

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (3): 530.doi: 10.7503/cjcu20190516
卓孟宁1,李飞2,蒋浩3,陈倩文1,李鹏3,王立章1,*
收稿日期:2019-10-10
出版日期:2020-02-26
发布日期:2019-12-25
通讯作者:
王立章
作者简介:王立章, 男, 博士, 教授, 主要从事电化学方面的研究. E-mail: wlzh0731@126.c
基金资助:ZHUO Mengning1,LI Fei2,JIANG Hao3,CHEN Qianwen1,LI Peng3,WANG Lizhang1,*
Received:2019-10-10
Online:2020-02-26
Published:2019-12-25
Contact:
Lizhang WANG
Supported by:摘要:
采用低温水热合成法制备了碳纸基底的SnO2气体扩散电极(SnO2/GDE), 并对其物化特性与催化还原CO2产甲酸性能进行了研究. 扫描电子显微镜、 X射线衍射及X射线光电子能谱表征结果表明, 在60, 75, 100 ℃下制备的催化剂均为分散性良好的纳米SnO2粉体, 其粒径分别为7.9, 11.8和12.9 nm. 循环伏安、 线性扫描伏安和电化学交流阻抗测试结果显示电极均具有优异的电催化活性, 其电化学活性表面积分别为150, 470, 240 cm 2, 通过等效电路拟合后电阻分别为8.5, 3.9, 6.6 Ω·cm 2. 在-1.8 V(vs. SCE)电位下电解, 通入电量500 C时, 电极都具有较高电催化还原CO2产甲酸性能, 而75 ℃下制备的电极性能最佳, 产甲酸电流密度为22.8 mA/cm 2 , 产甲酸法拉第效率高达93.5%; 该电极经过20 h长时间电解后, 产甲酸电流密度可维持在12.8 mA/cm 2 , 产甲酸法拉第效率稳定在约65%.
中图分类号:
TrendMD:
卓孟宁,李飞,蒋浩,陈倩文,李鹏,王立章. SnO2/GDE阴极的制备及电催化还原CO2产甲酸性能. 高等学校化学学报, 2020, 41(3): 530.
ZHUO Mengning,LI Fei,JIANG Hao,CHEN Qianwen,LI Peng,WANG Lizhang. Preparation of SnO2/GDE Cathodes and Their Electrocatalytic Reduction of CO2 to Produce Formic Acid †. Chem. J. Chinese Universities, 2020, 41(3): 530.
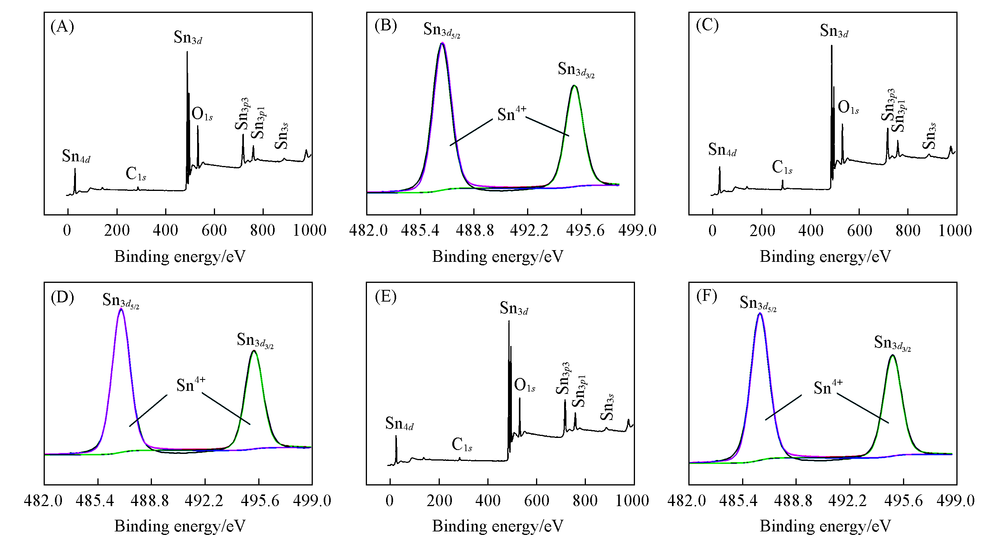
Fig.3 XPS survey(A, C, E) and high resolution spectra of Sn3d(B, D, F) of SnO2-T/GDE (A), (B) SnO2-60/GDE; (C), (D) SnO2-75/GDE; (E), (F) SnO2-100/GDE.
