

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (1): 148.doi: 10.7503/cjcu20200500
所属专题: 分子筛功能材料 2021年,42卷,第1期
收稿日期:2020-07-31
出版日期:2021-01-10
发布日期:2021-01-12
通讯作者:
徐君,邓风
E-mail:xujun@wipm.ac.cn;dengf@wipm.ac.cn
基金资助:
QI Guodong1, YE Xiaodong1,3, XU Jun1,2( ), DENG Feng1(
), DENG Feng1( )
)
Received:2020-07-31
Online:2021-01-10
Published:2021-01-12
Contact:
XU Jun,DENG Feng
E-mail:xujun@wipm.ac.cn;dengf@wipm.ac.cn
Supported by:摘要:
葡萄糖、 果糖和木糖等糖类是一类重要的绿色生物质资源, 其高效利用是生物质转化的重要研究方向. 具有Lewis酸性的分子筛在糖类催化转化中表现出优异的性能, 对其活性中心结构、 性质以及反应机理的认识是糖类高效转化研究中亟待解决的关键科学问题. 核磁共振是分子筛上活性中心表征和反应机理研究的重要手段. 本文讨论了先进核磁共振技术与方法在分子筛上糖类转化反应中的应用, 包括催化剂活性中心表征、 催化转化反应机理研究和催化反应产物分析3个方面, 总结了核磁共振在糖类转化反应研究中所取得的新进展并对其未来发展方向进行了展望.
中图分类号:
TrendMD:
齐国栋, 叶晓栋, 徐君, 邓风. 分子筛上糖类催化转化的核磁共振研究. 高等学校化学学报, 2021, 42(1): 148.
QI Guodong, YE Xiaodong, XU Jun, DENG Feng. Progress in NMR Studies of Carbohydrates Conversion on Zeolites. Chem. J. Chinese Universities, 2021, 42(1): 148.
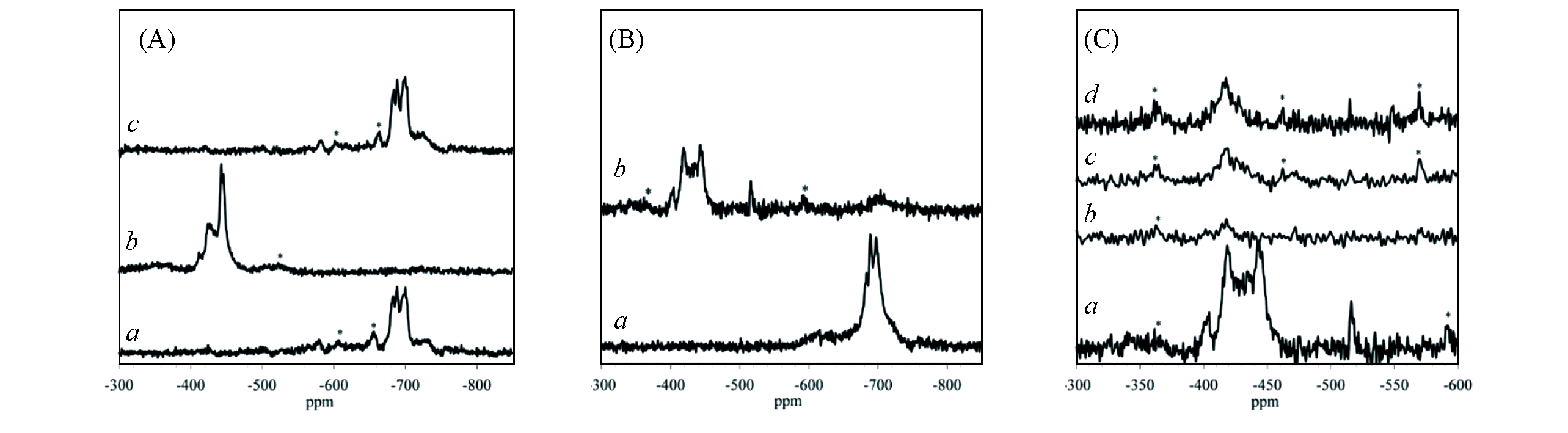
Fig.1 119Sn solid?state NMR spectra of Sn?Beta[13](A) Sn-Beta with different treatments. a. calcined; b. dehydrated after calcination; c. rehydrated after step(b). (B) Sn-Beta as a function of hydration. a. Sample was calcined at more humid conditions and exposed to ambient conditions; b. sample shown in curve a after vacuum drying at 393 K. (C) 119Sn MAS(a) and CP MAS NMR(b—d) spectra for dehydrated Sn-Beta. The cross- polarization contact times from 1H to 119Sn were varied: b. 0.2 ms; c. 1.0 ms; d. 2.0 ms. Spinning sidebands are marked by *.Copyright 2012, National Academy of Sciences.
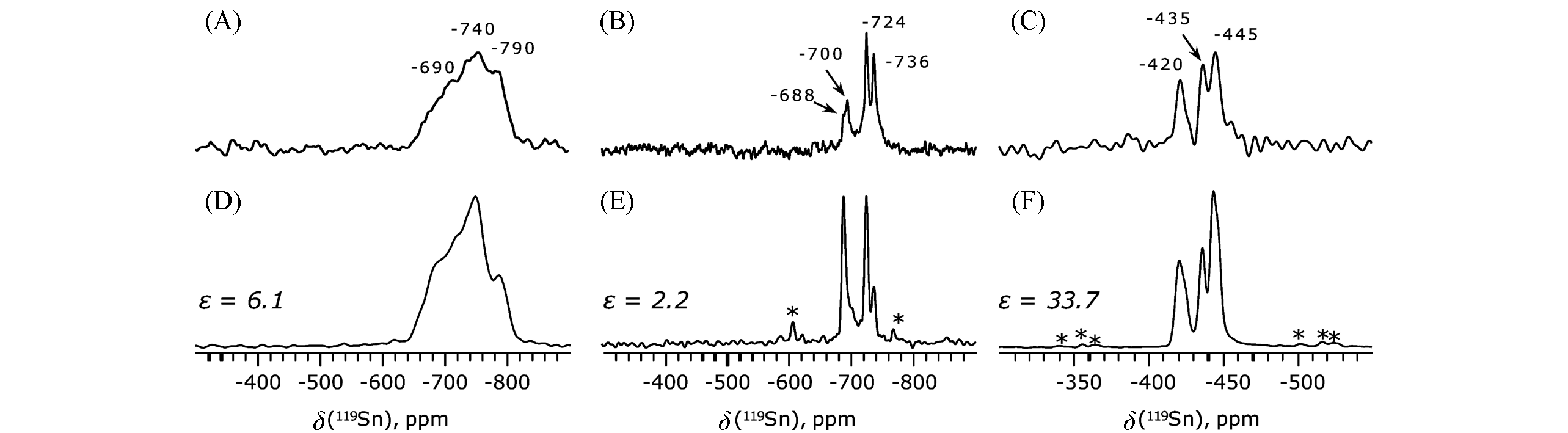
Fig.2 119Sn MAS NMR spectra of Sn?Beta[29](A), (D) 119Sn-BEA(syn); (B), (E) 119Sn-BEA(hyd); (C), (F) 119Sn-BEA(vac) recorded by using DP-HE[(A)—(C)], CP-CPMG, N=25[(D), (E)] and DP-CPMG, N=2000(F) pulse sequences. Asterisks mark spinning sidebands. Sensitivity gain(ε) is equal to(SNR)/(SNR0), where SNR0 is the signal-to-noise ratio of spectra obtained by Hahn-echo(DP-HE).Copyright 2016, American Chemical Society.
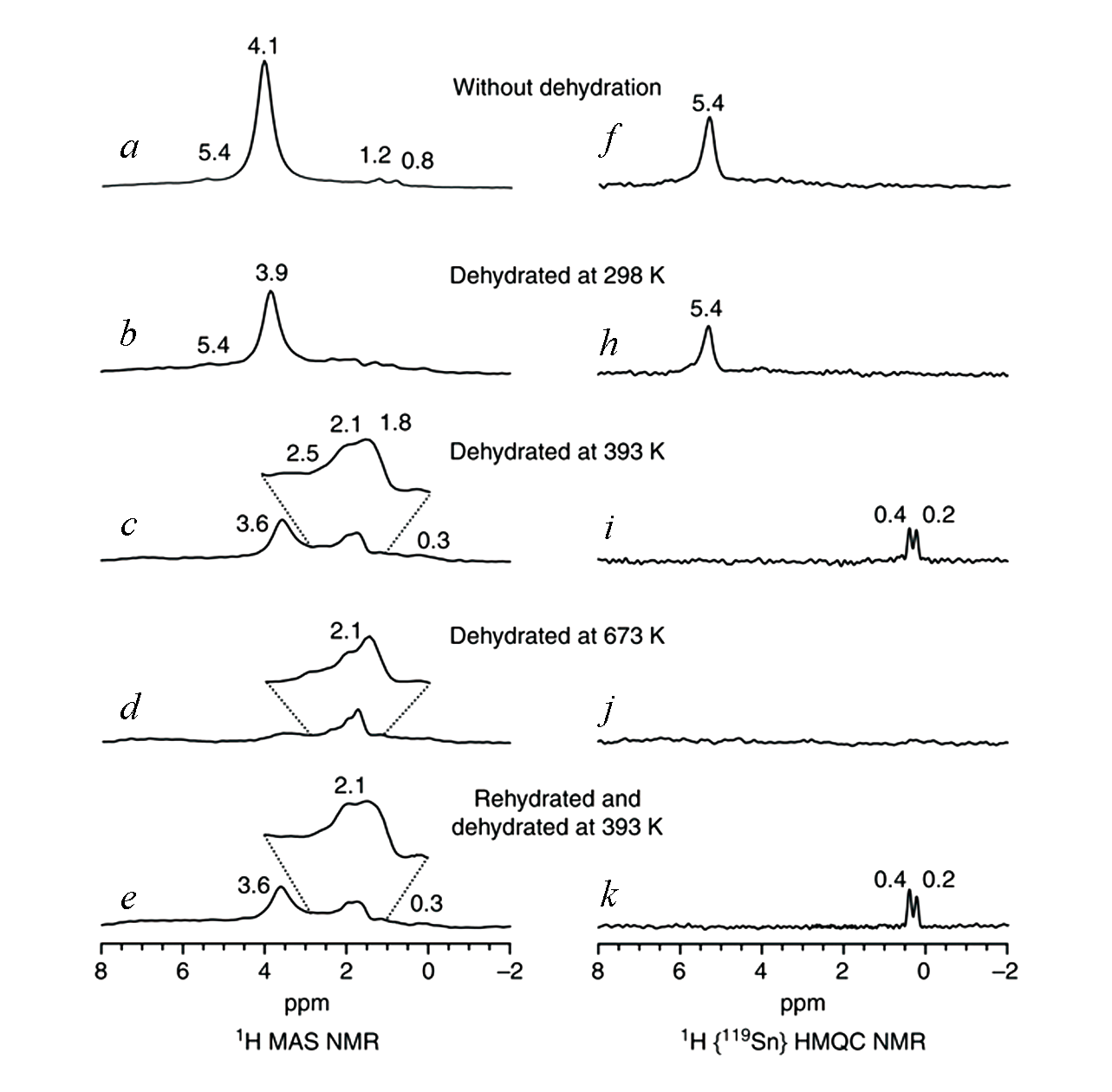
Fig.5 Solid?state proton NMR of open Sn sites in Sn?Betazeolite[38]1H MAS NMR spectra(a—e) and 1H/119Sn D-HMQC MAS NMR spectra(f—k) of 119Sn-Beta with different dehydration and rehydration treatments.Copyright 2018, Springer Nature.
