

Chem. J. Chinese Universities ›› 2022, Vol. 43 ›› Issue (2): 20210614.doi: 10.7503/cjcu20210614
• Physical Chemistry • Previous Articles Next Articles
Received:2021-08-25
Online:2022-02-10
Published:2021-10-13
Contact:
ZHU Haochen
E-mail:haochen_zhu@tongji.edu.cn
Supported by:CLC Number:
TrendMD:
HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem[J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614.
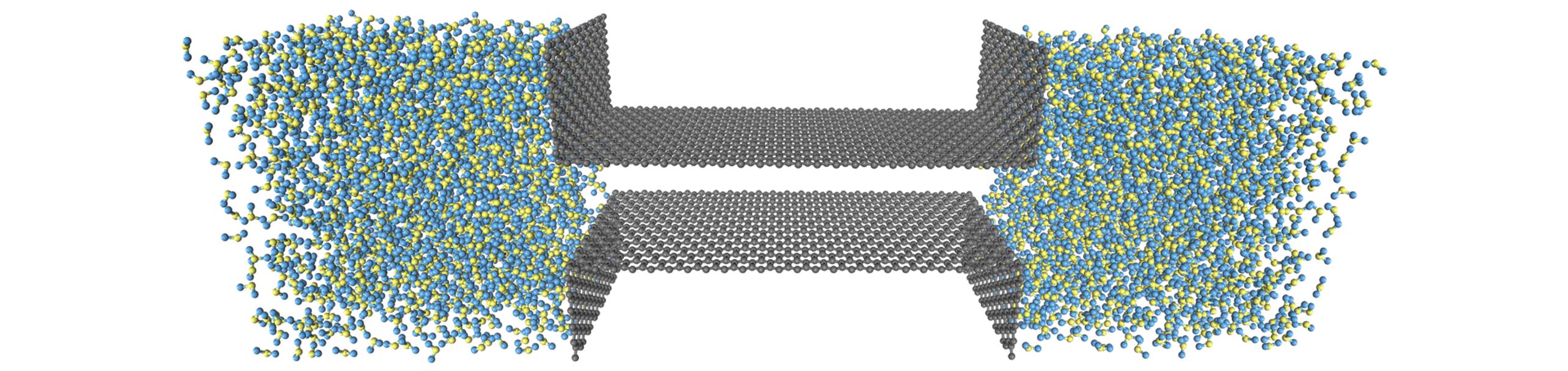
Fig.1 3D image of a nanochannel formed by two layers of graphene flanked by two storage poolsThe O and H atoms are represented by yellow and blue spheres, respectively.
| Atom | σ/nm | ε/(kJ?mol-1) | q/e |
|---|---|---|---|
| Ow | 0.3159 | 3.2436 | 0 |
| Hw | 0 | 0 | 0.5564 |
| Oh | 0.3070 | 0.7116 | -0.585 |
| Hh | 0 | 0 | 0.435 |
| C(C—C) | 0.3851 | 0.4395 | 0 |
| Ch | 0.3550 | 0.2930 | 0.150 |
Table 1 LJ potential and charge of the atoms used in the simulation*
| Atom | σ/nm | ε/(kJ?mol-1) | q/e |
|---|---|---|---|
| Ow | 0.3159 | 3.2436 | 0 |
| Hw | 0 | 0 | 0.5564 |
| Oh | 0.3070 | 0.7116 | -0.585 |
| Hh | 0 | 0 | 0.435 |
| C(C—C) | 0.3851 | 0.4395 | 0 |
| Ch | 0.3550 | 0.2930 | 0.150 |
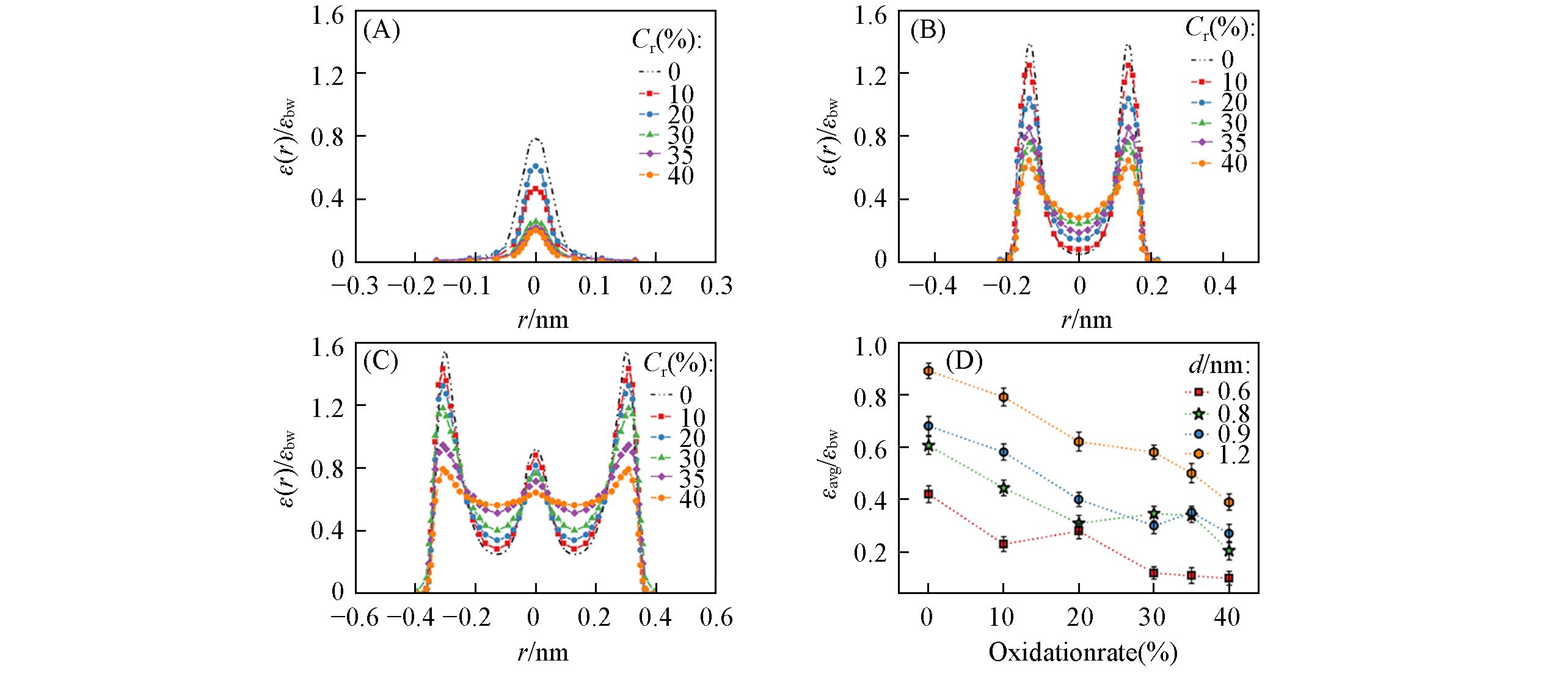
Fig.2 Distribution of the ratio of the radial permittivity[ε(r)] of confined aqueous solution to that of water in the bulk system with the degree of oxidation in the double?layer graphene channels with d=0.6 nm(A), 0.9 nm(B) and 1.2 nm(C), the ratio of their mean radial permittivity(εavg) to εbw with the degree of oxidation(D)
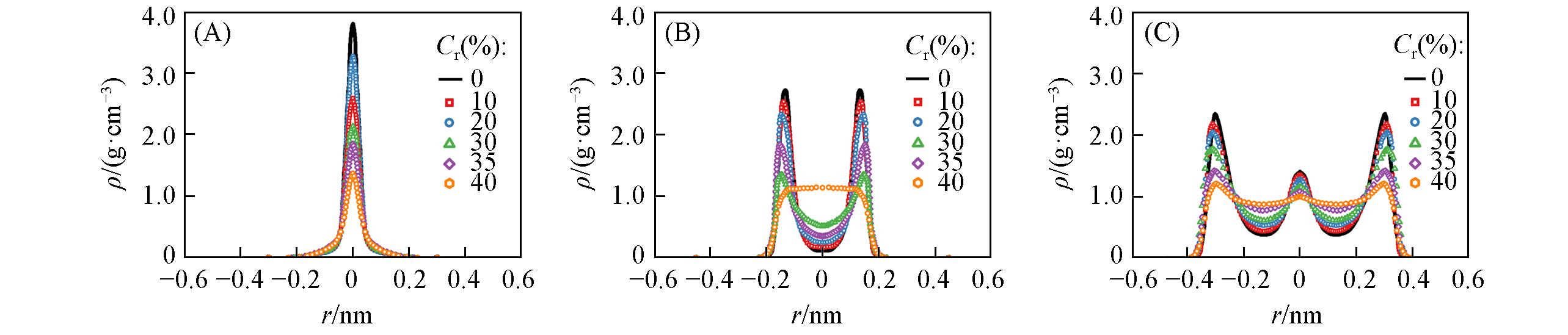
Fig.3 Radial profile of the water density as a function of various oxidation concentration in the double?layer graphene channels with d=0.6 nm(A), 0.9 nm(B) and 1.2 nm(C)
