

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (2): 523.doi: 10.7503/cjcu20200582
收稿日期:2020-08-21
出版日期:2021-02-10
发布日期:2021-02-05
通讯作者:
宫勇吉
E-mail:yongjigong@buaa.edu.cn
基金资助:
HE Qianqian, WANG Zhe, MENG Lingjia, CHEN Qian, GONG Yongji( )
)
Received:2020-08-21
Online:2021-02-10
Published:2021-02-05
Contact:
GONG Yongji
E-mail:yongjigong@buaa.edu.cn
Supported by:摘要:
解决全球气候变化和能源危机的有效途径之一是用氢能源(H2)代替传统的化石能源. 析氢反应(Electrochemical hydrogen evolution reaction, HER) 被认为是绿色环保的可持续产氢途径, 通常电解过程需要催化剂以降低电化学电位, 提高能量利用效率. 目前最先进的催化剂仍然依赖于贵金属, 但是研究表明, 过渡金属二硫族化物(Transition metal dichalcogenides, TMDs)同样具有优异的催化活性, 与贵金属相比, TMDs产量大、 价格低、 催化活性好、 便于调控和修饰, 有望替代贵金属在催化领域的应用. 基于此, 本文讨论了近年来TMDs在析氢方面的研究情况以及TMDs材料的性能调控, 包含原子工程、 相工程和异质结. 并总结和展望了TMDs催化材料的挑战与机遇.
中图分类号:
TrendMD:
何倩倩, 王哲, 孟令佳, 陈乾, 宫勇吉. 基于过渡金属二硫族化物析氢催化的研究进展. 高等学校化学学报, 2021, 42(2): 523.
HE Qianqian, WANG Zhe, MENG Lingjia, CHEN Qian, GONG Yongji. Recent Advances of Hydrogen Evolution Reaction Catalysis Based on Transition Metal Dichalcogenides. Chem. J. Chinese Universities, 2021, 42(2): 523.
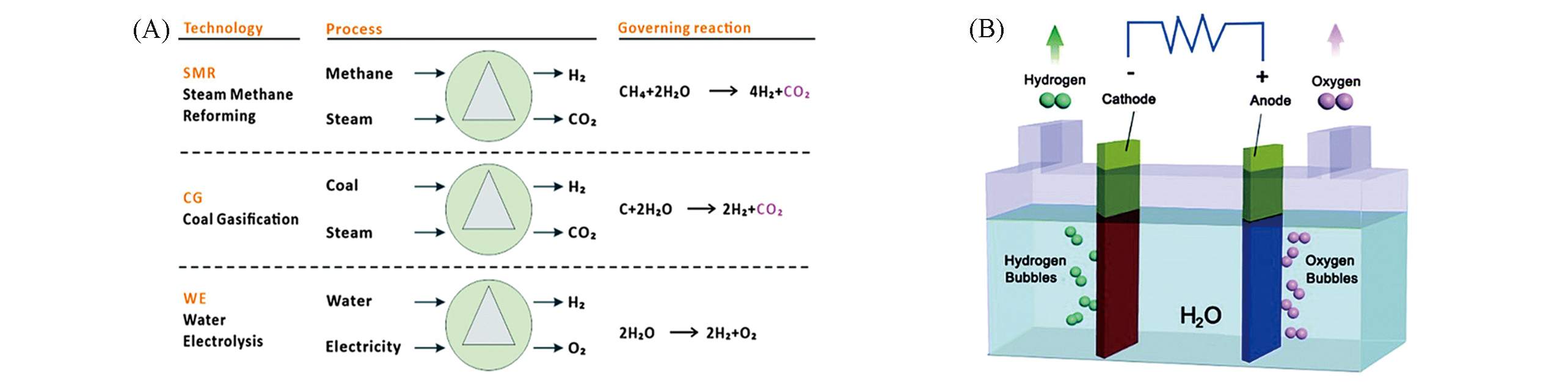
Fig.1 Three main pathways for industrial hydrogen production(A) and schematic diagram of an electrolyzer(B)[3]Copyright 2015, Royal Society of Chemistry.
| TMDs | Strategy | Electrolyte | Tafel slope/ (mV·dec-1) | Overpotential (onset, η/mV) | jx/(mA·cm-2) | Stability (cycle/time) | Ref. |
|---|---|---|---|---|---|---|---|
| 2H?MoS2 | Strained/S?vacancies | H2SO4(pH=2) | 60 | 170 | — | — | [ |
| MoS2 | Defects | 0.5 mol/L H2SO4 | 117 | 300 | — | — | [ |
| SL?MoS2?CNTs | S?vacancy | 0.1 mol/L H2SO4 | 63 | 40 | j0=1.25×10?2 | Stable(10000) | [ |
| TaS2 | S?vacancy | 0.5 mol/L H2SO4 | 142 | 200 | — | — | [ |
| MoS1.65 NCs | S?vacancy | 0.5 mol/L H2SO4 | 29 | <60 | j200=52.13 | Stable(3000) | [ |
| 2H?MoS2 | S?vacancy | 0.5 mol/L H2SO4 | 102 | — | — | — | [ |
| Bowl?like MoS2 | Defects | 0.5 mol/L H2SO4 | 59 | 113 | j150=40 | — | [ |
| MoS2 | Grain boundaries | 0.5 mol/L H2SO4 | 54 | 25 | — | — | [ |
| Pt?MoS2 | Doping | 0.1 mol/L H2SO4 | 96 | 60 | — | Stable(5000) | [ |
| Pd DR?MoS2 | Defects/ doping | 0.5 mol/L H2SO4 | 41 | 40 | j300=83 | Stable(1000) | [ |
| Co‐Pd‐MoS2 | Doping | 0.5 mol/L H2SO4 | 43.2 | 49.3 | — | Stable(10000) | [ |
| NiO@1T?MoS2 | Doping | 1.0 mol/L KOH | 52 | 46 | j0=0.44 | Stable(3000) | [ |
| SA Co?D 1T MoS2 | Phase change/doping | 0.5 mol/L H2SO4 | 32 | 42 | j100>40 | Stable(10000) | [ |
| PdxNbS2 | Interlayer | 0.5 mol/L H2SO4 | 50 | 157 | j199=50 | Stable(2000) | [ |
| 1T?WS2 | Phase change | 0.5 mol/L H2SO4 | 55 | 80 | j0=2×10?5 | — | [ |
| 1T?MoS2 | Phase change | 0.5 mol/L H2SO4 | 40 | 100 | — | — | [ |
| RexMo1–xS2 | Phase change/doping | 0.5 mol/L H2SO4 | 56 | 90 | — | Stable(3000) | [ |
| MoSe2 | Phase change/doping | 0.5 mol/L H2SO4 | 46 | 130 | — | 12 h | [ |
| 1T?2H MoS2 | Crytal?phase heterostructure | 0.5 mol/L H2SO4 | 46 | 234 | j300=48 | Stable(1000) | [ |
| 1T?2H MoS2 | Crytal?phase heterostructure | 1 mol/L KOH | 65 | 320 | — | Stable(1000) | [ |
| 1T?2H MoS2 | Crytal?phase heterostructure | 0.5 mol/L H2SO4 | 73 | 200 | j0=0.57×10-1 | 200 h | [ |
| MoS2/WSe2 | Heterostructures | 0.5 mol/L H2SO4 | 76 | 116 | — | 20 h | [ |
| 1T?MoS2QS/Ni(OH)2 | Heterostructures | 1 mol/L KOH | 30 | 57 | j200>500 | Stable(1000) | [ |
| H?NbS2 H?TaS2 | Phase change | 0.5 mol/L H2SO4 | 30—37 | 50—60 | — | — | [ |
| 3R?NbS2 | Phase change | 0.5 mol/L H2SO4 | 97 | 182 | — | Stable(20000) | [ |
| 2H Nb1+xS2 | Interlayer | 0.5 mol/L H2SO4 | 30 | — | j500>5000 | — | [ |
