

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (10): 2131.doi: 10.7503/cjcu20140508
• Physical Chemistry • Previous Articles Next Articles
WANG Chengjie, TANG Chunmei*( ), ZHANG Yijie, GAO Fengzhi
), ZHANG Yijie, GAO Fengzhi
Received:2014-06-04
Online:2014-10-10
Published:2014-09-15
Contact:
TANG Chunmei
E-mail:tcmnj@163.com
Supported by:CLC Number:
TrendMD:
WANG Chengjie, TANG Chunmei, ZHANG Yijie, GAO Fengzhi. Hydrogen Storage of the Different Kinds of Metal Atoms Coated Fullerene C20M(M=Li, Ti, Fe)†[J]. Chem. J. Chinese Universities, 2014, 35(10): 2131.
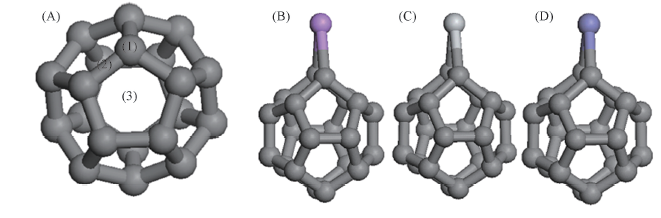
Fig.1 Three different positions of an exohedral atom in C20(A), optimized geometry of C20Li(B), C20Ti(C) and C20Fe(D) (1) The top site of C atom; (2) the bridge site of C—C bond; (3) the hollow site of the five-membered ring.
| C20M | C20Li(1) | C20Li(2) | C20Li(3) | C20Ti(1) | C20Ti(2) | C20Ti(3) | C20Fe(1) | C20Fe(2) | C20Fe(3) |
|---|---|---|---|---|---|---|---|---|---|
| Eb/eV | 1.70 | 2.14 | 1.59 | 4.94 | 5.03 | 4.42 | 1.96 | 2.67 | 2.43 |
| Eg/eV | 0.20 | 0.21 | 0.21 | 0.58 | 0.60 | 0.35 | 0.52 | 0.67 | 0.55 |
Table 1 Eb and Eg of C20M(M=Li, Ti, Fe)
| C20M | C20Li(1) | C20Li(2) | C20Li(3) | C20Ti(1) | C20Ti(2) | C20Ti(3) | C20Fe(1) | C20Fe(2) | C20Fe(3) |
|---|---|---|---|---|---|---|---|---|---|
| Eb/eV | 1.70 | 2.14 | 1.59 | 4.94 | 5.03 | 4.42 | 1.96 | 2.67 | 2.43 |
| Eg/eV | 0.20 | 0.21 | 0.21 | 0.58 | 0.60 | 0.35 | 0.52 | 0.67 | 0.55 |
| Species | C20Li | C20Li-H2 | C20Li-2H2 | C20Li-3H2 | C20Li-4H2 | C20Li-5H2 | C20Li-6H2 | C20Li-7H2 |
|---|---|---|---|---|---|---|---|---|
| Ead/eV | 0.46 | 0.43 | 0.36 | 0.30 | 0.26 | 0.25 | 0.22 | |
| Ec/eV | 0.46 | 0.20 | 0.23 | 0.12 | 0.10 | 0.16 | 0.05 | |
| Eg/eV | 0.21 | 0.38 | 0.38 | 0.39 | 0.39 | 0.38 | 0.38 | 0.38 |
| QM/e | 0.89 | 0.81 | 0.71 | 0.60 | 0.55 | 0.58 | 0.55 | 0.57 |
| QH/e | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 | |
| Species | C20Ti | C20Ti-H2 | C20Ti-2H2 | C20Ti-3H2 | C20Ti-4H2 | C20Ti-5H2 | C20Ti-6H2 | C20Ti-7H2 |
| Ead/eV | 0.44 | 0.69 | 0.64 | 0.58 | 0.55 | 0.53 | 0.46 | |
| Ec/eV | 0.44 | 0.94 | 0.54 | 0.40 | 0.44 | 0.41 | 0.08 | |
| Eg/eV | 0.60 | 0.80 | 0.96 | 0.98 | 0.97 | 1.02 | 1.02 | 1.02 |
| QM/e | 0.78 | 0.88 | 0.58 | 0.21 | -0.20 | -0.69 | -1.18 | -1.18 |
| QH/e | 0.01 | 0.02 | 0.07 | 0.09 | 0.11 | 0.14 | 0.12 | |
| Species | C20Fe | C20Fe-H2 | C20Fe-2H2 | C20Fe-3H2 | C20Fe-4H2 | C20Fe-5H2 | ||
| Ead/eV | 0.70 | 0.76 | 0.80 | 0.80 | 0.67 | |||
| Ec/eV | 0.70 | 0.82 | 0.93 | 0.84 | 0.07 | |||
| Eg/eV | 0.67 | 0.78 | 0.45 | 0.81 | 0.94 | 0.94 | ||
| QM/e | 0.71 | 0.58 | 0.28 | -0.44 | -1.01 | -1.01 | ||
| QH/e | 0.03 | 0.06 | 0.13 | 0.17 | 0.13 |
Table 2 Ead, Ec, Eg, QM and QH of C20Li-nH2(n=1—7), C20Ti-nH2(n=1—7), C20Fe-nH2(n=1—5)
| Species | C20Li | C20Li-H2 | C20Li-2H2 | C20Li-3H2 | C20Li-4H2 | C20Li-5H2 | C20Li-6H2 | C20Li-7H2 |
