

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (1): 100.doi: 10.7503/cjcu20150472
• Physical Chemistry • Previous Articles Next Articles
ZHAO Han1,2, ZHOU Lina1,3, WEI Dongshan1,*( ), ZHOU Xinjian2, SHI Haofei1
), ZHOU Xinjian2, SHI Haofei1
Received:2015-06-16
Online:2016-01-10
Published:2015-12-20
Contact:
WEI Dongshan
E-mail:dswei@cigit.ac.cn
Supported by:CLC Number:
TrendMD:
ZHAO Han, ZHOU Lina, WEI Dongshan, ZHOU Xinjian, SHI Haofei. Effects of External Electric Field on Hydrogen Storage Performance of Li-decorated Graphene Oxide†[J]. Chem. J. Chinese Universities, 2016, 37(1): 100.
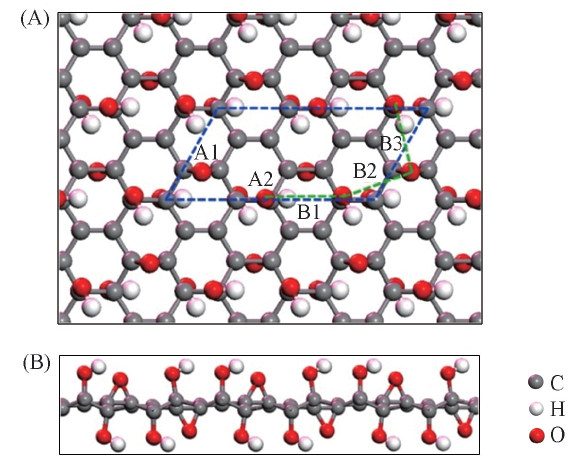
Fig.1 Top view(A) and side view(B) of model GO structure^The dashed line represents 2× supercell. A1, A2, B1, B2 and B3 represent the possible Li anchor sites(B1, B2 and B3 lie in the green dashed line).
| Site | Eb /eV | d(Li—OE)/nm | d(Li—OH)/nm | d(Li—C1)/nm | d(Li—C2)/nm |
|---|---|---|---|---|---|
| A1 | -2.994 | 0.1810 | 0.2047 | 0.2552 | 0.2323 |
| A2 | -2.994 | 0.1808 | 0.2026 | 0.2546 | 0.2332 |
| B1 | -1.892 | 0.2126 | 0.1833 | 0.2525 | 0.2263 |
| B2 | -3.080 | 0.1889 | 0.2141 | 0.2675 | 0.2305 |
| B3 | -2.427 | 0.2202 | 0.2035 | 0.2701 | 0.2371 |
Table 1 Binding energy, Li—O and Li—C distances of possible Li binding sites on GO after fully optimized
| Site | Eb /eV | d(Li—OE)/nm | d(Li—OH)/nm | d(Li—C1)/nm | d(Li—C2)/nm |
|---|---|---|---|---|---|
| A1 | -2.994 | 0.1810 | 0.2047 | 0.2552 | 0.2323 |
| A2 | -2.994 | 0.1808 | 0.2026 | 0.2546 | 0.2332 |
| B1 | -1.892 | 0.2126 | 0.1833 | 0.2525 | 0.2263 |
| B2 | -3.080 | 0.1889 | 0.2141 | 0.2675 | 0.2305 |
| B3 | -2.427 | 0.2202 | 0.2035 | 0.2701 | 0.2371 |
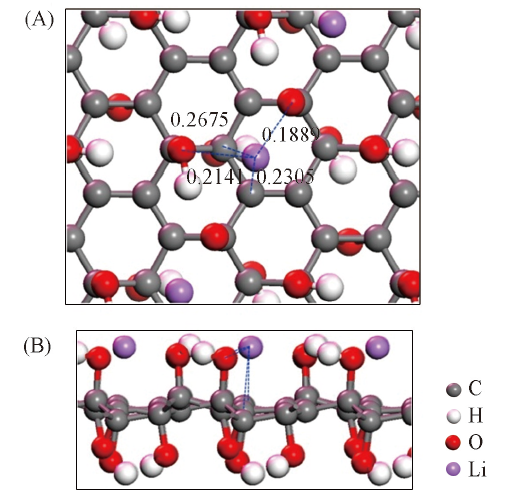
Fig.2 Top view(A) and side view(B) of optimized geometry of Li@GO(initial state: B2) structure^The dashed line represents the distance(nm) between different atoms.