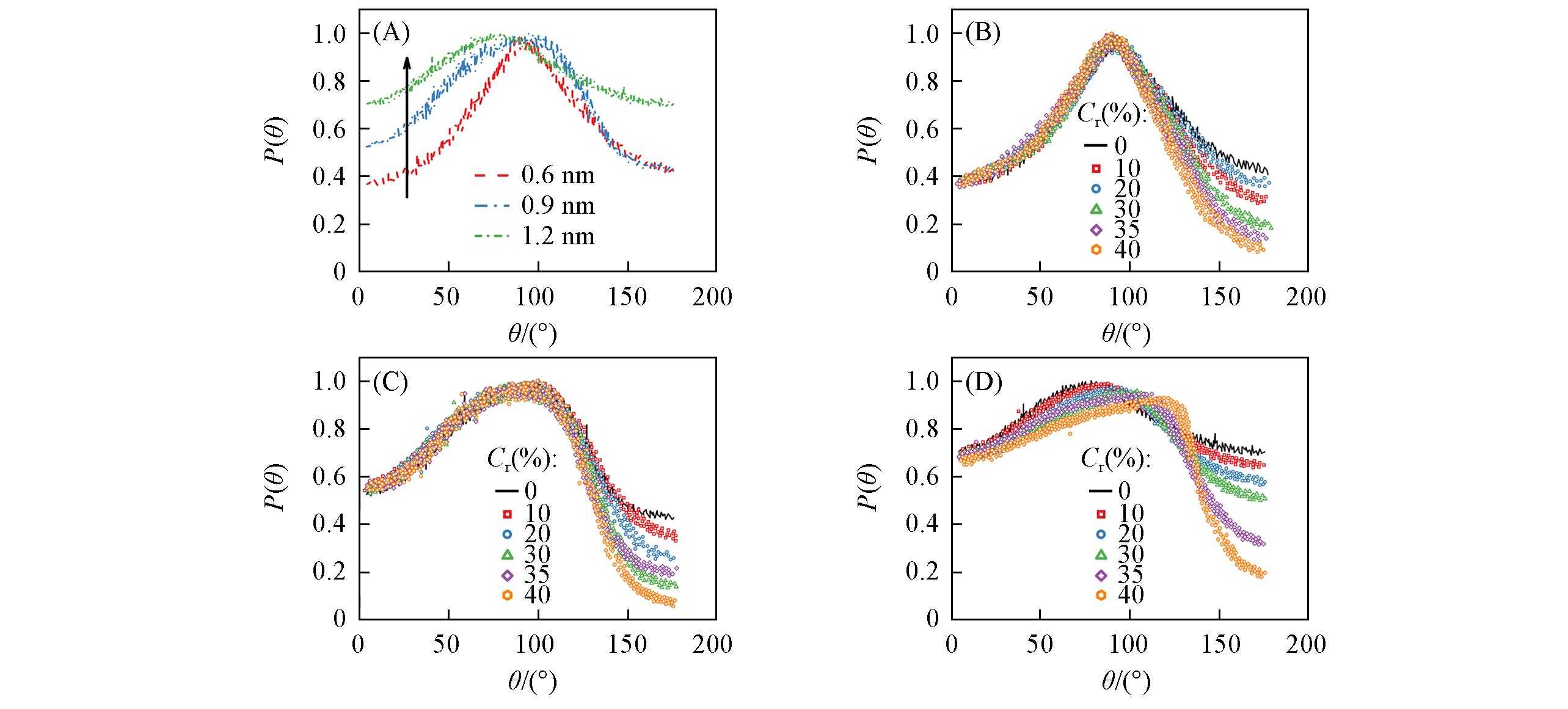
Fig.4 Probability of angular distribution of water molecules for PG nanochannels(A) and GO nanochannels with a size of 0.6 nm(B), 0.9 nm(C) and 1.2 nm(D)
| d/nm | PG | GO?10% | GO?20% | GO?30% | GO?35% | GO?40% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | |
| 0.6 | 0.25 | 3.23 | 0.37 | 4.12 | 0.33 | 3.71 | 0.51 | 5.14 | 0.53 | 5.25 | 0.55 | 5.35 |
| 0.9 | 0.10 | 1.21 | 0.23 | 1.84 | 0.30 | 2.79 | 0.42 | 3.58 | 0.38 | 3.33 | 0.44 | 3.98 |
| 1.2 | 0.04 | 0.72 | 0.10 | 1.21 | 0.24 | 2.10 | 0.30 | 2.75 | 0.35 | 3.19 | 0.39 | 3.71 |
Table 2 Number of hydrogen bond per water molecule between GO substrate and water and their corresponding dipolar relaxation time(τHB) for both PG and GO nanochannels with various oxidation degree
| d/nm | PG | GO?10% | GO?20% | GO?30% | GO?35% | GO?40% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | nHB/H2O | τHB/ps | |
| 0.6 | 0.25 | 3.23 | 0.37 | 4.12 | 0.33 | 3.71 | 0.51 | 5.14 | 0.53 | 5.25 | 0.55 | 5.35 |
| 0.9 | 0.10 | 1.21 | 0.23 | 1.84 | 0.30 | 2.79 | 0.42 | 3.58 | 0.38 | 3.33 | 0.44 | 3.98 |
| 1.2 | 0.04 | 0.72 | 0.10 | 1.21 | 0.24 | 2.10 | 0.30 | 2.75 | 0.35 | 3.19 | 0.39 | 3.71 |
| 1 | Schwarzenbach R. P., Egli T., Hofstetter T. B., von Gunten U., Wehrli B., Annu. Rev. Env. Resour., 2010, 35, 109―136 |
| 2 | Burn S., Hoang M., Zarzo D., Olewniak F., Campos E., Bolto B., Barron O., Desalination, 2015, 364, 2―16 |
| 3 | Chaibi M. T., Desalination, 2000, 127(2), 119―133 |
| 4 | Qasim M., Darwish N. A., Sarp S., Hilal N., Desalination, 2015, 374, 47―69 |