Table 1 Summary of hydrogen evolution catalytic performance of some TMDs under different control methods
| TMDs | Strategy | Electrolyte | Tafel slope/ (mV·dec-1) | Overpotential (onset, η/mV) | jx/(mA·cm-2) | Stability (cycle/time) | Ref. |
|---|---|---|---|---|---|---|---|
| 2H?MoS2 | Strained/S?vacancies | H2SO4(pH=2) | 60 | 170 | — | — | [ |
| MoS2 | Defects | 0.5 mol/L H2SO4 | 117 | 300 | — | — | [ |
| SL?MoS2?CNTs | S?vacancy | 0.1 mol/L H2SO4 | 63 | 40 | j0=1.25×10?2 | Stable(10000) | [ |
| TaS2 | S?vacancy | 0.5 mol/L H2SO4 | 142 | 200 | — | — | [ |
| MoS1.65 NCs | S?vacancy | 0.5 mol/L H2SO4 | 29 | <60 | j200=52.13 | Stable(3000) | [ |
| 2H?MoS2 | S?vacancy | 0.5 mol/L H2SO4 | 102 | — | — | — | [ |
| Bowl?like MoS2 | Defects | 0.5 mol/L H2SO4 | 59 | 113 | j150=40 | — | [ |
| MoS2 | Grain boundaries | 0.5 mol/L H2SO4 | 54 | 25 | — | — | [ |
| Pt?MoS2 | Doping | 0.1 mol/L H2SO4 | 96 | 60 | — | Stable(5000) | [ |
| Pd DR?MoS2 | Defects/ doping | 0.5 mol/L H2SO4 | 41 | 40 | j300=83 | Stable(1000) | [ |
| Co‐Pd‐MoS2 | Doping | 0.5 mol/L H2SO4 | 43.2 | 49.3 | — | Stable(10000) | [ |
| NiO@1T?MoS2 | Doping | 1.0 mol/L KOH | 52 | 46 | j0=0.44 | Stable(3000) | [ |
| SA Co?D 1T MoS2 | Phase change/doping | 0.5 mol/L H2SO4 | 32 | 42 | j100>40 | Stable(10000) | [ |
| PdxNbS2 | Interlayer | 0.5 mol/L H2SO4 | 50 | 157 | j199=50 | Stable(2000) | [ |
| 1T?WS2 | Phase change | 0.5 mol/L H2SO4 | 55 | 80 | j0=2×10?5 | — | [ |
| 1T?MoS2 | Phase change | 0.5 mol/L H2SO4 | 40 | 100 | — | — | [ |
| RexMo1–xS2 | Phase change/doping | 0.5 mol/L H2SO4 | 56 | 90 | — | Stable(3000) | [ |
| MoSe2 | Phase change/doping | 0.5 mol/L H2SO4 | 46 | 130 | — | 12 h | [ |
| 1T?2H MoS2 | Crytal?phase heterostructure | 0.5 mol/L H2SO4 | 46 | 234 | j300=48 | Stable(1000) | [ |
| 1T?2H MoS2 | Crytal?phase heterostructure | 1 mol/L KOH | 65 | 320 | — | Stable(1000) | [ |
| 1T?2H MoS2 | Crytal?phase heterostructure | 0.5 mol/L H2SO4 | 73 | 200 | j0=0.57×10-1 | 200 h | [ |
| MoS2/WSe2 | Heterostructures | 0.5 mol/L H2SO4 | 76 | 116 | — | 20 h | [ |
| 1T?MoS2QS/Ni(OH)2 | Heterostructures | 1 mol/L KOH | 30 | 57 | j200>500 | Stable(1000) | [ |
| H?NbS2 H?TaS2 | Phase change | 0.5 mol/L H2SO4 | 30—37 | 50—60 | — | — | [ |
| 3R?NbS2 | Phase change | 0.5 mol/L H2SO4 | 97 | 182 | — | Stable(20000) | [ |
| 2H Nb1+xS2 | Interlayer | 0.5 mol/L H2SO4 | 30 | — | j500>5000 | — | [ |
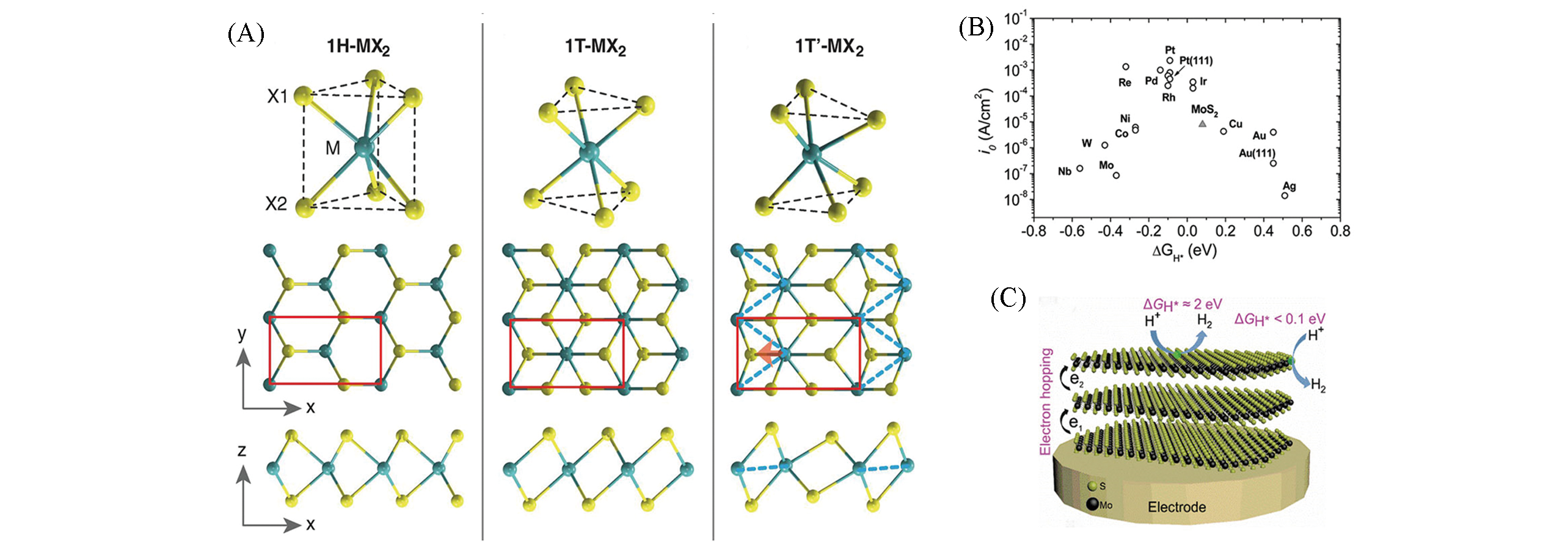
Fig.3 Schematics of 1H?MX2, 1T?MX2, and T′?MX2(A)[18], volcano plot of the exchange current density as a function of ΔGH* for nanoparticulate MoS2 and the pure metals(B)[28], electron hopping in multilayered 2H?MoS2 and the ΔGH* at basal plane and edge sites(C)[14](A) The unit cell is indicated by red rectangles. Copyright 2014, American Association for the Advancement of Science(AAAS); (B) Copyright 2007, American Association for the Advancement of Science(AAAS); (C) Copyright 2020, Wiley?VCH.
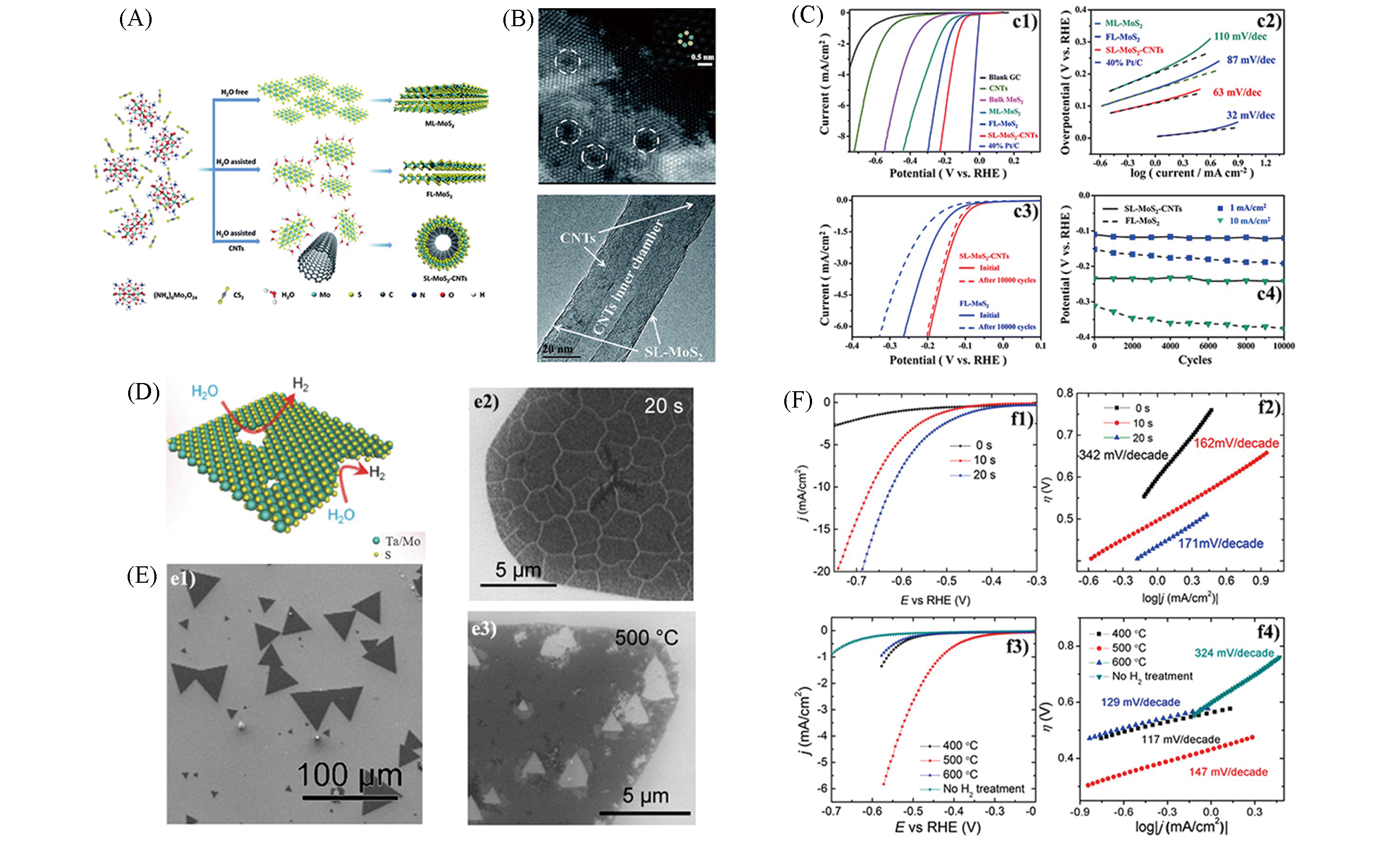
Fig.4 Schematic illustration for the direct chemical synthesis of different layer MoS2(A), HAADF?STEM image of defects in FL?MoS2 nanosheet(up) and HRTEM images of SL?MoS2?CNTs(down)(B), electrochemical measurement results of different layer MoS2(C)[42], schematic description of plasma treatment of TMDs nanosheets(D)[34], morphology of pristine monolayer MoS2(e1) and monolayer MoS2 treated by O2 plasma(e2) and annealed by H2(e3)(E), electrochemical measurement results of MoS2 before and after treatment(F)[43](A—C) Copyright 2014, Royal Society of Chemistry; (D) Copyright 2016, Wiley?VCH; (E,F) Copyright 2016, American Chemical Society.
