

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (2): 475.doi: 10.7503/cjcu20200652
收稿日期:2020-09-04
出版日期:2021-02-10
发布日期:2020-12-28
通讯作者:
于然波
E-mail:ranboyu@ustb.edu.cn
基金资助:Received:2020-09-04
Online:2021-02-10
Published:2020-12-28
Contact:
YU Ranbo
E-mail:ranboyu@ustb.edu.cn
Supported by:摘要:
电催化水分解制氢是可以形成闭环的生产过程, 起始原料与副产物均为水、 过程清洁无污染, 是极具希望的产氢策略. 目前制约其发展的瓶颈之一是价格昂贵的Pt基贵金属催化剂. 为推动电催化分解水制氢的普及, 亟待开发低成本非贵金属催化剂. 在众多备选非贵金属催化材料中, 纳米层状结构二硫化钼(MoS2)因催化效果可期、 价格低而获得了广泛关注. 然而, 通常条件下易于获得的层状结构2H相MoS2大面积的基面部分显示惰性, 仅在片层边缘处存在少量活性位点, 且导电性较差, 因而尚不能替代Pt基催化剂, 而如何增加其活性位点数量和提高其导电性成为亟待解决的问题; 另一方面, 1T相MoS2虽然活性高、 导电性好, 但却存在制备困难及稳定性差的问题. 鉴于此, 研究者通过对纳米MoS2进行掺杂改性实现了其活性与稳定性的有效提升. 本文对非贵金属纳米MoS2催化剂掺杂改性的方法、 机理及其电催化水解制氢性能的相关研究进行了总结与讨论. 作为典型的非贵金属电解水析氢催化剂, MoS2具有巨大发展潜力, 本文能够对相关非贵金属催化剂的研发提供有益的参考.
中图分类号:
TrendMD:
陈晓煜, 于然波. 纳米二硫化钼的掺杂及催化电解水产氢的研究进展. 高等学校化学学报, 2021, 42(2): 475.
CHEN Xiaoyu, YU Ranbo. Research Progress on Doping of Molybdenum Disulfide and Hydrogen Evolution Reaction. Chem. J. Chinese Universities, 2021, 42(2): 475.
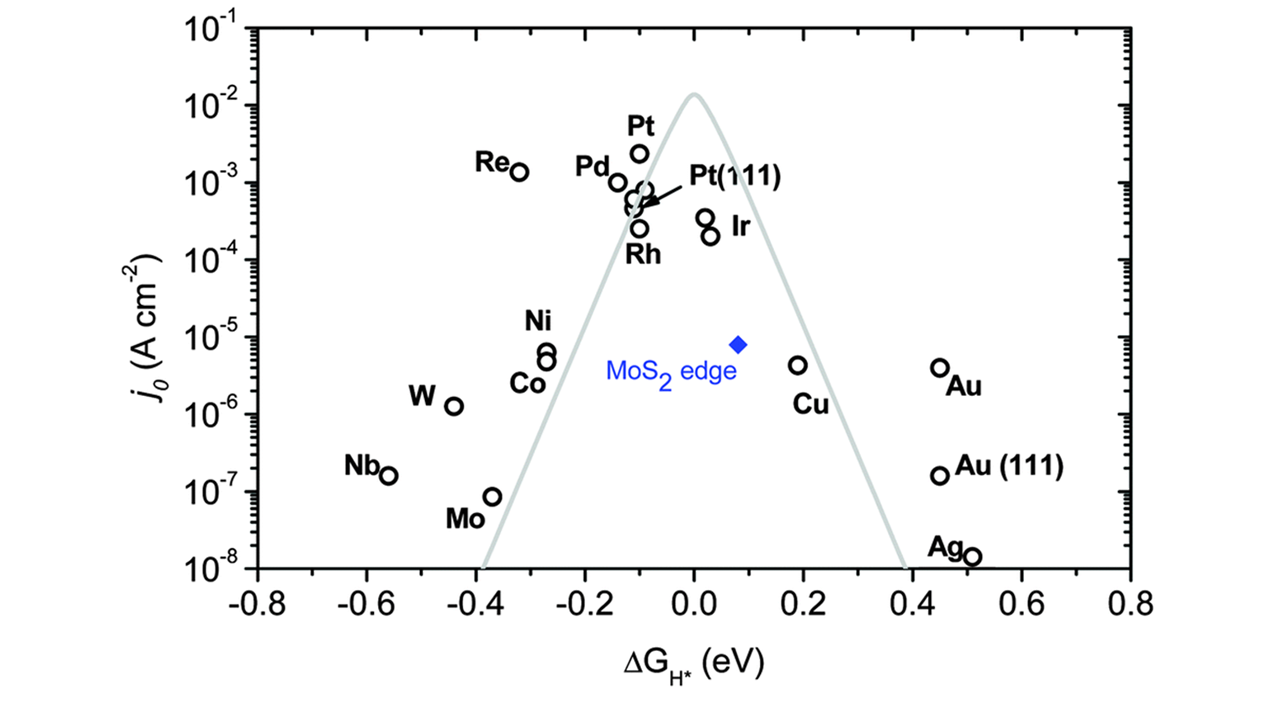
Fig.1 Relationship between the exchange current density of different HER catalyst materials and hydrogen adsorption free energy(ΔGH*)[43]Copyright 2014, Royal Society of Chemisty.
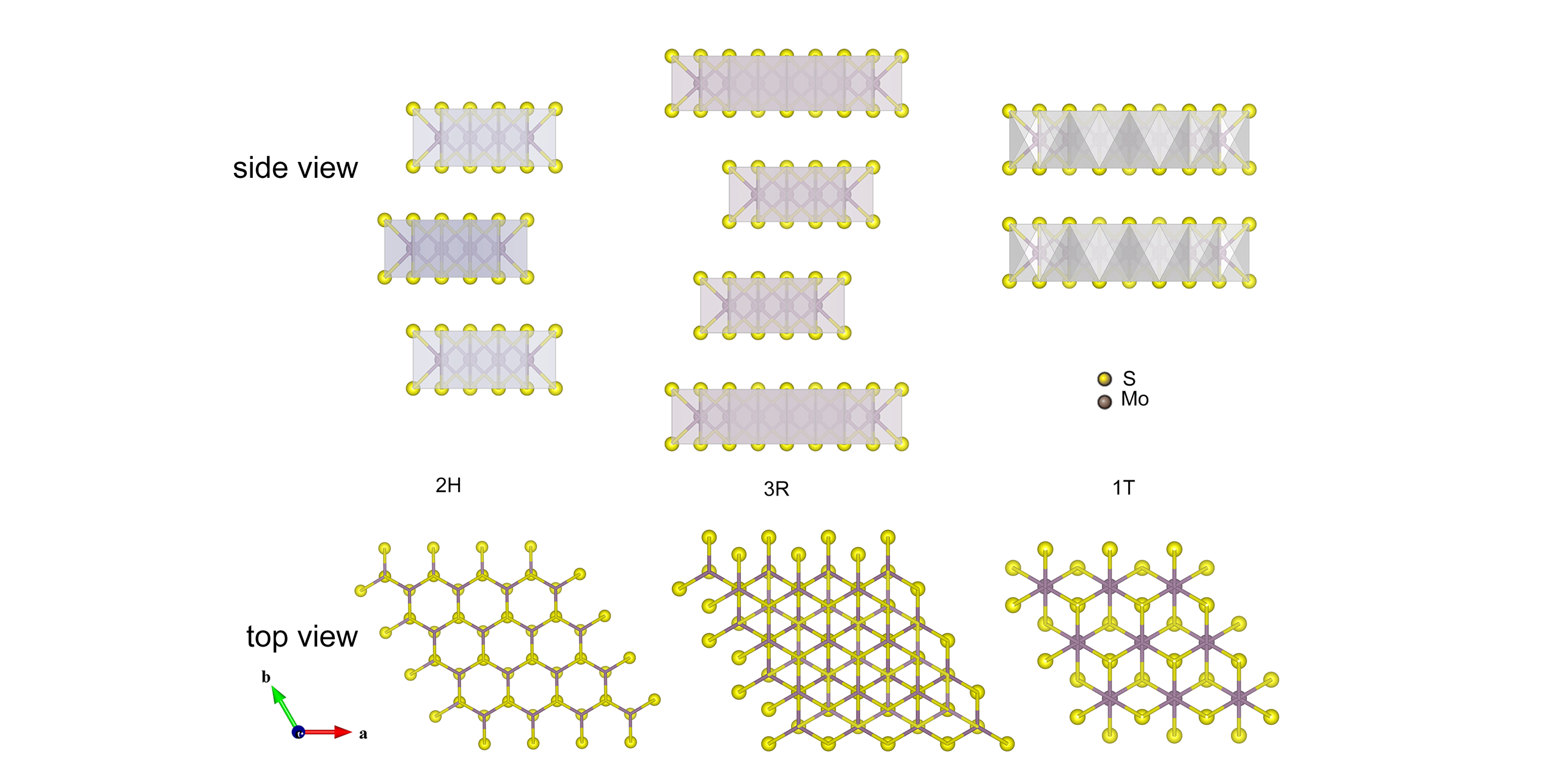
Fig.2 Side view and top view of the structural polytypes of 2H(hexagonal symmetry), 3R(rhombohedral symmetry) and 1T MoS2(rhombohedral symmetry)[71]Dark purple and yellow atoms are indicated as Mo and S, respectively. Copyright 2012, SpringerNature.