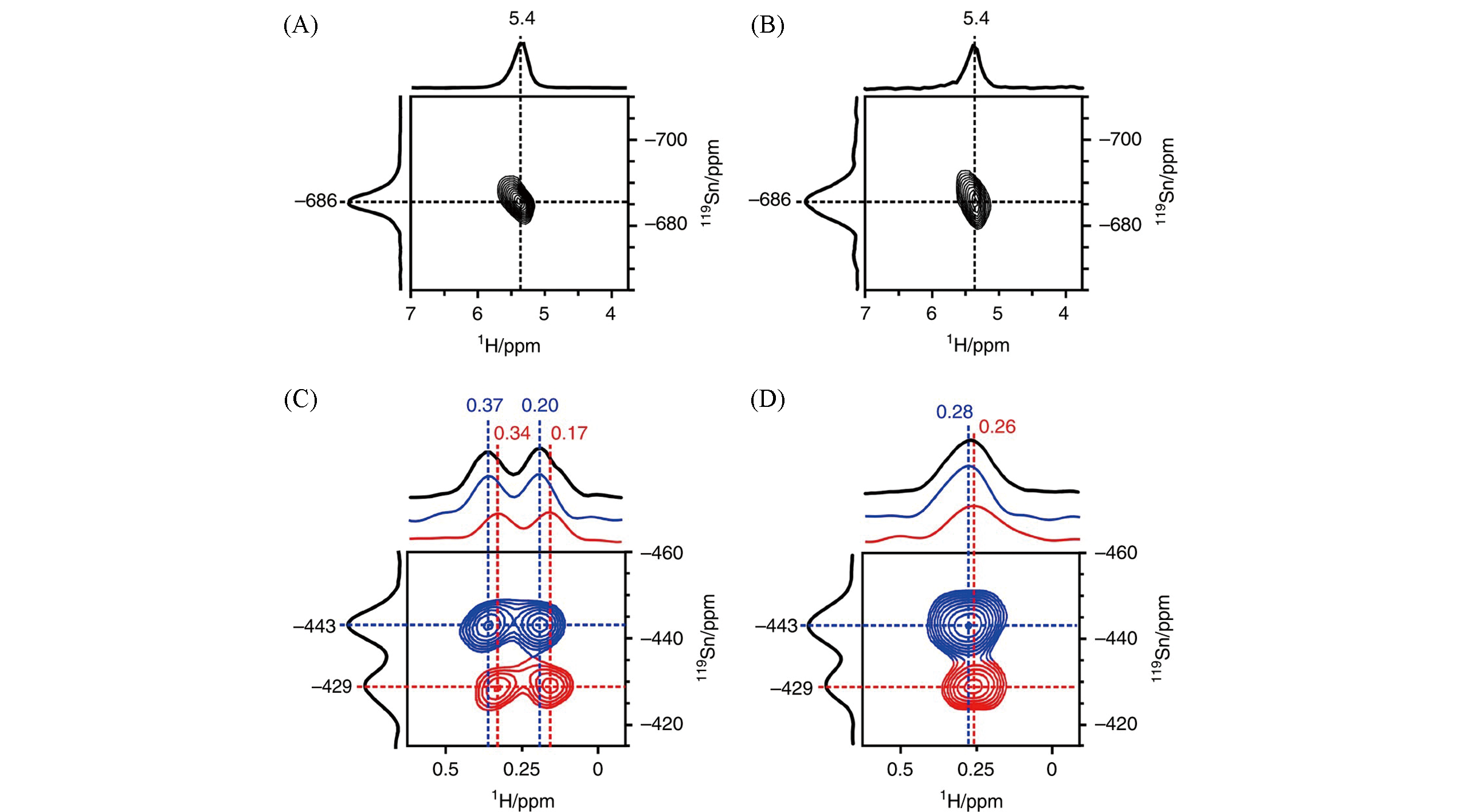
Fig.6 Identification of open Sn sites by proton?detected 1H/119Sn correlation NMR[38]Two-dimensional 1H-119Sn HMQC MAS NMR spectra of 119Sn-β without dehydration(A), dehydrated at 298?K(B), dehydrated at 393?K without 119Sn decoupling(C), and dehydrated at 393?K with 119Sn decoupling(D). Projections of 1H and 119Sn dimensions are shown in black. Representative slices along δ -429(red) and δ -443(blue) in the F1 dimension are also displayed in (C) and (D).Copyright 2018, Springer Nature.
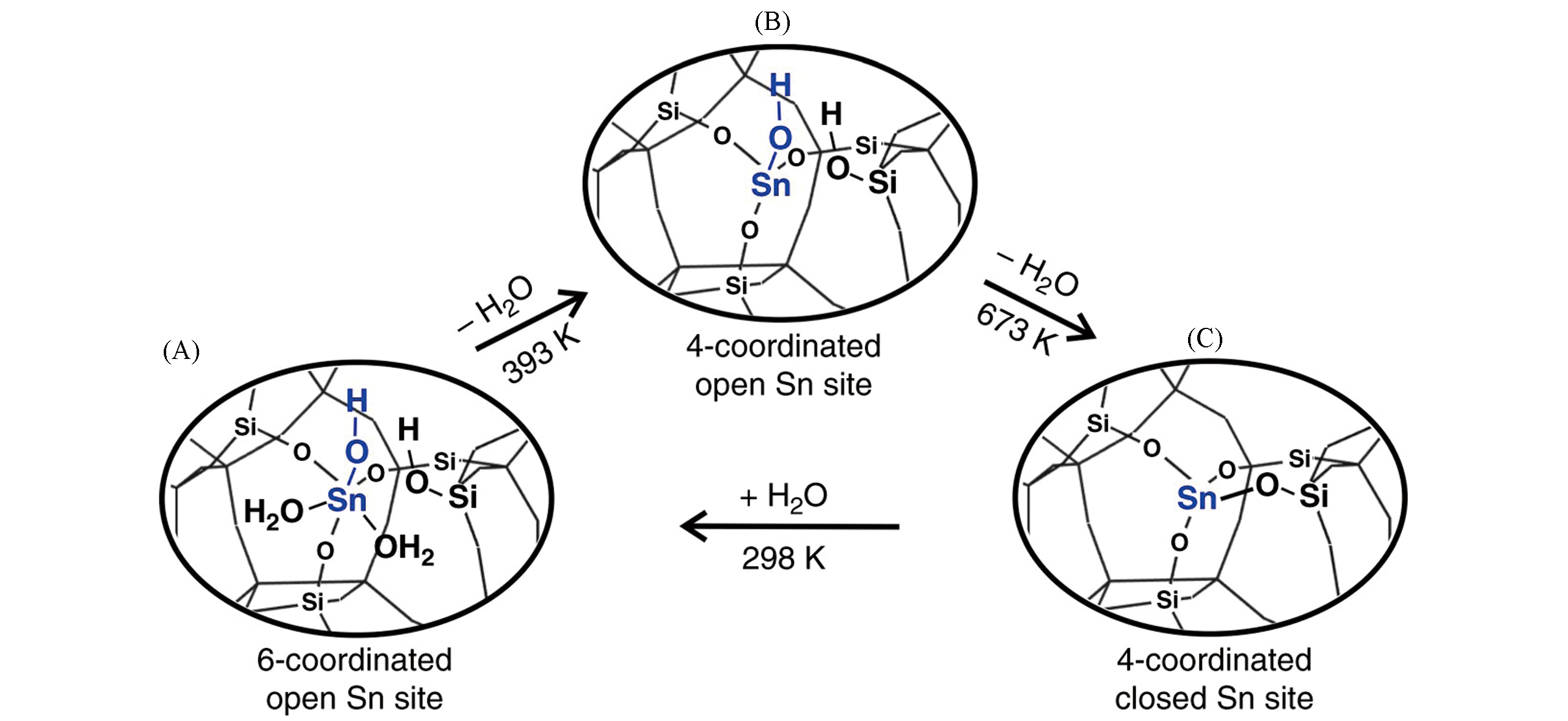
Fig.7 Proposed model for interconversion between open and closed Sn sites in Sn?Beta zeolite[38](A) 6-Coordinated open Sn site; (B) 4-coordinated open Sn site; (C) 4-coordinated closed Sn site. Copyright 2018, Springer Nature.
