Cu?SAPO?18 | 0.102 | 0.086 | 0.071 | 0.259 |
| 0.4K?Cu?SAPO?18?650 | 0.084 | 0.059 | 0.051 | 0.174 |
| 0.4K?Cu?SAPO?18?750 | 0.013 | 0.018 | 0.011 | 0.042 |
| 0.4K?Cu?SAPO?18?850 | 0.011 | 0.012 | 0.010 | 0.033 |
| Cu?SAPO?18?850 | 0.120 | 0.092 | 0.091 | 0.313 |
Table 3 NH3-TPD results of all samples
| Sample | Acidity site/(mmol?g?1) | Amount/(mmol?g?1) | ||
|---|---|---|---|---|
| Weak | Moderate | Strong | ||
| Cu?SAPO?18 | 0.201 | 0.103 | 0.302 | 0.636 |
| 0.4K?Cu?SAPO?18 | 0.102 | 0.086 | 0.071 | 0.259 |
| 0.4K?Cu?SAPO?18?650 | 0.084 | 0.059 | 0.051 | 0.174 |
| 0.4K?Cu?SAPO?18?750 | 0.013 | 0.018 | 0.011 | 0.042 |
| 0.4K?Cu?SAPO?18?850 | 0.011 | 0.012 | 0.010 | 0.033 |
| Cu?SAPO?18?850 | 0.120 | 0.092 | 0.091 | 0.313 |
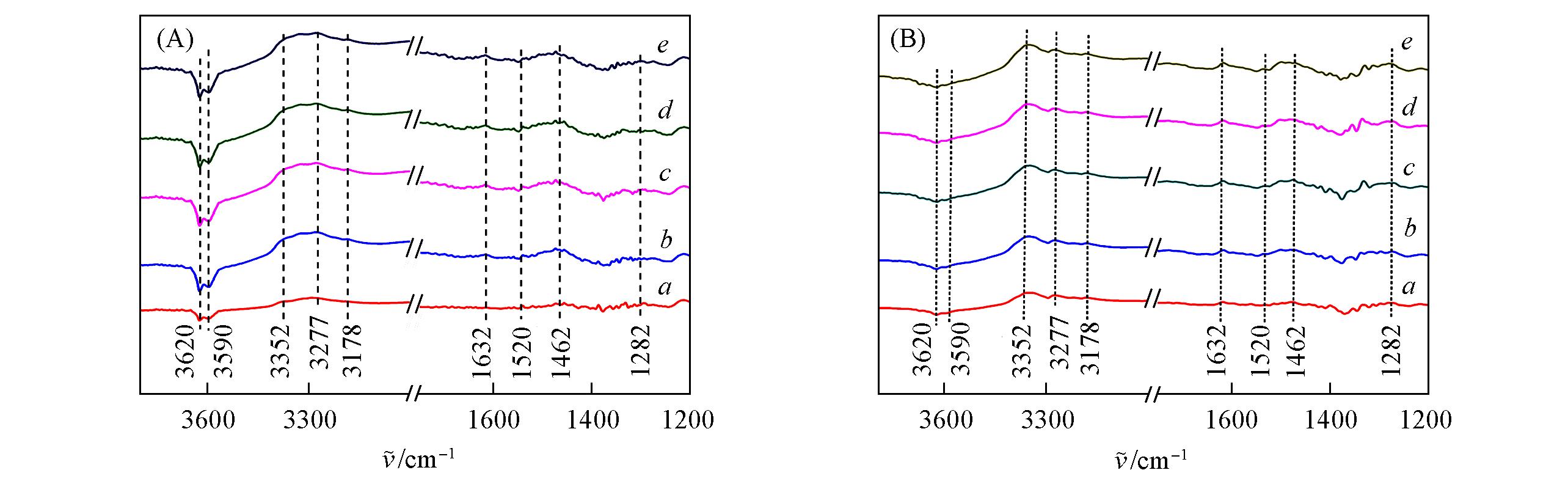
Fig.7 In situ FTIR spectra of Cu?SAPO?18(A) and 0.4K?Cu?SAPO?18(B) samples adsorbed by NH3 at 150 ℃a. 10 min; b. 20 min; c. 30 min; d. 40 min; e. 60 min.