| Catalyst | Method | Electrolyte | Overpotential*/ mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|---|
| Ni?MoS2 | One?step hydrothermal method | 0.5 mol/L H2SO4 | 173 | 69 | [ |
| Re?MoS2 | CVD | 0.5 mol/L H2SO4 | 169 | 56 | [ |
| MoS2 | — | 0.5 mol/L H2SO4 | 300 | 103 | [ |
| Co?MoS2 | Exfoliation doping | 0.5 mol/L H2SO4 | 220 | 92 | [ |
| Ni?MoS2 | Exfoliation doping | 0.5 mol/L H2SO4 | 353 | 103 | [ |
| Ni?Co doped 2H?MoS2 | Co?precipitation hydrothermal method | 1 mol/L KOH | 87 | 40.3 | [ |
| Ni?Co doped 1T?MoS2 | Co?precipitation hydrothermal method | 1 mol/L KOH | 70 | 38.1 | [ |
Table 1 HER performance parameters of Ni, Co atomic doped MoS2
| Catalyst | Method | Electrolyte | Overpotential*/ mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|---|
| Ni?MoS2 | One?step hydrothermal method | 0.5 mol/L H2SO4 | 173 | 69 | [ |
| Re?MoS2 | CVD | 0.5 mol/L H2SO4 | 169 | 56 | [ |
| MoS2 | — | 0.5 mol/L H2SO4 | 300 | 103 | [ |
| Co?MoS2 | Exfoliation doping | 0.5 mol/L H2SO4 | 220 | 92 | [ |
| Ni?MoS2 | Exfoliation doping | 0.5 mol/L H2SO4 | 353 | 103 | [ |
| Ni?Co doped 2H?MoS2 | Co?precipitation hydrothermal method | 1 mol/L KOH | 87 | 40.3 | [ |
| Ni?Co doped 1T?MoS2 | Co?precipitation hydrothermal method | 1 mol/L KOH | 70 | 38.1 | [ |
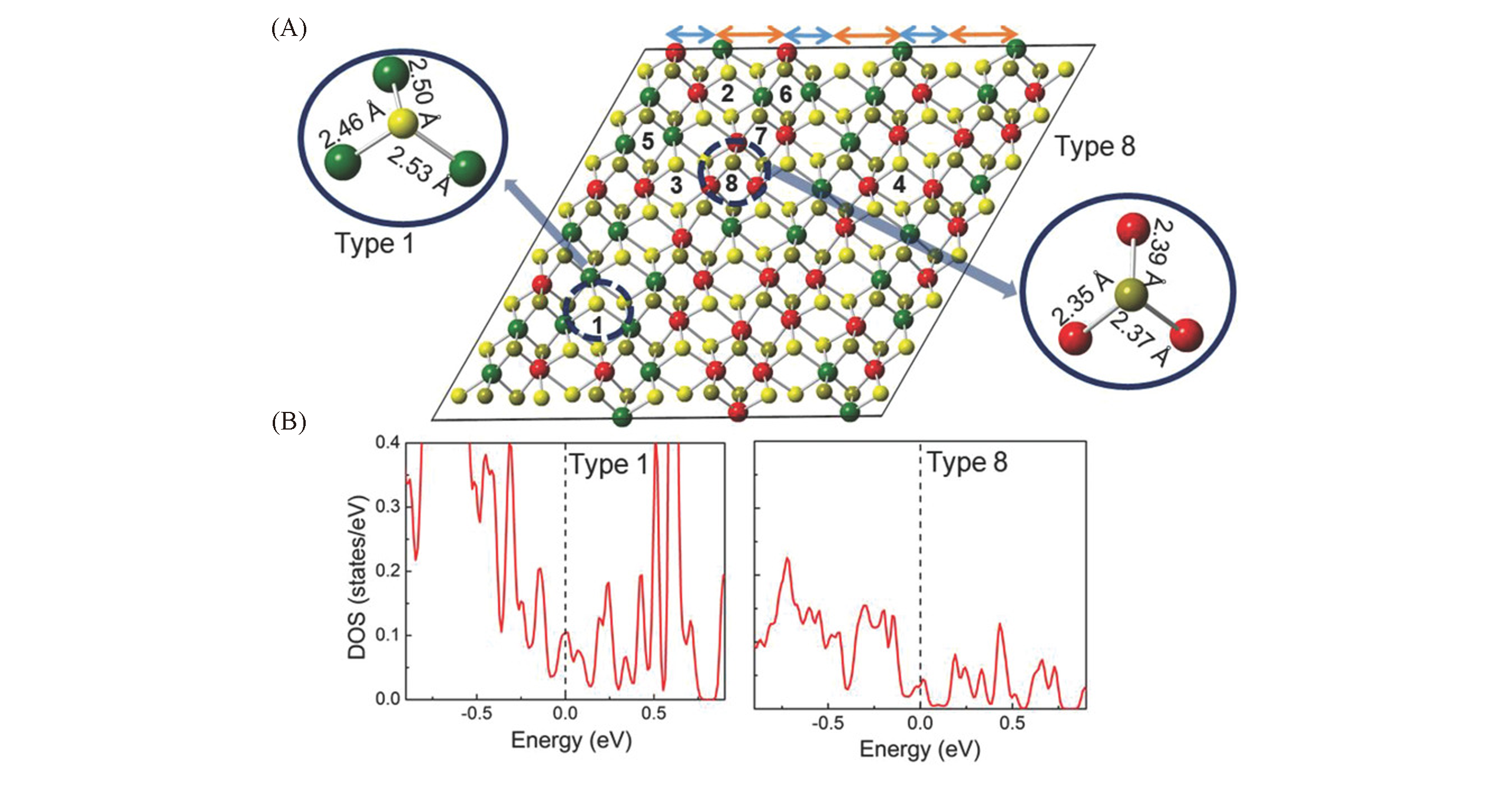
Fig.4 Electron structure of the local bonding environment and active site in RexMo1-xS2 alloy[77](A) RexMo1-xS2 structure model alloy monolayer with random distribution of Re atoms. Mo atom is green, Re atom is red, S atom is yellow. (B) As shown in type 1 and type 8, they are the most active and the least active points, respectively(according to DOS calculation).Copyright 2018, Wiley-VCH.
