

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (10): 2138.doi: 10.7503/cjcu20140478
• Physical Chemistry • Previous Articles Next Articles
WANG Xiujun*( ), QI Qiuhong, CHEN Li
), QI Qiuhong, CHEN Li
Received:2014-05-23
Online:2014-10-10
Published:2014-09-15
Contact:
WANG Xiujun
E-mail:xjwangcn@scut.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Xiujun, QI Qiuhong, CHEN Li. Theoretical Studies on Suppression of Carbon Deposition over Titania Supported Monometallic Nickel(Platinum) Catalysts in Methane Dissociation†[J]. Chem. J. Chinese Universities, 2014, 35(10): 2138.
| Adsorption system | Eads/(kJ·mol-1) | ||||
|---|---|---|---|---|---|
| Ni-B1 | 347.16 | 0.1904 | 0.2056 | 0.2774 | 0.2658 |
| Pt-B1 | 315.90 | 0.2059(0.204)b | 0.2778(0.278)b | 0.2826(0.279)b | |
| Ni-B3 | 311.61 | 0.1844 | 0.1862 | 0.2315 | |
| Pt-B4 | 309.60 | 0.2015 | 0.2353 | ||
| Ni-B2 | 244.22 | 0.1765 | 0.2419 | ||
| Pt-B2 | 235.35 | 0.1972 | 0.2498 |
Table 1 Optimazated chemisorptions energies and structural parameters of Ni(Pt) atom adsorbed on anatase TiO2(101) surface
| Adsorption system | Eads/(kJ·mol-1) | ||||
|---|---|---|---|---|---|
| Ni-B1 | 347.16 | 0.1904 | 0.2056 | 0.2774 | 0.2658 |
| Pt-B1 | 315.90 | 0.2059(0.204)b | 0.2778(0.278)b | 0.2826(0.279)b | |
| Ni-B3 | 311.61 | 0.1844 | 0.1862 | 0.2315 | |
| Pt-B4 | 309.60 | 0.2015 | 0.2353 | ||
| Ni-B2 | 244.22 | 0.1765 | 0.2419 | ||
| Pt-B2 | 235.35 | 0.1972 | 0.2498 |
| Adsorption site | qNi(Pt)/e | /e | /e | /e | /e |
|---|---|---|---|---|---|
| Ni-B1 | 0.49 | -0.66 | -0.70 | 1.30 | 1.25 |
| Ni-B2 | 0.43 | -0.65 | 1.06 | ||
| Ni-B3 | 0.46 | -0.66 | -0.72 | 1.11 | |
| Pt-B1 | 0.18 | -0.64 | 1.30 | 1.24 | |
| Pt-B2 | 0.18 | -0.62 | 1.17 | ||
| Pt-B4 | 0.21 | -0.57 | 1.09 |
Table 2 Mulliken charge population of Ni(Pt) atom adsorbed on TiO2(101) surface
| Adsorption site | qNi(Pt)/e | /e | /e | /e | /e |
|---|---|---|---|---|---|
| Ni-B1 | 0.49 | -0.66 | -0.70 | 1.30 | 1.25 |
| Ni-B2 | 0.43 | -0.65 | 1.06 | ||
| Ni-B3 | 0.46 | -0.66 | -0.72 | 1.11 | |
| Pt-B1 | 0.18 | -0.64 | 1.30 | 1.24 | |
| Pt-B2 | 0.18 | -0.62 | 1.17 | ||
| Pt-B4 | 0.21 | -0.57 | 1.09 |
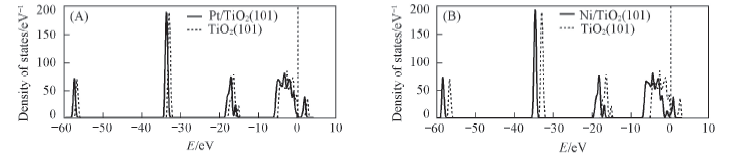
Fig.4 Density of states for the TiO2(101) surface and the most stable structural of Pt/TiO2(101) surface(A) and Ni/TiO2(101) surface(B) The vertical dot lines at the energy zero represent the Fermi level.