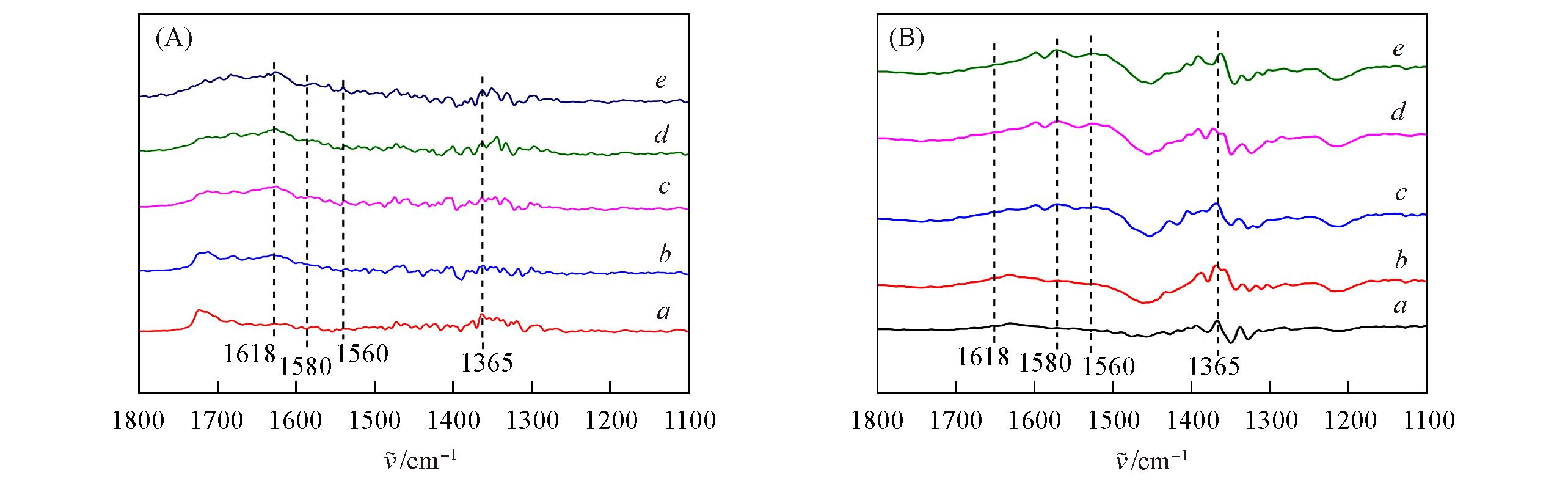
Fig.8 In situ FTIR spectra of NO+O2 adsorption on Cu?SAPO?18(A) and 0.4K?Cu?SAPO?18(B) samples at 150 ℃a. 10 min; b. 20 min; c. 30 min; d. 40 min; e. 60 min.