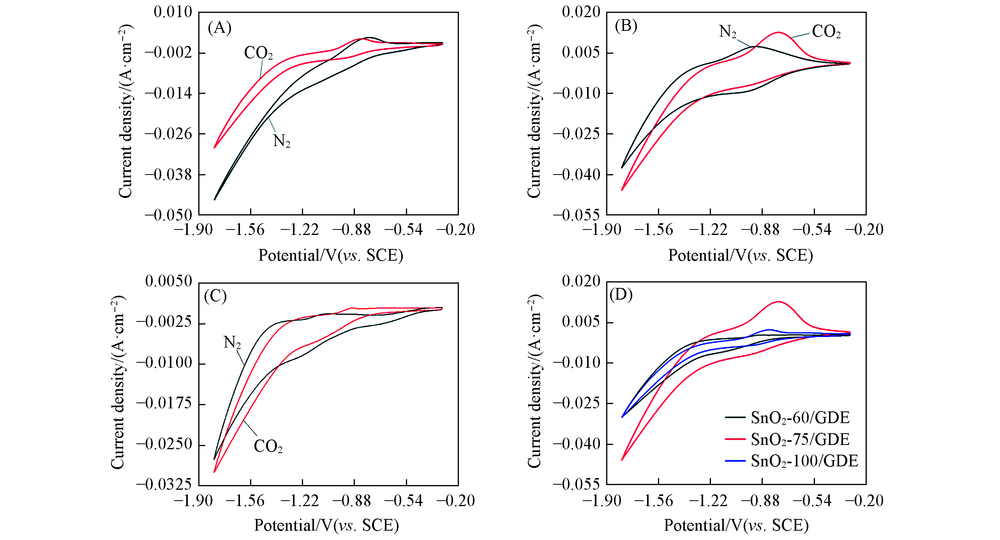
Fig.4 Cyclic voltammetric scan of SnO2-T/GDE in N2 and CO2 environments Scanning rate: 50 mV/s; potential window: -0.3—1.8 V(vs. SCE); electrolyte: 0.1 mol/L KHCO3 solution. (A) SnO2-60/GDE; (B) SnO2-75/GDE; (C) SnO2-100/GDE; (D) SnO2-T/GDE in the CO2 environment.
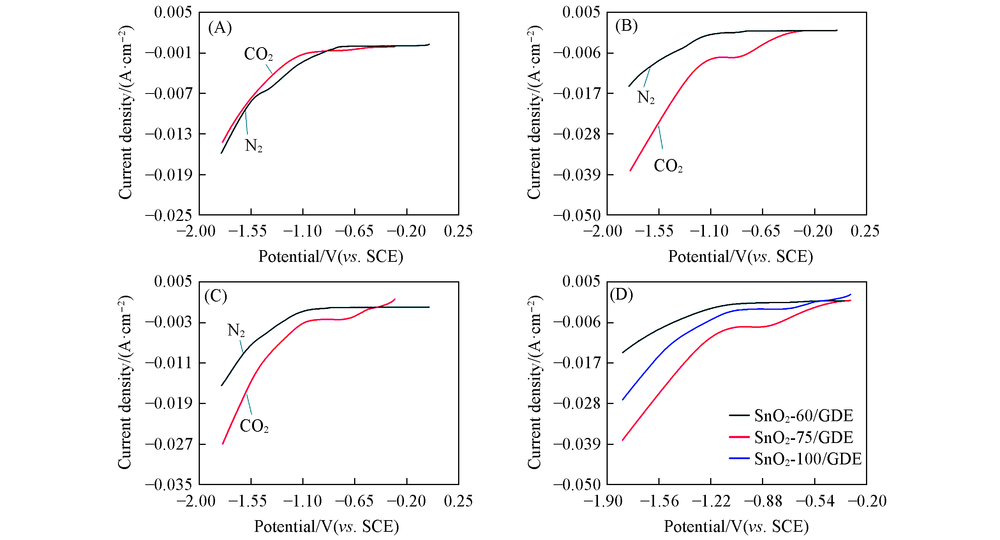
Fig.5 Linear sweep voltammetry of SnO2-T/GDE in N2 and CO2 environments Scanning rate: 50 mV/s; Potential window: -0.3—1.8 V vs. SCE; electrolyte: 0.1 mol/L KHCO3 solution. (A) SnO2-60/GDE; (B) SnO2-75/GDE; (C) SnO2-100/GDE; (D) SnO2-T/GDE in the CO2 environment.