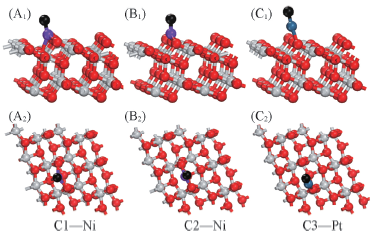
Fig.7 Side views(A1—C1) and top views(A2—C2)of the optimized configuration about the carbon atoms on Ni(Pt)/TiO2(101) surface Black balls indicate C atoms.
| Adsorption site | Eads/(kJ·mol-1) | RC—Ni(Pt)/nm | qC/e | qNi(Pt)/e | ||||
|---|---|---|---|---|---|---|---|---|
| C1—Ni | 474.19 | 0.1623 | 0.1986* | 0.2236 | -0.16 | 0.60 | -0.69 | -0.72 |
| C2—Ni | 462.63 | 0.1621 | 0.1939* | -0.15 | 0.56 | -0.71 | ||
| C3—Pt | 570.08 | 0.1754 | 0.2201 | -0.10 | 0.10 | -0.71 |
Table 3 Adsorption energies(Eads), geometric parameters of different configurations about carbon atom adsorbed on Ni(Pt)/TiO2(101) surface(R) and the mulliken population for C atom adsorbed on Ni(Pt)/TiO2(101) surface(q)
| Adsorption site | Eads/(kJ·mol-1) | RC—Ni(Pt)/nm | qC/e | qNi(Pt)/e | ||||
|---|---|---|---|---|---|---|---|---|
| C1—Ni | 474.19 | 0.1623 | 0.1986* | 0.2236 | -0.16 | 0.60 | -0.69 | -0.72 |
| C2—Ni | 462.63 | 0.1621 | 0.1939* | -0.15 | 0.56 | -0.71 | ||
| C3—Pt | 570.08 | 0.1754 | 0.2201 | -0.10 | 0.10 | -0.71 |
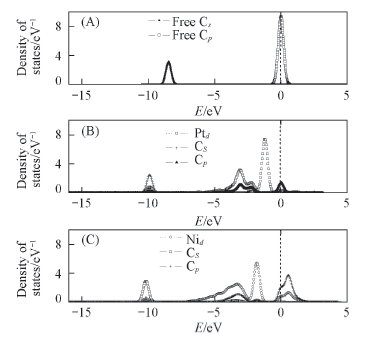
Fig.8 Partial density of the states for the most stable structural of C adsorbed on Ni(Pt)/TiO2(101) surface (A) Free C; (B) C on the Pt/TiO2(101) surface; (C) C adsorbed on the Ni/TiO2(101) surface.
| [1] | Luntz A. C., Bethune D. S., J. Chem. Phys., 1989, 90(2), 1274—1280 |
| [2] | San Jose-Alonso D., Illan-Gomez M. J., Roman-Martinez M. C., Int. J. Hydrog. Energy,2013, 38(5), 2230—2239 |
| [3] | Lv X. Y., Chen J. F., Tan Y. S., Zhang Y., Catal. Commun., 2012, 20(5), 6—11 |
| [4] | Solymosi F., Erdohelyi A., Cserenyi J., Felvegi A., J. Catal., 1994, 147(1), 272—278 |
| [5] | Mateos-Pedrero C., Gonzalez-Carrazan S. R., Soria M. A., Ruiz P., Catal. Today,2013, 203(30), 158—162 |
| [6] | Zhang Z. L., Verykios X. E., App. Catal. A,1996, 138(1), 109—133 |
| [7] | Nagaoka K., Seshan K., Aika K., Lercher J. A., J. Catal., 2001, 197(1), 34—42 |
| [8] | Bitter J., Seshan K., Lercher J., J. Catal., 1998, 176(1), 93—101 |
| [9] | Li G. S., Li L. P., Boerio-Goates J., Woodfield B. F., J. Am. Chem. Soc., 2005, 127(24), 8659—8666 |
| [10] | Lazzeri M., Vittadini A., Selloni A., Phys. Rev. B,2002, 65(11), 119901 |
| [11] | Herman G. S., Dohnalek Z., Ruzycki N., Diebold U., J. Phys. Chem. B,2003, 107(12), 2788—2795 |
| [12] | Hengerer R., Bolliger B., Erbudak M., Gratzel M., Surf. Sci., 2000, 460(1/3), 162—169 |
| [13] | Lazzeri M., VittadiniA., Selloni A., Phys. Rev. B,2001, 63(15), 155409—155418 |
| [14] | Wang F. C., Wan H. L., Tsai K. R., Wang S. J., Xu F. C., Catal. Lett., 1992, 12(1/3), 319—325 |
| [15] | Stakheev A. Y., Gololobov A. M., Beck I. E., Bragina G. O., Zaikovsky V. I., Ayupov A. B., Telegina N. S., Bukhtiyarov V. I., Russ. Chem. Bull., 2010, 59(9), 1713—1719 |
| [16] | Zou J. J., He H., Cui L., Du H. Y., Int. J. Hydrog. Energy,2007, 32(12), 1762—1770 |
| [17] | Bitter J. H., Seshan K., Lercher J. A., J. Catal., 1999, 183(2), 336—343 |
| [18] | Li C. L., Chen W., Yuan J., Shangguan W. F., Acta Phys. Chim. Sinica,2012, 28(2), 450—456 |
| (李曹龙, 陈威, 袁坚, 上官文峰. 物理化学学报, 2012, 28(2), 450—456) | |
| [19] | Bradford M. C., Vannice M. A., Appl. Catal. A,1996, 142(1), 73—96 |
