

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (11): 2018.doi: 10.7503/cjcu20160449
杨丙星1,2, 叶丽萍1,2( ), 顾慧劼1,2, 徐华胜2,3, 罗勇1,2, 李会英4
), 顾慧劼1,2, 徐华胜2,3, 罗勇1,2, 李会英4
收稿日期:2016-06-22
出版日期:2016-11-10
发布日期:2016-10-18
作者简介:联系人简介: 叶丽萍, 女, 博士, 高级工程师, 主要从事催化新材料研究. E-mail:基金资助:
YANG Bingxing1,2, YE Liping1,2,*( ), GU Huijie1,2, XU Huasheng2,3, LUO Yong1,2, LI Huiying4
), GU Huijie1,2, XU Huasheng2,3, LUO Yong1,2, LI Huiying4
Received:2016-06-22
Online:2016-11-10
Published:2016-10-18
Contact:
YE Liping
E-mail:ylp_by@126.com
摘要:
采用密度泛函理论(DFT)方法, 考察了八面沸石(FAU)型分子筛β笼孔道结构内含氧化合物(甲醇、 二甲醚、 丙醛)的吸附, 并进一步计算研究了Zn, Ca同晶置换改性的作用机理. 研究结果表明, β笼孔道结构内, Al原子为甲醇、 二甲醚和丙醛的吸附活性位, Si原子无吸附活性. Zn, Ca掺杂的β笼结构内, 正2价的Zn和Ca掺杂替换正3价的Al, 导致邻近的Si原子位置形成缺电子空穴, 增强了甲醇、 二甲醚和丙醛的吸附, 而杂原子Zn和Ca本身并没有吸附活性.
TrendMD:
杨丙星, 叶丽萍, 顾慧劼, 徐华胜, 罗勇, 李会英. 同晶置换改性FAU分子筛结构及吸附性能的密度泛函理论研究. 高等学校化学学报, 2016, 37(11): 2018.
YANG Bingxing, YE Liping, GU Huijie, XU Huasheng, LUO Yong, LI Huiying. Theoretical Studies on the Structure and Adsorption Properties of Isomorphously Substituted FAU Zeolite†. Chem. J. Chinese Universities, 2016, 37(11): 2018.
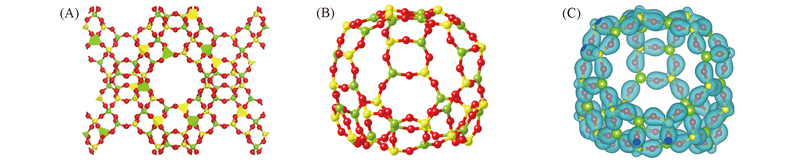
Fig.1 Calculated structure of FAU zeolite(A), β cage(B) and the isosurface(50 e/nm3) of calculated spin charge densities(C) The Si, Al and O atoms are represented by balls in yellow, green and red, respectively.
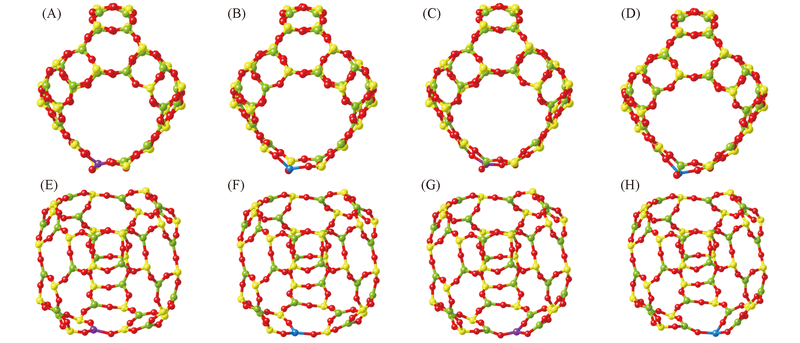
Fig.2 Calculated structures of Al(A, B), Si(C, D), Al'(E, F) and Si'(G, H) atoms substituted by Zn(A, C, E, G) and Ca(B, D, F, H) atoms The Zn and Ca atoms are represented by balls in purple and blue, respectively.