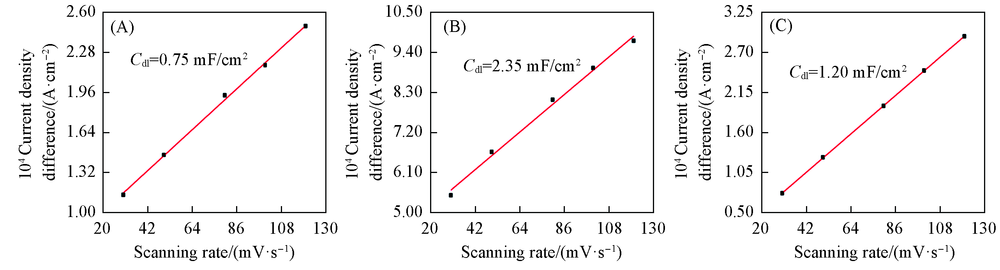
Fig.6 Linear relationship between current density difference and scan rate of SnO2-T/GDE at -0.4 V(vs. SCE) Potential window: -0.3—-0.5 V(vs. SCE); electrolyte: N2 satarated 0.1 mol/L KHCO3 solution. (A) SnO2-60/GDE; (B) SnO2-75/GDE; (C) SnO2-100/GDE.
| Electrode | Rs/(Ω·cm2) | Rct/(Ω·cm2) | Error(%) |
|---|---|---|---|
| SnO2-60/GDE | 4.8 | 8.5 | 1.6 |
| SnO2-75/GDE | 4.8 | 3.9 | 1.3 |
| SnO2-100/GDE | 4.5 | 6.6 | 1.5 |
Table 1 EIS equivalent circuit fitting of SnO2-T/GDE under -1.5 V(vs. SCE)
| Electrode | Rs/(Ω·cm2) | Rct/(Ω·cm2) | Error(%) |
|---|---|---|---|
| SnO2-60/GDE | 4.8 | 8.5 | 1.6 |
| SnO2-75/GDE | 4.8 | 3.9 | 1.3 |
| SnO2-100/GDE | 4.5 | 6.6 | 1.5 |
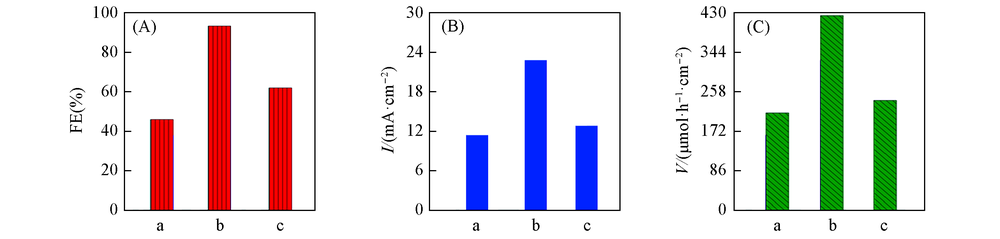
Fig.8 Faradic efficiency(A), current density(B) and production yield(C) of formic acid in CO2 saturared 0.1 mol/L KHCO3 solution over SnO2-T/GDE at -1.8 V(vs. SCE) a. SnO2-60/GDE; b. SnO2-75/GDE; c. SnO2-100/GDE.