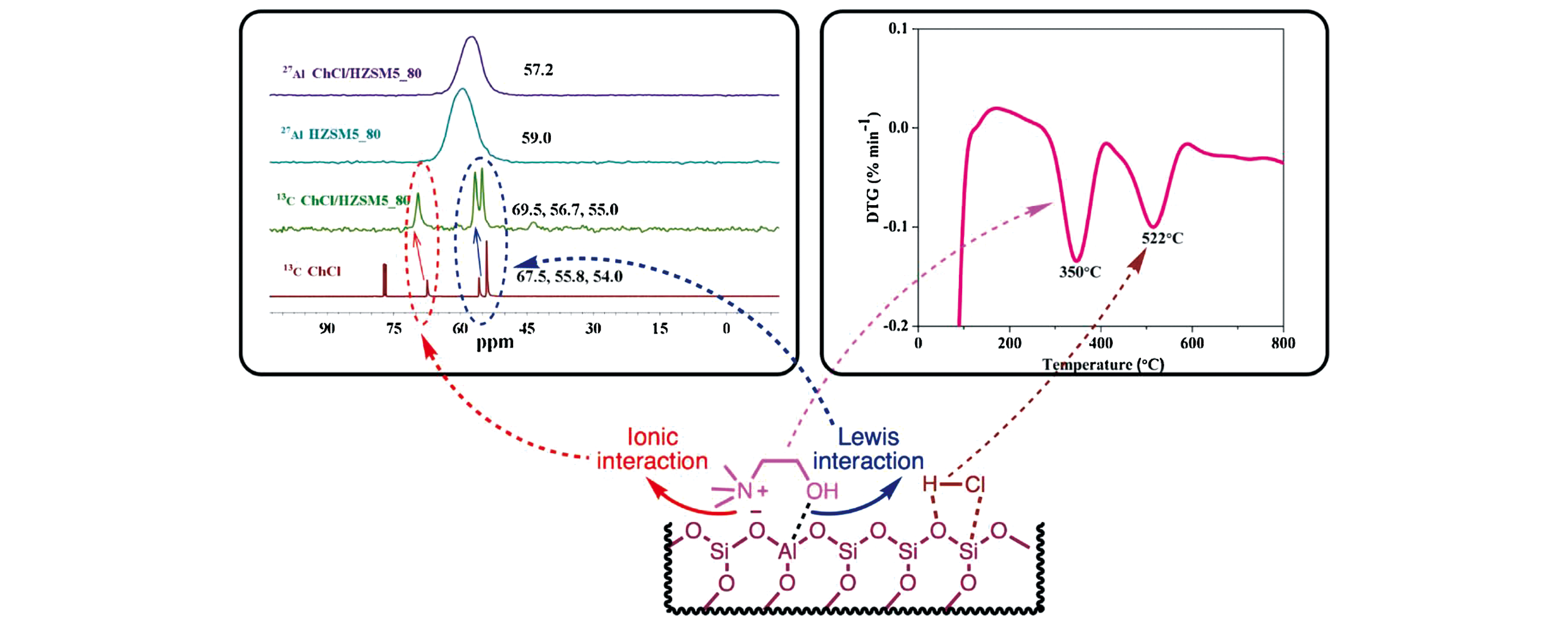
Fig.8 Pictorial representation of the interactions between ChCl and the zeolite as observed from SS?NMR and thermo?gravimetric analysis[44]Copyright 2019, Elsevier.
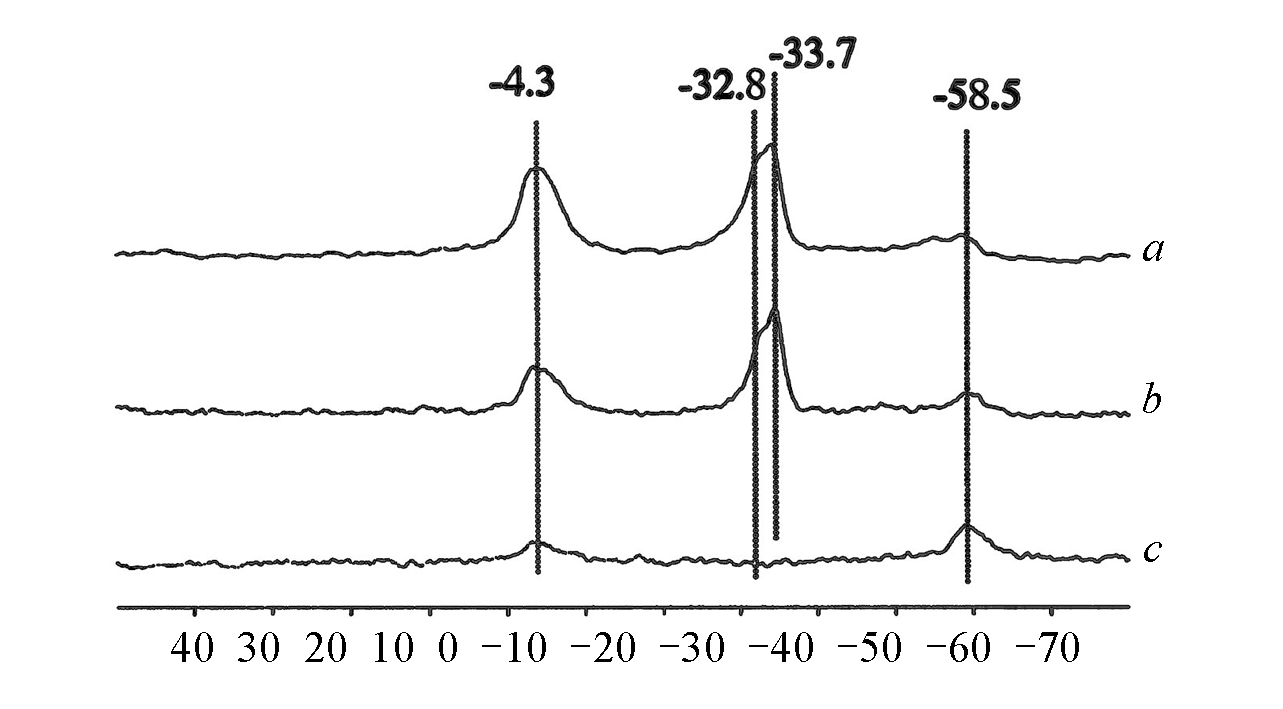
Fig.9 31P MAS NMR spectra of TMP in TS?1 with higher(a) and lower(b) Ti content in the zeolite and silicalite?1(c)[52]Copyright 2002, Springer Nature.
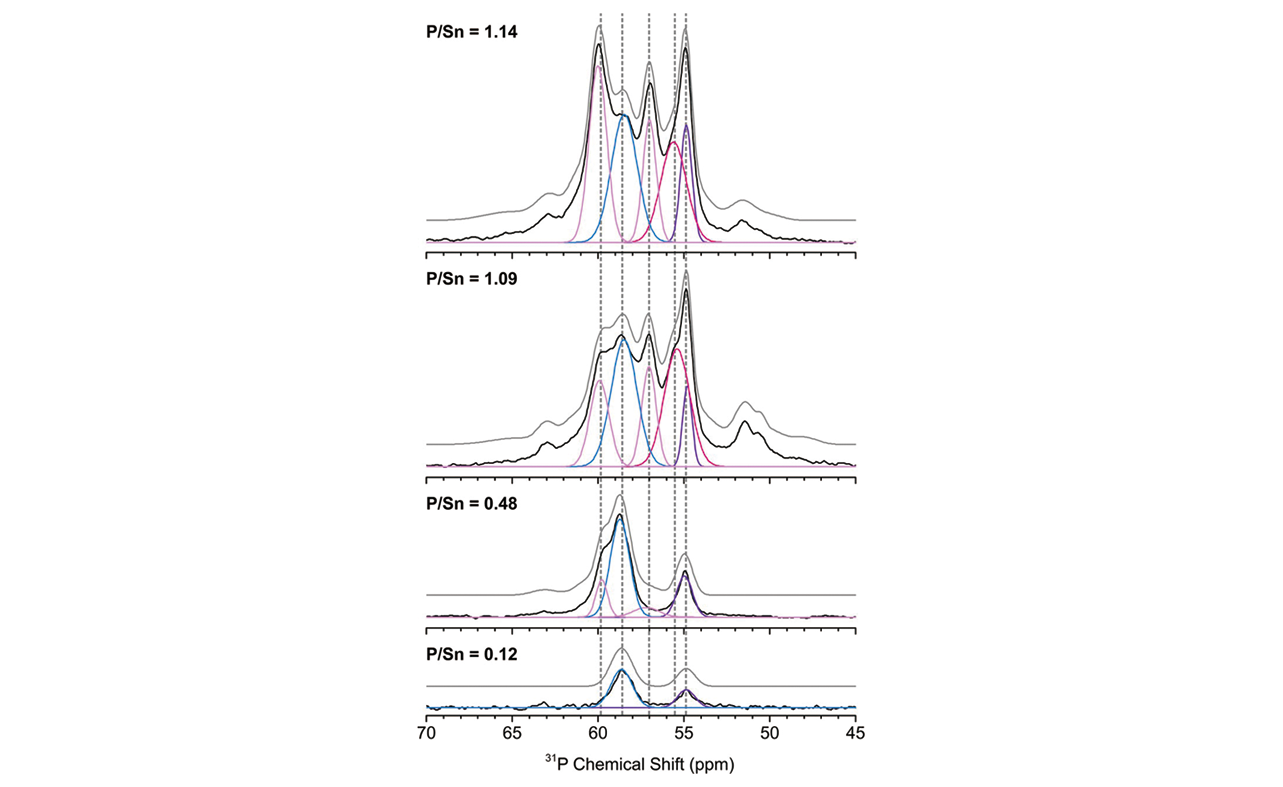
Fig.10 31P MAS NMR spectra of TMPO adsorbed on Sn?Beta at di?erent loading levels[53]Experimental NMR spectra are shown in black(lower traces), while the simulated spectra are shown in gray(upper traces). The gray dashed lines indicate the main resonance positions with corresponding Gaussian peak curves shown in light pink(δiso: 59.9 and 57.2), blue(δiso: 58.6), pink(δiso: 55.8), and violet(δiso: 54.9).Copyright 2018, American Chemical Society.