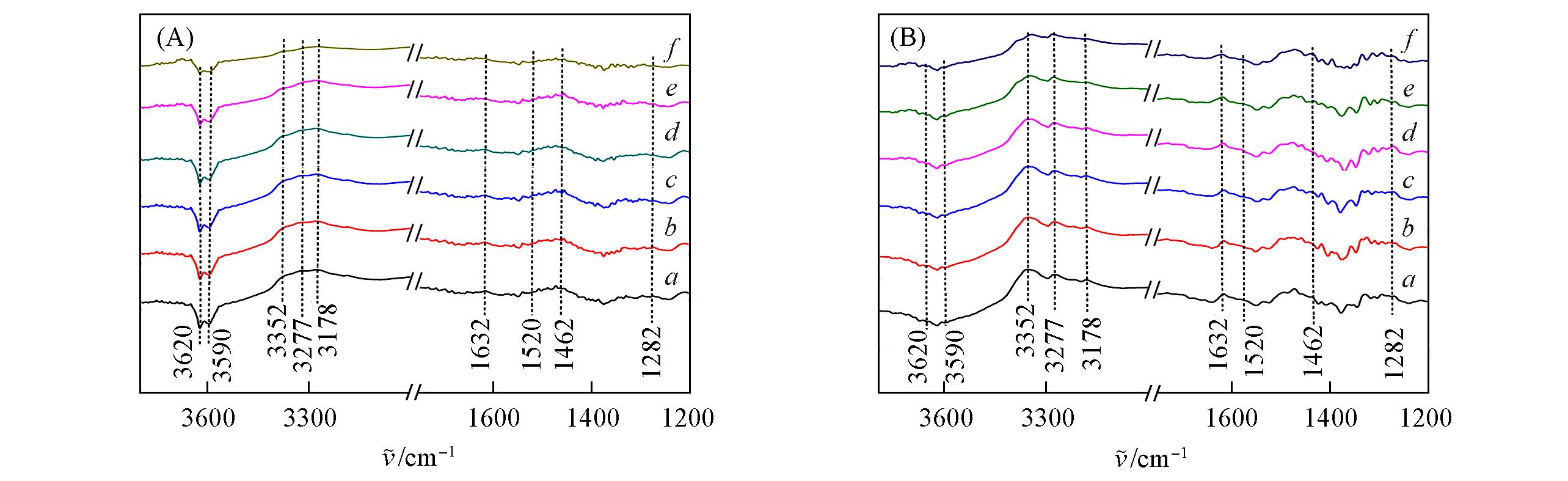
Fig.9 In situ FTIR spectra of reacting NO+O2 with pre?adsorbed NH3 of Cu?SAPO?18(A) and 0.4K?Cu?SAPO?18(B) samples at 150 ℃(A) a. NH3; b. NO-O2, 1 min; c. NO+O2, 5 min; d. NO+O2, 10 min; e. NO+O2, 20 min; f. NO+O2, 30 min.(B) a. NH3; b. NO+O2, 1 min; c. NO+O2, 5 min; d. NO+O2, 10 min; e. NO+O2, 20 min; f. NO+O2, 30 min.
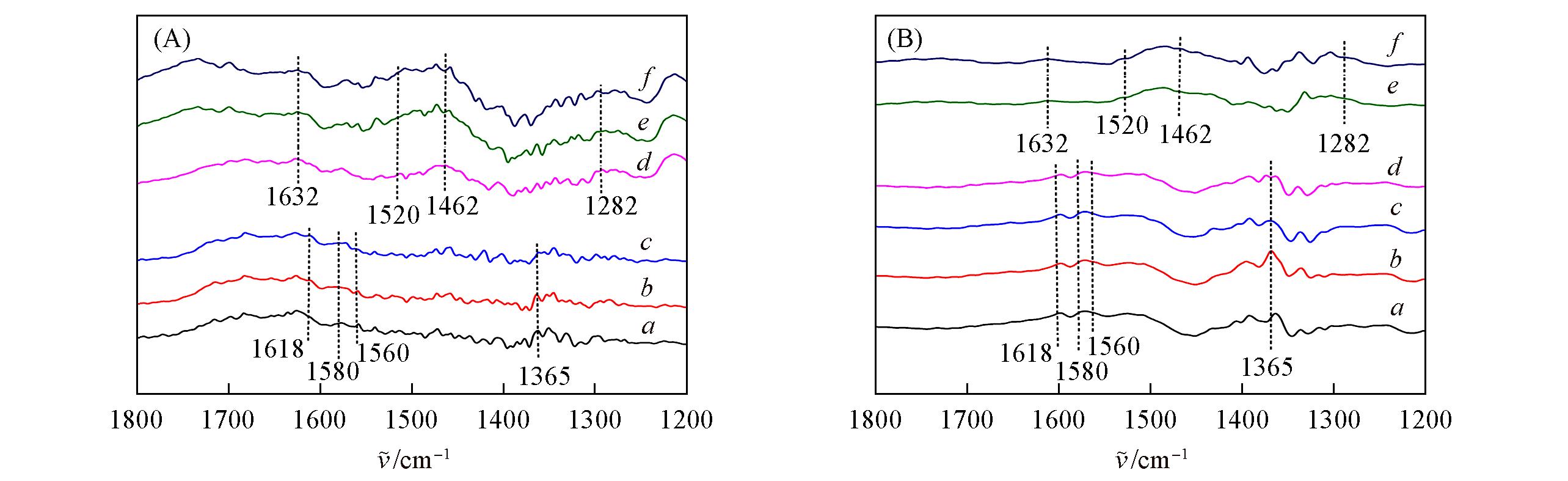
Fig.10 In situ FTIR spectra of reacting with NH3 and preadsorbed NO+O2 of Cu?SAPO?18(A) and 0.4K?Cu?SAPO?18(B) samples at 150 ℃a. NO+O2; b. NH3, 1 min; c. NH3, 5 min; d. NH3, 10 min; e. NH3, 20 min;f. NH3, 30 min.