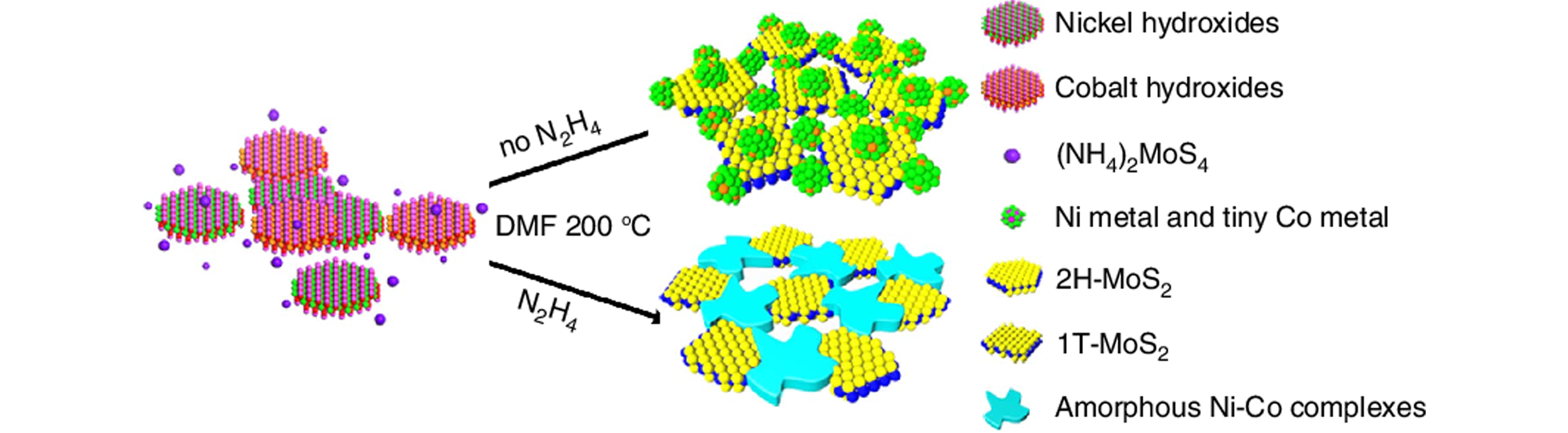
Fig.5 Schematic representation of the formation of porous hybrid nanostructures combining amorphous Ni?Co complexes with 1T phase MoS2[84]Copyright 2017, Springer Nature.
| Catalyst | Method | Onset potential/mV | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|---|
| P?MoS2 | Calcination | — | 120 | 57 | [ |
| P/O?MoS2 | Electrochemical deposition and heat treatment | 130 | 217 | 49 | [ |
| O?MoS2 | Hydrothermal method | 120 | — | 55 | [ |
| N?MoS2 | One?step sintering | 35 | — | 41 | [ |
| Amorphous P?MoS2 | Hydrothermal method | 167 | 219 | 39 | [ |
Table 2 HER performance parameters of non-metal atomic doped MoS2a
| Catalyst | Method | Onset potential/mV | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|---|
| P?MoS2 | Calcination | — | 120 | 57 | [ |
| P/O?MoS2 | Electrochemical deposition and heat treatment | 130 | 217 | 49 | [ |
| O?MoS2 | Hydrothermal method | 120 | — | 55 | [ |
| N?MoS2 | One?step sintering | 35 | — | 41 | [ |
| Amorphous P?MoS2 | Hydrothermal method | 167 | 219 | 39 | [ |
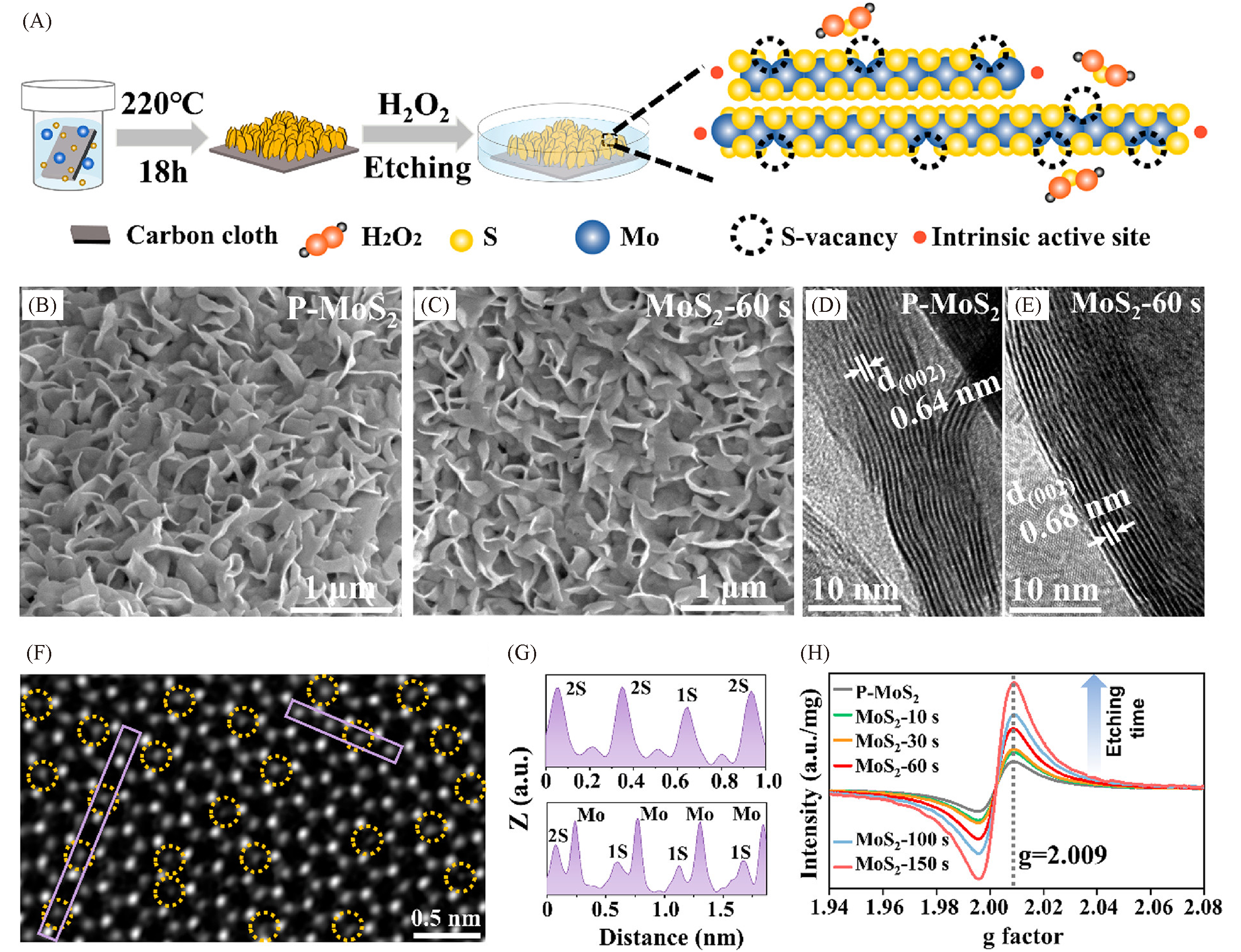
Fig.8 Schematic of the chemical etching process to introduce single S?vacancies(A), SEM and HRTEM images of P?MoS2(B, D) and MoS2?60 s(C, E), STEM image together with the line profiles extracted from the areas marked with purple rectangles of a CVD?grown monolayer MoS2 flake film after etching(yellow dotted circles represent the S?vacancies)(F, G) and EPR spectra of etched MoS2 with different etching durations compared to P?MoS2(H)[107]Copyright 2020, American Chemical Society.