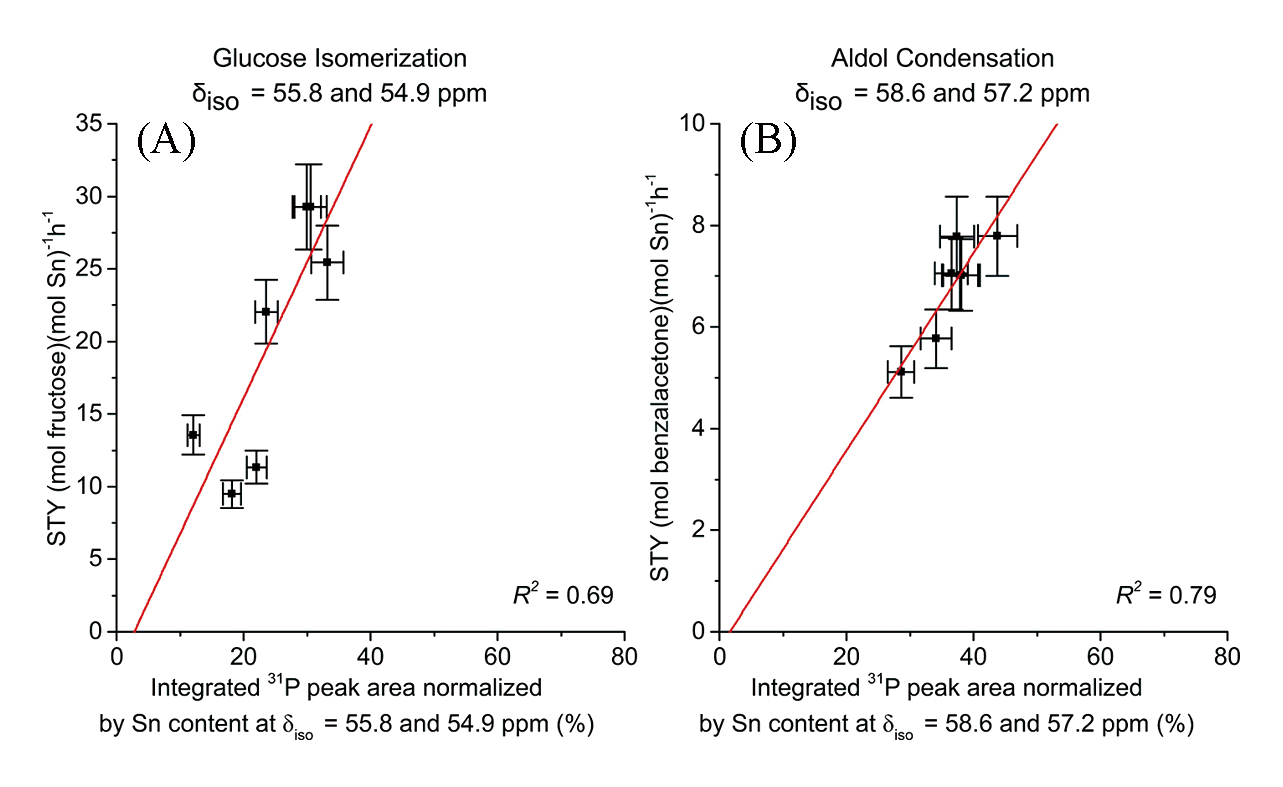
Fig.11 Site time yield(STY) for glucose isomerization(A) and aldol condensation(B) of benzaldehyde and acetone catalyzed by different Sn?Beta catalysts plotted against the integrated 31P peak area normalized by P and Sn content[53]Copyright 2018, American Chemical Society.
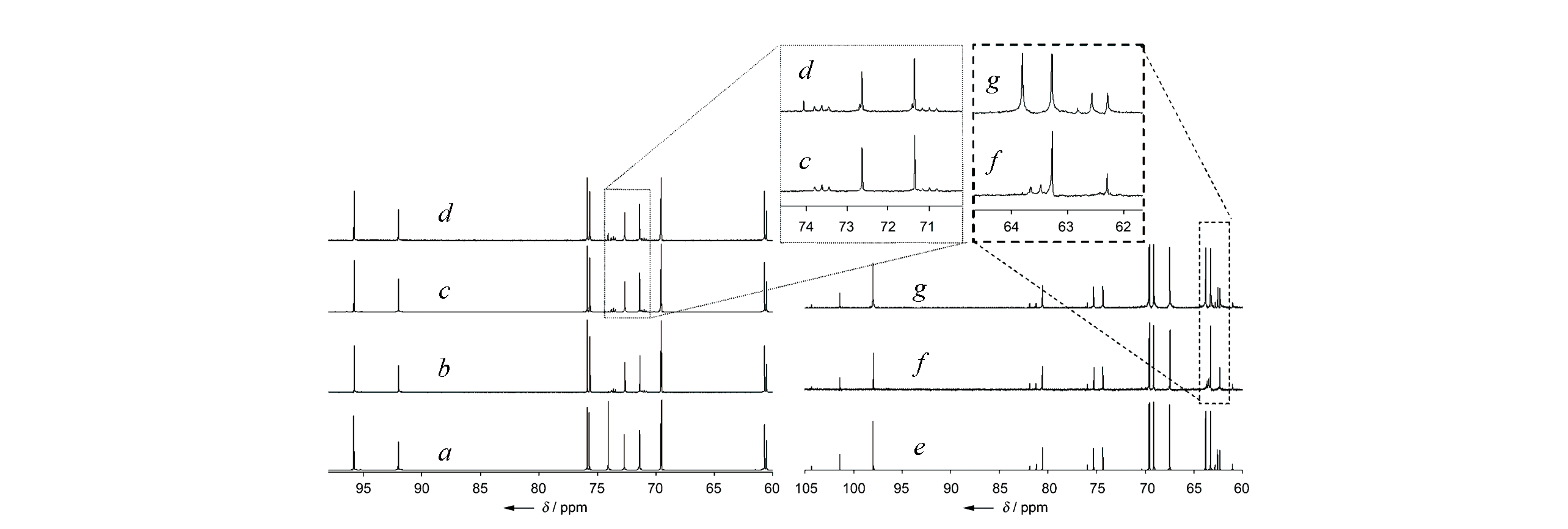
Fig.12 13C NMR spectra of glucose?D2 isomerization on Sn?Beta[12]a. Unlabeled glucose; b. labeled glucose-D2; c. glucose fraction obtained after reacting glucose-D2 with Sn-Beta; d. glucose fraction obtained after reacting labeled glucose-D2 with NaOH; e. unlabeled fructose; f. fructose fraction obtained after reacting labeled glucose-D2 with Sn-Beta; g. fructose fraction obtained after reacting labeled glucose-D2 with NaOH.Copyright 2010, John Wiley & Sons, Inc..
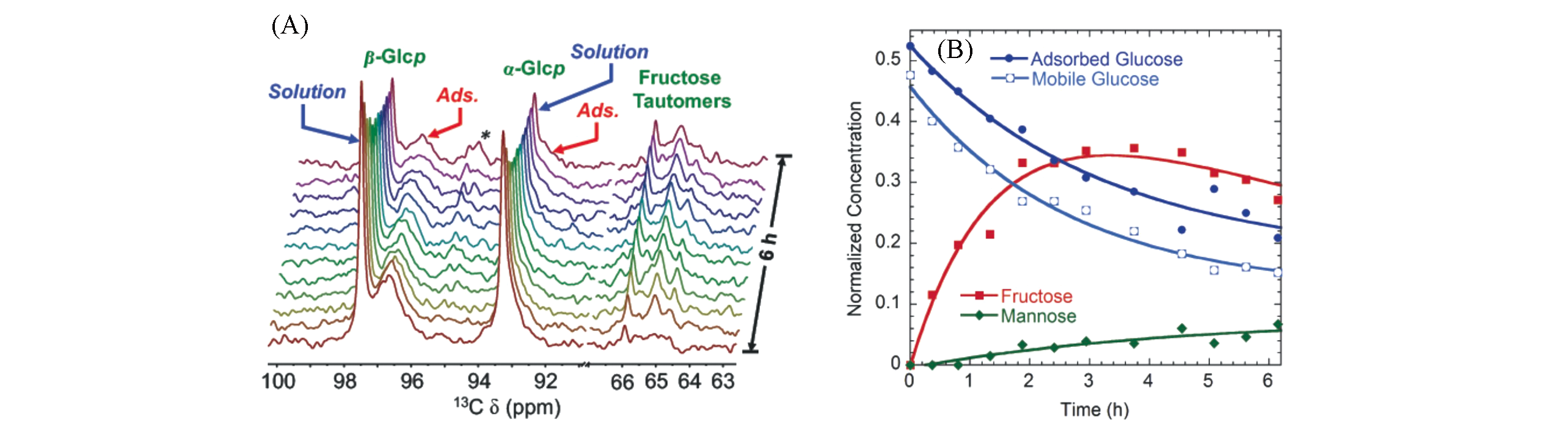
Fig.14 13C MAS NMR study of a mixture of Na?X zeolite with a solution of glucose?1?13C in GVL at 393 K[62](A) Time-resolved operando spectra, showing glucose conversion to fructose; (B) kinetic profiles(points) for glucose adsorbed in the zeolite, glucose present in the solution phase, total fructose, and total mannose.Copyright 2017, American Chemical Society.
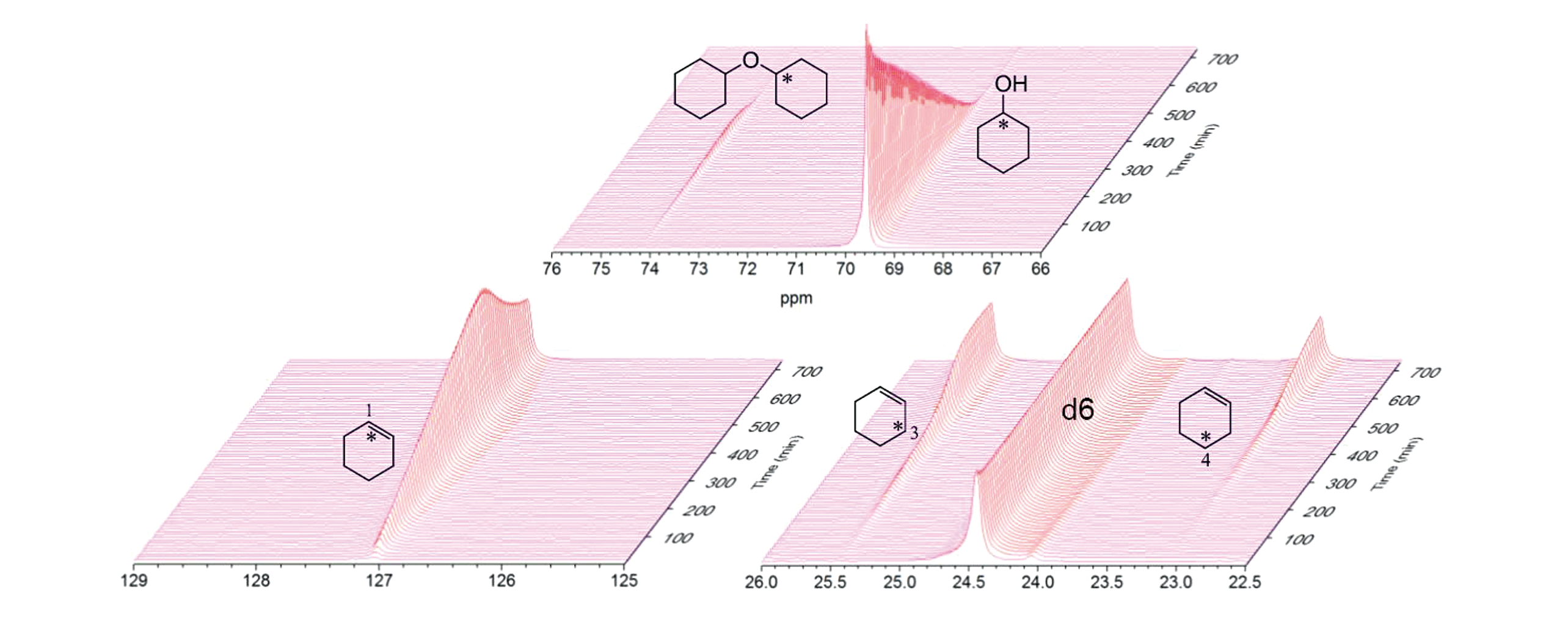
Fig.16 Stacked plot of in situ 13C MAS NMR spectra of 1?13C?cyclohexanol dehydration in decalin as a function of reaction time[68]Only the main detectable compounds are shown. The d6 peak at δ 24.5 is from decalin.Copyright 2018, Springer Nature.