| 29 | Wang J. C., Peng Z. L., Chen Y., Bao W. R., Chang L. P., Feng G., Chem. Eng. J., 2015, 263, 9―19 |
| 30 | Chen Z., Fan C., Pang L., Ming S. J., Liu P., Li T., Appl. Catal. B: Environ., 2018, 237, 116―127 |
| 31 | Wang J., Yu T., Wang X. Q., Qi G. S., Xue J. J., Shen M. Q., Li W., Appl. Catal. B: Environ., 2012, 127, 137―147 |
| 32 | Izadbakhsh A., Farhadi F., Khorasheh F., Sahebdelfar S., Asadi M., Feng Y. Z., Appl. Catal. A: Gen., 2009, 364(1/2), 48―56 |
| 33 | Costa P. D., Modén B., Meitzner G. D., Lee D. K., Iglesia E., Phys. Chem. Chem. Phys., 2002, 4(18), 4590―4601 |
| 34 | Wang D., Zhang L., Kamasamudram K., Epling W. S., ACS Catal., 2013, 3(5), 871―881 |
| 35 | Zhu H. Y., Kwak J. H., Peden C. H. F., Szanyi J., Catal. Today, 2013, 205, 16―23 |
| 36 | Chen W. S., Hu F. L., Qin L. B., Han J., Zhao B., Tu Y. Z., Yu F., Catal., 2019, 9(1), 90―102 |
| 37 | Wang X. T., Hu H. P., Zhang X. Y., Su X. X., Yang X. D., Environ. Sci. Pollut. R., 2019, 26(2), 1706―1715 |
| 38 | Li X. S., Li K. Z., Peng Y., Li X., Zhang Y. N., Wang D., Chen J. J., Li J. H., Chem. Eng. J., 2018, 347, 173―183 |
| 39 | Underwood G. M., Miller T. M., Grassian V. H., J. Phys. Chem. A, 1999, 103(31), 6184―6190 |
| 40 | Zhu N., Shan W. P., Shan Y. L., Du J. P., Lian Z. H., Zhang Y., He H., Chem. Eng. J., 2020, 388, 124250―124260 |
| 1 | Han L., Cai S. X., Gao M., Hasegawa J. Y., Wang P. L., Zhang J. P., Shi L. Y., Zhang D. S., Chem. Rev., 2019, 119(19), 10916―10976 |
| 2 | Richter A., Burrows J. P., Nüß H., Granier C., Niemeier U., Nature, 2005, 437(7055), 129―132 |
| 3 | Bishop G. A., Hottor-Raguindin R., Stedman D. H., Environ. Sci. Technol., 2015, 49(3), 1639―1645 |
| 4 | Xu J. Q., Chen G. R., Guo F., Xie J. Q., Chem. Eng. J., 2018, 353, 507―518 |
| 5 | Chen L., Si Z. C., Wu X. D., Weng D., Ran R., Yu J., J. Rare Earth, 2014, 32(10), 907―917 |
| 6 | Han S., Cheng J., Zheng C. K., Ye Q., Cheng S.Y., Kang T. F., Dai H. X., Appl. Surf. Sci., 2017, 419, 382―392 |
| 7 | Awate V., Tiwari R., Shrivastava A. K., Dubey V., J. Mater. Sci.: Mater. in Electron., 2018, 29(6), 4391―4401 |