| Defect | Substitution atom | d(Al/Si/Zn/Ca—O)/nm | rRMS/nm | Esub/eV | Figure |
|---|---|---|---|---|---|
| Clean | Al | 0.1701, 0.1698, 0.1701 | 1(B) | ||
| Si | 0.1641, 0.1647, 0.1643 | 1(B) | |||
| Al' | 0.1685, 0.1699, 0.1696 | 1(B) | |||
| Si' | 0.1646, 0.1629, 0.1641 | 1(B) | |||
| Zn doped | Al | 0.1842, 0.1999, 0.1863 | 0.0213 | 4.89 | 2(A) |
| Si | 0.1888, 0.1860, 0.1929 | 0.0250 | 5.67 | 2(C) | |
| Al' | 0.1975, 0.1852, 0.1859 | 0.0211 | 5.05 | 2(E) | |
| Si' | 0.1900, 0.1833, 0.1909 | 0.0244 | 5.72 | 2(G) | |
| Ca doped | Al | 0.2133, 0.2112, 0.2254 | 0.0470 | 0.92 | 2(B) |
| Si | 0.2147, 0.2129, 0.2169 | 0.0505 | 1.86 | 2(D) | |
| Al' | 0.2103, 0.2141, 0.2254 | 0.0477 | 1.00 | 2(F) | |
| Si' | 0.2181, 0.2096, 0.2162 | 0.0509 | 1.90 | 2(H) |
Table 1 Calculated lengths of Al/Si/Zn/Ca-O bonds, rRMS of different substituted atoms and substitution energies of Zn/Ca doped β cage
| Defect | Substitution atom | d(Al/Si/Zn/Ca—O)/nm | rRMS/nm | Esub/eV | Figure |
|---|---|---|---|---|---|
| Clean | Al | 0.1701, 0.1698, 0.1701 | 1(B) | ||
| Si | 0.1641, 0.1647, 0.1643 | 1(B) | |||
| Al' | 0.1685, 0.1699, 0.1696 | 1(B) | |||
| Si' | 0.1646, 0.1629, 0.1641 | 1(B) | |||
| Zn doped | Al | 0.1842, 0.1999, 0.1863 | 0.0213 | 4.89 | 2(A) |
| Si | 0.1888, 0.1860, 0.1929 | 0.0250 | 5.67 | 2(C) | |
| Al' | 0.1975, 0.1852, 0.1859 | 0.0211 | 5.05 | 2(E) | |
| Si' | 0.1900, 0.1833, 0.1909 | 0.0244 | 5.72 | 2(G) | |
| Ca doped | Al | 0.2133, 0.2112, 0.2254 | 0.0470 | 0.92 | 2(B) |
| Si | 0.2147, 0.2129, 0.2169 | 0.0505 | 1.86 | 2(D) | |
| Al' | 0.2103, 0.2141, 0.2254 | 0.0477 | 1.00 | 2(F) | |
| Si' | 0.2181, 0.2096, 0.2162 | 0.0509 | 1.90 | 2(H) |
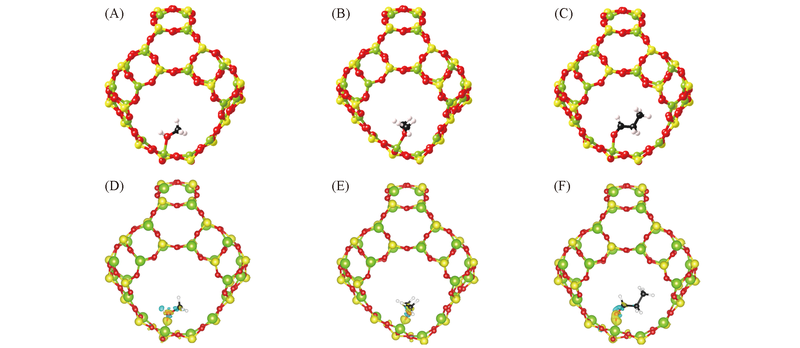
Fig.3 Calculated structures of methanol(A), dimethyl ether(B) and propionaldehyde adsorption(C) at Al site of β cage and isosurfaces(6 e/nm3)(D—F) of charge redistribution of (A—C), respectively The yellow and blue isosurfaces denote charge gain and miss, repectively. The C and H atoms are represented by balls in black and white, respectively.