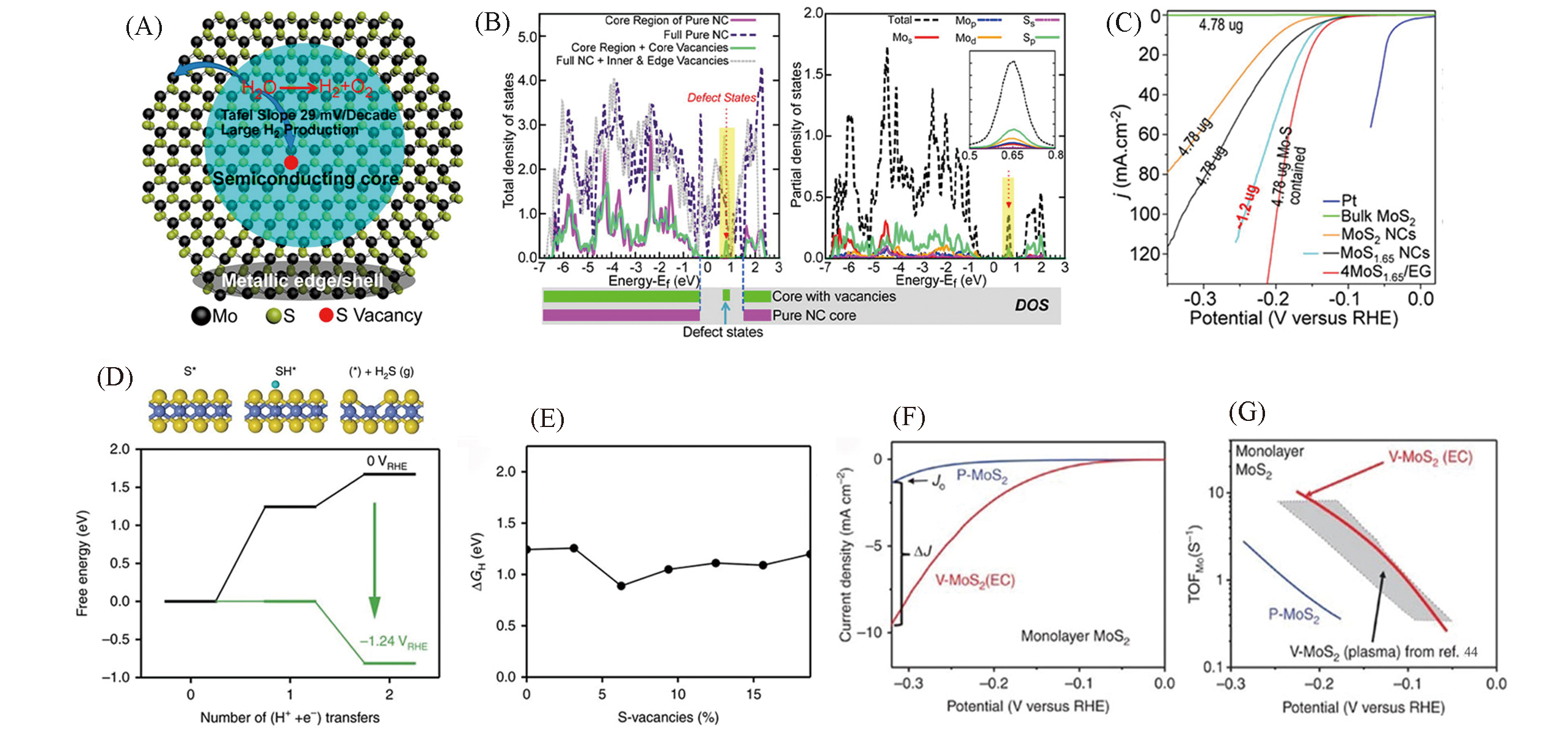
Fig.5 Schematic showing the metallic edge, near?edge regions, and semiconducting core of the MoS2 NCs(A), calculated density of state(DOS) of MoS2 in the core and/or edge region, and the entire NC(left) and decomposition of the total DOS of MoS2 with S vacancies(S depletion) in the core and edge regions into partial DOS of the Mo and S orbitals(right)(B), polarization curves of different catalysts in 0.5 mol/L H2SO4(C)[45], free energy diagram for the protonation and removal of S(D), hydrogen adsorption free energy onto a sulfur atom on the basal plane for each concentration of S?vacancies(E), polarization curves(F) and Tafel plots of monolayer MoS2(G)[46](A—C) Copyright 2016, American Chemical Society; (D—G) Copyright 2017, Springer Nature.
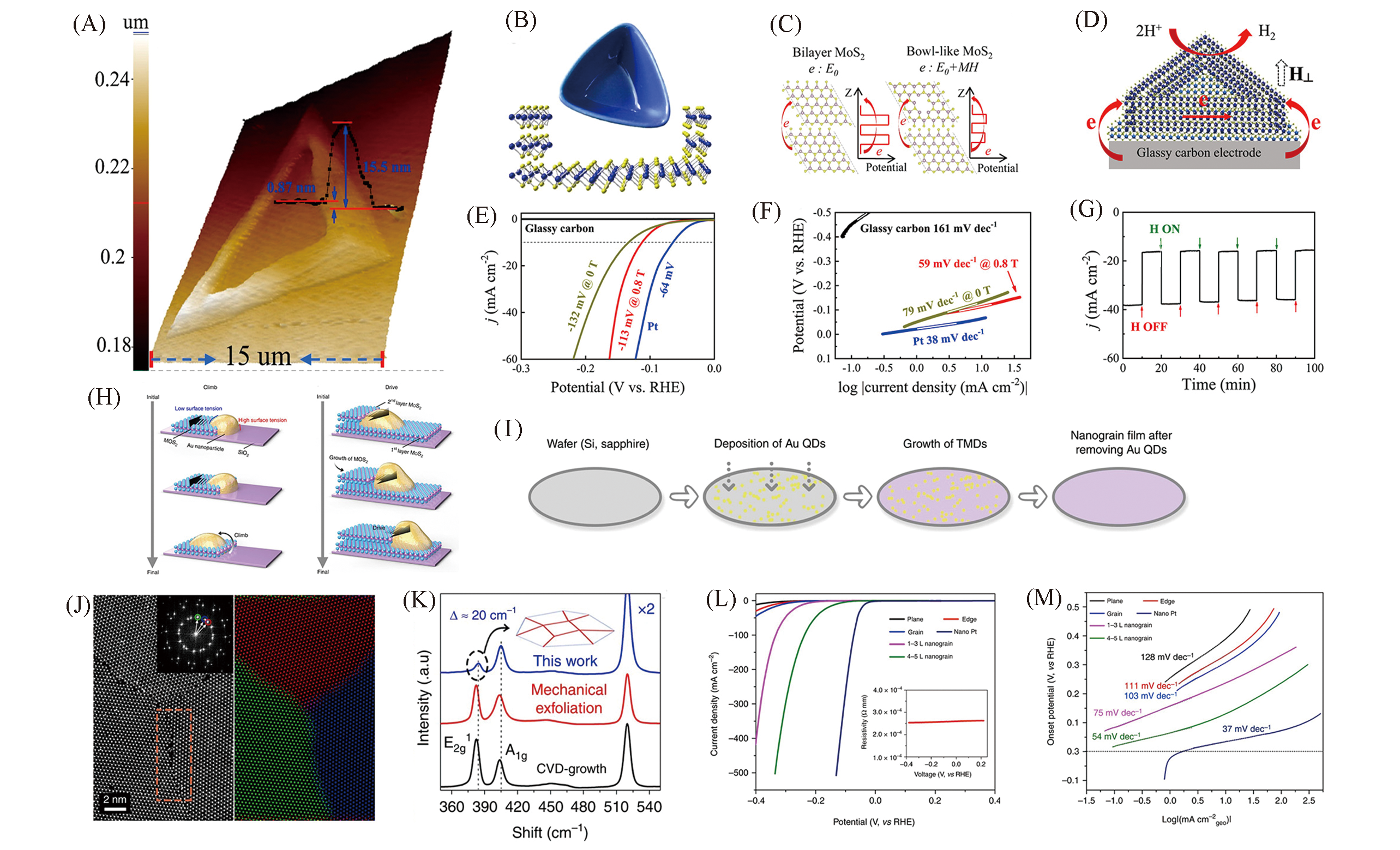
Fig.6 3D AFM image and cross?sectional step height profile of a bowl?like MoS2 flake(A), schematic diagram of a bowl?like MoS2 structure(B), schematic of electron transfer in bilayer MoS2 and bowl?like MoS2 under an external magnetic field(C), schematic of electron transfer in bowl?like MoS2 flakes during HER(D), polarization curves(E) and Tafel plots of different situation in 0.5 mol/L H2SO4(F), chronoamperometric responses(j?t) recorded from bowl?like MoS2 flakes at a constant overpotential of -150 mV(vs. RHE) under pulsed turn?on and turn?off magnetic field(G)[47], schematic of the climb stage and schematic of the drive stage(H), schematic of the wafer?scale growth of TMD nanograin films(I), HAADF STEM investigation of MoS2 grains(J), Raman spectra acquired from the MoS2 film(K), polarization curves(L) and Tafel plots of MoS2 devices in 0.5 mol/L H2SO4(M)[48](A—G) Copyright 2020, American Chemical Society; (H—M) Copyright 2020, Springer Nature.