| [20] | Bradford M. C., Vannice M. A., Appl. Catal. A,1996, 142(1), 97—122 |
| [21] | Yan Q. G., Weng W.Z., Wan H. L., Toghiani H., Toghiani R. K., Pittman C. U., Appl. Catal. A,2003, 239(1/2), 43—58 |
| [22] | Takanabe K., Nagaoka K., Nariai K., Aika K., J. Catal., 2005, 232(2), 268—275 |
| [23] | Wanbayor R., Ruangpornvisuti V., Appl. Surf. Sci., 2012, 258(7), 3298—3301 |
| [24] | Zhou Y., Muhich C. L., Neltner B. T., Weimer A. W., Musgrave C. B., J. Phys. Chem. C,2012, 116(22), 12114—12123 |
| [25] | Celik V., Unal H., Mete E., Ellialtioglu S., Phys. Rev. B,2010, 82(20), 205113—205125 |
| [26] | Zhang R. G., Song L. Z., Wang Y. H., Appl. Surf. Sci., 2012, 258(18), 7154—7160 |
| [27] | He P. L., Mao Y. L., Sun L. Z., Zhong J. X., J. Comput. Theor. Nanosci., 2010, 7(10), 2063—2067 |
| [28] | Escamilla-Roa E., Timon V., Hernandez-Laguna A., Comput. Theor. Chem., 2012, 981(1), 59—67 |
| [29] | Yang Z. X., He B. L., Lu Z. S., Hermansson K. T., J. Phys. Chem. C,2010, 114(10), 4486—4494 |
| [30] | Cheng D. J., Lan J. H., Cao D. P., Wang W. C., Appl. Catal. B,2010, 106(3/4), 510—519 |
| [31] | Delley B., J. Phys. Chem., 1996, 100(15), 6107—6110 |
| [32] | Delley B., J. Chem. Phys., 1990, 92(1), 508—517 |
| [33] | Delley B., Phys. Rev. B, 2002, 66(15), 155125—155134 |
| [34] | Perdew J. P., Chevary J. A., Vosko S. H., Jackson K. A., Pederson M. R., Singh D. J., Fiolhais C., Phys. Rev. B,1992, 46(11), 6671—6687 |
| [35] | Ernzerhof M., Scuseria G. E., J. Chem. Phys., 1999, 110(11), 5029—5036 |
| [36] | Iwaszuk A., Mulheran P. A., Nolan M., J. Mater. Chem. A,2013, 1(7), 2515—2525 |
| [37] | Burdett J. K., Hughbanks T., Miller G. J., Richardson J. W. Jr, Smith J. V., J. Am. Chem. Soc., 1987, 109(12), 3639—3646 |
| [38] | Goldwasser M. R., Rivas M. E., Pietri E., Perez-Zurita M. J., Cubeiro M. L., Gingembre L., Leclercq L., Leclercq G., Appl. Catal. A,2003, 255(1), 45—57 |
| [39] | Ding K. N., Zhang Y. F., Li Y., Li J. J., J. Fuzhou. Univ. Nat. Sci. Ed., 2005, 33(4), 528—532 |
| (丁开宁, 章永凡, 李奕, 李俊籛. 福州大学学报自然科学版, 2005, 33(4), 528—532) | |
| [40] | Han Y., Liu C. J., Ge Q. F., J. Phys. Chem. B,2006, 110(14), 7463—7472 |
| [41] | Li S. R., Lu X. Q., Guo W. Y., Zhu H. Y., Li M., Zhao L. M., Li Y., Shan H. H., J. Organomet. Chem., 2012, 704(1), 38—48 |
| [42] | Chen Y. F., Zhang M. H., Jiang H. X., Mol. Catal.(China), 2007, 21(4), 351—355 |
| (陈毅飞, 张敏华, 姜浩锡. 分子催化, 2007, 21(4), 351—355) | |
| [43] | Zhao Y., Teng B. T., Wen X. D., Zhao Y., Zhao L. H., Luo M. F., Catal. Commun., 2012, 27(5), 63—68 |
| [44] | Zhao Y., Teng B. T., Yang Z. X., Zhao Y., Zhao L. H., Luo M., J. Phys. Chem. C,2011, 115(33), 16461—16466 |
| [45] | Zhang W. D., Liu B. S., Zhu C., Tian Y. L., Appl. Catal. A,2005, 292(18), 138—143 |
| [46] | Fan C., Zhu Y. A., Zhou X. G., Chem. Re. Eng. Technol., 2012, 28(2), 117—121 |
| [47] | Xu Y., Ruban A. V., Mavrikakis M., J. Am. Chem. Soc., 2004, 126(14), 4717—4725 |
| [48] | Greeley J., Norskov J. K., Surf. Sci., 2005, 592(1/3), 104—111 |
| [49] | Tomishige K., Kanazawa S., Suzuki K., Asadullah M., Sato M., Ikushima K., Kunimori K., Appl. Catal. A,2002, 233(1/2), 35—44 |
| [50] | Wang S. B., Lu G. Q., Millar G. J., Energ. Fuel,1996, 10(4), 896—904 |