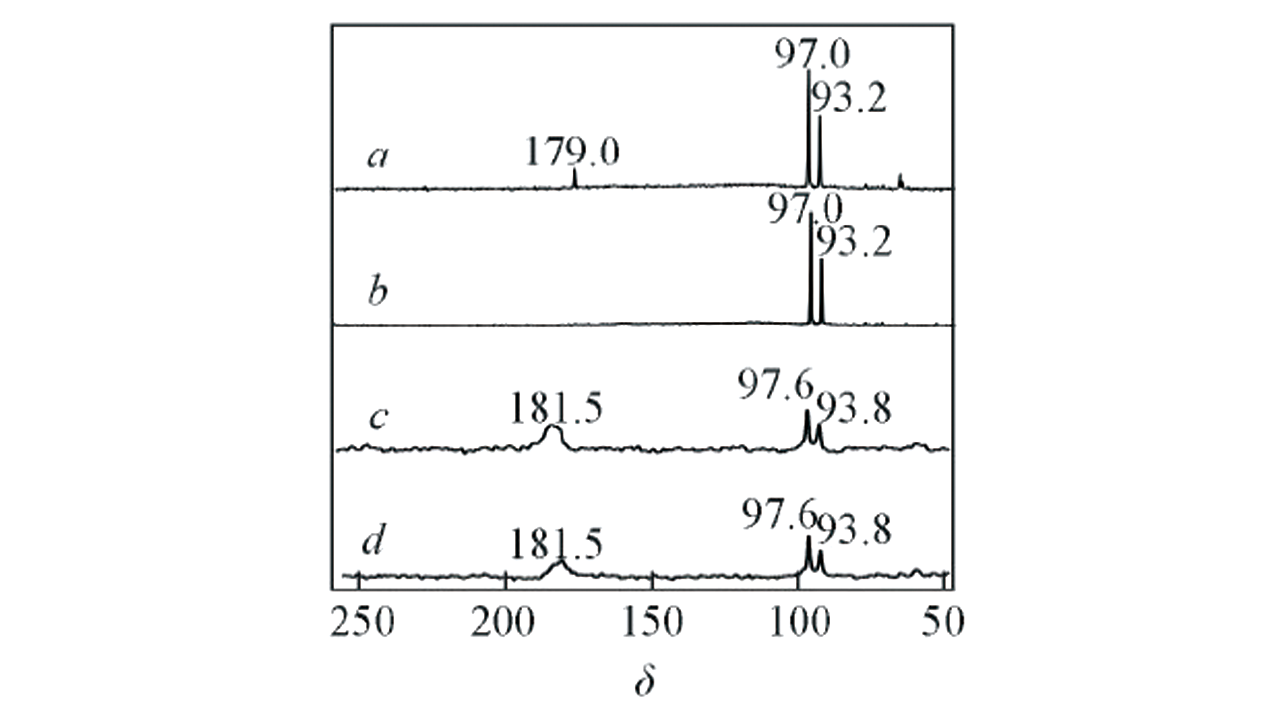
Fig.17 In situ NMR investigation of glucose oxidation on Au?Al/SBA?15[49]13C MAS NMR spectra(a, b) and 1H-13C CP MAS NMR spectra(c, d) with(a, c) and without(b, d) NaOH.Copyright 2020, Higher Education Press.
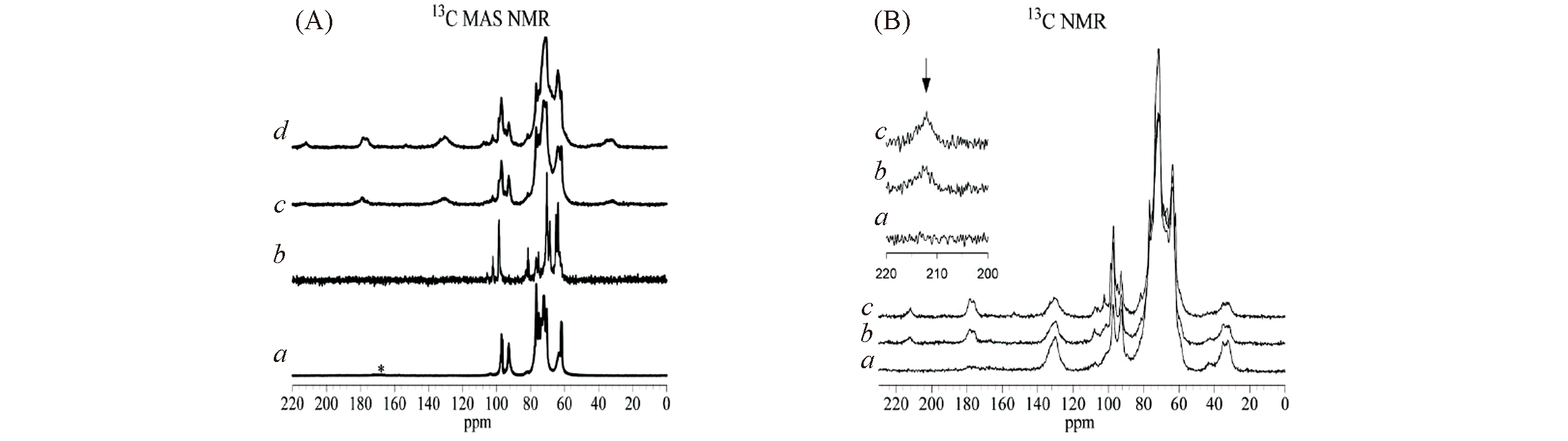
Fig.18 13C NMR spectra of sugars adsorbed in zeolites[13](A) a. Glucose adsorbed into Si-Beta; b. fructose adsorbed into Si-Beta; c. glucose adsorbed into Sn-Beta; d. fructose adsorbed into Sn-Beta. (B) Spectra from fructose adsorbed into Sn-Beta: a. cross polarization contact time of 0.1 ms; b. cross polarization contact time of 1.0 ms; c. no cross polarization.Copyright 2012, National Academy of Sciences.
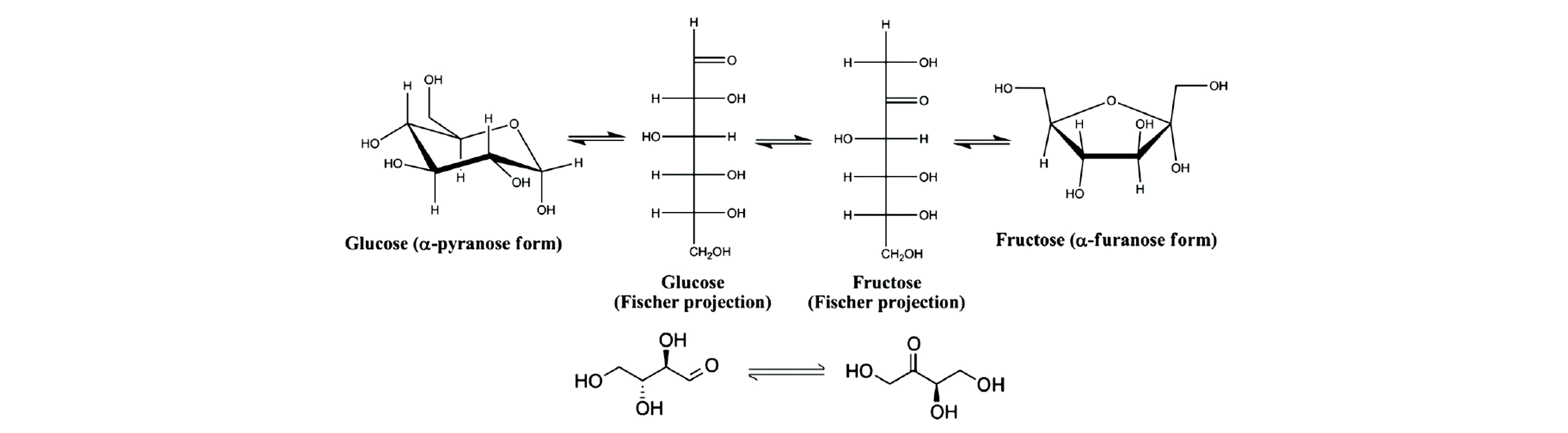
Fig.19 Schematic representations of the isomerization of glucose to fructose(top) and erythrose to erythrulose(bottom)[13]Copyright 2012, National Academy of Sciences.