|---|---|---|---|---|---|---|---|---|
| Ead/eV | 0.46 | 0.43 | 0.36 | 0.30 | 0.26 | 0.25 | 0.22 | |
| Ec/eV | 0.46 | 0.20 | 0.23 | 0.12 | 0.10 | 0.16 | 0.05 | |
| Eg/eV | 0.21 | 0.38 | 0.38 | 0.39 | 0.39 | 0.38 | 0.38 | 0.38 |
| QM/e | 0.89 | 0.81 | 0.71 | 0.60 | 0.55 | 0.58 | 0.55 | 0.57 |
| QH/e | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 | |
| Species | C20Ti | C20Ti-H2 | C20Ti-2H2 | C20Ti-3H2 | C20Ti-4H2 | C20Ti-5H2 | C20Ti-6H2 | C20Ti-7H2 |
| Ead/eV | 0.44 | 0.69 | 0.64 | 0.58 | 0.55 | 0.53 | 0.46 | |
| Ec/eV | 0.44 | 0.94 | 0.54 | 0.40 | 0.44 | 0.41 | 0.08 | |
| Eg/eV | 0.60 | 0.80 | 0.96 | 0.98 | 0.97 | 1.02 | 1.02 | 1.02 |
| QM/e | 0.78 | 0.88 | 0.58 | 0.21 | -0.20 | -0.69 | -1.18 | -1.18 |
| QH/e | 0.01 | 0.02 | 0.07 | 0.09 | 0.11 | 0.14 | 0.12 | |
| Species | C20Fe | C20Fe-H2 | C20Fe-2H2 | C20Fe-3H2 | C20Fe-4H2 | C20Fe-5H2 | ||
| Ead/eV | 0.70 | 0.76 | 0.80 | 0.80 | 0.67 | |||
| Ec/eV | 0.70 | 0.82 | 0.93 | 0.84 | 0.07 | |||
| Eg/eV | 0.67 | 0.78 | 0.45 | 0.81 | 0.94 | 0.94 | ||
| QM/e | 0.71 | 0.58 | 0.28 | -0.44 | -1.01 | -1.01 | ||
| QH/e | 0.03 | 0.06 | 0.13 | 0.17 | 0.13 |
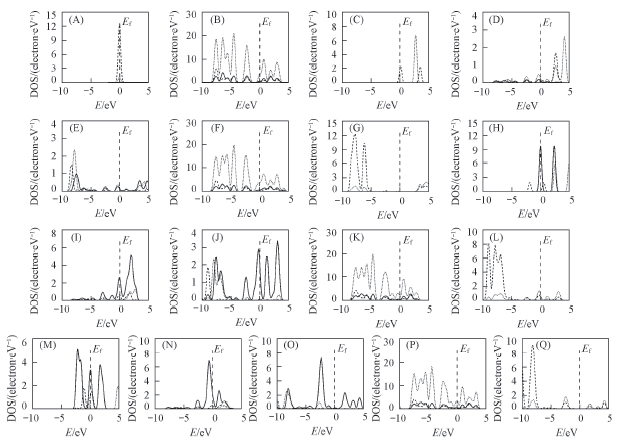
Fig.3 Partial density of states of H2(A), C20(B), isolated Li(C), Li in C20Li(D), Li in C20Li-nH2(E), C20 in C20Li-nH2(F), H in C20Li-6H2(G), isolated Ti(H), Ti in C20Ti(I), Ti in C20Ti-nH2(J), C20 in C20Ti-nH2(K), H in C20Ti-6H2(L), isolated Fe(M), Fe in C20Fe(N), Fe in C20Fe-nH2(O) and C20 in C20Fe-nH2(P), H in C20Fe-4H2(Q)
| [1] | Wang Q.,Sun Q., Puru J., Yoshiyuki K., J. Chem.The.Com., 2009, 374(5), 374—379 |
| [2] | Sun Q., Puru J., Wang Q., Manuel M., J. Am. Chem. Soc., 2006, 128, 9741—9745 |
| [3] | Guo Y. J., Liu Z. G., Liu S. Q., Zhao X. H., Huang K. L., Appl. Phys. Lett., 2011, 98, 023107-1—023107-3 |
| [4] | |
| [5] | Kubas G.J., Metal Dihydrogen and σ-Bond Complexes, Kluwer Academic/Plenum Publishing, New York, 2001 |
| [6] | Kiran B., Anil K. K., Jena P., J. Chem.Phys., 2006, 124, 224703-1—224703-6 |
| [7] | Deng W Q., Xu X., Goddard W. A., Phys. Rev. Lett., 2004, 92, 166103-1—166103-4 |
| [8] | Zhao Y. F., Kim Y. H., Dillon A. C., Heben M. J., Zhang S. B., Phys. Rev. Lett., 2005, 94, 155504-1—155504-4 |
| [9] | Dillon A.C., Parilla P. A ., Gennet T., Gilbert K. E. H., Blackburn J. L., Kim Y. H., Zhao Y., Zhang S. B., Alleman J. L., Jones K. M., McDonald T., Heben M., Discovering the Mechanism of H2 Adsorption on Aromatic Carbon Nanostructures to Develop Adsorbents for Vehicular Applications, DOE Hydrogen Program, FY Progress Report, 2003 |
| [10] | Delley B., J. Chem. Phys., 1990, 92, 508—517 |
| [11] | Tan C. L., Cai W., Tian X. H., Chin. Phys. B,2006, 15(11), 2718—2723 |
| [12] | San D., Dmol., Biosym. Technologies, San Diego CA, 1996 |