| Catalyst | Method | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|
| S vacancy in MoS2 | Plasma reduction | 183 | 77.6 | [ |
| S vacancy in MoS2 | Hydrothermal?etching | 131 | 48 | [ |
| V?MoS2/VGN@CP | Plasma deposition?solvothermal method | 128 | 50 | [ |
Table 3 HER performance parameters of atomic-level vacancies doped MoS2a
| Catalyst | Method | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|
| S vacancy in MoS2 | Plasma reduction | 183 | 77.6 | [ |
| S vacancy in MoS2 | Hydrothermal?etching | 131 | 48 | [ |
| V?MoS2/VGN@CP | Plasma deposition?solvothermal method | 128 | 50 | [ |
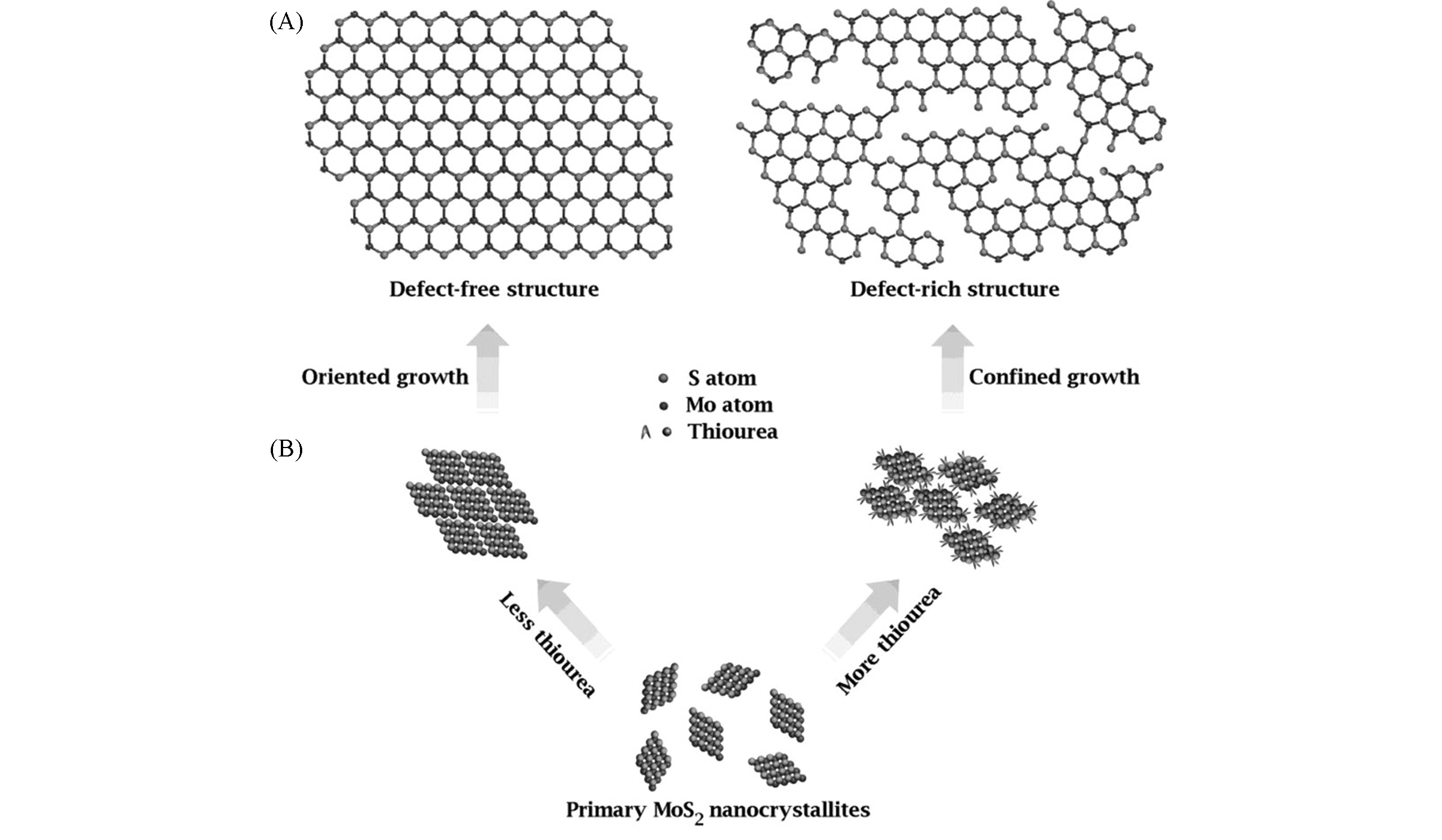
Fig.9 Structural models of defect?free and defect?rich structures(A) and as?designed synthetic pathways to obtain the above two structures(B)[110]Copyright 2013, Wiley-VCH.
| Catalyst | Method | Onset potential/mV | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|---|
| Defect rich MoS2 | Hydrothermal method | 120 | — | 50 | [ |
| Mesoporous MoS2 | Template method | 150—200 | — | 50 | [ |
| Porous 1T?MoS2 | Liquid ammonia assisted lithiation | — | 154 | 43 | [ |
| Defect rich MoS2 | Ball milling | — | 176 | 63 | [ |
Table 4 HER performance parameters of MoS2 with in-plane defectsa
| Catalyst | Method | Onset potential/mV | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|---|
| Defect rich MoS2 | Hydrothermal method | 120 | — | 50 | [ |
| Mesoporous MoS2 | Template method | 150—200 | — | 50 | [ |
| Porous 1T?MoS2 | Liquid ammonia assisted lithiation | — | 154 | 43 | [ |
| Defect rich MoS2 | Ball milling | — | 176 | 63 | [ |
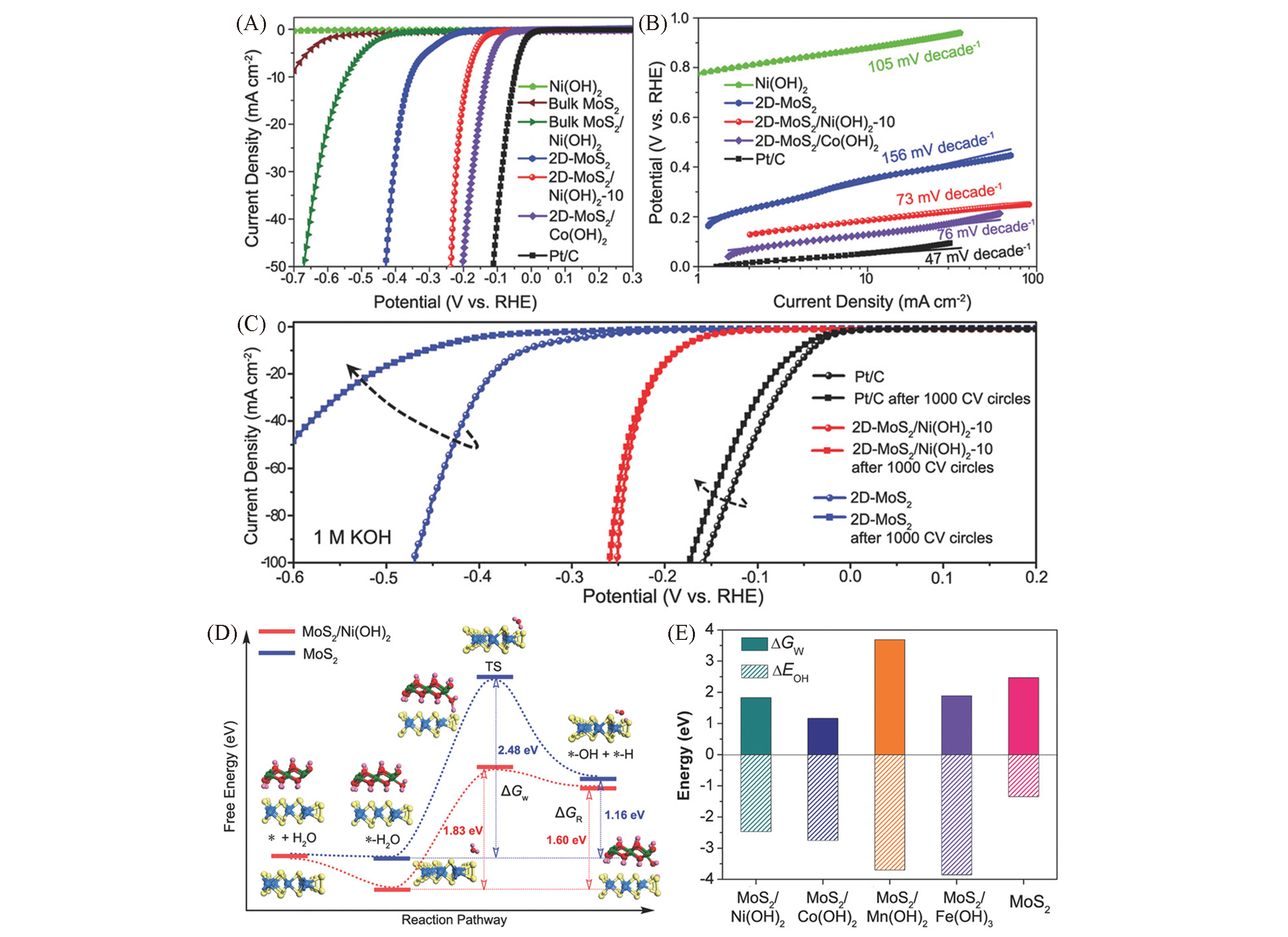
Fig.10 HER performance of different samples in alkaline media(A), corresponding Tafel slopes(B), stability tests of different samples(C) and free energy of each step of water splitting: initial state, intermediate state, final state and transition state(D), ΔGW and ΔGOH(the chemisorption energies of hydro-xides on MoS2 and hybrids)(E)[121](D) ΔGR presents the free energy change of the reaction, ΔGw represents water splitting kinetic energy barrier. Mo, S, Ni, O, H represented by light blue, yellow, green, red, pink, respectively.Copyright 2018, Wiley-VCH.