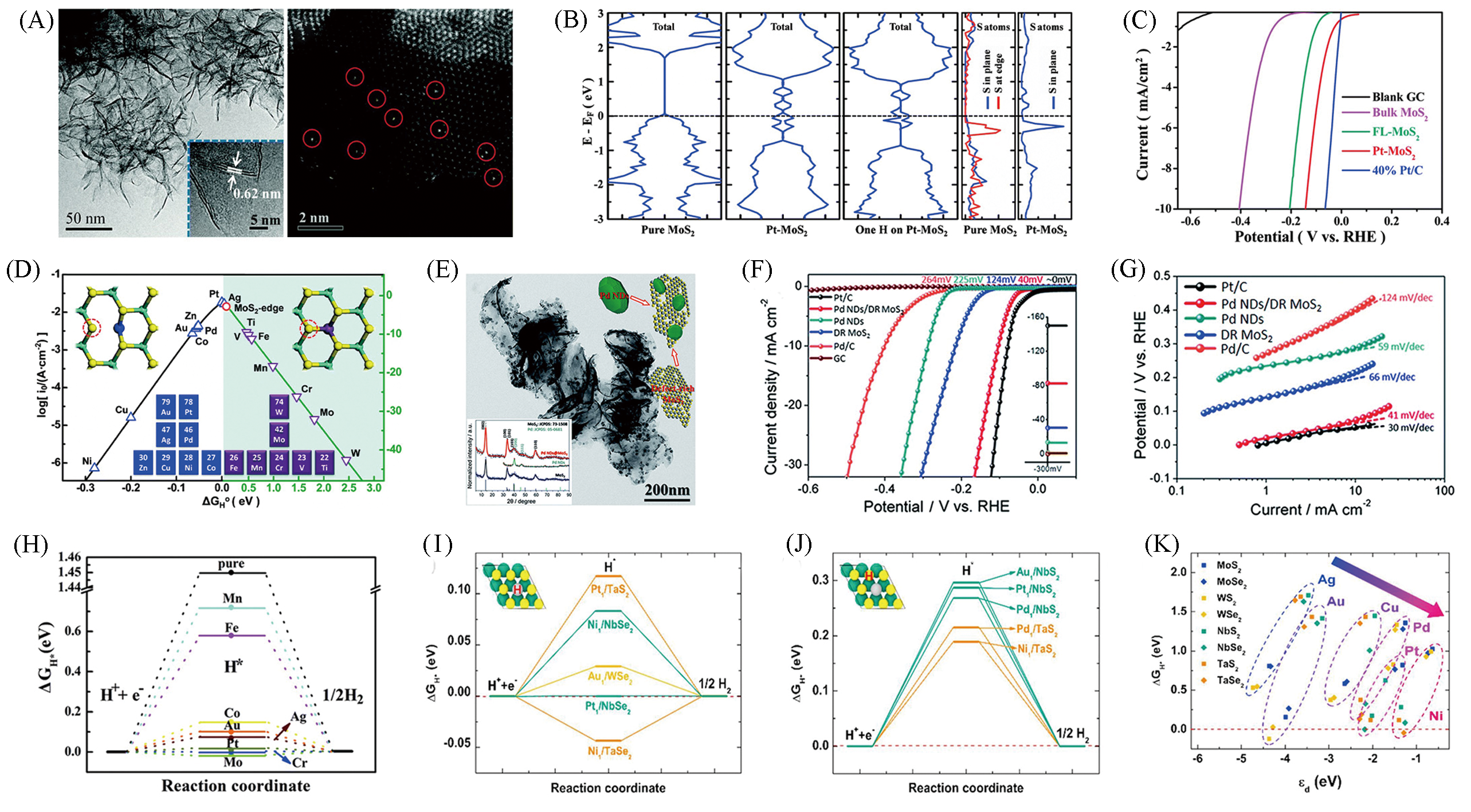
Fig.7 TEM image and HAADF?STEM images of Pt?MoS2(A), total DOS and projected DOS of pure MoS2 and Pt?MoS2, respectively(B), HER polarization curves of Pt?MoS2(C), volcano curve of different atom doped(D)[51], TEM image of Pd ND/DR?MoS2(E), polarization curves(F) and Tafel plots(G) of Pd ND/DR MoS2[52], ΔGH*vs. the reaction coordinate of HER for TM doped ReS2(H)[53], ΔGH* diagram of H adsorption on single atom(I) and on ligand next to single atom(J) and relations between the ΔGH* on single atom as a function of d?band center of single atom bound on a support(K)[54](A—D) Copyright 2015, Royal Society of Chemistry; (E—G) Copyright 2016, Royal Society of Chemistry; (H) Copyright 2019, Royal Society of Chemistry; (I—K) Copyright 2019, Elsevier.
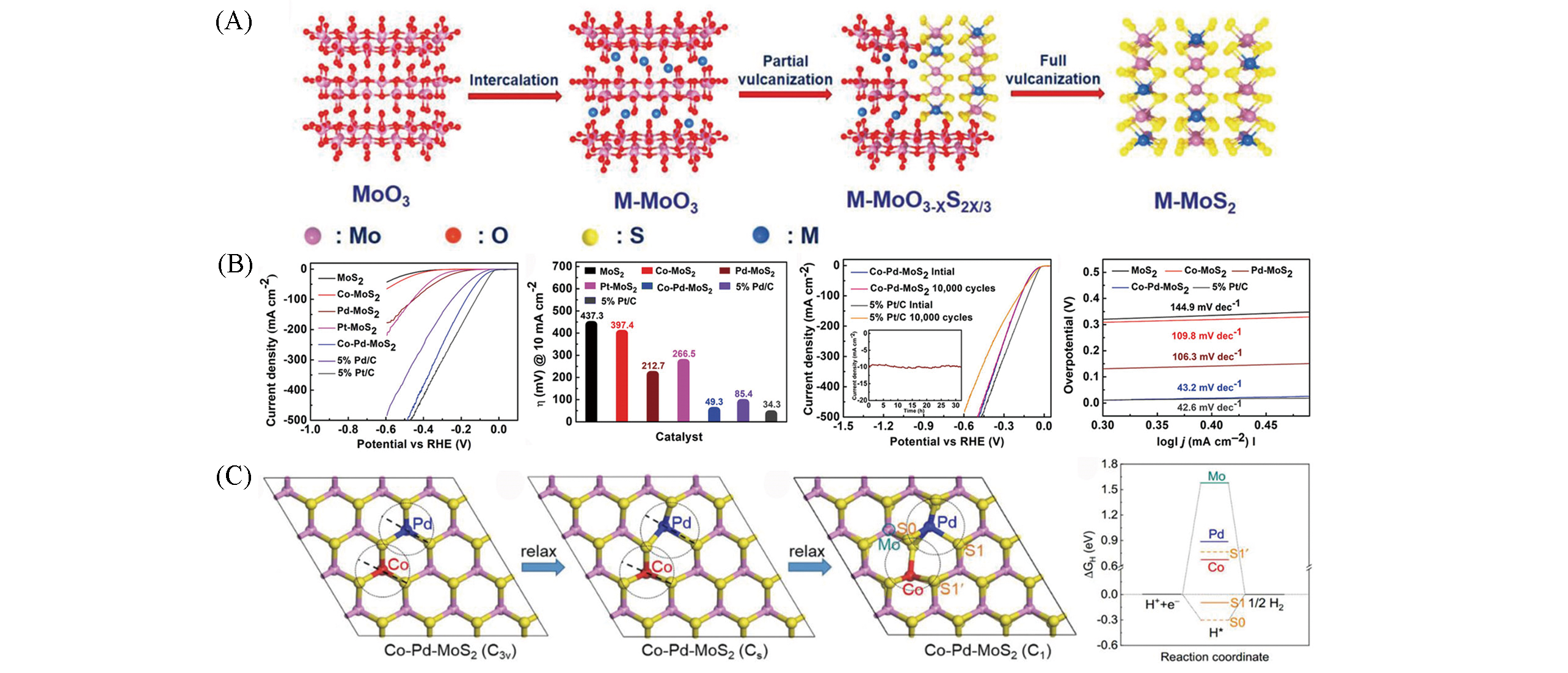
Fig.8 Schematic illustration of the synthesis of M‐MoS2(A), electrochemical evaluation of HER performance for pristine, doped MoS2 and performance comparison in 0.5 mol/L H2SO4(B), Co‐Pd‐MoS2 with different local symmetries and ΔGH diagrams of different catalytic sites of most stable doped structures in Co‐Pd‐MoS2(C)[55]Copyright 2020, Wiley?VCH.
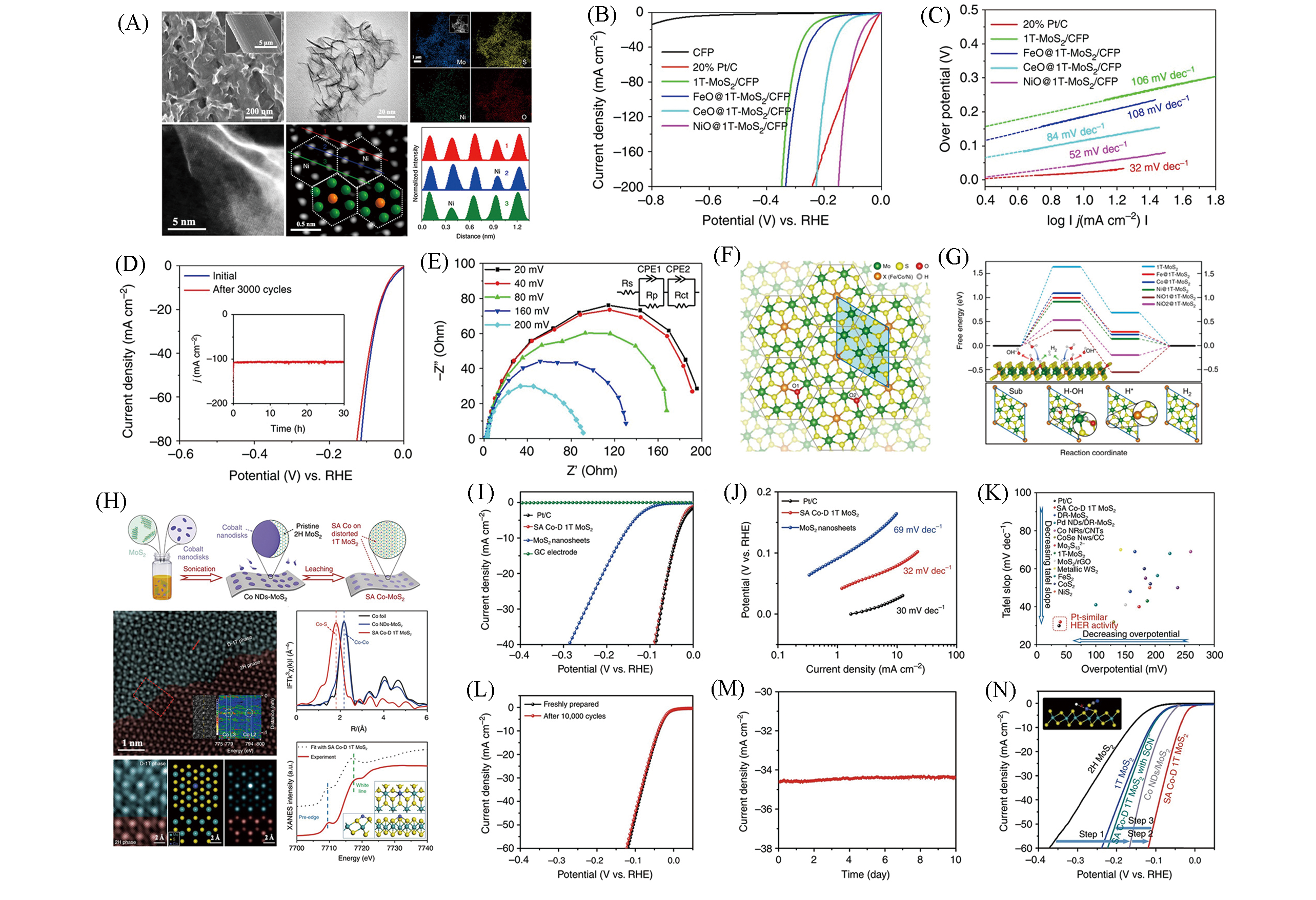
Fig.9 Structure characterizations of NiO@1T?MoS2:SEM, TEM, EDX mappings, HAADF?STEM and intensity profiles(green: Mo; orange: Ni)(A), polarization curves(B) and Tafel plots(C), stability tests(D) and electrochemical impedance spectroscopy(E) of NiO@1T?MoS2, first?principles calculations of the doping effect on HER performance(F, G)[56], schematic illustration of synthetic method for SA Co?D 1T MoS2 and its characterization(H), HER performance of SA Co?D 1T MoS2(I—N)[57](A―G) Copyright 2019, Springer Nature; (H―N) Copyright 2019, Springer Nature.