| Defect | Adsorption molecular | Before adsorption Al/Si—O/nm | After adsorption Al/Si—O/nm | rRMS/nm | Figure |
|---|---|---|---|---|---|
| Clean | Ethanol | 0.1701, 0.1698, 0.1701 | 0.1726, 0.1736, 0.1718 | 0.0028 | 3(A) |
| Dimethylether | 0.1727, 0.1728, 0.1707 | 0.0023 | 3(B) | ||
| Propionaldehyde | 0.1733, 0.1737, 0.1727 | 0.0033 | 3(C) | ||
| Zn doped | Ethanol | 0.1550, 0.1590, 0.1589 | 0.1614, 0.1618, 0.1640 | 0.005 | 4(G) |
| Dimethyl ether | 0.1592, 0.1612, 0.1631 | 0.0037 | 4(H) | ||
| Propionaldehyde | 0.1619, 0.1625, 0.1665 | 0.0063 | 4(I) | ||
| Ca doped | Ethanol | 0.1595, 0.1602, 0.1552 | 0.1620, 0.1622, 0.1557 | 0.0019 | 4(D) |
| Dimethyl ether | 0.1625, 0.1626, 0.1559 | 0.0023 | 4(E) | ||
| Propionaldehyde | 0.1629, 0.1630, 0.1579 | 0.003 | 4(F) |
Table 2 Calculated lengths of Al/Si—O bonds, rRMS of methanol, dimethyl ether and propionaldehyde adsorption at Al/Si site of β cage
| Defect | Adsorption molecular | Before adsorption Al/Si—O/nm | After adsorption Al/Si—O/nm | rRMS/nm | Figure |
|---|---|---|---|---|---|
| Clean | Ethanol | 0.1701, 0.1698, 0.1701 | 0.1726, 0.1736, 0.1718 | 0.0028 | 3(A) |
| Dimethylether | 0.1727, 0.1728, 0.1707 | 0.0023 | 3(B) | ||
| Propionaldehyde | 0.1733, 0.1737, 0.1727 | 0.0033 | 3(C) | ||
| Zn doped | Ethanol | 0.1550, 0.1590, 0.1589 | 0.1614, 0.1618, 0.1640 | 0.005 | 4(G) |
| Dimethyl ether | 0.1592, 0.1612, 0.1631 | 0.0037 | 4(H) | ||
| Propionaldehyde | 0.1619, 0.1625, 0.1665 | 0.0063 | 4(I) | ||
| Ca doped | Ethanol | 0.1595, 0.1602, 0.1552 | 0.1620, 0.1622, 0.1557 | 0.0019 | 4(D) |
| Dimethyl ether | 0.1625, 0.1626, 0.1559 | 0.0023 | 4(E) | ||
| Propionaldehyde | 0.1629, 0.1630, 0.1579 | 0.003 | 4(F) |
| Site | Eads/eV | ||
|---|---|---|---|
| Ethanol | Dimethyl ether | Propionaldehyde | |
| Al | 0.83 | 0.97 | 0.76 |
| Si | 0.54 | ||
Table 3 Calculated adsorption energies of ethanol, dimethyl ether and propionaldehyde at Al and Si sites
| Site | Eads/eV | ||
|---|---|---|---|
| Ethanol | Dimethyl ether | Propionaldehyde | |
| Al | 0.83 | 0.97 | 0.76 |
| Si | 0.54 | ||
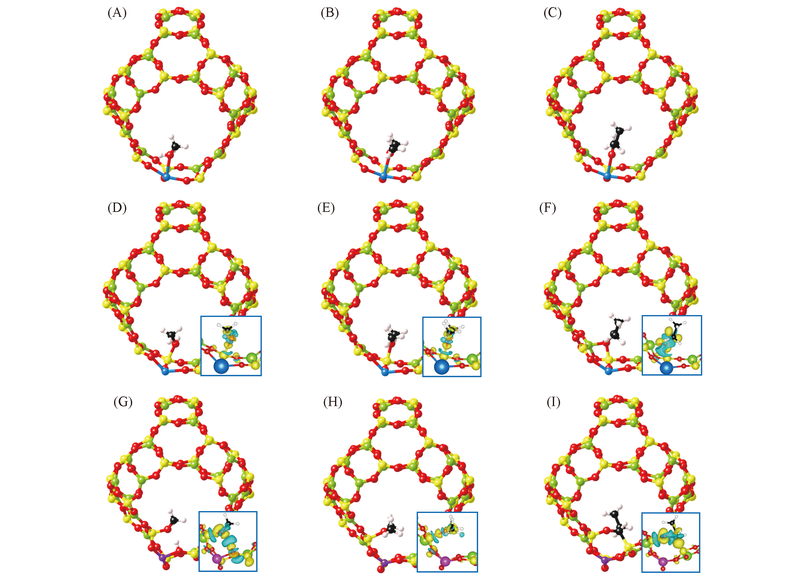
Fig.4 Calculated structures of ethanol(A, D, G), dimethyl ether(B, E, H) and propionaldehyde(C, F, I) adsorption in Ca-doped(D—F) and Zn-doped(G—I) β cageThe insets denote the isosurfaces(6 e/nm3) of charge redistribution.