| Catalyst | Method | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|
| 1T‐MoS2/NiS2 | Hydrothermal method | 116 | 72 | [ |
| MoS2/Co(OH)2 | Li ion exfoliation and hydrothermal method | 128 | 76 | [ |
| 1T‐MoS2/SWNT | Solvothermal | 108 | 36 | [ |
| MoS2‐Ni3S2/NF | Two step hydrothermal method | 98 | 61 | [ |
| MoS2/Au | LPCVD | — | 61 | [ |
| MoS2‐Ni3S2/C | Solvothermal method and heat treatment | 233 | 95 | [ |
Table 5 HER performance parameters of MoS2 composited with heterostructuresa
| Catalyst | Method | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|
| 1T‐MoS2/NiS2 | Hydrothermal method | 116 | 72 | [ |
| MoS2/Co(OH)2 | Li ion exfoliation and hydrothermal method | 128 | 76 | [ |
| 1T‐MoS2/SWNT | Solvothermal | 108 | 36 | [ |
| MoS2‐Ni3S2/NF | Two step hydrothermal method | 98 | 61 | [ |
| MoS2/Au | LPCVD | — | 61 | [ |
| MoS2‐Ni3S2/C | Solvothermal method and heat treatment | 233 | 95 | [ |
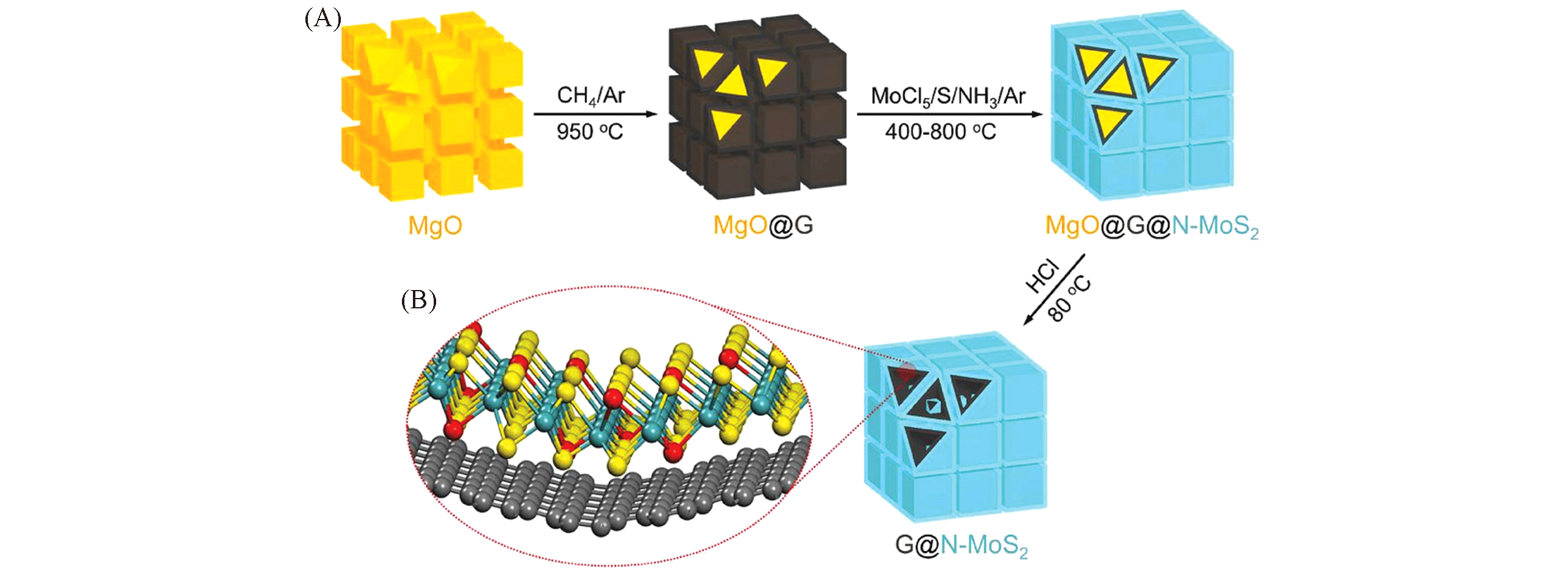
Fig.12 Schematic diagram of the synthesis of 3D mesoporous G@N‐MoS2 heterostructures[134](A) Two‐step CVD method, including CVD growth of mesoporous graphene on porous MgO templates at a high temperature, and then the in situ deposition of N‐MoS2 at a low temperature; (B) schematic representation of G@N‐MoS2. C, Mo, S and N atoms are marked by gray, green, yellow, red balls, respectively. Copyright 2018, Wiley-VCH.
| Catalyst | Method | Onset potential/mV | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|---|
| Mesoporous G@N?MoS2 | CVD | — | 243 | 82.5 | [ |
| CoS2@MoS2/RGO | Hydrothermal method | — | 98 | 37.4 | [ |
| MoS2 /N?RGO | Hydrothermal method | 5 | 56 | 41.3 | [ |
| N?rGO/MoS2/Ni(OH)2 | Two step hydrothermal method | — | 223 | 86.0 | [ |
Table 6 HER performance parameters of MoS2 composited with multiple materialsa
| Catalyst | Method | Onset potential/mV | Overpotentialb/mV | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|---|
| Mesoporous G@N?MoS2 | CVD | — | 243 | 82.5 | [ |
| CoS2@MoS2/RGO | Hydrothermal method | — | 98 | 37.4 | [ |
| MoS2 /N?RGO | Hydrothermal method | 5 | 56 | 41.3 | [ |
| N?rGO/MoS2/Ni(OH)2 | Two step hydrothermal method | — | 223 | 86.0 | [ |
| 57 | Topsøe H., Clausen B. S., Massoth F. E.; Eds.: Anderson J. R., Boudart M., Catalysis: Science and Technology, Springer Berlin Heidelberg, Berlin, 1996, 1—269 |
| 58 | Wilcoxon J. P., J. Phys. Chem. B,2000, 104, 7334—7343 |
| 59 | Tributsch H., Bennett J. C., J. Electroanal. Chem. Interfacial Electrochem.,1977, 81, 97—111 |
| 60 | Nørskov J. K., Bligaard T., Logadottir A., Kitchin J. R., Chen J. G., Pandelov S., Stimming U., J. Electrochem. Soc.,2005, 152, J23 |
| 61 | Bonde J., Moses P. G., Jaramillo T. F., Nørskov J. K., Chorkendorff I., Faraday Discuss.,2009, 140, 219—231 |
| 62 | Jaramillo T. F., Jorgensen K. P., Bonde J., Nielsen J. H., Horch S., Chorkendorff I., Science,2007, 317, 100—102 |
| 63 | Bruix A., Fuchtbauer H. G., Tuxen A., Walton A. S., Andersen M., Porsgaard S., Besenbacher F., Hammer B., Lauritsen J. V., ACS Nano,2015, 9, 9322—9330 |
| 64 | Alexiev V., Prins R., Weber T., Phys. Chem. Chem. Phys.,2001, 3, 5326—5336 |
| 65 | Dong L., Guo S., Wang Y., Zhang Q., Gu L., Pan C., Zhang J., J. Mater. Chem. A,2019, 7, 27603—27611 |
| 66 | Li H., Tsai C., Koh A. L., Cai L., Contryman A. W., Fragapane A. H., Zhao J., Han H. S., Manoharan H. C., Abild⁃Pedersen F., Nørskov J. K., Zheng X., Nat. Mater.,2016, 15, 48—53 |
| 67 | Ambrosi A., Sofer Z., Pumera M., Chem. Commun.,2015, 51, 8450—8453 |
| 68 | Tang Q., Jiang D. E., ACS Catal.,2016, 6, 4953—4961 |
| 69 | Ma F., Liang Y., Zhou P., Tong F., Wang Z., Wang P., Liu Y., Dai Y., Zheng Z., Huang B., Mater. Chem. Phys.,2020, 244, 122642 |
| 70 | Hao Y., Wang Y. T., Xu L. C., Yang Z., Liu R. P., Li X. Y., Appl. Surf. Sci.,2019, 469, 292—297 |
| 71 | Ji L., Yan P., Zhu C., Ma C., Wu W., Wei C., Shen Y., Chu S., Wang J., Du Y., Chen J., Yang X., Xu Q., Appl. Catal. B,2019, 251, 87—93 |
| 72 | Wang D., Zhang X., Bao S., Zhang Z., Fei H., Wu Z., J. Mater. Chem. A,2017, 5, 2681—2688 |
| 73 | Ambrosi A., Pumera M., Chem. Eur. J.,2018, 24, 18551—18555 |
| 74 | Xiong Q., Zhang X., Wang H., Liu G., Wang G., Zhang H., Zhao H., Chem. Commun.,2018, 54, 3859—3862 |
| 75 | Luo R., Luo M., Wang Z., Liu P., Song S., Wang X., Chen M., Nanoscale,2019, 11, 7123—7128 |
| 76 | Wang D., Zhang X., Shen Y., Wu Z., RSC Adv.,2016, 6, 16656—16661 |
| 77 | Yang S. Z., Gong Y., Manchanda P., Zhang Y. Y., Ye G., Chen S., Song L., Pantelides S. T., Ajayan P. M., Chisholm M. F., Zhou W., Adv. Mater.,2018, 30, 1803477 |
| 78 | Shi Y., Zhou Y., Yang D. R., Xu W. X., Wang C., Wang F. B., Xu J. J., Xia X. H., Chen H. Y., J. Am. Chem. Soc.,2017, 139, 15479—15485 |
| 79 | Chen M., Jian X., Wu H., Huang J., Liu W., Liu Y., Nanotechnology,2020, 31, 205403 |
| 80 | Tadi K. K., Palve A. M., Pal S., Sudeep P. M., Narayanan T. N., Nanotechnology,2016, 27, 275402 |