| Preparation condition | Electrolysis potential/V | Electrolysis time/h | FE(%) | Current density/(mA·cm-2) | Ref. |
|---|---|---|---|---|---|
| 180 ℃, 24 h | -1.7(vs. SHE) a | 1 | 62 | 12.5 | [ |
| 120 ℃, 6 h | -1.8(vs. SCE) a | 1 | 60 | 9 | [ |
| 100 ℃, 8 h | -1.5(vs. SHE) a | 1 | 87.1 | 10 | [ |
| 60 ℃, 10 h | -1.8(vs. SCE) | 1.21 b | 46.5 | 11.4 | This work |
| 75 ℃, 10 h | -1.8(vs. SCE) | 1.76 b | 93.5 | 22.8 | This work |
| 100 ℃, 10 h | -1.8(vs. SCE) | 1.57 b | 62.1 | 12.8 | This work |
Table 2 Comparison of the electrode performances between the reported results and this work with SnO2 as catalyst
| Preparation condition | Electrolysis potential/V | Electrolysis time/h | FE(%) | Current density/(mA·cm-2) | Ref. |
|---|---|---|---|---|---|
| 180 ℃, 24 h | -1.7(vs. SHE) a | 1 | 62 | 12.5 | [ |
| 120 ℃, 6 h | -1.8(vs. SCE) a | 1 | 60 | 9 | [ |
| 100 ℃, 8 h | -1.5(vs. SHE) a | 1 | 87.1 | 10 | [ |
| 60 ℃, 10 h | -1.8(vs. SCE) | 1.21 b | 46.5 | 11.4 | This work |
| 75 ℃, 10 h | -1.8(vs. SCE) | 1.76 b | 93.5 | 22.8 | This work |
| 100 ℃, 10 h | -1.8(vs. SCE) | 1.57 b | 62.1 | 12.8 | This work |
| [1] | Koo Y., Malik R., Alvarez N., White L., Shanov V N., Schulz M., Collins B., Sankar J., Yun Y ., RSC Adv., 2014, 4( 31), 16362— 16367 |
| [2] | Zhang Q., Xu W. T., Liu Y. Y., Zhang J. J ., Chin. J. Nat., 2017, 39( 4), 242— 250 |
| ( 张琪, 许武韬, 刘予宇, 张久俊 . 自然杂志, 2017, 39( 4), 242— 250) | |
| [3] | Zhang S., Kang P., Meyer T. J ., J. Am. Chem. Soc., 2014, 136( 5), 1734— 1737 |
| [4] | Reis A., Mert S ., Int. J. Hydrog. Energy, 2015, 40( 37), 12776— 12783 |
| [5] | Lu X., Leung D., Wang H., Leung M., Xuan J ., ChemElectroChem, 2014, 1( 5), 836— 849 |
| [6] | Bashir S., Hossain M., Hossain S., Rahman S., Ahmed S., Al-Ahmed A ., J. CO2 Util., 2016, 16, 346— 353 |
| [7] | Scialdone O., Galia A., Nero G. L., Proietto F., Sabatino S., Schiavo B ., Electrochim. Acta, 2016, 199( 199), 331— 332 |
| [8] | Lv W., Zhang R., Gao P., Lei L ., J. Power Sources, 2014, 253, 276— 281 |
| [9] | Asadi M., Kumar B., Behranginia A., Rosen B., Baskin A., Repnin N., Pisasale D., Phillips P., Zhu W., Haasch R., Klie R F., Král P., Abiade J., Salehi-Khojin A ., Nat. Commun., 2014, 5, 4470 |
| [10] | Kim D., Resasco J., Yu Y., Asiri A., Yang P ., Nat. Commun., 2014, 5, 5948 |
| [11] | Li Q., Rao X., Sheng J., Xu J., Yi J., Liu Y., Zhang J ., J. CO2 Util., 2018, 27, 48— 59 |