| [1] | REN Shijie, QIAO Sicong, LIU Chongjing, ZHANG Wenhua, SONG Li. Synchrotron Radiation X-Ray Absorption Spectroscopy Research Progress on Platinum Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220466. |
| [2] | HOU Congcong, WANG Huiying, LI Tingting, ZHANG Zhiming, CHANG Chunrui, AN Libao. Preparation and Electrochemical Properties of N-CNTs/NiCo-LDH Composite [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220351. |
| [3] | CHU Mingyue, LI Fengbo, GAO Ning, YANG Xin, YU Tingting, MA Huiyuan, YANG Guixin, PANG Haijun. Construction of a Coronal Polyoxometalate-based Composite Film for Determination of Nitrite [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210579. |
| [4] | FAN Ye, HAN Huihui, FANG Yun, FENG Ruiqin, XIA Yongmei. Facile Synthesis of Hollow Nickel Submicrospheres with Hierarchical Nano-structure and Its Catalytic Hydrogenation of Phenol [J]. Chem. J. Chinese Universities, 2021, 42(6): 1801. |
| [5] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [6] | WANG Yimeng, LIU Kai, WANG Baoguo. Coating Strategies of Ni-rich Layered Cathode in LIBs [J]. Chem. J. Chinese Universities, 2021, 42(5): 1514. |
| [7] | XIAO Zhaozhong, MA Zhi, PIAO Lingyu. Co-catalytic Effect of Ni2P on Photocatalytic Formic Acid Dehydrogenation over Different Semiconductors [J]. Chem. J. Chinese Universities, 2021, 42(12): 3692. |
| [8] | REN Yushuang, GUO Yuanyuan, LIU Xueyi, SONG Jie, ZHANG Chuan. Platinum(Ⅳ) Prodrug-grafted Phosphorothioate DNA and Its Self-assembled Nanostructure for Targeted Drug Delivery [J]. Chem. J. Chinese Universities, 2020, 41(8): 1721. |
| [9] | ZHAO Guoqing, YUAN Zhao, WANG Lian, GUO Zhuo. Preparation of Ni2P/N, S co-Doped Reduced Graphene Oxide Composites and Their Electrocatalytic Properties for Hydrogen Evolution† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1575. |
| [10] | SUN Qiangqiang, CAO Baoyue, ZHOU Chunsheng, ZHANG Guochun, WANG Zenglin. Enhancing Hydrogen Evolution Performance of a Regular Cube NiCu Nanocrystalline Electrocatalyst Fabricated by Normal Pluse Electrodeposition † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1287. |
| [11] | LIU Lu,WU Hanyue,LI Jing,SHE Lan. Tuning Microstructures of Iron-Nickel Alloy Catalysts for Efficient Oxygen Evolution Reaction † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1083. |
| [12] | JIANG Yuanyuan, LI Boyu, LU Yizhong, WU Tongshun, HAN Dongxue. Oxygen Evolution Reaction Electrocatalytic Performance Analysis of Electroless Plated Ni-Bx [J]. Chem. J. Chinese Universities, 2020, 41(12): 2774. |
| [13] | WU Hao, WANG Changzhen, QIU Yuan, TIAN Yani, ZHAO Yongxiang. Effect of Steric Confinement Dimension on Metal Site Anti-carbon Deposition Ability of Ni-SiO2 Catalysts in CH4-CO2 Reforming [J]. Chem. J. Chinese Universities, 2020, 41(11): 2488. |
| [14] | XIA Xiaoli,TAN Jingjing,WEI Caiyun,ZHAO Yongxiang. Molybdenum Modified Nickel Phyllosilicates Catalyst for Maleic Anhydride Hydrogenation† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1207. |
| [15] | ZHANG Xiaoying,DU Guifang,ZHU Bo,GUAN Wei. Theoretical Mechanistic Study on Nickel-Catalyzed Cycloaddition of Azetidinone with Butadiene† [J]. Chem. J. Chinese Universities, 2019, 40(4): 770. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||