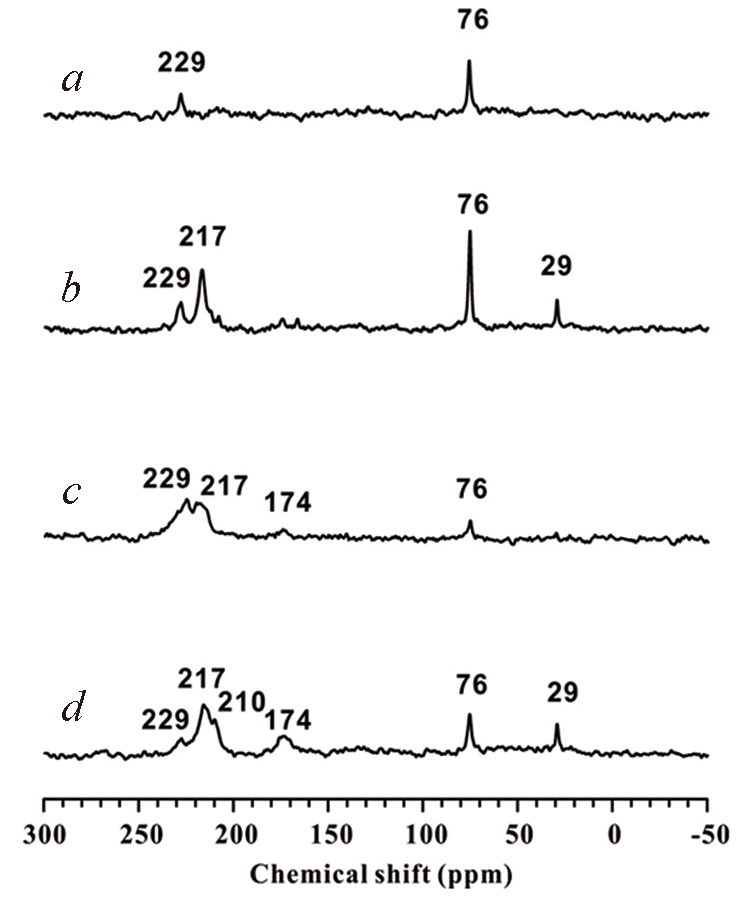
Fig.20 1H?13C CP MAS NMR spectra[77]30(a) and 100 mmol/g(b) of 2-13C acetone adsorbed on Sn-Beta zeolite dehydrated at 393 K; 30(c) and 100 mmol/g(d) of 2-13C acetone adsorbed on Sn-Beta zeolite dehydrated at 673 K.Copyright 2020, John Wiley & Sons, Inc.
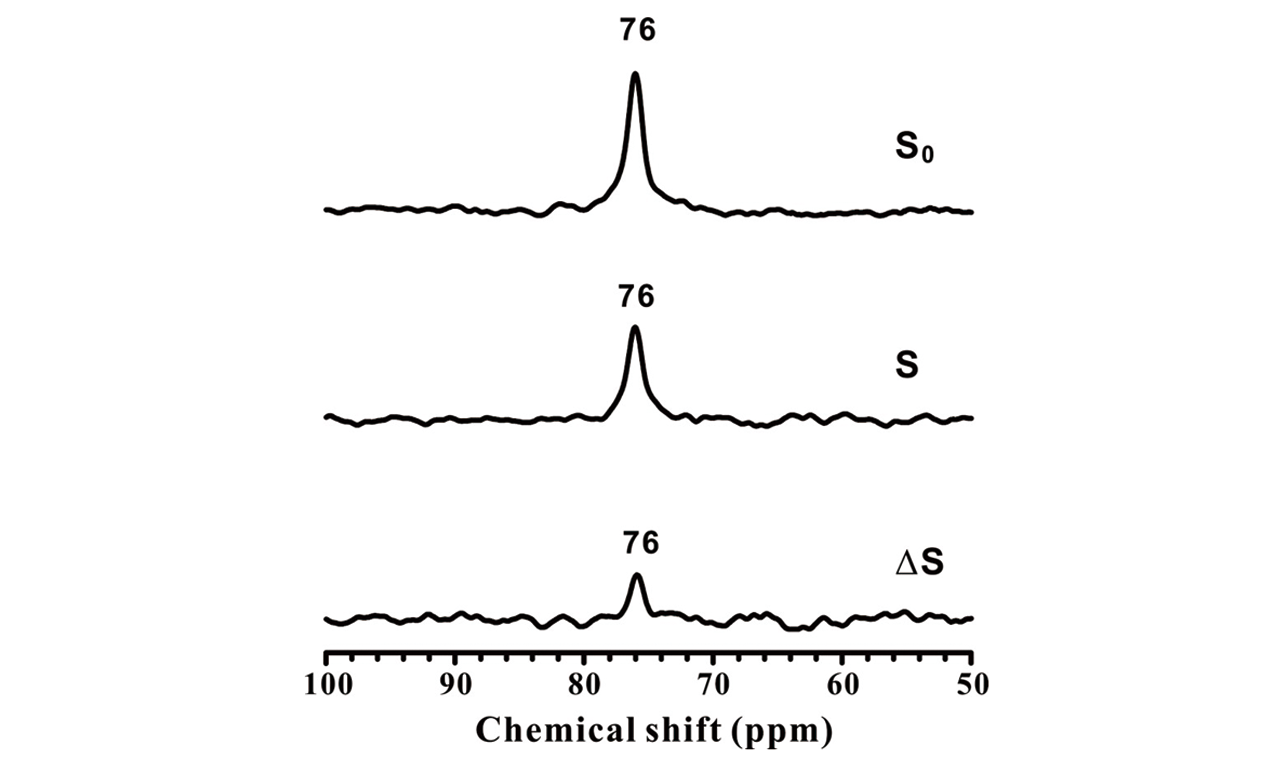
Fig.21 13C?119Sn S?REDOR spectra[77]S0, S, and ΔS spectra of 100 mmol/g of 2-13C acetone adsorbed on 119Sn-Beta dehydrated at 393 K probing the spatial proximity between tin atoms and the gem-diol-type group.Copyright 2020, John Wiley & Sons, Inc.
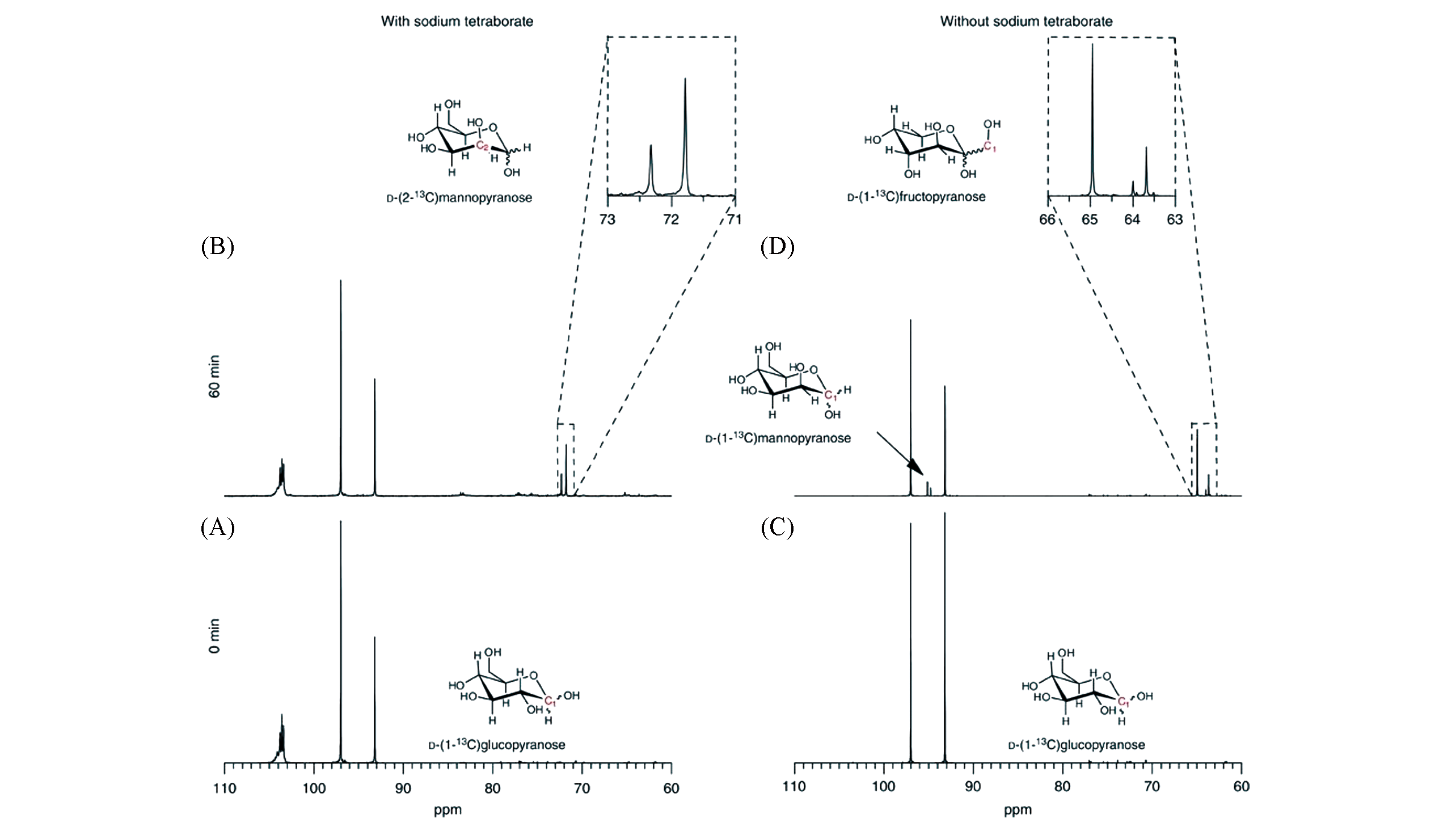
Fig.23 Solution 13C NMR spectra of reactants and products in epimerization and isomerization reactions[43](A) D-(1-13C) glucose with SB in a glucose/SB ratio of 4∶1; (B) product mixture after reacting D-(1-13C) glucose with SB in a glucose/SB ratio of 4∶1 and Sn-Beta; (C) D-(1-13C) glucose; (D) product mixture after reacting D-(1-13C) glucose with Sn-Beta.Copyright 2012, Springer Nature.