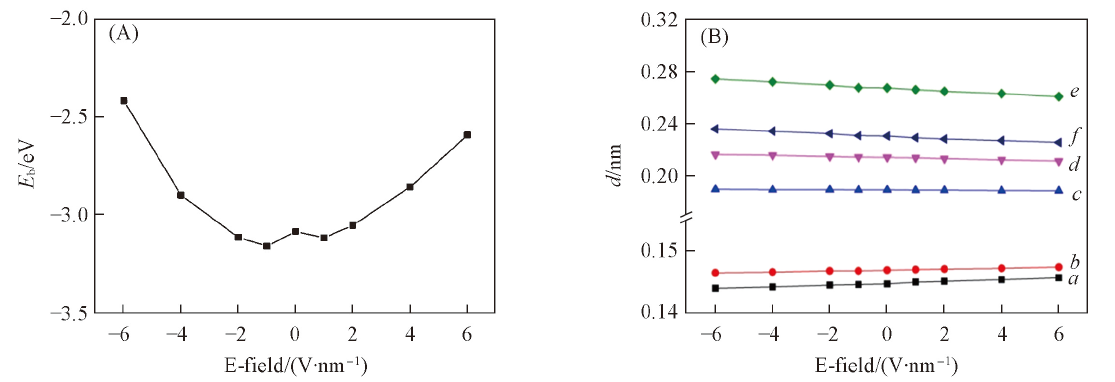
Fig.3 Binding energy() of Li(A) and the length of C—O bond and the distance between Li and its neighboring atoms under different electric fields(B)^ a. d(CE—OE); b. d(CH—OH); c. d(Li—OE); d. d(Li—OH); e. d(Li—C1); f. d(Li—C2).
| E-field/(V·nm-1) | Net charge/e | ||||||
|---|---|---|---|---|---|---|---|
| Li | OE | OH | C1 | C2 | CE | CH | |
| -6 | +0.86 | -1.13 | -1.15 | +0.46 | -0.13 | +0.55 | +0.46 |
| 0 | +0.88 | -1.08 | -1.13 | +0.47 | -0.01 | +0.53 | +0.43 |
| 6 | +0.89 | -1.10 | -1.15 | +0.46 | -0.05 | +0.51 | +0.42 |
Table 2 Net charge of Li, O and C under different electric fields from Bader charge analysis
| E-field/(V·nm-1) | Net charge/e | ||||||
|---|---|---|---|---|---|---|---|
| Li | OE | OH | C1 | C2 | CE | CH | |
| -6 | +0.86 | -1.13 | -1.15 | +0.46 | -0.13 | +0.55 | +0.46 |
| 0 | +0.88 | -1.08 | -1.13 | +0.47 | -0.01 | +0.53 | +0.43 |
| 6 | +0.89 | -1.10 | -1.15 | +0.46 | -0.05 | +0.51 | +0.42 |
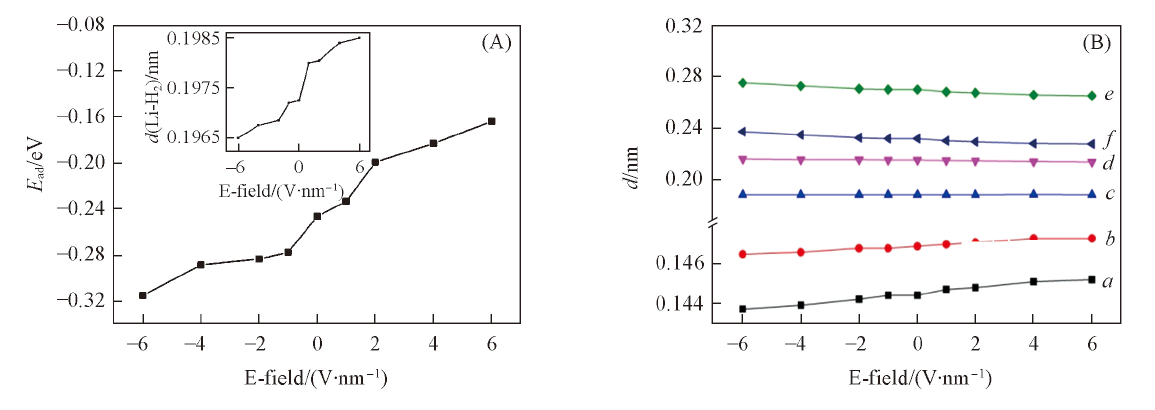
Fig.6 Adsorption energy() of H2(A) and the length of C—O bond and the distance between Li and its neighboring atoms in the presence of an external electric field(B)^ Inset of (A) is the intensity-dependent distance between Li and H2. a. d(CE—OE); b. d(CH—OH); c. d(Li—OE); d. d(Li—OH); e. d(Li—C1); f. d(Li—C2).