| [12] | Gao D., Zhou H., Wang J., Miao S., Yang F., Wang G., Wang J., Bao X ., J. Am. Chem. Soc., 2015, 137( 13), 4288— 4291 |
| [13] | Xie J., Huang Y., Yu H ., Front. Environ. Sci. Eng., 2015, 9( 5), 861— 866 |
| [14] | Jing Z. G., Fan G., Zhang D. F ., Southern Metals, 2007, 5, 9—11, 17 |
| ( 荆忠国, 樊刚, 张德丰 . 南方金属, 2007, 5, 9—11, 17) | |
| [15] | Vemury S., Pratsinis S., Kibbey L ., J. Mater. Res., 1997, 12( 4), 1031— 1042 |
| [16] | Pan Q. Y., Dong X. W., Zhang J. P ., J. Inorg. Mater., 1997, 12( 4), 494— 498 |
| ( 潘庆谊, 董晓雯, 张剑平 . 无机材料学报, 1997, 12( 4), 494— 498) | |
| [17] | Wang D. X., Zhong J. M., Sun B. S ., Chin. J. Inorga. Chem., 2008, 24( 6), 892— 896 |
| ( 王东新, 钟景明, 孙本双 . 无机化学学报, 2008, 24( 6), 892— 896) | |
| [18] | Zhang J. R., Gao L ., Acta Chimica Sinica, 2003, 12, 1965—1968 |
| ( 张建荣, 高濂 . 化学学报, 2003, 12, 1965—1968) | |
| [19] | Cheng H. M., Ma J. M ., Chem. J. Chinese Universities, 1996, 17( 6), 833— 83 |
| ( 程虎民, 马季铭 . 高等学校化学学报, 1996, 17( 6), 833— 83) | |
| [20] | Hu X. Y., Wang N., Hao Y. T., Xu Z. Q., Wang M. H., Shi G. Q., Yang H. M., Liang Z. H ., Chem. J. Chinese Universities, 2018, 39( 10), 2265— 2271 |
| ( 胡雪艳, 王娜, 郝玉婷, 许志庆, 王明慧, 师改琴, 杨慧敏, 梁镇海 . 高等学校化学学报, 2018, 39( 10), 2265— 2271) | |
| [21] | Fu Y., Li Y., Zhang X., Qiao J ., Chin. J. Cataly, 2016, 7( 37), 1081— 1088 |
| [22] | Li H., Oloman C ., J. Appli. Electrochem., 2005, 35( 10), 955— 965 |
| [23] | Fan M., Bai Z., Zhang Q ., RSC Adv., 2014, 4( 84), 44583— 44591 |
| [24] | Li Y., Qiao J., Zhang X., Lei T., Girma A., Liu Y., Zhang J ., ChemElectroChem, 2016, 3( 10), 1618— 1628 |
| [25] | Zhang Z. N ., J. Zhejiang Univ. Technol., 2002, 1, 33— 37 |
| ( 张泽南 . 浙江工业大学学报, 2002, 1, 33— 37) | |
| [26] | Wang L., Du J., Xing G. J., Xiong Y. H., Yuan P., Yu X. Y., ., Proceedings of the Twelfth Chinese Conference on Solid State Ionology, 2004, 543— 545 |
| ( 王磊, 杜军, 刑光健, 熊玉华, 苑鹏, 尉秀英 . 第十二届中国固态离子学学术会议论文集, 2004, 543— 545) | |
| [27] | Wu J. F., Zhao N., Xu X. H ., China Conference on Functional Materials and Applications, 2007, 81— 85 |
| ( 吴建锋, 赵娜, 徐晓虹 . 中国功能材料及其应用学术会议, 2007, 81— 85) | |
| [28] | Zhang J., Ma Z., Jiang W., Zou Y., Wang Y., Lu C ., J. Electroanaly. Chem., 2016, 767, 49— 55 |
| [29] | Wu J., Risalvato F., Ma S., Zhou X ., J. Mater. Chem. A, 2014, 2( 6), 1647— 1651 |
| [30] | Li C. W., Kanan M ., J. Am. Chem. Soc., 2012, 134( 17), 7231— 7234 |