| 5 | Kim Y. K., Lee S. Y., Kim D. H., Lee B. S., Nam S. Y., Rhim J. W., Desalination, 2010, 250(2), 865―867 |
| 6 | Cho Y. H., Kim H. W., Lee H. D., Shin J. E., Yoo B. M., Park H. B., J. Membrane Sci., 2017, 544, 425―435 |
| 7 | Sui X., Ding H. R., Yuan Z. W., Leong C. F., Goh K. L., Li W., Yang N., D'Alessandro D. M., Chen Y., Carbon, 2019, 148, 277―289 |
| 8 | Sun M., Li J. H., Nano Today, 2018, 20, 121―137 |
| 9 | Li X. Y., Roth C. C., Mohr D., Int. J. Plasticity, 2019, 118, 320―344 |
| 10 | Wang S. F., Wu Y. Z., Zhang N., He G. W., Xin Q. P., Wu X. Y., Wu H., Cao X. Z., Guiver M. D., Jiang Z. Y., Energ. Environ. Sci., 2016, 9(10), 3107―3112 |
| 11 | Zhao M. Y., Yang X. P., Yang X. N., Acta Physico⁃Chimica Sinica, 2015, 31(8), 1489―1498 |
| 12 | Wei Y., Zhang Y. S., Gao X. L., Ma Z., Wang X. J., Gao C. J., Carbon, 2018, 139, 964―981 |
| 13 | Yang Q., Su Y., Chi C., Cherian C. T., Huang K., Kravets V. G., Wang F. C., Zhang J. C., Pratt A., Grigorenko A. N., Guinea F., Geim A. K., Nair R. R., Nat. Mater., 2017, 16(12), 1198 |
| 14 | Ding M. X., Ghoufi A., Szymczyk A., Desalination, 2014, 343, 48―53 |
| 15 | Ding M. X., Szymczyk A., Ghoufi A., Desalination, 2015, 368, 76―80 |
| 16 | Renou R., Ding M. X., Zhu H. C., Szyrnczyk A., Malfreyt P., Ghoufi A., J. Phys. Chem. B, 2014, 118(14), 3931―3940 |
| 17 | Renou R., Szymczyk A., Ghoufi A., J. Chem. Phys., 2014, 140(4), 044704 |
| 18 | Renou R., Szymczyk A., Ghoufi A., Phys. Rev. E, 2015, 91(3), 032411 |
| 19 | Renou R., Szymczyk A., Ghoufi A., Nanoscale, 2015, 7(15), 6661―6666 |
| 20 | Zhu H., Ghoufi A., Szymczyk A., Balannec B., Morineau D., Phys. Rev. Lett., 2012, 109(10), 107801 |
| 21 | Zhu H. C., Hu B., Hu H., Zhu Y. J., He W. Z., Huang J. W., Li G. M., J. Mol. Liq., 2020, 317, 113995 |