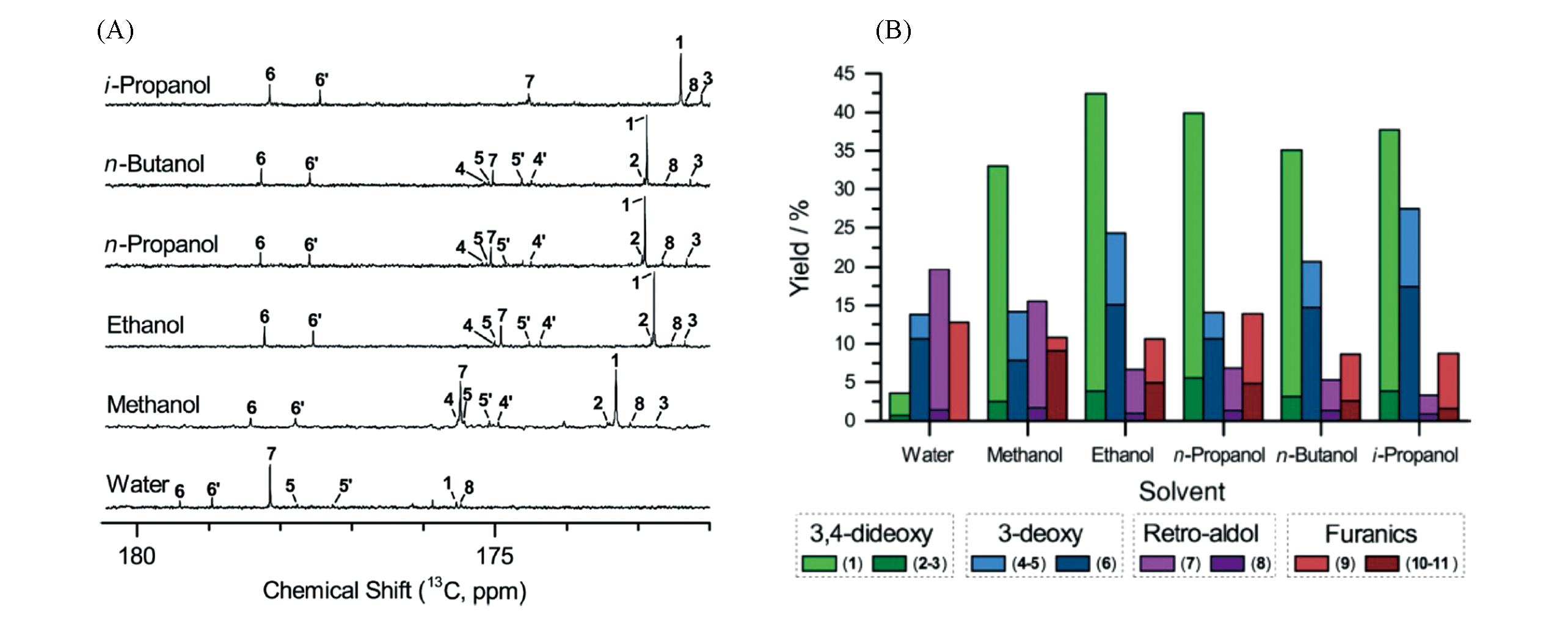
Fig.25 Solution 13C NMR spectra of product stream from xylose conversion catalyzed by Sn?Beta(A) and the product distribution of converting xylose(B)[83]Copyright 2017, John Wiley & Sons, Inc..
| 1 | Rogelj J., Nabel J., Chen C., Hare W., Markmann K., Meinshausen M., Schaeffer M., Macey K., Höhne N., Nature,2010,464(7292), 1126—1128 |
| 2 | Ragauskas A. J., Williams C. K., Davison B. H., Britovsek G., Cairney J., Eckert C. A., Frederick W. J., Hallett J. P., Leak D. J., Liotta C. L., Mielenz J. R., Murphy R., Templer R., Tschaplinski T., Science, 2006, 311(5760), 484—489 |
| 3 | Zhou C. H., Xia X., Lin C. X., Tong D. S., Beltramini J., Chem. Soc. Rev., 2011, 40(11), 5588—5617 |
| 4 | Liu X. R., Wang X. C., Yao S. X., Jiang Y. J., Guan J., Mu X. D., RSC Adv., 2014, 4(90), 49501—49520 |
| 5 | Alvira P., Tomás⁃Pejó E., Ballesteros M., Negro M. J., Bioresource. Technol., 2010, 101(13), 4851 |
| 6 | Román⁃Leshkov Y., Chheda J. N., Dumesic J. A., Science, 2006, 312(5782), 1933—1937 |
| 7 | Roman⁃Leshkov Y., Barrett C. J., Liu Z. Y., Dumesic J. A., Nature,2007, 447(7147), 982—985 |
| 8 | Gu M. Y., Xia Q. N., Liu X. H., Guo Y., Wang Y. Q., ChemSusChem, 2017, 10(20), 4102—4108 |
| 9 | Zhao H., Holladay J. E., Brown H., Zhang Z. C., Science, 2007, 316(5831), 1597—1600 |
| 10 | Wu L., Song J., Zhang B., Zhou B., Zhou H., Fan H., Yang Y., Han B., Green Chem., 2014, 16(8), 3935—3941 |
| 11 | Moliner M., Román⁃Leshkov Y., Davis M. E., Proc. Natl. Acad. Sci. USA, 2010, 107(14), 6164—6168 |
| 12 | Román⁃Leshkov Y., Moliner M., Labinger J. A., Davis M. E., Angew. Chem. Int. Ed.,2010, 49(47), 8954—8957 |
| 13 | Bermejo⁃Deval R., Assary R. S., Nikolla E., Moliner M., Roman⁃Leshkov Y., Hwang S. J., Palsdottir A., Silverman D., Lobo R. F., Curtiss L. A., Davis M. E., Proc. Natl. Acad. Sci. USA, 2012, 109(25), 9727—9732 |
| 14 | Nikolla E., Roman⁃Leshkov Y., Moliner M., Davis M. E., ACS Catal., 2011, 1(4), 408—410 |
| 15 | Holm M. S., Saravanamurugan S., Taarning E., Science, 2010, 328(5978), 602—605 |
| 16 | Zhang X., Wilson K., Lee A. F., Chem. Rev., 2016, 116(19), 12328—12368 |
| 17 | Mika L. T., Csefalvay E., Nemeth A., Chem. Rev., 2018, 118(2), 505—613 |
| 18 | Ennaert T., van Aelst J., Dijkmans J., de Clercq R., Schutyser W., Dusselier M., Verboekend D., Sels B. F., Chem. Soc. Rev., 2016, 45(3), 584—611 |
| 19 | Dapsens P. Y., Mondelli C., Chem. Soc. Rev., 2015, 44(20), 7025—7043 |
| 20 | Kubička D., Kubičková I., Čejka J., Cata. Rev.,2013, 55(1), 1—78 |
| 21 | Bare S. R., Kelly S. D., Sinkler W., Low J. J., Modica F. S., Valencia S., Corma A., Nemeth L. T., J. Am. Chem. Soc., 2005, 127(37), 12924—12932 |
| 22 | Boronat M., Concepcion P., Corma A., Renz M., Valencia S., J. Catal., 2005, 234(1), 111—118 |
| 23 | Corma A., Nemeth L. T., Renz M., Valencia S., Nature, 2001, 412(6845), 423—425 |
| 24 | Bermejo⁃Deval R., Gounder R., Davis M. E., ACS Catal.,2012, 2(12), 2705—2713 |
| 25 | Roy S., Bakhmutsky K., Mahmoud E., Lobo R. F., Gorte R. J., ACS Catal.,2013, 3(4), 573—580 |
| 26 | Tang B., Dai W., Wu G., Guan N., Li L., Hunger M., ACS Catal.,2014, 4(8), 2801—2810 |
| 27 | Wang L., Zhang J., Wang X., Zhang B., Ji W., Meng X., Li J., Su D. S., Bao X., Xiao F. S., J. Mater. Chem. A, 2014, 2(11), 3725—3729 |
| 28 | Dijkmans J., Dusselier M., Gabriëls D., Houthoofd K., Magusin P. C. M. M., Huang S., Pontikes Y., Trekels M., Vantomme A., Giebeler L., Oswald S., Sels B. F., ACS Catal.,2015, 5(2), 928—940 |
| 29 | Kolyagin Y. G., Yakimov A. V., Tolborg S., Vennestrøm P. N. R., Ivanova I. I., J. Phys. Chem. Lett.,2016, 7(7), 1249—1253 |
| 30 | Padovan D., Botti L., Hammond C., ACS Catal., 2018, 8(8), 7131—7140 |
| 31 | Gunther W. R., Michaelis V. K., Caporini M. A., Griffin R. G., Roman⁃Leshkov Y., J. Am. Chem. Soc.,2014, 136(17), 6219—6222 |
| 32 | Wolf P., Valla M., Rossini A. J., Comas⁃Vives A., Nunez⁃Zarur F., Malaman B., Lesage A., Emsley L., Copéret C., Hermans I., Angew. Chem. Int. Ed., 2014, 53(38), 10179—10183 |
| 33 | Wolf P., Liao W. C., Ong T. C., Valla M., Harris J. W., Gounder R., van der Graaff W. N. P., Pidko E. A., Hensen E. J. M., Ferrini P., Dijkmans J., Sels B., Hermans I., Copéret C., Helv. Chim. Acta, 2016, 99(12), 916—927 |
| 34 | Wolf P., Valla M., Núñez⁃Zarur F., Comas⁃Vives A., Rossini A. J., Firth C., Kallas H., Lesage A., Emsley L., Copéret C., Hermans I., ACS Catal.,2016, 6(7), 4047—4063 |
| 35 | Xu J., Wang Q., Deng F., Acc. Chem. Res.,2019, 52(8), 2179—2189 |
| 36 | Gao P., Wang Q., Xu J., Qi G., Wang C., Zhou X., Zhao X., Feng N., Liu X., Deng F., ACS Catal., 2018, 8(1), 69—74 |
| 37 | Qi G., Wang Q., Xu J., Trébosc J., Lafon O., Wang C., Amoureux J. P., Deng F., Angew. Chem. Int. Ed.,2016, 55(51), 15826—15830 |
| 38 | Qi G., Wang Q., Xu J., Wu Q., Wang C., Zhao X., Meng X., Xiao F., Deng F., Commun. Chem., 2018, 1(1), 22 |
| 39 | Khokhlova E. A., Kachala V. V., Ananikov V. P., Russ. Chem. Bull., 2013, 62(3), 830—835 |
| 40 | Li Y. N., Wang J. Q., He L. N., Yang Z. Z., Liu A. H., Yu B., Luan C. R., Green Chem., 2012, 14(10), 2752—2758 |
| 41 | Siankevich S., Fei Z., Scopelliti R., Laurenczy G., Katsyuba S., Yan N., Dyson P. J., ChemSusChem, 2014, 7(6), 1647—1654 |
| 42 | Abou⁃Yousef H., Hassan E. B., J. Ind. Eng. Chem., 2014, 20(4), 1952—1957 |
| 43 | Gunther W. R., Wang Y. R., Ji Y. W., Michaelis V. K., Hunt S. T., Griffin R. G., Roman⁃Leshkov Y., Nat. Commun., 2012, 3(1), 1109 |
| 44 | Peela N. R., Yedla S. K., Velaga B., Kumar A., Golder A. K., Appl. Catal. A: Gen., 2019, 580, 59—70 |
| 45 | Hutchings G. J., Gold. Bull., 2004, 37(1), 3—11 |
| 46 | Mirescu A., Berndt H., Martin A., Prüße U., Appl. Catal. A: Gen.,2007, 317(2), 204—209 |