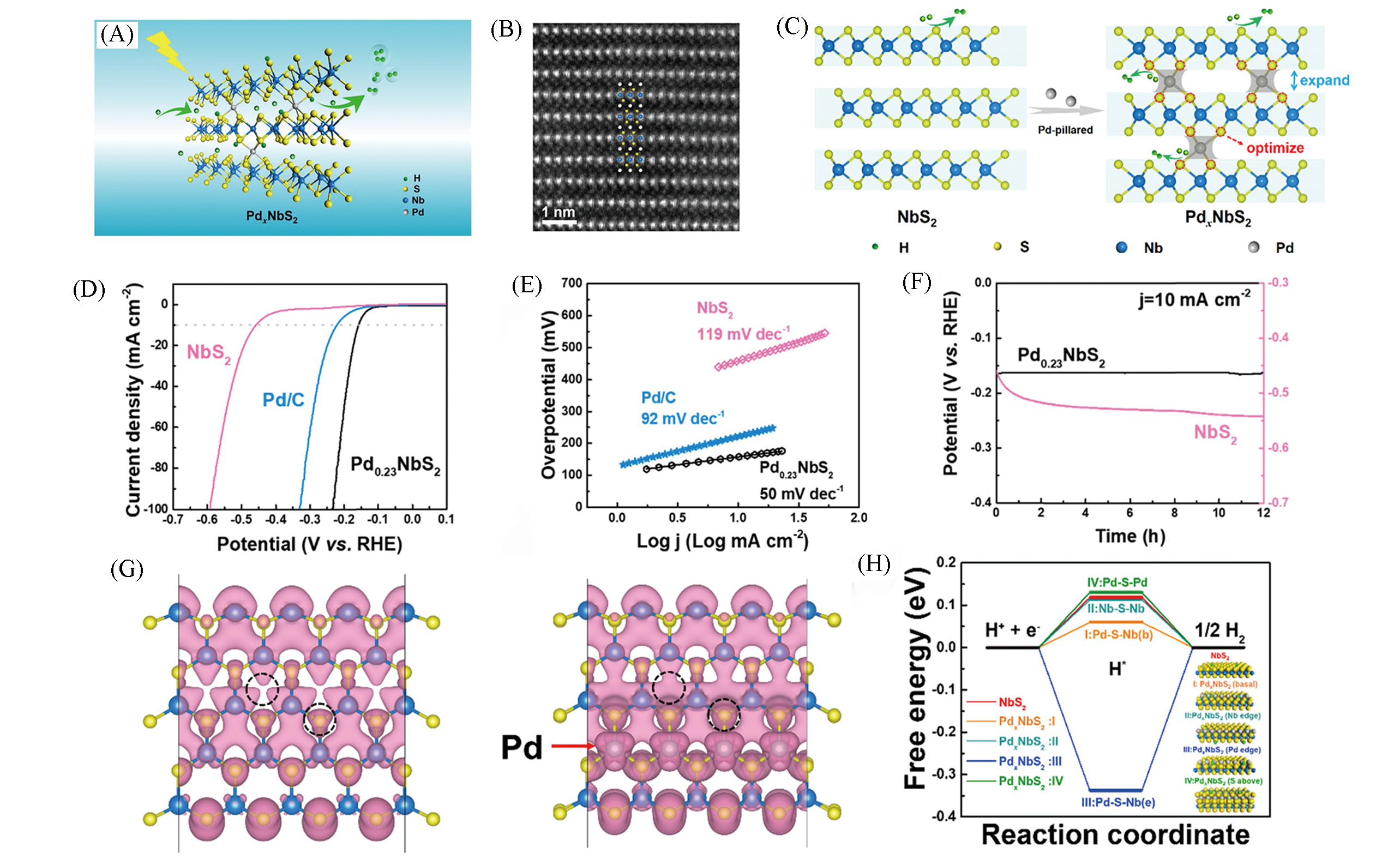
Fig.10 Schematic of electron transfer in PdxNbS2 during HER(A), HAADF?STEM image of Pd0.23NbS2 along(B), schematic illustration of atomic?pillar effect of Pd in PdxNbS2(C), polarization curves(D), Tafel plots(E) and time?dependent potential curves(F) of Pd0.23NbS2, partial charge density of NbS2 and PdxNbS2 single layers within 1.0 eV below Fermi level(G), calculated free energy diagram of hydrogen evolution for NbS2 and PdxNbS2 with different atomic configurations(H)[58]Copyright 2019, American Chemical Society.
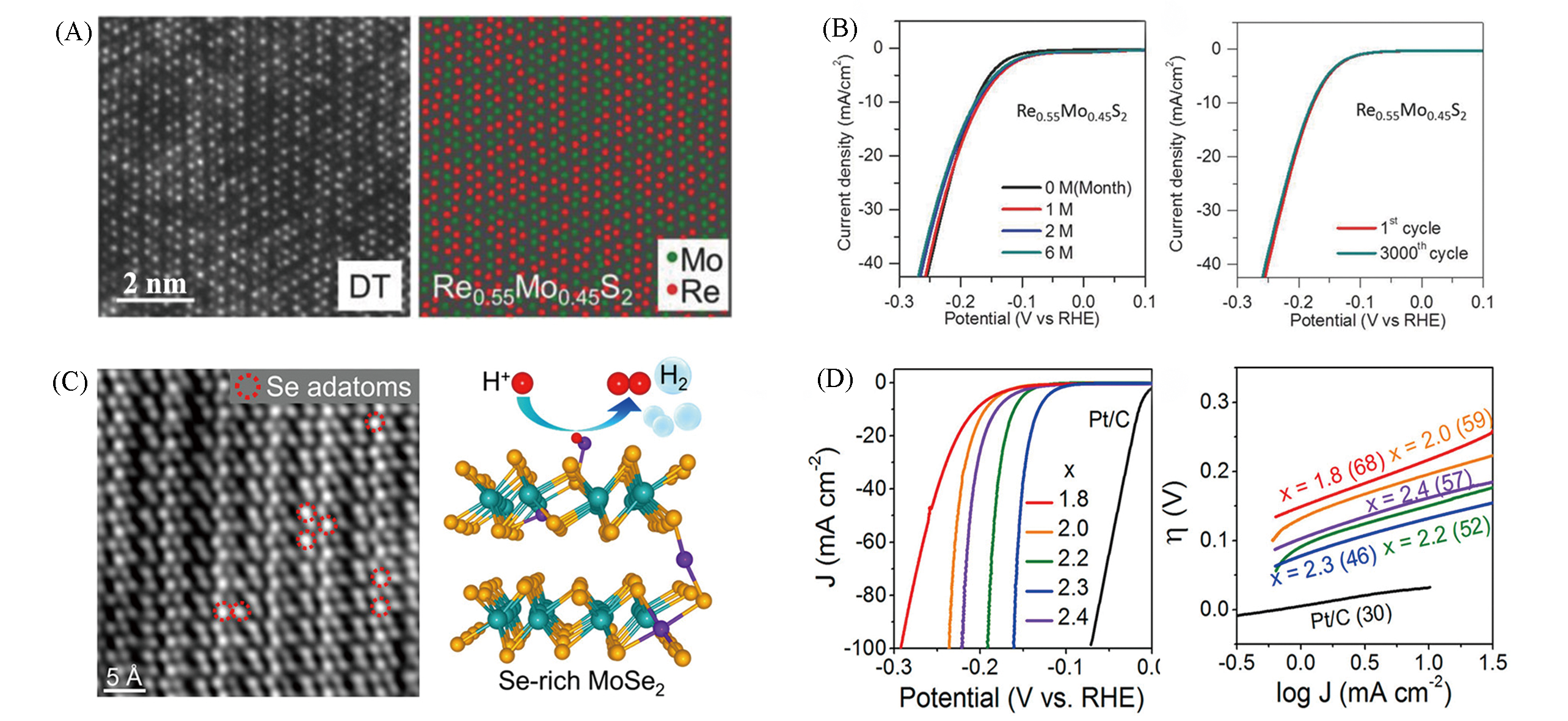
Fig.12 Structure of Re0.55Mo0.45S2 alloy monolayers(A), polarization curves and stability test of Re0.55Mo0.45S2 alloy(B)[62], HAADF?STEM of MoSe2.3 and DFT calculation of HER pathways(C), polarization curves and Tafel plots of MoSex(D)[36](A, B) Copyright 2018, John Wiley?VCH; (C, D) Copyright 2020, American Chemical Society.