| 22 | Fumagalli L., Esfandiar A., Fabregas R., Hu S., Ares P., Janardanan A., Yang Q., Radha B., Taniguchi T., Watanabe K., Gomila G., Novoselov K. S., Geim A. K., Science, 2018, 360(6395), 1339 |
| 23 | Garbin H. D., Estimation of Partial Decoupling of Cavity Events, Sandia National Labs., Albuquerque, 1993 |
| 24 | Abascal J. L. F., Vega C., J. Chem. Phys., 2005, 123(23), 234505 |
| 25 | MacKerell A. D., Bashford D., Bellott M., Dunbrack R. L., Evanseck J. D., Field M. J., Fischer S., Gao J., Guo H., Ha S., Joseph⁃ McCarthy D., Kuchnir L., Kuczera K., Lau F. T. K., Mattos C., Michnick S., Ngo T., Nguyen D. T., Prodhom B., Reiher W. E., Roux B., Schlenkrich M., Smith J. C., Stote R., Straub J., Watanabe M., Wiorkiewicz⁃Kuczera J., Yin D., Karplus M., J. Phys. Chem. B, 1998,102(18), 3586―3616 |
| 26 | Buneman O., Siam. Rev., 1983, 25(3), 425―426 |
| 27 | Liu B., Wu R. B., Baimova J. A., Wu H., Law A. W. K., Dmitriev S. V., Zhou K., Phys. Chem. Chem. Phys., 2016, 18(3), 1886―1896 |
| 28 | Werder T., Walther J. H., Jaffe R. L., Halicioglu T., Koumoutsakos P., J. Phys. Chem. B, 2003, 107(6), 1345―1352 |
| 29 | Allen M. P., Tildesley D. J., Computer Simulation of Liquids, Oxford University Press, London, 2017 |
| 30 | Ryckaert J. P., Ciccotti G., Berendsen H. J. C., J. Comput. Phys., 1977, 23(3), 327―341 |
| 31 | Hoover W. G., Phys. Rev. A, 1985, 31(3), 1695―1697 |
| 32 | Nose S., J. Chem. Phys., 1984, 81(1), 511―519 |
| 33 | Bonthuis D. J., Gekle S., Netz R. R., Phys. Rev. Lett., 2011, 107(16), 166102 |
| 34 | Ballenegger V., Hansen J. P., J. Chem. Phys., 2005, 122(11), 114711 |