| 47 | Qi P., Chen S., Chen J., Zheng J., Zheng X., Yuan Y., ACS Catal., 2015, 5(4), 2659—2670 |
| 48 | Odrozek K., Maresz K., Koreniuk A., Prusik K., Mrowiec⁃Białoń J., Appl. Catal. A: Gen., 2014, 475, 203—210 |
| 49 | Ye X. D., Qi G. D., Xu J., Deng F., Chem. J. Chinese. Universities, 2020, 41(5), 960—966 |
| 50 | Haw J. F., Phys. Chem. Chem. Phys.,2002, 4(22), 5431—5441 |
| 51 | Zheng A., Liu S. B., Deng F., Solid State Nucl. Magn. Reson, 2013, 55/56, 12—27 |
| 52 | Zhuang J., Yan Z., Liu X., Liu X., Han X., Bao X., Mueller U., Catal. Lett., 2002, 83(1), 87—91 |
| 53 | Lewis J. D., Ha M., Luo H., Faucher A., Michaelis V. K., Roman⁃Leshkov Y., ACS Catal., 2018, 8(4), 3076—3086 |
| 54 | Zhang J., Weitz E., ACS Catal.,2012, 2(6), 1211—1218 |
| 55 | Hannah N., Nikolakis V., Vlachos D. G., ACS Catal., 2016,6(3), 1497—1504 |
| 56 | Tosi I., Elliot S. G., Jessen B. M., Riisager A., Taarning E., Meier S., Top. Catal.,2019, 62(7—11), 669—677 |
| 57 | Saravanamurugan S., Riisager A., Taarning E., Meier S., Chem. Commun.,2016, 52(86), 12773—12776 |
| 58 | Han X. W., Yan Z. M., Zhang W. P., Bao X. H., Curr. Org. Chem.,2001, 5(10), 1017—1037 |
| 59 | Hunger M., Catal. Today, 2004, 97(1), 3—12 |
| 60 | Xu J., Zheng A. M., Wang X. M., Qi G. D., Su J. H., Du J. F., Gan Z. H., Wu J. F., Wang W., Deng F., Chem. Sci., 2012, 3(10), 2932—2940 |
| 61 | Zhou X., Wang C., Chu Y., Xu J., Wang Q., Qi G., Zhao X., Feng N., Deng F., Nat. Commun., 2019, 10(1), 1961 |
| 62 | Qi L., Alamillo R., Elliott W. A., Andersen A., Hoyt D. W., Walter E. D., Han K. S., Washton N. M., Rioux R. M., Dumesic J. A., Scott S. L., ACS Catal., 2017, 7(5), 3489—3500 |
| 63 | Sun Q., Wang S., Aguila B., Meng X., Ma S., Xiao F. S., Nat. Commun.,2018, 9(1), 3236 |
| 64 | Hu J. Z., Hu M. Y., Zhao Z., Xu S., Vjunov A., Shi H., Camaioni D. M., Peden C. H. F., Lercher J. A., Chem. Commun., 2015, 51(70), 13458—13461 |
| 65 | Prodinger S., Vjunov A., Hu J. Z., Fulton J. L., Camaioni D. M., Derewinski M. A., Lercher J. A., Chem. Mater.,2018, 30(3), 888—897 |
| 66 | Wang M., Jaegers N. R., Lee M. S., Wan C., Hu J. Z., Shi H., Mei D., Burton S. D., Camaioni D. M., Gutiérrez O. Y., Glezakou V. A., Rousseau R., Wang Y., Lercher J. A., J. Am. Chem. Soc., 2019, 141(8), 3444—3455 |
| 67 | Jaegers N. R., Mueller K. T., Wang Y., Hu J. Z., Acc. Chem. Res., 2020, 53(3), 611—619 |
| 68 | Liu Y., Baráth E., Shi H., Hu J., Camaioni D. M., Lercher J. A., Nat. Catal., 2018, 1(2), 141—147 |
| 69 | Boronat M., Corma A., Renz M., J. Phys. Chem. B, 2006, 110(42), 21168—21174 |
| 70 | Bermejo⁃Deval R., Orazov M., Gounder R., Hwang S. J., Davis M. E., ACS Catal.,2014, 4(7), 2288—2297 |
| 71 | Harris J. W., Cordon M. J., Di Iorio J. R., Vega⁃Vila J. C., Ribeiro F. H., Gounder R., J. Catal., 2016, 335, 141—154 |
| 72 | Van de Vyver S., Román⁃Leshkov Y., Angew. Chem. Int. Ed., 2015, 54(43), 12554—12561 |
| 73 | Van de Vyver S., Odermatt C., Romero K., Prasomsri T., Román⁃Leshkov Y., ACS Catal., 2015, 5(2), 972—977 |
| 74 | Rai N., Caratzoulas S., Vlachos D. G., ACS Catal., 2013, 3(10), 2294—2298 |
| 75 | Li Y. P., Head⁃Gordon M., Bell A. T., ACS Catal., 2014, 4(5), 1537—1545 |
| 76 | Hunger M., Weitkamp J., Angew. Chem. Int. Ed., 2001, 40(16), 2954—2971 |
| 77 | Qi G., Chu Y., Wang Q., Wang X., Li Y., Trébosc J., Lafon O., Xu J., Deng F., Angew. Chem. Int. Ed., 2020, 59(44), 19532—19538 |
| 78 | Saha B., De S., Fan M., Fuel,2013, 111, 598—605 |
| 79 | Ren Q., Huang Y., Ma H., Wang F., Gao J., Xu J., Bioresources,2013, 8(2), 1563—1572 |
| 80 | Armstrong R. D., Hirayama J., Knight D. W., Hutchings G. J., ACS Catal., 2019, 9(1), 325—335 |
| 81 | Armstrong R. D., Kariuki B. M., Knight D. W., Hutchings G. J., Eur. J. Org. Chem.,2017, 2017(45), 6811—6814 |
| 82 | Norton A. M., Hannah N., Xiao N. L., Vlachos D. G., RSC Adv., 2018, 8(31), 17101—17109 |
| 83 | Elliot S. G., Tolborg S., Sadaba I., Taarning E., Meier S., ChemSusChem,2017, 10(14), 2990—2996 |
| 84 | Elliot S. G., Tosi I., Riisager A., Taarning E., Meier S., Top. Catal.,2019, 62(7—11), 590—598 |
| 85 | Xu J., Deng F., Acta Phys. Sin., 2020, 36(4), 1912074 |
| [1] | 姚伊婷, 吕佳敏, 余申, 刘湛, 李昱, 李小云, 苏宝连, 陈丽华. 等级孔微孔-介孔Fe2O3/ZSM-5中空分子筛催化材料的制备及催化苄基化性能[J]. 高等学校化学学报, 2022, 43(8): 20220090. |
| [2] | 周紫璇, 杨海艳, 孙予罕, 高鹏. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220235. |
| [3] | 杨丹, 刘旭, 戴翼虎, 祝艳, 杨艳辉. 金团簇电催化二氧化碳还原反应的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220198. |
| [4] | 任娜娜, 薛洁, 王治钒, 姚晓霞, 王繁. 热力学数据对1, 3-丁二烯燃烧特性的影响[J]. 高等学校化学学报, 2022, 43(6): 20220151. |
| [5] | 陈玮琴, 吕佳敏, 余申, 刘湛, 李小云, 陈丽华, 苏宝连. 有机杂化介孔Beta分子筛的合成及在苯甲醇烷基化反应中的应用[J]. 高等学校化学学报, 2022, 43(6): 20220086. |
| [6] | 李志光, 齐国栋, 徐君, 邓风. Sn-Al-β分子筛酸性在葡萄糖转化反应中作用的固体NMR研究[J]. 高等学校化学学报, 2022, 43(6): 20220138. |
| [7] | 李加富, 张凯, 王宁, 孙启明. 分子筛限域单原子金属催化剂的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220032. |
| [8] | 孟祥龙, 杨歌, 郭海玲, 刘晨光, 柴永明, 王纯正, 郭永梅. 纳米分子筛的合成及硫化氢吸附性能[J]. 高等学校化学学报, 2022, 43(3): 20210687. |
| [9] | 周颖, 贺培楠, 丰海松, 张欣. 双原子位点M-N-C电催化剂在CO2还原反应中活性位点的最佳分布[J]. 高等学校化学学报, 2022, 43(2): 20210640. |
| [10] | 孙翠红, 吕立强, 刘迎, 王妍, 杨静, 张绍文. 硝酸异丙酯与Cl原子、 OH和NO3自由基反应的机理及动力学[J]. 高等学校化学学报, 2022, 43(2): 20210591. |
| [11] | 魏李娜, 彭莉, 朱锋, 顾鹏飞, 顾学红. 中空纤维Au-CeZr/FAU催化膜的制备及在富氢气氛CO选择性氧化反应中的应用[J]. 高等学校化学学报, 2022, 43(10): 20220175. |
| [12] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [13] | 孟繁伟, 高琦, 叶青, 李晨曦. Cu-SAPO-18催化剂氨选择性催化还原NOx钾中毒机理的研究[J]. 高等学校化学学报, 2021, 42(9): 2832. |
| [14] | 罗强强, 金少青, 孙洪敏, 杨为民. 液相酸溶液后补钛合成Ti-MWW分子筛[J]. 高等学校化学学报, 2021, 42(9): 2742. |
| [15] | 王美银, 黄道丰, 陈欣, 周俊夫, 任远航, 叶林, 岳斌, 贺鹤勇. 介孔磷钨酸铯盐的液相组装及酸性研究[J]. 高等学校化学学报, 2021, 42(9): 2734. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||