| [31] | Popczyk M., Serek A., Budniok A ., Nanotechnology, 2003, 14( 2), 341— 346 |
| [32] | Chen W. M., Sun G. Q., Zhao X. S., Sun P. C., Yang S. H., Xin Q ., Chem. J. Chinese Universities, 2007, 28( 5), 928— 931 |
| ( 陈维民, 孙公权, 赵新生, 孙丕昌, 杨少华, 辛勤 . 高等学校化学学报, 2007, 28( 5), 928— 931) | |
| [33] | Zhang H ., Study on the Electrocatalytic Reduction of CO2 to Formic Acid with SnO2 and Bi-Based Catalysts, Central China Normal University, Wuhan, 2014 |
| ( 张慧 . SnO2和Bi基催化剂电催化还原CO2至甲酸的研究, 武汉: 华中师范大学, 2014) | |
| [34] | Wang Q., Dong H., Yu H ., J. Power Sources, 2014, 271, 278— 284 |
| [35] | Li H., Oloman C ., J. Appl. Electrochem., 2006, 36( 10), 1105— 1115 |
| [36] | Zhang R., Lv W., Lei L ., Appl. Surf. Sci., 2015, 356, 24— 29 |
| [37] | Zhang B., Sun L., Wang Y., Chen S., Zhang J ., J. Energy Chem., 2020, 41, 7— 14 |
| [1] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [2] | 吴玉, 李轩, 杨恒攀, 何传新. 钴单原子的双重限域制备策略及高效CO2电还原性能[J]. 高等学校化学学报, 2022, 43(9): 20220343. |
| [3] | 王新天, 李攀, 曹越, 洪文浩, 耿忠璇, 安志洋, 王昊宇, 王桦, 孙斌, 朱文磊, 周旸. 单原子材料在二氧化碳催化中的技术经济分析与产业化应用前景[J]. 高等学校化学学报, 2022, 43(9): 20220347. |
| [4] | 崔伟, 赵德银, 白文轩, 张晓东, 余江. CO2在非质子溶剂与铁基离子液体复合体系中的吸收[J]. 高等学校化学学报, 2022, 43(8): 20220120. |
| [5] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [6] | 王征文, 高凤翔, 曹瀚, 刘顺杰, 王献红, 王佛松. 基于二氧化碳共聚物的紫外光固化高分子材料的制备与性能[J]. 高等学校化学学报, 2022, 43(7): 20220236. |
| [7] | 黄孝舜, 马海英, 柳淑娟, 王斌, 王红利, 钱波, 崔新江, 石峰. 二氧化碳间接转化制化学品的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220222. |
| [8] | 宋德文, 汪明旺, 王亚旎, 焦振梅, 宁汇, 吴明铂. 二氧化碳电还原制草酸研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220248. |
| [9] | 赵润瑶, 纪桂鹏, 刘志敏. 吡咯氮配位单原子铜催化剂的电催化二氧化碳还原性能[J]. 高等学校化学学报, 2022, 43(7): 20220272. |
| [10] | 郭志强, 杨博如, 席婵娟. 硼氢化试剂在二氧化碳还原官能化反应中的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220199. |
| [11] | 张昕昕, 许狄, 王艳秋, 洪昕林, 刘国亮, 杨恒权. CO2加氢制低碳醇CuFe基催化剂中的Mn助剂效应[J]. 高等学校化学学报, 2022, 43(7): 20220187. |
| [12] | 周紫璇, 杨海艳, 孙予罕, 高鹏. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220235. |
| [13] | 彭奎霖, 李桂林, 江重阳, 曾少娟, 张香平. 电解液调控CO2电催化还原性能微观机制的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220238. |
| [14] | 张振, 邓煜, 张琴芳, 余达刚. 可见光促进二氧化碳参与的羧基化反应[J]. 高等学校化学学报, 2022, 43(7): 20220255. |
| [15] | 王丽君, 李欣, 洪崧, 詹新雨, 王迪, 郝磊端, 孙振宇. 调节氧化镉-炭黑界面高效电催化CO2还原生成CO[J]. 高等学校化学学报, 2022, 43(7): 20220317. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||