| [1] | Schlapbach L., Züttel A., Nature,2001, 414(6861), 353—358 |
| [2] | Schlapbach L., Nature, 2009, 460(7257), 809—811 |
| [3] | Wang Y., Meng Z., Liu Y., You D., Wu K., Lü J., Wang X., Deng K., Rao D., Lu R., Applied Physics Letters, 2015, 106(6), 063901 |
| [4] | Fichtner M., Advanced Engineering Materials, 2005, 7(6), 443—455 |
| [5] | Grochala W., Edwards P. P., Chemical Reviews, 2004, 104(3), 1283—1316 |
| [6] | Eberle U., Felderhoff M., Schueth F., Angewandte Chemie International Edition, 2009, 48(36), 6608—6630 |
| [7] | Chen P., Wu X., Lin J., Tan K., Science,1999, 285(5424), 91—93 |
| [8] | Rosi N. L., Eckert J., Eddaoudi M., Vodak D. T., Kim J., O’Keeffe M., Yaghi O. M., Science,2003, 300(5622), 1127—1129 |
| [9] | Yang W. J., Huang R. Z., Liu L., J. Mater. Sci. Eng., 2012, 30(3), 449—452 |
| (杨文静, 黄仁忠, 刘柳, 材料科学与工程学报,2012, 30(3), 449—452) | |
| [10] | Wang L., Lee K., Sun Y. Y., Lucking M., Chen Z., Zhao J. J., Zhang S. B., ACS Nano, 2009, 3(10), 2995—3000 |
| [11] | Burress J. W., Gadipelli S., Ford J., Simmons J. M., Zhou W., Yildirim T., Angewandte Chemie International Edition, 2010, 49(47), 8902—8904 |
| [12] | Kostoglou N., Tzitzios V., Kontos A. G., Giannakopoulos K., Tampaxis C., Papavasiliou A., Charalambopoulou G., Steriotis T., Li Y., Liao K., International Journal of Hydrogen Energy,2015, 40(21), 6844—6852 |
| [13] | Zhou H., Liu X., Zhang J., Yan X., Liu Y., Yuan A., International Journal of Hydrogen Energy,2014, 39(5), 2160—2167 |
| [14] | Chen C., Zhang J., Zhang B., Duan H. M., J. Phys. Chem. C, 2013, 117(9), 4337—4344 |
| [15] | Sun Q., Jena P., Wang Q., Marquez M., Journal of the American Chemical Society,2006, 128(30), 9741—9745 |
| [16] | Guo Y., Jiang K., Xu B., Xia Y., Yin J., Liu Z., J. Phys. Chem. C, 2012, 116(26), 13837—13841 |
| [17] | Liu W., Zhao Y., Li Y., Jiang Q., Lavernia E., J. Phys. Chem. C, 2009, 113(5), 2028—2033 |
| [18] | Lu R., Rao D., Lu Z., Qian J., Li F., Wu H., Wang Y., Xiao C., Deng K., Kan E., J. Phys. Chem. C, 2012, 116(40), 21291—21296 |
| [19] | Ao Z., Peeters F., Phys. Rev. B, 2010, 81(20), 205406 |
| [20] | Zhou J., Wang Q., Sun Q., Jena P., Chen X. S., Proceedings of the National Academy of Science,2010, 107(7), 2801—2806 |
| [21] | Zhang L., Zhang S., Wang P., Liu C., Huang S., Tian H., Computational & Theoretical Chemistry, 2014, 1035(9), 68—75 |
| [22] | Kohn W., Sham L. J., Phys. Rev., 1965, 140(4A), A1133—A1138 |
| [23] | Kresse G., Furthmüller J., Phys. Rev. B, 1996, 54(16), 11169—11186 |
| [24] | Kresse G., Hafner J., Phys. Rev. B, 1993, 47(1), 558—561 |
| [25] | Kresse G., Hafner J., Phys. Rev. B, 1994, 49(20), 14251—14269 |
| [26] | Kresse G., Furthmüller J., Computational Materials Science, 1996, 6(1), 15—50 |
| [27] | Kresse G., Joubert D., Phys. Rev. B, 1999, 59(3), 1758—1775 |
| [28] | Perdew J. P., Chevary J., Vosko S., Jackson K. A., Pedersenm M., Singh D., Fiolhais C., Phys. Rev. B, 1992, 46(1), 6671—6687 |