| 8 | Aguilar⁃Romero M., Camposeco R., Castillo S., Marín J., Rodríguez⁃González V., García⁃Serrano L.A., Mejía⁃Centeno I., Fuel, 2017, 198, 123―133 |
| 9 | He Y. Y., Ford M. E., Zhu M. H., Liu Q. C., Tumuluri C., Wu Z. L., Wachs I. E., Appl. Catal. B: Environ., 2016, 193, 141―150 |
| 10 | Kim M. H., Park S. W., Catal. Commun., 2016, 86, 82―85 |
| 11 | Xu W. J., Zhang G. X., Chen H. W., Zhang G. M., Han Y., Chang Y. C., Chang P., Chinese J. Catal., 2018, 39(1), 118―127 |
| 12 | Xia Y., Zhan W. C., Guo Y., Guo Y. L., Lu G. Z., Chinese J. Catal., 2016, 37(12), 2069―2078 |
| 13 | van Kooten W. E. J., Krijnsen H. C., van den Bleek C. M., Calis H. P. A., Applied Catal. B: Environ., 2000, 25(2/3), 125―135 |
| 14 | Ma L., Su W. K., Li Z. G., Li J. H., Fu L. X., Hao J. M., Catal. Today, 2015, 245, 16―21 |
| 15 | Kwak J. H., Tran D., Szanyi J., Peden C. H. F., Lee J. H., Catal. Lett., 2012, 142(3), 295―301 |
| 16 | Fan C., Chen Z., Pang L., Ming S. J., Dong C. Y., Albert K. B., Liu P., Wang J. Y., Zhu D. J., Chen H. P., Li T., Chem. Eng. J., 2018, 334, 344―354 |
| 17 | Xie K. P., Woo J., Bernin D., Kumar A., Kamasamudram K., Olsson L., Appl. Catal. B: Environ., 2019, 241, 205―216 |
| 18 | Albert K. B., Fan C., Pang L., Chen Z., Ming S. J., Albert T., Li T., Appl. Surf. Sci., 2019, 479, 1200―1211 |
| 19 | Martínez-Franco R., Moliner M., Corma A., J. Catal., 2014, 319, 36―43 |
| 20 | Gao F., Wang Y. L., Washton N. M., Kollar M., Szanyi J., Peden C. H. F., ACS Catal., 2015, 5(11), 6780―6791 |
| 21 | Richter M., Fait M. J.G., Eckelt R, Schneider M., Radnik J., Heidemann D., Fricke R., J. Catal., 2007, 245(1), 11―24 |
| 22 | Xie L. J., Liu F. D., Ren L. M., Shi X.Y., Xiao F. S., He H., Environ. Sci. Technol., 2013, 48(1), 566―572 |
| 23 | Wang C., Wang C., Wang J. Q., Shen M. Q., Li W., J. Environ. Sci., 2018, 70, 20―28 |
| 24 | Gao Q., Ye Q., Han S., Dai H. X., Chemistry Select, 2020, 5(43), 13477―13486 |
| 25 | Kim Y. J., Lee J. K., Min K. M., Hong S. B., Nam I.S., Cho B. K., J. Catal., 2014, 311, 447―457 |