| 35 | Algara⁃Siller G., Lehtinen O., Kaiser U., Nature, 2015, 528(7583), E3 |
| 36 | Wei N., Peng X. S., Xu Z. P., ACS Appl. Mater. Inter., 2014, 6(8), 5877―5883 |
| 37 | Giri A. K., Teixeira F., Cordeiro M. N. D. S., Desalination, 2019, 460, 1―14 |
| 38 | Chandra A., Chowdhuri S., J. Phys. Chem. B, 2002, 106(26), 6779―6783 |
| [1] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [2] | YU Bin, CHEN Xiaoyan, ZHAO Yue, CHEN Weichang, XIAO Xinyan, LIU Haiyang. Graphene Oxide-based Cobalt Porphyrin Composites for Electrocatalytic Hydrogen Evolution Reaction [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210549. |
| [3] | WANG Xueli, SONG Xiangwei, XIE Yanning, DU Niyang, WANG Zhenxin. Preparation, Characterization of Partially Reduced Graphene Oxide and Its Killing Effect on Human Cervical Cancer Cells [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210595. |
| [4] | YANG Junge, GAO Chengqian, LI Boxin, YIN Dezhong. Preparation of High Thermal Conductivity Phase Change Monolithic Materials Based on Pickering Emulsion Stabilized by Surface Modified Graphene Oxide [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210593. |
| [5] | ZHANG Zhibo, SHANG Han, XU Wenxuan, HAN Guangdong, CUI Jinsheng, YANG Haoran, LI Ruixin, ZHANG Shenghui, XU Huan. Self-Assembly of Graphene Oxide at Poly(3-hydroxybutyrate) Microparticles Toward High-performance Intercalated Nanocomposites [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210566. |
| [6] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [7] | LEI Xiaotong, JIN Yiqing, MENG Xuanyu. Prediction of the Binding Site of PIP2 in the TREK-1 Channel Based on Molecular Modeling [J]. Chem. J. Chinese Universities, 2021, 42(8): 2550. |