| Site | Eads/eV | ||
|---|---|---|---|
| Ethanol | Dimethyl ether | Propionaldehyde | |
| Zn | |||
| Si(Zn) | 2.28 | 1.08 | 3.93 |
| Ca | 0.45 | 0.25 | 0.15 |
| Si(Ca) | 0.77 | 0.68 | 2.29 |
| Site | Eads/eV | ||
|---|---|---|---|
| Ethanol | Dimethyl ether | Propionaldehyde | |
| Zn | |||
| Si(Zn) | 2.28 | 1.08 | 3.93 |
| Ca | 0.45 | 0.25 | 0.15 |
| Si(Ca) | 0.77 | 0.68 | 2.29 |
| Si number | Charge/e | ||
|---|---|---|---|
| Clean | Ca-doped | Zn-doped | |
| 1 | 0.83 | 0.76 | 0.00 |
| 2 | 0.82 | 0.00 | 0.76 |
| 3 | 0.82 | 0.78 | 0.78 |
Table 5 Calculated Bader charge of different Si sites
| Si number | Charge/e | ||
|---|---|---|---|
| Clean | Ca-doped | Zn-doped | |
| 1 | 0.83 | 0.76 | 0.00 |
| 2 | 0.82 | 0.00 | 0.76 |
| 3 | 0.82 | 0.78 | 0.78 |
| [1] | 夏思奇, 王鹏飞, 徐华胜, 胡杰, 吕待清. 精细化工, 2014, 31( 12), 1476- 1479 |
| Xia S., Q. , Wang P., F. , Xu H., S. , Hu, J. , Lv D., Q. , Fine Chem., 2014, 31( 12), 1476- 1479 ( | |
| [2] | Luiz K., C. , Juliana J., R. , Jose R., Z. , Geraldo, N. , Cristiano M., B. , Romulo S., A. , Carlos E., F. , Powder Techn., 2012, 229, 1- 6 |
| [3] | Sheng X., L. , Zhou Y., M. , Duan Y., Z. , Xue M., W. , J. Porous Mat., 2011, 18( 6), 677- 683 |
| [4] | Yan, B. , Mahmood, A. , Liang, Y. , Xu B., Q. , Catal. Today, 2016, 269( 1), 65- 73 |
| [5] | 张艳青, 郑华艳, 章日光, 李忠, 王宝俊, 赵秋勇. 高等学校化学学报, 2015, 36( 10), 1945- 1953 |
| Zhang Y., Q. , Zheng H., Y. , Zhang R., G. , Li, Z. , Wang B., J. , Zhao Q., Y. , Chem. J. Chinese Universities, 2015, 36( 10), 1945- 1953 ( | |
| [6] | Popovych N., O. , Kyriienko P., I. , Soloviev S., O. , Orlyk S., M. , Dzwigaj, S. , Microp. Mesop. Mater., 2016, 226( 15), 10- 18 |
| [7] | Najar, H. , Zina M., S. , Ghorbel, A. , React Kinet. Mech. Catal., 2010, 100( 2), 385- 398 |
| [8] | Lim W., T. , Seo S., M. , Lee O., S. , Wang L., Z. , Lu G., Q. , J. Incl. Phenom. Macrocycl. Chem., 2010, 67( 3), 261- 269 |
| [9] | Li H., C. , Zhou D., H. , Tian D., X. , Shi, C. , Muller, U. , Feyen, M. , Yilmaz, B. , Gies, H. , Xiao F., S. , de Vos, D. , ChemPhysChem, 2014, 15( 8), 1700- 1707 |
| [10] | Meeprasert, J. , Jungsuttiwong, S. , Namuangruk, S. , Microp. Mesop. Mater., 2013, 175, 99- 106 |