| 26 | Li Y. H., Deng J. L., Song W. Y., Liu J., Zhao Z., Gao M. L., Wei Y. C., Zhao L., J. Phys. Chem. C, 2016, 120(27), 14669―14680 |
| 27 | Sultana A., Nanba T., Sasaki M., Haneda M., Suzuki K., Hamada H., Catal. Today, 2011, 164(1), 495―499 |
| 28 | Wang J., Fan D. Q., Yu T., Wang J. Q., Hao T., Hao X. Q., Shen M. Q., Li W., J. Catal., 2015, 322, 84―90 |
| 41 | Wang C., Yan W. J., Wang Z. X., Chen Z. X., Wang J. Q., Wang J., Wang J. M., Shen M. Q., Kang X., Catal. Today, 2020, 355, 482―492 |
| 42 | Wang C., Wang J., Wang J. Q., Wang Z. X., Chen Z. X., Li X. L., Shen M. Q., Yan W. J., Kang X., Catal., 2018, 8(12), 593 |
| [1] | 郭彪, 赵晨灿, 刘芯辛, 于洲, 周丽景, 袁宏明, 赵震. 表面水热碳层对磁性NiFe2O4八面体光催化活性的影响[J]. 高等学校化学学报, 2022, 43(11): 20220472. |
| [2] | 董妍红, 鲁新环, 杨璐, 孙凡棋, 段金贵, 郭昊天, 张钦峻, 周丹, 夏清华. 双功能金属有机骨架材料的制备及催化烯烃环氧化性能[J]. 高等学校化学学报, 2022, 43(11): 20220458. |
| [3] | 王祖民, 孟程, 于然波. 过渡金属磷化物析氢催化剂的掺杂调控[J]. 高等学校化学学报, 2022, 43(11): 20220544. |
| [4] | 何建云 蒋云波 张爱敏 唐振艳 李鸿鹏. 新型卟啉基多孔有机聚合物COP-180负载钯催化剂的制备及应用研究[J]. 高等学校化学学报, 0, (): 20220535. |
| [5] | 夏文文 于洪晶 王时野 姚丽 李象远. 燃烧反应机理构建的极小反应网络方法—芳香烃燃烧[J]. 高等学校化学学报, 0, (): 20220616. |
| [6] | 李怀科 岳贵初 谢海韵 刘静 高松伟 侯兰兰 李帅 苗贝贝 王女 白杰 崔志民 赵勇. 静电纺丝中空纳米纤维在催化领域的应用[J]. 高等学校化学学报, 0, (): 20220625. |
| [7] | 匡华艺 陈晨. 贵金属纳米框架催化剂的设计合成及电催化性能研究[J]. 高等学校化学学报, 0, (): 20220586. |
| [8] | 王雅芝 贾显枝 张宏港 刘璐 赵彬然. 介质阻挡放电等离子制备5Ni-5La/SiO2催化剂用于甲烷干重整反应[J]. 高等学校化学学报, 0, (): 20220503. |
| [9] | 朱佶鹏 刘润辉 宋恭华. 双噁唑啉接枝的氨基酸聚合物作为手性催化中心在不对称Henry 反应中的应用[J]. 高等学校化学学报, 0, (): 20220569. |
| [10] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [11] | 孙金时, 陈鹏, 景丽萍, 孙福兴, 刘佳. 多级孔芳香骨架材料的合成及固载硫脲催化剂的研究[J]. 高等学校化学学报, 2022, 43(10): 20220171. |
| [12] | 李学宇, 王朝, 陈雅, 李可可, 李建全, 金顺敬, 陈丽华, 苏宝连. 等离激元共振光转热增强负载纳米金对丁二烯选择性加氢的催化性能[J]. 高等学校化学学报, 2022, 43(10): 20220174. |
| [13] | 宋佳欣, 崔静, 范晓强, 孔莲, 肖霞, 解则安, 赵震. 介孔二氧化硅负载高分散钒催化剂的制备及乙烷选择氧化性能研究[J]. 高等学校化学学报, 0, (): 20220532. |
| [14] | 唐全骏, 刘颖馨, 孟蓉炜, 张若天, 凌国维, 张辰. 单原子催化在海洋能源领域的应用[J]. 高等学校化学学报, 2022, 43(9): 20220324. |
| [15] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||