| [29] | Perdew J. P., Burke K., Ernzerhof M., Physical Review Letters, 1996, 77(18), 3865—3868 |
| [30] | Jiang D. E., Sumpter B. G., Dai S., J. Chem. Phys., 2007, 126(13), 134701 |
| [31] | Tkatchenko A., Scheffler M., Physical Review Letters, 2009, 102(7), 073005 |
| [32] | Chan K. T., Neaton J. B., Cohen M. L., Phys. Rev. B, 2008, 77(23), 235430 |
| [33] | Sun C., Searles D. J., J. Phys. Chem. C, 2012, 116(50), 26222—26226 |
| [34] | Bellert D., Breckenridge W. H., J. Chem. Phys., 2001, 114(7), 2871—2874 |
| [35] | Chen C., Kong W., Duan H., Zhang J., Physical Chemistry Chemical Physics, 2015, 17(20), 13654—13658 |
| [36] | Wei D., Wang F., Surface Science, 2012, 606(3/4), 485—489 |
| [37] | Henkelman G., Arnaldsson A., Jónsson H., Computational Materials Science, 2006, 36(3), 354—360 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | JIANG Hongbin, DAI Wenchen, ZHANG Rao, XU Xiaochen, CHEN Jie, YANG Guang, YANG Fenglin. Research on Co3O4/UiO-66@α-Al2O3 Ceramic Membrane Separation and Catalytic Spraying Industry VOCs Waste Gas [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220025. |
| [3] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [4] | LIU Jiaqi, LI Tianbao. Preparation and Photoelectrochemical Performance of BiVO4/CuBi2O4 Thin Film Photoanodes [J]. Chem. J. Chinese Universities, 2022, 43(4): 20220017. |
| [5] | YANG Junge, GAO Chengqian, LI Boxin, YIN Dezhong. Preparation of High Thermal Conductivity Phase Change Monolithic Materials Based on Pickering Emulsion Stabilized by Surface Modified Graphene Oxide [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210593. |
| [6] | ZHANG Zhibo, SHANG Han, XU Wenxuan, HAN Guangdong, CUI Jinsheng, YANG Haoran, LI Ruixin, ZHANG Shenghui, XU Huan. Self-Assembly of Graphene Oxide at Poly(3-hydroxybutyrate) Microparticles Toward High-performance Intercalated Nanocomposites [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210566. |
| [7] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [8] | YU Bin, CHEN Xiaoyan, ZHAO Yue, CHEN Weichang, XIAO Xinyan, LIU Haiyang. Graphene Oxide-based Cobalt Porphyrin Composites for Electrocatalytic Hydrogen Evolution Reaction [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210549. |
| [9] | WANG Xueli, SONG Xiangwei, XIE Yanning, DU Niyang, WANG Zhenxin. Preparation, Characterization of Partially Reduced Graphene Oxide and Its Killing Effect on Human Cervical Cancer Cells [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210595. |
| [10] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [11] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [12] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [13] | LIU Kun, ZUO Jie, LI Hua, XIANG Hongfu, RAN Congfu, YANG Minghao, GENG Wenqiang. Effects of Electron Energy on the Chemical Products of Surface Dielectric Barrier Discharge Plasma [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220249. |
| [14] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [15] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||