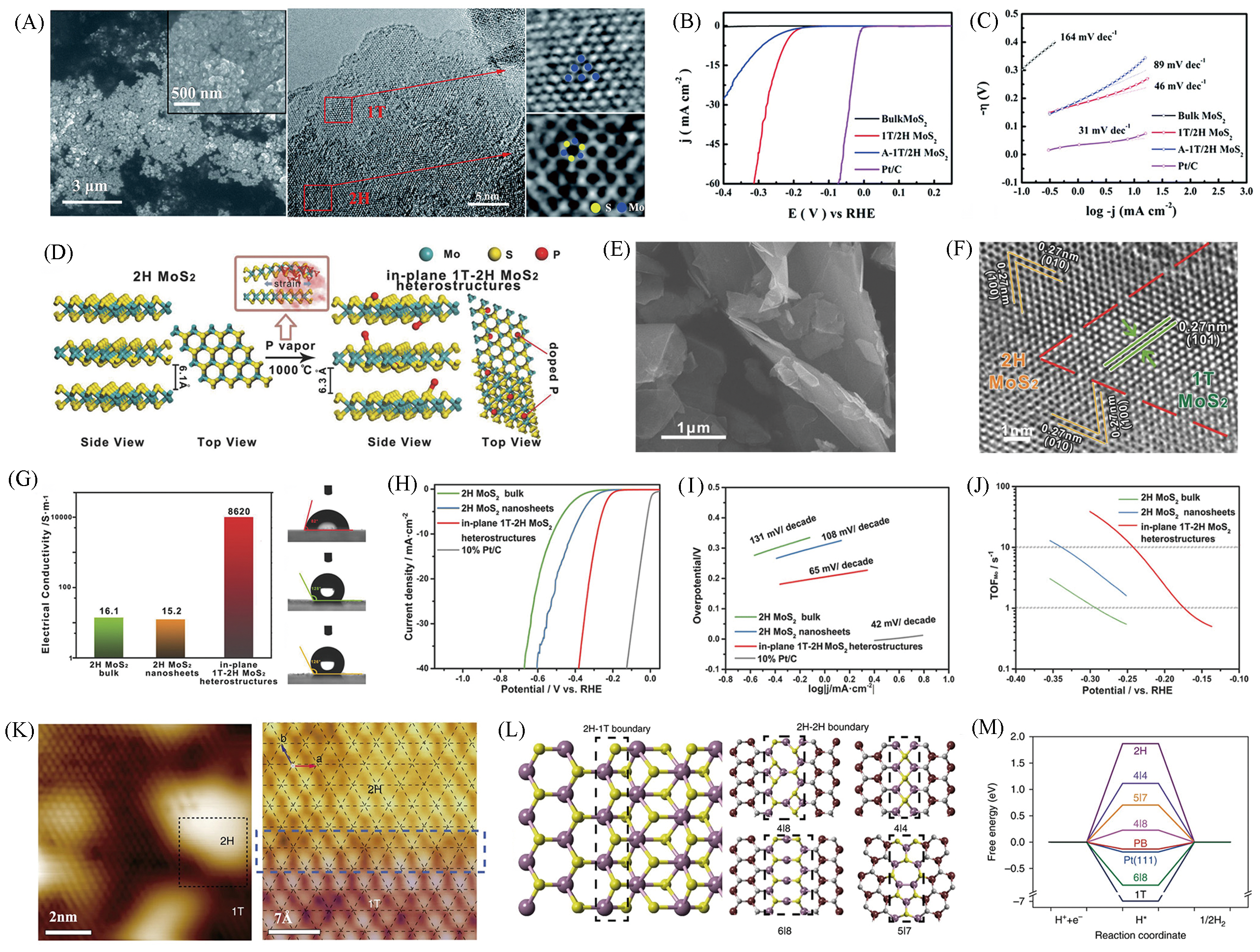
Fig.13 SEM and HRTEM images of the as?prepared 1T/2H MoS2(A), polarization curves(B), Tafel slopes of 1T/2H MoS2(C)[63], schematic for the partial phase transition process of semiconducting 2H MoS2 to metallic 1T MoS2 induced by phosphorus atoms(D), SEM(E) and HRTEM images(F) of in?plane 1T?2H MoS2 heterostructures, electrical conductivity and contact angle of water droplets on the surface of in?plane 1T?2H MoS2 heterostructures, 2H MoS2 bulk and 2H MoS2 nanosheets, respectively(G), polarization curves(H), Tafel slopes of 1T?2H MoS2 heterostructures(I), comparative TOF values(J)[64], STM topography of as?treated MoS2 showing mixed 2H(bright) and 1T(dark) domains(K) and theoretical simulations(L, M)[65](A―C) Copyright 2017, Royal Society of Chemistry; (D―J) Copyright 2018, Wiley?VCH; (K―M) Copyright 2019, Springer Nature.
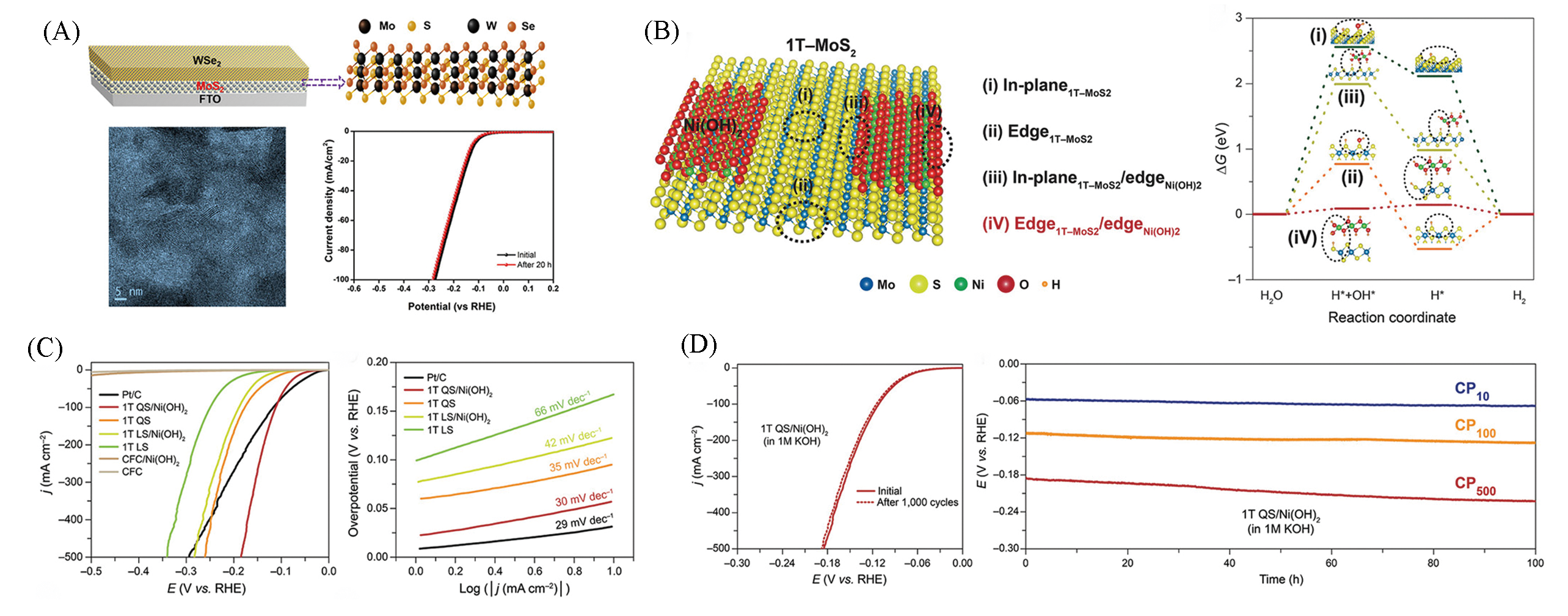
Fig.14 Structure and performance characterization of WSe2/MoS2 heterostructure on FTO substrate(A)[70], theoretical investigation of 1T?MoS2QS/Ni(OH)2(B), HER performance of 1T?MoS2QS/Ni(OH)2 in 1 mol/L KOH(C, D)[71](A) Copyright 2019, Elsevier; (B―D) Copyright 2020, Wiley?VCH.
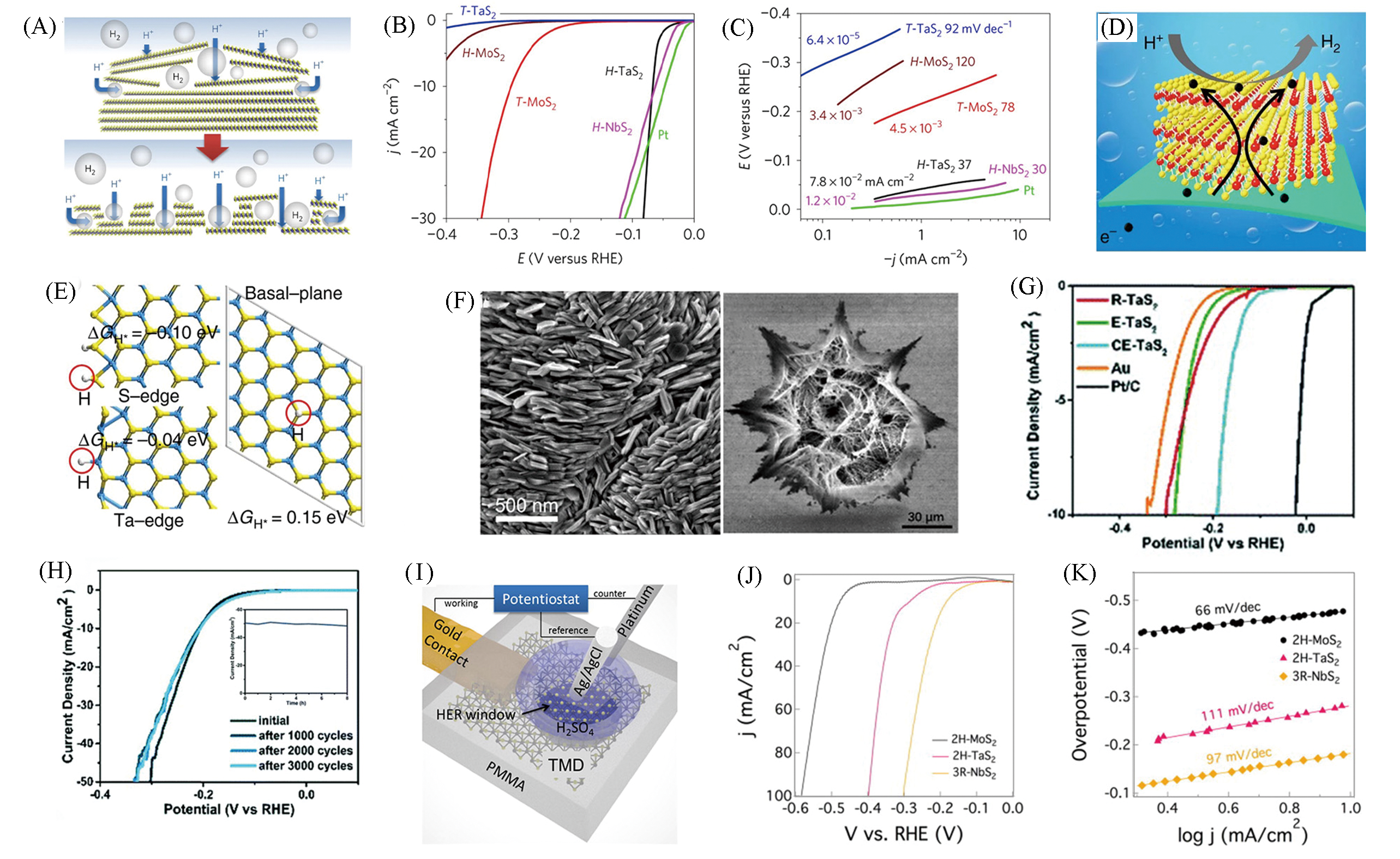
Fig.15 Schematic of the proposed mechanism for the morphology change(A), polarization curves(B) and Tafel plots(C) of H?TaS2, H?NbS2[73], schematic illustration of the HER process of 2H?TaS2/Au foils(D), hydrogen adsorption energies at S?edge, Ta?edge, and basal?plane of 2H?TaS2, respectively(yellow, cyan, and grey balls represent S, Ta, and adsorbed H atoms, respectively)(E)[74], morphology of 3R?TaS2(left) and cracked eight?awn star TaS2(CE?TaS2)(right)(F)[75], polarization curves(G) and durability test(H) of CE?TaS2[76], schematic illustration of the local electrochemical measurement setup(I), the HER performance of the 2H?MoS2, 2H?TaS2(J, K)[77](A—C) Copyright 2017, Springer Nature.(D,E) Copyright 2017, Springer Nature; (F) Copyright 2018, American Chemical Society; (G,H) Copyright 2019, Royal Society of Chemistry; (I—K) Copyright 2019, Elsevier.