| 81 | Lau T. H. M., Lu X., Kulhavý J., Wu S., Lu L., Wu T. S., Kato R., Foord J. S., Soo Y. L., Suenaga K., Tsang S. C. E., Chem. Sci.,2018, 9, 4769—4776 |
| 82 | Kong X., Wang N., Zhang Q., Liang J., Wang M., Wei C., Chen X., Zhao Y., Zhang X., ChemistrySelect,2018, 3, 9493—9498 |
| 83 | Wang Y., Sun W., Ling X., Shi X., Li L., Deng Y., An C., Han X., Chem. Eur. J., 2020, 26, 4097—4103 |
| 84 | Li H., Chen S., Jia X., Xu B., Lin H., Yang H., Song L., Wang X., Nat. Commun.,2017, 8, 15377 |
| 85 | Tsai C., Abild⁃Pedersen F., Nørskov J. K., Nano Lett.,2014, 14, 1381—1387 |
| 86 | Guo J., Liu C., Sun Y., Sun J., Zhang W., Si T., Lei H., Liu Q., Zhang X., J. Solid State Chem.,2018, 263, 84—87 |
| 87 | Chen A., He Y., Cui R., Zhang J., Pu Y., Yang J., Li X. A., Mater. Lett.,2018,233, 246—249 |
| 88 | Deng Y., Liu Z., Wang A., Sun D., Chen Y., Yang L., Pang J., Li H., Li H., Liu H., Zhou W., Nano Energy,2019, 62, 338—347 |
| 89 | Liu Y., Wang Y. M., Yakobson B. I., Wood B. C., Phys. Rev. Lett.,2014, 113, 028304 |
| 90 | Ye R., del Angel⁃Vicente P., Liu Y., Arellano⁃Jimenez M. J., Peng Z., Wang T., Li Y., Yakobson B. I., Wei S. H., Yacaman M. J., Tour J. M., Adv. Mater.,2016, 28, 1427—1432 |
| 91 | Huang X., Leng M., Xiao W., Li M., Ding J., Tan T. L., Lee W. S. V., Xue J., Adv. Funct. Mater.,2017, 27, 1604943 |
| 92 | Xie J., Zhang J., Li S., Grote F., Zhang X., Zhang H., Wang R., Lei Y., Pan B., Xie Y., J. Am. Chem. Soc.,2013, 135, 17881—17888 |
| 93 | Chen Z., Ha Y., Liu Y., Wang H., Yang H., Xu H., Li Y., Wu R., ACS Appl. Mater. Interfaces,2018, 10, 7134—7144 |
| 94 | Li Y., Wei X., Chen L., Shi J., He M., Nat. Commun.,2019, 10, 5335 |
| 95 | Xiao W., Liu P., Zhang J., Song W., Feng Y. P., Gao D., Ding J., Adv. Energy Mater.,2017, 7, 1602086 |
| 96 | Wang D., Xie Y., Wu Z., Nanotechnology,2019, 30, 205401 |
| 97 | Nowotny J., Alim M. A., Bak T., Idris M. A., Ionescu M., Prince K., Sahdan M. Z., Sopian K., Mat Teridi M. A., Sigmund W., Chem. Soc. Rev.,2015, 44, 8424—8442 |
| 98 | Ouyang Y., Ling C., Chen Q., Wang Z., Shi L., Wang J., Chem. Mater.,2016, 28, 4390—4396 |
| 99 | Jiao S., Fu X., Zhang L., Zeng Y. J., Huang H., Nano Today,2020, 31, 100833 |
| 100 | Shu H., Zhou D., Li F., Cao D., Chen X., ACS Appl. Mater. Interfaces,2017, 9, 42688—42698 |
| 101 | Asahi R., Morikawa T., Irie H., Ohwaki T., Chem. Rev.,2014, 114, 9824—9852 |
| 1 | Norskov J. K., Christensen C. H., Science,2006, 312, 1322 |
| 2 | Wang J., Wang K., Wang F. B., Xia X. H., Nat. Commun.,2014, 5, 5285 |
| 3 | Bae S. Y., Jeon I. Y., Mahmood J., Baek J. B., Chem. Eur. J.,2018, 24, 18158—18179 |
| 102 | Deng J., Li H., Xiao J., Tu Y., Deng D., Yang H., Tian H., Li J., Ren P., Bao X., Energy Environ. Sci.,2015, 8, 1594—1601 |
| 103 | Shi Y., Chen G., Liu F., Yue X., Chen Z., ACS Energy Lett.,2018, 3, 1683—1692 |
| 104 | Liu Z., Hu J., Jiao H., Li L., Zheng G., Chen Y., Huang Y., Zhang Q., Shen C., Chen Q., Zhou H., Adv. Mater.,2017, 29, 1606774 |
| 4 | Zhao G. Q., Yuan Z., Wang L., Guo Z., Chem. J. Chinese Universities, 2020, 41(7), 1575—1581(赵国庆, 袁钊, 王连, 郭卓, 高等学校化学学报, 2020, 41(7), 1575—1581) |
| 5 | Liu K., Zhong H., Meng F., Zhang X., Yan J., Jiang Q., Mater. Chem. Front.,2017, 1, 2155—2173 |
| 105 | Cheng C. C., Lu A. Y., Tseng C. C., Yang X., Hedhili M. N., Chen M. C., Wei K. H., Li L. J., Nano Energy,2016, 30, 846—852 |
| 106 | Wang X., Zhang Y., Si H., Zhang Q., Wu J., Gao L., Wei X., Sun Y., Liao Q., Zhang Z., Ammarah K., Gu L., Kang Z., Zhang Y., J. Am. Chem. Soc.,2020, 142, 4298—4308 |
| 107 | Ye G., Gong Y., Lin J., Li B., He Y., Pantelides S. T., Zhou W., Vajtai R., Ajayan P. M., Nano Lett.,2016, 16, 1097—1103 |
| 108 | Li L., Qin Z., Ries L., Hong S., Michel T., Yang J., Salameh C., Bechelany M., Miele P., Kaplan D., Chhowalla M., Voiry D., ACS Nano,2019, 13, 6824—6834 |
| 6 | Sharma S., Ghoshal S. K., Renew. Sust. Energ. Rev.,2015, 43, 1151—1158 |
| 7 | Liu X., Dai L., Nat. Rev. Mater.,2016, 1, 16064 |
| 109 | Zhao Y., Tang M. T., Wu S., Geng J., Han Z., Chan K., Gao P., Li H., J. Catal.,2020, 382, 320—328 |
| 110 | Xie J., Zhang H., Li S., Wang R., Sun X., Zhou M., Zhou J., Lou X. W., Xie Y., Adv. Mater.,2013, 25, 5807—5813 |
| 8 | Yan B., Liu D., Feng X., Shao M., Zhang Y., Chem. Res. Chinese Universities,2020, 36(3), 425—430 |
| 9 | Yu X., Yu Z. Y., Zhang X. L., Li P., Sun B., Gao X., Yan K., Liu H., Duan Y., Gao M. R., Wang G., Yu S. H., Nano Energy,2020, 71, 104652 |
| 111 | Yin Y., Han J., Zhang Y., Zhang X., Xu P., Yuan Q., Samad L., Wang X., Wang Y., Zhang Z., Zhang P., Cao X., Song B., Jin S., J. Am. Chem. Soc.,2016, 138, 7965—7972 |
| 112 | Li Y., Yin K., Wang L., Lu X., Zhang Y., Liu Y., Yan D., Song Y., Luo S., Appl. Catal. B,2018, 239, 537—544 |
| 10 | Yan Y., He T., Zhao B., Qi K., Liu H., Xia B. Y., J. Mater. Chem. A,2018, 6, 15905—15926 |