| [11] | Ma, J. , Qiang L., S. , Wang J., F. , Tang X., B. , Tang D., Y. , J. Porous Mat., 2011, 18( 5), 607- 614 |
| [12] | Wang, Q. , Wu Y., J. , Wang, J. , Lin, X. , Acta Phys. Chim. Sin., 2012, 28( 9), 2108- 2114 |
| [13] | Hernandez-Morales, V. , Nava, R. , Acosta-Silva Y., J. , Pawelec, B. , Microp. Mesop. Mater., 2012, 160, 133- 142 |
| [14] | Tao, L. , Li G., S. , Yin S., F. , Au C., T. , React Kinet. Mech. Catal., 2011, 103( 1), 191- 207 |
| [15] | 张瑞珍, 王翠, 邢普, 温少波, 王剑, 赵亮富, 李玉平. 高等学校化学学报, 2015, 36( 4), 725- 732 |
| Zhang R., Z. , Wang, C. , Xing, P. , Wen S., B. , Wang, J. , Zhao L., F. , Li Y., P. , Chem. J. Chinese Universities, 2015, 36( 4), 725- 732 ( | |
| [16] | Su X., F. , Wang G., L. , Bai X., F. , Wu, W. , Xiao L., F. , Fang Y., J. , Zhang J., W. , Chem. Eng. J., 2016, 293, 365- 375 |
| [17] | Jin Y., J. , Asaoka, S. , Zhang S., D. , Li, P. , Zhao S., L. , Fuel Proc. Techn., 2013, 115, 34- 41 |
| [18] | Omata, K. , Yamazaki, Y. , Watanabe, Y. , Kodama, K. , Yamada, M. , Ind. Eng. Chem. Res., 2009, 48( 13), 6256- 6261 |
| [19] | 王瑜, 吴伟, 李程, 杨杰, 周亚静. 石油学报, 2011, 27( 5), 682- 686 |
| Wang, Y. , Wu, W. , Li, C. , Yang, J. , Zhou Y., J. , Acta Petrolei Sin., 2011, 27( 5), 682- 686 ( | |
| [20] | Geraldo, E. , Stevie H., L. , Ana C., R. , Antonio S., A. , Valter, J. , J. Mater. Sci., 2010, 45( 4), 1117- 1122 |
| [21] | Shamzhy, M. , Shvets, O. , Opanasenko, M. , Cejka, J. , J. Mater. Chem., 2012, 22( 31), 15793- 15803 |
| [22] | Ahmed, S. , J. Porous Mat., 2012, 19( 1), 111- 117 |
| [23] | Wang J., H. , Xie J., Y. , Zhou, Y. , Wang, J. , Microp. Mesop. Mater., 2013, 171, 87- 93 |
| [24] | Yang C., G. , Qiu M., H. , Hu S., W. , Chen X., Q. , Zeng G., F. , Liu Z., Y. , Sun Y., H. , Microp. Mesop. Mater., 2016, 231, 110- 116 |
| [25] | Zhou, Y. , Jin Y., H. , Wang, M. , Zhang, W. , Xie J., Y. , Gu, J. , Wen H., M. , Wang, J. , Peng L., M. , Chem. Eur. J., 2015, 21( 43), 15412- 15420 |
| [26] | Kang L., H. , Zhang, T. , Liu Z., M. , Han K., L. , J. Phys. Chem. C, 2008, 112( 14), 5526- 5532 |
| [27] | Kresse, G. , Furthmü, ller J. , Comp. Mater. Sci., 1996, 6, 15- 50 |