| 1 | Balat M., Int. J. Hydrog. Energy, 2008, 33(15), 4013—4029 |
| 2 | International Energy Outlook 2016, Office of Energy Analysis, U.S. Energy Information Administration, Washington DC, 2016 |
| 3 | Zou X., Zhang Y., Chem. Soc. Rev., 2015, 44(15), 5148—5180 |
| 4 | Qu D. W., Li X., Chen G. M., Chem. J. Chinese Universities, 2019, 40(4), 617—623(曲大伟, 李昕, 陈光明. 高等学校化学学报, 2019, 40(4), 617—623) |
| 5 | Dresselhaus M. S., Thomas I. L., Nature, 2001, 414(6861), 332—337 |
| 6 | Dincer I., Int. J. Hydrog. Energy, 2012, 37(2), 1954—1971 |
| 7 | Turner J. A., Science, 2004, 305(5686), 972—974 |
| 8 | Troostwijk P. V., Deiman J. R., Obs. Phys., 1789, 35, 369—370 |
| 9 | Levie R. D., J. Electroanal., Chem., 1999, 476, 92—93 |
| 10 | Tributsch H., Z. Naturforsch. A, 1977, 32(9), 972—985 |
| 11 | Morales⁃Guio C. G., Stern L. A., Hu X., Chem. Soc. Rev., 2014, 43(18), 6555—6569 |
| 12 | Lu Q., Yu Y., Ma Q., Chen B., Zhang H., Adv. Mater., 2016, 28(10), 1917—1933 |
| 13 | Zhang X., Lai Z., Ma Q., Zhang H., Chem. Soc. Rev., 2018, 47(9), 3301—3338 |
| 14 | Lin L., Sherrell P., Liu Y., Lei W., Zhang S., Zhang H., Wallace G. G., Chen J., Adv. Energy Mater., 2020, 10(16), 1903870 |
| 15 | Hinnemann B., Moses P. G., Bonde J., Kristina P. J., Jane H. N., Horch S., Chorkendorff I. B., Jens K. N., J. Am. Chem. Soc., 2005, 127(15), 5308—5309 |
| 16 | Conway B. E., Tilak B. V., Electrochim. Acta, 2002, 47(22/23), 3571—3594 |
| 17 | Meng L. J., Ma Y., Si K. P., Xu S. Y., Wang J. L., Gong Y. J., Tungsten, 2019, 1(1), 46—58 |
| 18 | Qian X., Liu J., Fu L., Li J., Science, 2014, 346(6215), 1344—1347 |
| 19 | Manzeli S., Ovchinnikov D., Pasquier D., Yazyev O. V., Kis A., Nat. Rev. Mater., 2017, 2(8),17033 |
| 20 | Wang J., Wei Y., Li H., Huang X., Zhang H., Sci. China Chem., 2018, 61(10), 1227—1242 |
| 21 | Huan Y., Shi J., Zou X., Zou X., Gong Y., Xie C., Yang Z., Zhang Z., Gao Y., Shi Y., Li M., Yang P., Jiang S., Hong M., Gu L., Zhang Q., Yan X., Zhang Y., J. Am. Chem. Soc., 2019, 141(47), 18694—18703 |
| 22 | Yu Y., Nam G. H., He Q., Wu X. J., Zhang K., Yang Z., Chen J., Ma Q., Zhao M., Liu Z., Ran F. R., Wang X., Li H., Huang X., Li B., Xiong Q., Zhang Q., Liu Z., Gu L., Du Y., Huang W., Zhang H., Nat. Chem., 2018, 10(6), 638—641 |
| 23 | Lukowski M. A., Daniel A. S., Meng F., Forticaux A., Li L., Jin S., J. Am. Chem. Soc., 2013, 135(28), 10274—10277 |
| 24 | Voiry D., Yamaguchi H., Li J., Silva R., Alves D. C. B., Fujita T., Chen M., Asefa T., Shenoy V. B., Eda G., Chhowalla M., Nat. Mater., 2013, 12(9), 850—855 |
| 25 | Greeley J., Jaramillo T. F., Bonde J., Chorkendorff I. B., Nørskov J. K., Nat. Mater., 2006, 5(11), 909—913 |
| 26 | Lin X. Y., Wang J., Acta Chim. Sin., 2017, 75(10), 979—990(林潇羽, 王璟. 化学学报, 2017, 75(10), 979—990) |
| 27 | Tsai C., Chan K., Nørskov J. K., Frank A. P., Surf. Sci., 2015, 640, 133—140 |
| 28 | Jaramillo T. F., Jørgensen K. P., Bonde J., Nielsen J. H., Horch S., Chorkendorff I. B., Science, 2007, 317(5834), 100—102 |
| 29 | Kibsgaard J., Chen Z., Reinecke B. N., Jaramillo T. F., Nat. Mater., 2012, 11(11), 963—969 |
| 30 | Deng D., Novoselov K. S., Fu Q., Zheng N. F., Tian Z. Q., Bao X. H., Nat. Nanotechnol., 2016, 11(3), 218—230 |
| 31 | Tsai C., Chan K., Abild⁃Pedersen F., Nørskov J., Phys. Chem. Chem. Phys., 2014, 16(26), 13156—13164 |
| 32 | Qian X., Xie K., Guo S., Liang Q., Zhang S., Xiong Z., Zhan H., Liu C., Yang X., Zhu J., Li D., Chem. Commun., 2020, 56(51), 7005—7008 |
| 33 | Wang L., Sofer Z., Luxa J., Sedmidubský D., Ambrosi A., Pumera M., Electrochem. Commun., 2016, 63, 39—43 |
| 34 | Li H., Tan Y., Liu P., Guo C., Luo M., Han J., Lin T., Huang F., Chen M., Adv. Mater., 2016, 28(40), 8945—8949 |
| 35 | Gao J., Li L., Tan J., Sun B., Idrobo J. C., Singh C. V., Lu T. M., Koratkar N., Nano Lett., 2016, 16(6), 3780—3787 |
| 36 | Kwon I. S., Kwak I. H., Debela T. T., Abbas H. G., Park Y. C., Ahn J. P., Park J., Kang H. S., ACS Nano, 2020, 14(5), 6295—6304 |
| 37 | Navarro⁃Moratalla E., Island J. O., Manas⁃Valero S., Pinilla⁃Cienfuegos E., Castellanos⁃Gomez A., Quereda J., Rubio⁃Bollinger G., Chirolli L., Silva⁃Guillén J. A., Agraït N., Steele G. A., Guinea F., vander Zant H. S. J., Coronado E., Nat. Commun., 2016, 7(1), 1—7 |
| 38 | Tan Y., Liu P., Chen, L., Cong W., Ito Y., Han J., Guo X., Tang Z., Fujita T., Hirata A., Chen M. W., Adv. Mater., 2014, 26(47), 8023—8028 |
| 39 | Li Y., Wang H., Xie L., Liang Y., Hong G., Dai H., J. Am. Chem. Soc., 2011, 133(19), 7296—7299 |
| 40 | Yang J., Voiry D., Ahn S. J., Kang D., Kim A. Y., Chhowalla M., Shin H. S., Angew. Chem. Int. Ed., 2013, 52(51), 13751—13754 |
| 41 | Yu Y., Huang S. Y., Li Y., Steinmann S. N., Yang W., Cao L., Nano Lett., 2014, 14(2), 553—558 |
| 42 | Deng J., Yuan W., Ren P., Wang Y., Deng D., Zhang Z., Bao X., RSC Adv., 2014, 4(66), 34733—34738 |
| 43 | Ye G., Gong Y., Lin J., Lin J., Li B., He Y., Pantelides S. T., Zhou W., Vajtai R., Ajayan P. M., Nano Lett., 2016, 16(2), 1097—1103 |
| 44 | Li H., Tsai C., Koh A.L., Cai L., Contryman A. W., Fragapane A. H., Zhao J., Han H. S., Manoharan H. C., Abild⁃Pedersen F., Nørskov J. K., Zheng X., Nat. Mater., 2016, 15(1), 48—53 |
| 45 | Lin L., Miao N., Wen Y., Zhang S., Ghosez P., Sun Z., Allwood D. A., ACS Nano, 2016, 10(9), 8929—8937 |
| 46 | Tsai C., Li H., Park S., Park J., Han H. S., Nørskov J. K., Zheng X., Abild⁃Pedersen F., Nat. Commun., 2017, 8(1), 1—8 |
| 47 | Zhou W., Chen M., Guo M., Guo M., Hong A., Yu T., Luo X., Yuan C., Lei W., Wang S., Nano Lett., 2020, 20(4), 2923—2930 |
| 48 | He Y., Tang P., Hu Z., He Q., Zhu C., Wang L., Zeng Q., Golani P., Gao G., Fu W., Huang Z., Gao C., Xia J., Wang X., Wang X., Zhu C., Ramasse Q. M., Zhang A., An B., Zhang Y., Martí⁃Sánchez S., Morante J. R., Wang L., Tay B. K., Yakobson B. I., Trampert A., Zhang H., Wu M., Wang Q. J., Arbiol J., Liu Z., Nat. Commun., 2020, 11(1), 1—12 |