| [13] | Zhao J. Y., Zhao F. Q., Xu S. Y., Ju X. H., J. Phys. Chem. A,2013, 117(10), 2213—2222 |
| [14] | Wang Z. Q., Day P., Pachter R., Chem. Phys. Lett., 1996, 248, 121—126 |
| [15] | An Y. P., Yang C. L., Wang M. S., Ma X. G., Wang D. H., Cur. Appl. Phys., 2010, 10, 260—265 |
| [16] | Lu G. L.,Yuan Y. B., Deng K. M., Wu H. P., Yang J. L., Wang X., Chem. Phys. Lett., 2006, 424, 142—145 |
| [17] | Aihara J. I., Chem. Phys. Lett., 2001, 343, 465—469 |
| [18] | Sun Q., Wang Q., Jena P., Kawazoe Y., J. Am. Chem. Soc., 2005, 127, 14582—14583 |
| [19] | Huang H. S., Wang X. M., Zhao D. Q., Wu L. F., Huang X. W., Li Y. C., Acta Phys. Sinica,2012, 61(7), 073101-1—073101-7 |
| (黄海深, 王小满, 赵冬秋, 伍良福, 黄晓伟, 李蕴才. 物理学报, 2012, 61(7), 073101-1—073101-7) | |
| [20] | Schleyer P. v. R., Maerker C., Dransfeld A., J. Am. Chem. Soc., 1996, 118(26), 6317—6318 |
| [21] | SchSeyer P. v. R., Manohasan M., Wang Z. X., Org.Lett., 2001, 3(16), 2465—2468 |
| [22] | Wu G. F., Wang J. L., Zhang X. Y., Zhu L.Y., J. Phys. Chem.C,2009, 113(17), 7052—7057 |
| [23] | Lu Q. L., Wan J. G., J. Chem. Phys., 2010, 132, 224308-1—224308-5 |
| [24] | Mokkath J. H., Schwingenschlogl U., J. Phys. Chem. C,2014, 118(9), 4885—4889 |
| [25] | Crabtree R.H., The Organometallic Chemistry of the Transition Metals, 3rd ed.; Wiley Interscience, New York, 2001 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [3] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [4] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [5] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [6] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [7] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [8] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [9] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [10] | YING Fuming, JI Chenru, SU Peifeng, WU Wei. λ-DFCAS: A Hybrid Density Functional Complete Active Space Self Consistent Field Method [J]. Chem. J. Chinese Universities, 2021, 42(7): 2218. |
| [11] | ZHENG Ruoxin, ZHANG Igor Ying, XU Xin. Development and Benchmark of Lower Scaling Doubly Hybrid Density Functional XYG3 [J]. Chem. J. Chinese Universities, 2021, 42(7): 2210. |
| [12] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [13] | HU Wei, LIU Xiaofeng, LI Zhenyu, YANG Jinlong. Surface and Size Effects of Nitrogen-vacancy Centers in Diamond Nanowires [J]. Chem. J. Chinese Universities, 2021, 42(7): 2178. |
| [14] | YANG Yiying, ZHU Rongxiu, ZHANG Dongju, LIU Chengbu. Theoretical Study on Gold-catalyzed Cyclization of Alkynyl Benzodioxin to 8-Hydroxy-isocoumarin [J]. Chem. J. Chinese Universities, 2021, 42(7): 2299. |
| [15] | LIU Changhui, LIANG Guojun, LI Yanlu, CHENG Xiufeng, ZHAO Xian. Density Functional Theory Study of NH3 Adsorption on Boron Nanotubes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2263. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||