| [28] | Kresse, G. , Furthmü, ller J. , Phys. Rev. B, 1996, 54( 16), 11169- 11186 |
| [29] | Perdew J., P. , Burke, K. , Ernzerhof, M. , Phys. Rev. Lett., 1996, 77( 18), 3865- 3868 |
| [30] | Kresse, G. , Joubert, D. , Phys. Rev. B, 1999, 59( 3), 1758- 1775 |
| [31] | Blö, chl P. E. , Phys. Rev. B, 1994, 50( 24), 17953- 17979 |
| [32] | Monkhorst H., J. , Pack J., D. , Phys. Rev. B, 1976, 13, 5188- 5192 |
| [33] | Wang H., F. , Gong X., Q. , Guo Y., L. , Guo, Y. , Lu G., Z. , Hu, P. , J. Phys. Chem. C, 2009, 113( 23), 10229- 10232 |
| [34] | Li H., Y. , Wang H., F. , Gong X., Q. , Guo Y., L. , Guo, Y. , Lu G., Z. , Hu, P. , Phys. Rev. B, 2009, 79( 19), 193401- 193405 |
| [1] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [2] | 程前, 杨博龙, 吴文依, 向中华. S掺杂Fe-N-C高活性氧还原反应催化剂[J]. 高等学校化学学报, 2022, 43(9): 20220341. |
| [3] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [4] | 蒋小康, 周琦, 周恒为. Gd2ZnTiO6∶Dy3+, Eu3+单基质白光荧光粉的制备与发光性能[J]. 高等学校化学学报, 2022, 43(6): 20220029. |
| [5] | 宋有为, 安江伟, 王征, 王旭慧, 权燕红, 任军, 赵金仙. Ag,Zn,Pd掺杂对铜基催化剂草酸二甲酯选择性加氢反应的影响[J]. 高等学校化学学报, 2022, 43(6): 20210842. |
| [6] | 姜宏斌, 代文臣, 张娆, 徐晓晨, 陈捷, 杨光, 杨凤林. Co3O4/UiO-66@α-Al2O3陶瓷膜对VOCs废气的分离催化性能[J]. 高等学校化学学报, 2022, 43(6): 20220025. |
| [7] | 戴卫, 侯华, 王宝山. 七氟异丁腈负离子结构与反应活性的理论研究[J]. 高等学校化学学报, 2022, 43(6): 20220044. |
| [8] | 龚妍熹, 王建兵, 柴歩瑜, 韩元春, 马云飞, 贾超敏. 钾掺杂g-C3N4薄膜光阳极的制备及光电催化氧化降解水中双氯芬酸钠性能[J]. 高等学校化学学报, 2022, 43(6): 20220005. |
| [9] | 郝宏蕾, 孟繁雨, 李若钰, 李迎秋, 贾明君, 张文祥, 袁晓玲. 生物质基氮掺杂多孔炭材料的制备及对水中亚甲基蓝的吸附性能[J]. 高等学校化学学报, 2022, 43(6): 20220055. |
| [10] | 黄汉浩, 卢湫阳, 孙明子, 黄勃龙. 石墨炔原子催化剂的崭新道路:基于自验证机器学习方法的筛选策略[J]. 高等学校化学学报, 2022, 43(5): 20220042. |
| [11] | 孙雪峰, 热娜古丽·阿不都热合曼, 杨通胜, 杨倩婷. Cr,In共掺杂MgGa2O4小尺寸近红外长余辉纳米颗粒的制备及发光性能[J]. 高等学校化学学报, 2022, 43(4): 20210850. |
| [12] | 王红宁, 黄丽, 清江, 马腾洲, 蒋伟, 黄维秋, 陈若愚. 香蒲基生物炭的活化及对VOCs吸附的应用[J]. 高等学校化学学报, 2022, 43(4): 20210824. |
| [13] | 孟祥龙, 杨歌, 郭海玲, 刘晨光, 柴永明, 王纯正, 郭永梅. 纳米分子筛的合成及硫化氢吸附性能[J]. 高等学校化学学报, 2022, 43(3): 20210687. |
| [14] | 陈潇禄, 袁珍闫, 仲迎春, 任浩. 机械球磨制备三苯胺基PAF-106s及C2烃吸附性质[J]. 高等学校化学学报, 2022, 43(3): 20210771. |
| [15] | 靳科研, 白璞, 李小龙, 张佳楠, 闫文付. 新型Mg-Al吸附剂去除压水堆核电厂废水中高浓度硼[J]. 高等学校化学学报, 2022, 43(2): 20210516. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||