| 11 | Zhu Q., Qu Y., Liu D., Ng K. W., Pan H., ACS Appl. Nano Mater.,2020, 3, 6270—6296 |
| 113 | Kagkoura A., Tzanidis I., Dracopoulos V., Tagmatarchis N., Tasis D., Chem. Commun.,2019, 55, 2078—2081 |
| 114 | Zhang Z., Yue C., Hu J., Nano,2017, 12, 1750116 |
| 115 | Kibsgaard J., Chen Z., Reinecke B. N., Jaramillo T. F., Nat. Mater.,2012, 11, 963—969 |
| 116 | Zhang L. F., Ke X., Ou G., Wei H., Wang L. N., Wu H., Sci. China Mater.,2017, 60, 849—856 |
| 12 | Benck J. D., Hellstern T. R., Kibsgaard J., Chakthranont P., Jaramillo T. F., ACS Catal.,2014, 4, 3957—3971 |
| 13 | Lasia A., Int. J. Hydrogen Energy,2019, 44, 19484—19518 |
| 117 | Paton K. R., Varrla E., Backes C., Smith R. J., Khan U., O’Neill A., Boland C., Lotya M., Istrate O. M., King P., Higgins T., Barwich S., May P., Puczkarski P., Ahmed I., Moebius M., Pettersson H., Long E., Coelho J., O’Brien S. E., McGuire E. K., Sanchez B. M., Duesberg G. S., McEvoy N., Pennycook T. J., Downing C., Crossley A., Nicolosi V., Coleman J. N., Nat. Mater.,2014, 13, 624—630 |
| 118 | Cheng L., Huang W., Gong Q., Liu C., Liu Z., Li Y., Dai H., Angew. Chem. Int. Ed.,2014, 53, 7860—7863 |
| 14 | Barbir F.; Ed.: Barbir F., PEM Fuel Cells(Second Edition), Academic Press, Boston, 2013, 33—72 |
| 15 | Pinto A. M. F. R., Oliveira V. B., Falcão D. S.; Eds.: Pinto A. M. F. R., Oliveira V. B., Falcão D. S., Direct Alcohol Fuel Cells for Portable Applications, Academic Press, London, 2018, 17—80 |
| 119 | Zhang B. Q., Chen J. S., Niu H. L., Mao C. J., Song J. M., Nanoscale,2018, 10, 20266—20271 |
| 120 | Chen X., Wang Z., Wei Y., Zhang X., Zhang Q., Gu L., Zhang L., Yang N., Yu R., Angew. Chem. Int. Ed.,2019, 58, 17621—17624 |
| 16 | Voiry D., Yamaguchi H., Li J., Silva R., Alves D. C. B., Fujita T., Chen M., Asefa T., Shenoy V. B., Eda G., Chhowalla M., Nat. Mater.,2013, 12, 850—855 |
| 17 | Jiang N., Tang Q., Sheng M., You B., Jiang D. E., Sun Y., Catal. Sci. Technol., 2016, 6, 1077—1084 |
| 18 | Togano H., Asai K., Oda S., Ikeno H., Kawaguchi S., Oka K., Wada K., Yagi S., Yamada I., Mater. Chem. Front.,2020, 4, 1519—1529 |
| 121 | Zhu Z., Yin H., He C. T., Al-Mamun M., Liu P., Jiang L., Zhao Y., Wang Y., Yang H. G., Tang Z., Wang D., Chen X. M., Zhao H., Adv. Mater.,2018, 30, 1801171 |
| 122 | Zhu H., Du M., Zhang M., Zou M., Yang T., Fu Y., Yao J., J. Mater. Chem. A,2014, 2, 7680—7685 |
| 19 | Jia Y., Chen J., Yao X., Mater. Chem. Front.,2018, 2, 1250—1268 |
| 20 | Fang L., Jiang Z., Xu H., Liu L., guan Y., Gu X., Wang Y., J. Catal.,2018, 357, 238—246 |
| 123 | Yan Y., Xia B., Li N., Xu Z., Fisher A., Wang X., J. Mater. Chem. A,2015, 3, 131—135 |
| 124 | Wang H., Zhang Q., Yao H., Liang Z., Lee H. W., Hsu P. C., Zheng G., Cui Y., Nano Lett.,2014, 14, 7138—7144 |
| 21 | Li S., Yang N., Liao L., Luo Y., Wang S., Cao F., Zhou W., Huang D., Chen H., ACS Appl. Mater. Interfaces,2018, 10, 37038—37045 |
| 22 | Zhang W., Ma X., Zhong C., Ma T., Deng Y., Hu W., Han X., Front. Chem.,2018, 6, 569—578 |
| 125 | Ma Y., Dai Y., Guo M., Niu C., Huang B., Nanoscale,2011, 3, 3883—3887 |
| 126 | Liu Q., Fang Q., Chu W., Wan Y., Li X., Xu W., Habib M., Tao S., Zhou Y., Liu D., Xiang T., Khalil A., Wu X., Chhowalla M., Ajayan P. M., Song L., Chem. Mater.,2017, 29, 4738—4744 |
| 23 | Yang J., Shin H. S., J. Mater. Chem. A,2014, 2, 5979—5985 |
| 24 | Liu H., Ma X., Rao Y., Liu Y., Liu J., Wang L., Wu M., ACS Appl. Mater. Interfaces,2018, 10, 10890—10897 |
| 25 | Hao Q., Li S., Liu H., Mao J., Li Y., Liu C., Zhang J., Tang C., Catal. Sci. Technol.,2019, 9, 3099—3108 |
| 26 | Wu Z., Guo J., Wang J., Liu R., Xiao W., Xuan C., Xia K., Wang D., ACS Appl. Mater. Interfaces,2017, 9, 5288—5294 |
| 127 | Zhang X., Zhang Y., Li F., Easton C. D., Bond A. M., Zhang J., Appl. Catal. B,2019, 249, 227—234 |
| 128 | Miao J., Xiao F. X., Yang H. B., Khoo S. Y., Chen J., Fan Z., Hsu Y. Y., Chen H. M., Zhang H., Liu B., Sci. Adv.,2015, 1, e1500259 |
| 129 | Zhang W., Li D., Zhang L., She X., Yang D., J. Energy Chem.,2019, 39, 39—53 |
| 27 | Li H., Wen P., Itanze D. S., Kim M. W., Adhikari S., Lu C., Jiang L., Qiu Y., Geyer S. M., Adv. Mater.,2019, 31, 1900813 |
| 28 | Hu G., Tang Q., Jiang D. E., Phys. Chem. Chem. Phys.,2016, 18, 23864—23871 |
| 29 | Men Y., Li P., Zhou J., Cheng G., Chen S., Luo W., ACS Catal.,2019, 9, 3744—3752 |
| 130 | Yang Y., Zhang K., Lin H., Li X., Chan H. C., Yang L., Gao Q., ACS Catal.,2017, 7, 2357—2366 |
| 131 | Shi J., Ma D., Han G. F., Zhang Y., Ji Q., Gao T., Sun J., Song X., Li C., Zhang Y., Lang X. Y., Zhang Y., Liu Z., ACS Nano,2014, 8, 10196—10204 |
| 30 | Liu Q., Tang C., Lu S., Zou Z., Gu S., Zhang Y., Li C. M., Chem. Commun.,2018, 54, 12408—12411 |
| 31 | Xiong J., Cai W., Shi W., Zhang X., Li J., Yang Z., Feng L., Cheng H., J. Mater. Chem.,2017, 5, 24193—24198 |
| 132 | Wang C. P., Kong L. J., Sun H., Zhong M., Cui H. J., Zhang Y. H., Wang D. H., Zhu J., Bu X. H., ChemElectroChem,2019, 6, 5603—5609 |