| 49 | van der Zande A. M., Huang P. Y., Chenet D. A., Berkelbach T. C., You Y. M., Lee G. H., Heinz T. F., Reichman D. R., Muller D. A., Hone J., Nat. Mater., 2013, 12(6), 554—561 |
| 50 | Najmaei S., Liu Z., Zhou W., Zou X., Shi G., Lei S., Yakobson B. I., Idrobo J. C., Ajayan P. M., Lou J., Nat. Mater., 2013, 12(8), 754—759 |
| 51 | Deng J., Li H., Xiao J., Tu Y., Deng D., Yang H., Tian H., Li J., Ren P., Bao X., Energy Environ. Sci., 2015, 8(5), 1594—1601 |
| 52 | Qi K., Yu S., Wang Q., Zhang W., Fan J., Zhang W., Cui X., J. Mater. Chem. A, 2016, 4(11), 4025—4031 |
| 53 | Pan J., Wang R., Xu X., Hu J., Ma L., Nanoscale, 2019, 11(21), 10402—10409 |
| 54 | Hwang J., Noh S.H., Han B., Appl. Surf. Sci., 2019, 471, 545—552 |
| 55 | Yang W., Zhang S., Chen Q., Zhang C., Wei Y., Jiang H., Lin Y., Zhao M., He Q., Wang X., Du Y., Song L., Yang S., Nie A., Zou X.L., Gong Y. J., Adv. Mater., 2020, 2001167 |
| 56 | Huang Y., Sun Y., Zheng X., Aoki T., Pattengale B., Huang J., He X., Bian W., Younan S., Williams N., Hu J., Ge J., Pu N., Yan X., Pan X., Zhang L., Wei Y., Gu J., Nat. Commun., 2019, 10(1), 1—11 |
| 57 | Qi K., Cui X., Gu L., Yu S., Fan X., Luo M., Xu S., Li N., Zheng L., Zhang Q., Ma J., Gong Y., Lv F., Wang K., Huang H., Zhang W., Guo S., Zheng W., Liu P., Nat. Commun., 2019, 10(1), 1—9 |
| 58 | Huang C., Wang X., Wang D., Zhao W., Bu K., Xu J., Huang X., Bi Q., Huang J., Huang F., Chem. Mater., 2019, 31(13), 4726—4731 |
| 59 | Xu J., Zhang J., Zhang W., Zhang W., Lee C. S., Adv. Energy Mater., 2017, 7(23), 1700571 |
| 60 | Xu J., Huang Y., Cheng X., Liu T., Lu Y., Chen X., You Y., Zhang J., Inorg. Chem. Front., 2018, 5(12), 3140—3147 |
| 61 | Voiry D., Salehi M., Silva R., Fujita T., Chen M., Asefa T., Shenoy V. B., Eda G., Chhowalla M., Nano Lett., 2013, 13(12), 6222—6227 |
| 62 | Yang S. Z., Gong Y., Manchanda P., Zhang Y. Y., Ye G., Chen S., Song L., Pantelides S. T., Ajayan P. M., Chisholm M. F., Zhou W., Adv. Mater., 2018, 30(51), 1803477 |
| 63 | Wang D., Zhang X., Bao S., Zhang Z., Fei H., Wu Z., J. Mater. Chem. A, 2017, 5(6), 2681—2688 |
| 64 | Wang S., Zhang D., Li B., Zhang C., Du Z., Yin H., Bi X., Yang S., Adv. Energy. Mater., 2018, 8(25), 1801345 |
| 65 | Zhu J., Wang Z. C., Dai H., Wang Q., Yang R., Yu H., Liao M., Zhang J., Chen W., Wei Z., Li N., Du L., Shi D., Wang W., Zhang L., Jiang Y., Zhang G., Nat. Commun., 2019, 10(1), 1—7 |
| 66 | Ma Y., Ajayan P. M., Yang S., Gong Y. J., Small, 2018, 14(38), 1801606 |
| 67 | Aeschlimann S., Rossi A., Chávez⁃Cervantes M., Krause R., Arnoldi B., Stadtmüller B., Aeschlimann M., Forti S., Fabbri F., Coletti C., Gierz I., Sci. Adv., 2020, 6(20), eaay0761 |
| 68 | Tsai C., Abild⁃Pedersen F., Nørskov J. K., Nano Lett., 2014, 14(3), 1381—1387 |
| 69 | Vikraman D., Hussain S., Akbar K., Truong L., Kathalingam A., Chun S. H., Jung J., Park H. J., Kim H. S., ACS Sustain. Chem. Eng., 2018, 6(7), 8400—8409 |
| 70 | Vikraman D., Hussain S., Truong L., Karuppasamy K., Kim H. J., Maiyalagan T., Chun S. H., Jung J., Kim H. S., Appl. Surf. Sci., 2019, 480, 611—620 |
| 71 | Chen W., Gu J., Du Y., Song F., Bu F., Li J., Yuan Y., Luo R., Liu Q., Zhang D., Adv. Funct. Mater., 2020, 2000551 |
| 72 | Fujita T., Ito Y., Tan Y., Yamaguchi H., Hojo D., Hirata A., Voiry D., Chhowalla M., Chen M., Nanoscale, 2014, 6(21), 12458—12462 |
| 73 | Liu Y., Wu J., Hackenberg K. P., Zhang J., Wang M., Yang Y., Keyshar K., Gu J., Ogitsu T., Vajtai R., Lou J., Ajayan P. M., Wood B. C., Yakobson B. I., Nat. Energy, 2017, 2(9), 1—7 |
| 74 | Shi J., Wang X., Zhang S., Xiao L., Huan Y., Gong Y., Zhang Z., Li Y., Zhou X., Hong M., Fang Q., Zhang Q., Liu X., Gu L., Liu Z., Zhang Y., Nat. Commun., 2017, 8(1), 1—9 |
| 75 | Feng Y., Gong S., Du E., Chen X., Qi R., Yu K., Zhu Z., J. Phys. Chem. C, 2018, 122(4), 2382—2390 |
| 76 | Feng Y., Yu K., Zhu Z., CrystEngComm., 2019, 21(22), 3517—3524 |
| 77 | Zhang J., Wu J., Zou X., Hackenberg K., Zhou W., Chen W., Yuan J., Keyshar K., Gupta G., Mohite A., Ajayan P. M., Lou J., Mater. Today, 2019, 25, 28—34 |
| 78 | Yang J., Mohmad A. R., Wang Y., Fullon R., Song X., Zhao F., Bozkurt L., Augustin M., Santos E. J. G., Shin H. S., Zhang W., Voiry D., Jeong H. Y., Chhowalla M., Nat. Mater., 2019, 18(12), 1309—1314 |
| [1] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [2] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [3] | 杨丽君, 于洋, 张蕾. 双功能2D/3D杂化结构Co2P-CeO x 异质结一体化电极的构筑及电催化尿素氧化辅助制氢性能[J]. 高等学校化学学报, 2022, 43(6): 20220082. |
| [4] | 宋颖颖, 黄琳, 李庆森, 陈立妙. CuO/BiVO4光催化剂的制备及光催化CO2还原性能[J]. 高等学校化学学报, 2022, 43(6): 20220126. |
| [5] | 刘家琪, 李天保. BiVO4/CuBi2O4薄膜光电极的制备及光电性能[J]. 高等学校化学学报, 2022, 43(4): 20220017. |
| [6] | 王祖民, 孟程, 于然波. 过渡金属磷化物析氢催化剂的掺杂调控[J]. 高等学校化学学报, 2022, 43(11): 20220544. |
| [7] | 田润赛, 卢芊, 张洪滨, 张渤, 冯源源, 魏金香, 冯季军. 氮杂碳原位包覆Cu2O/Co3O4@C异质结构复合材料的设计构筑及高效储锂性能[J]. 高等学校化学学报, 2021, 42(8): 2592. |
| [8] | 武亚强, 刘思明, 金顺敬, 严永情, 王朝, 陈丽华, 苏宝连. 锌掺杂NiCoP多孔双层阵列电极材料的制备及电催化产氢性能[J]. 高等学校化学学报, 2021, 42(8): 2483. |
| [9] | 薛晋波, 高国翔, 申倩倩, 刘天武, 刘旭光, 贾虎生. 新型S型CdS-BiVO4异质结光电极的构筑及产氢性能研究[J]. 高等学校化学学报, 2021, 42(8): 2493. |
| [10] | 唐定, 衷水平. Bi1-xFexVO4薄膜光阳极的制备及光电化学性能[J]. 高等学校化学学报, 2021, 42(8): 2509. |
| [11] | 季小好, 王祖民, 陈晓煜, 于然波. 过渡金属磷化物的制备及电催化析氢性能提升策略[J]. 高等学校化学学报, 2021, 42(5): 1377. |
| [12] | 张楠, 韩阔, 李悦, 王春茹, 赵凤, 韩冬雪, 牛利. 尖晶石型过渡金属硫化物CuCo2S4与MoS2复合材料的制备及电催化析氢性能[J]. 高等学校化学学报, 2021, 42(4): 1307. |
| [13] | 史江维, 孟楠楠, 郭亚梅, 于一夫, 张兵 . 二维材料用于电催化析氢的研究进展[J]. 高等学校化学学报, 2021, 42(2): 492. |
| [14] | 王乙舒, 李雪, 闫丽, 徐红赟, 祝玉鑫, 宋艳华, 崔言娟. 二维Z型BCN/Sn3O4复合材料的光催化还原性能[J]. 高等学校化学学报, 2021, 42(12): 3722. |
| [15] | 赵国庆, 袁钊, 王连, 郭卓. 磷化镍/氮硫双掺杂石墨烯复合材料的制备及电催化析氢性能[J]. 高等学校化学学报, 2020, 41(7): 1575. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||