| [8] | LI Congcong, LIU Minghao, HAN Jiarui, ZHU Jingxuan, HAN Weiwei, LI Wannan. Theoretical Study of the Catalytic Activity of VmoLac Non-specific Substrates Based on Molecular Dynamics Simulations [J]. Chem. J. Chinese Universities, 2021, 42(8): 2518. |
| [9] | ZHU Deshuai, ZHAO Jianying, YANG Zhenghui, GUO Haiquan, GAO Lianxun. Graphene Oxide/Polyimide Composites with High Energy Storage Density Based on Multilayer Structure [J]. Chem. J. Chinese Universities, 2021, 42(8): 2694. |
| [10] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [11] | LI Peihong, ZHANG Chunling, DAI Xueyan, SUI Yanlong. Progress of Graphene Oxide/Polymer Composite Hydrogel [J]. Chem. J. Chinese Universities, 2021, 42(6): 1694. |
| [12] | LIU Aiqing, XU Wensheng, XU Xiaolei, CHEN Jizhong, AN Lijia. Molecular Dynamics Simulation of Polymer/rod Nanocomposite [J]. Chem. J. Chinese Universities, 2021, 42(3): 875. |
| [13] | MIAO Weijun, WU Feng, WANG Yong, WANG Zongbao. In⁃situ Study of the Epitaxial Crystallization of PCL/RGO at High Shear Rate [J]. Chem. J. Chinese Universities, 2021, 42(3): 910. |
| [14] | QI Renrui, LI Minghao, CHANG Hao, FU Xueqi, GAO Bo, HAN Weiwei, HAN Lu, LI Wannan. Theoretical Study on the Unbinding Pathway of Xanthine Oxidase Inhibitors Based on Steered Molecular Dynamics Simulation [J]. Chem. J. Chinese Universities, 2021, 42(3): 758. |
| [15] | HUANG Dongxue, ZHANG Ying, ZENG Ting, ZHANG Yuanyuan, WAN Qijin, YANG Nianjun. Transition Metal Sulfides Hybridized with Reduced Graphene Oxide for High-Performance Supercapacitors [J]. Chem. J. Chinese Universities, 2021, 42(2): 643. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