| 133 | Van Drunen J., Pilapil B. K., Makonnen Y., Beauchemin D., Gates B. D., Jerkiewicz G., ACS Appl. Mater. Interfaces,2014, 6, 12046—12061 |
| 134 | Tang C., Zhong L., Zhang B., Wang H. F., Zhang Q., Adv. Mater.,2018, 30, 1705110 |
| 135 | Guo Y., Gan L., Shang C., Wang E., Wang J., Adv. Funct. Mater.,2017, 27, 1602699 |
| 136 | Tang Y. J., Wang Y., Wang X. L., Li S. L., Huang W., Dong L. Z., Liu C. H., Li Y. F., Lan Y. Q., Adv. Energy Mater.,2016, 6, 1600116 |
| 137 | Pandey A., Mukherjee A., Chakrabarty S., Chanda D., Basu S., ACS Appl. Mater. Interfaces,2019, 11, 42094—42103 |
| 32 | Guan J., Li C., Zhao J., Yang Y., Zhou W., Wang Y., Li G. R., Appl. Catal. B,2020, 269, 118600 |
| 33 | Jin H., Liu X., Vasileff A., Jiao Y., Zhao Y., Zheng Y., Qiao S. Z., ACS Nano,2018, 12, 12761—12769 |
| 34 | Wei H., Xi Q., Chen X. A., Guo D., Ding F., Yang Z., Wang S., Li J., Huang S., Adv. Sci.,2018, 5, 1700733 |
| 35 | Huang Y., Gong Q., Song X., Feng K., Nie K., Zhao F., Wang Y., Zeng M., Zhong J., Li Y., ACS Nano,2016, 10, 11337—11343 |
| 36 | Han Q., Cui S., Pu N., Chen J., Liu K., Wei X., Int. J. Hydrogen Energy,2010, 35, 5194—5201 |
| 37 | Zhang X., Li Y., Guo Y., Hu A., Li M., Hang T., Ling H., Int. J. Hydrogen Energy,2019, 44, 29946—29955 |
| 38 | Su J., Yang Y., Xia G., Chen J., Jiang P., Chen Q., Nat. Commun.,2017, 8, 14969 |
| 39 | Krstajić N. V., Jović V. D., Gajić⁃Krstajić L., Jović B. M., Antozzi A. L., Martelli G. N., Int. J. Hydrogen Energy,2008, 33, 3676—3687 |
| 40 | Zhou Q., Li Z. Y., Wang F., Chem. J. Chinese Universiities, 2019, 40(8), 1717—1725(周琦, 李志洋, 汪帆, 高等学校化学学报,2019, 40(8), 1717—1725) |
| 41 | Voiry D., Fullon R., Yang J., de Carvalho Castro e Silva C., Kappera R., Bozkurt I., Kaplan D., Lagos M. J., Batson P. E., Gupta G., Mohite Aditya D., Dong L., Er D., Shenoy V. B., Asefa T., Chhowalla M., Nat. Mater.,2016, 15, 1003—1009 |
| 42 | Hinnemann B., Moses P. G., Bonde J., Jørgensen K. P., Nielsen J. H., Horch S., Chorkendorff I., Nørskov J. K., J. Am. Chem. Soc.,2005, 127, 5308—5309 |
| 43 | Rasamani K. D., Alimohammadi F., Sun Y., Mater. Today,2017, 20, 83—91 |
| 44 | Morales⁃Guio C. G., Stern L. A., Hu X., Chem. Soc. Rev.,2014, 43, 6555—6569 |
| 45 | Simon P., Gogotsi Y., Nat. Mater.,2008, 7, 845—854 |
| 46 | Aricò A. S., Bruce P., Scrosati B., Tarascon J. M., van Schalkwijk W., Nat. Mater.,2005, 4, 366—377 |
| 47 | Kim S. G., Kim S. H., Park J., Kim G. S., Park J. H., Saraswat K. C., Kim J., Yu H. Y., ACS Nano,2019, 13, 10294—10300 |
| 48 | Jiao Y., Hafez A. M., Cao D., Mukhopadhyay A., Ma Y., Zhu H., Small,2018, 14, 1800640 |
| 49 | Yang C., Wang H. F., Xu Q., Chem. Res. Chinese Universities,2020, 36(1), 10—23 |
| 50 | Toh R. J., Sofer Z., Luxa J., Sedmidubský D., Pumera M., Chem. Commun.,2017, 53, 3054—3057 |
| 51 | Wang Q. H., Kalantar⁃Zadeh K., Kis A., Coleman J. N., Strano M. S., Nat. Nanotechnol.,2012, 7, 699—712 |
| 52 | Nam G., He Q., Wang X., Yu Y., Chen J., Zhang K., Yang Z., Hu D., Lai Z., Li B., Adv. Mater.,2019, 31, 1807764 |
| 53 | Tan D., Willatzen M., Wang Z. L., Nano Energy,2019, 56, 512—515 |
| 54 | Acerce M., Voiry D., Chhowalla M., Nat. Nanotechnol.,2015,10, 313—318 |
| 55 | Shi Y., Zhang B., Inorg. Chem. Front.,2018, 5, 1490—1492 |
| 138 | Debata S., Banerjee S., Sharma P. K., Electrochim. Acta,2019, 303, 257—267 |
| 56 | Li X., Zhu H., J. Materiomics,2015, 1, 33—44 |
| [1] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [2] | 程前, 杨博龙, 吴文依, 向中华. S掺杂Fe-N-C高活性氧还原反应催化剂[J]. 高等学校化学学报, 2022, 43(9): 20220341. |
| [3] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [4] | 龚妍熹, 王建兵, 柴歩瑜, 韩元春, 马云飞, 贾超敏. 钾掺杂g-C3N4薄膜光阳极的制备及光电催化氧化降解水中双氯芬酸钠性能[J]. 高等学校化学学报, 2022, 43(6): 20220005. |
| [5] | 郝宏蕾, 孟繁雨, 李若钰, 李迎秋, 贾明君, 张文祥, 袁晓玲. 生物质基氮掺杂多孔炭材料的制备及对水中亚甲基蓝的吸附性能[J]. 高等学校化学学报, 2022, 43(6): 20220055. |
| [6] | 蒋小康, 周琦, 周恒为. Gd2ZnTiO6∶Dy3+, Eu3+单基质白光荧光粉的制备与发光性能[J]. 高等学校化学学报, 2022, 43(6): 20220029. |
| [7] | 宋有为, 安江伟, 王征, 王旭慧, 权燕红, 任军, 赵金仙. Ag,Zn,Pd掺杂对铜基催化剂草酸二甲酯选择性加氢反应的影响[J]. 高等学校化学学报, 2022, 43(6): 20210842. |
| [8] | 张义超, 赵付来, 王宇, 王亚玲, 沈永涛, 冯奕钰, 封伟. 基于多层二硒化钨的高性能场效应晶体管的实验优化和理论模拟[J]. 高等学校化学学报, 2022, 43(6): 20220113. |
| [9] | 孙雪峰, 热娜古丽·阿不都热合曼, 杨通胜, 杨倩婷. Cr,In共掺杂MgGa2O4小尺寸近红外长余辉纳米颗粒的制备及发光性能[J]. 高等学校化学学报, 2022, 43(4): 20210850. |
| [10] | 郑雪莲, 杨翠翠, 田维全. 全椅式边含薁缺陷石墨烯纳米片的二阶非线性光学性质[J]. 高等学校化学学报, 2022, 43(3): 20210806. |
| [11] | 俞彬, 谌小燕, 赵越, 陈卫昌, 肖新颜, 刘海洋. 氧化石墨烯基钴卟啉复合材料的电催化析氢反应[J]. 高等学校化学学报, 2022, 43(2): 20210549. |
| [12] | 王祖民, 孟程, 于然波. 过渡金属磷化物析氢催化剂的掺杂调控[J]. 高等学校化学学报, 2022, 43(11): 20220544. |
| [13] | 伍泽鑫, 朱渊杰, 王泓中, 王均安, 贺英. 甲基修饰的咔唑/二苯砜基AIE-TADF蓝光材料及其OLED器件[J]. 高等学校化学学报, 2022, 43(11): 20220371. |
| [14] | 姜珊, 申倩倩, 李琦, 贾虎生, 薛晋波. Pd增强缺陷态TiO2纳米管阵列的光催化产氢性能[J]. 高等学校化学学报, 2022, 43(10): 20220206. |
| [15] | 侯从聪, 王惠颖, 李婷婷, 张志明, 常春蕊, 安立宝. N-CNTs/NiCo-LDH复合材料的制备及电化学性能[J]. 高等学校化学学报, 2022, 43(10): 20220351. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
