

高等学校化学学报 ›› 2023, Vol. 44 ›› Issue (5): 20220724.doi: 10.7503/cjcu20220724
盛心茹1, 张壮壮1,2, 丁唐婧1, 廖家英1, 周小四1( )
)
收稿日期:2022-11-18
出版日期:2023-05-10
发布日期:2022-12-20
通讯作者:
周小四
E-mail:zhouxiaosi@njnu.edu.cn
基金资助:
SHENG Xinru1, ZHANG Zhuangzhuang1,2, DING Tangjing1, LIAO Jiaying1, ZHOU Xiaosi1( )
)
Received:2022-11-18
Online:2023-05-10
Published:2022-12-20
Contact:
ZHOU Xiaosi
E-mail:zhouxiaosi@njnu.edu.cn
Supported by:摘要:
随着能源与环境问题的日益加剧, 发展绿色能源存储与转化技术变得越来越重要. 作为一种环境友好型储能器件, 钠离子电池的快速发展激发了对高性能正极材料的需求. 在各类正极材料中, 无定形磷酸铁 (FePO4)因其较高的理论比容量和优异的电化学可逆性而受到了广泛关注. 基于此, 本文综合评述了无定形 FePO4作为钠离子电池正极材料的研究进展. 首先, 介绍了无定形FePO4的基本特征及其应用; 然后, 系统总结了其常见的合成方法, 如模板法、 水热法等; 介绍了增强无定形FePO4储钠性能的策略, 强调了形貌结构和性能之间的紧密联系; 最后, 对该领域进行了总结与展望.
中图分类号:
TrendMD:
盛心茹, 张壮壮, 丁唐婧, 廖家英, 周小四. 无定形FePO4作为钠离子电池正极材料的研究进展. 高等学校化学学报, 2023, 44(5): 20220724.
SHENG Xinru, ZHANG Zhuangzhuang, DING Tangjing, LIAO Jiaying, ZHOU Xiaosi. Recent Advances in Amorphous FePO4 for Sodium-Ion Battery Cathodes. Chem. J. Chinese Universities, 2023, 44(5): 20220724.
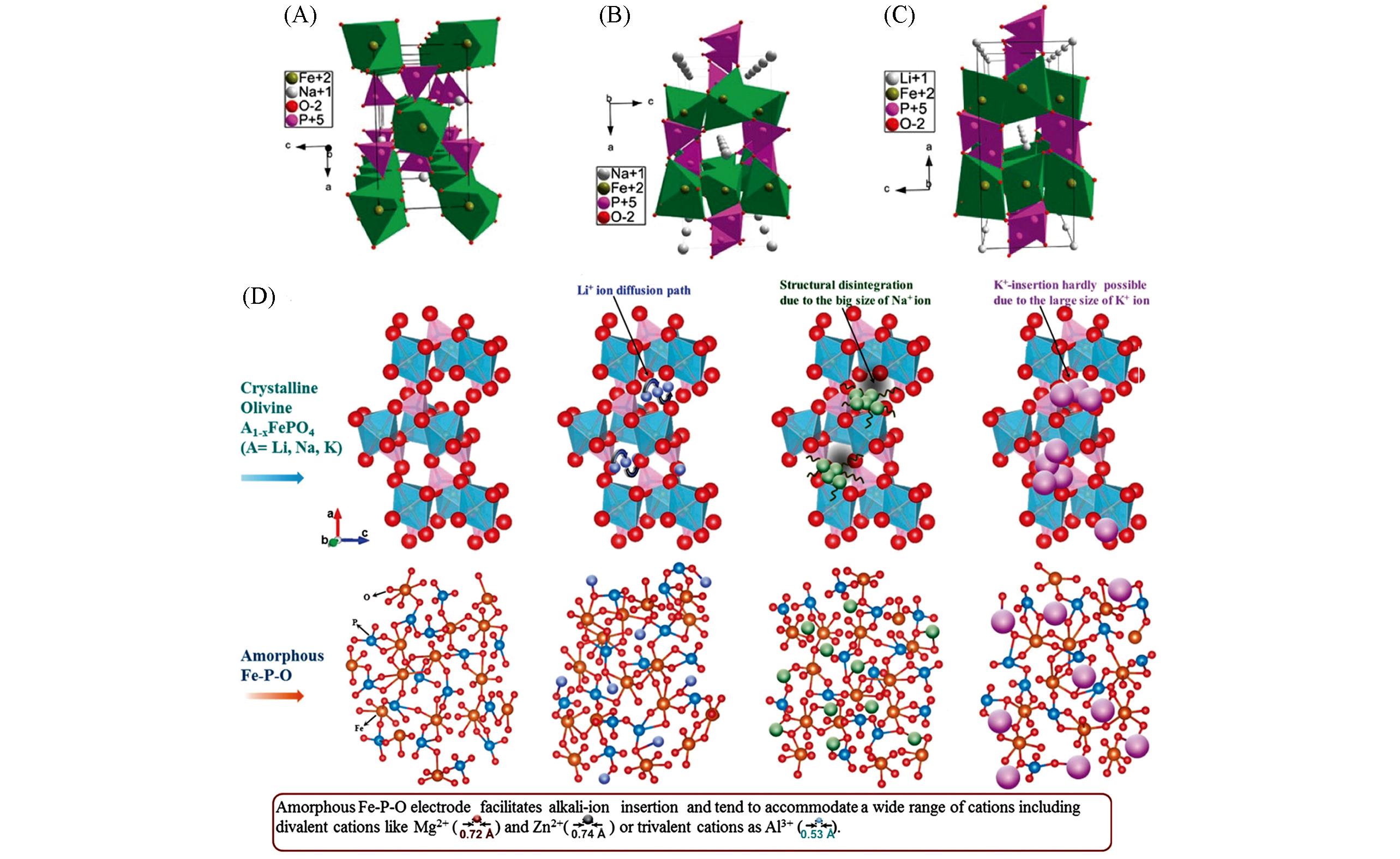
Fig.1 Structures of maricite NaFePO4(A), olivine NaFePO4(B), and olivine LiFePO4(C)[16], and schematic representation of alkali⁃ion insertion in crystalline and amorphous electrode hosts(D)[17](D) The feasibility of inserting alkali ions(Li/Na/K) in crystalline(layered, spinel and olivine structured) and amorphous hosts is presented in detail. (A—C) Copyright 2010, American Chemical Society; (D) Copyright 2014, Springer Nature.
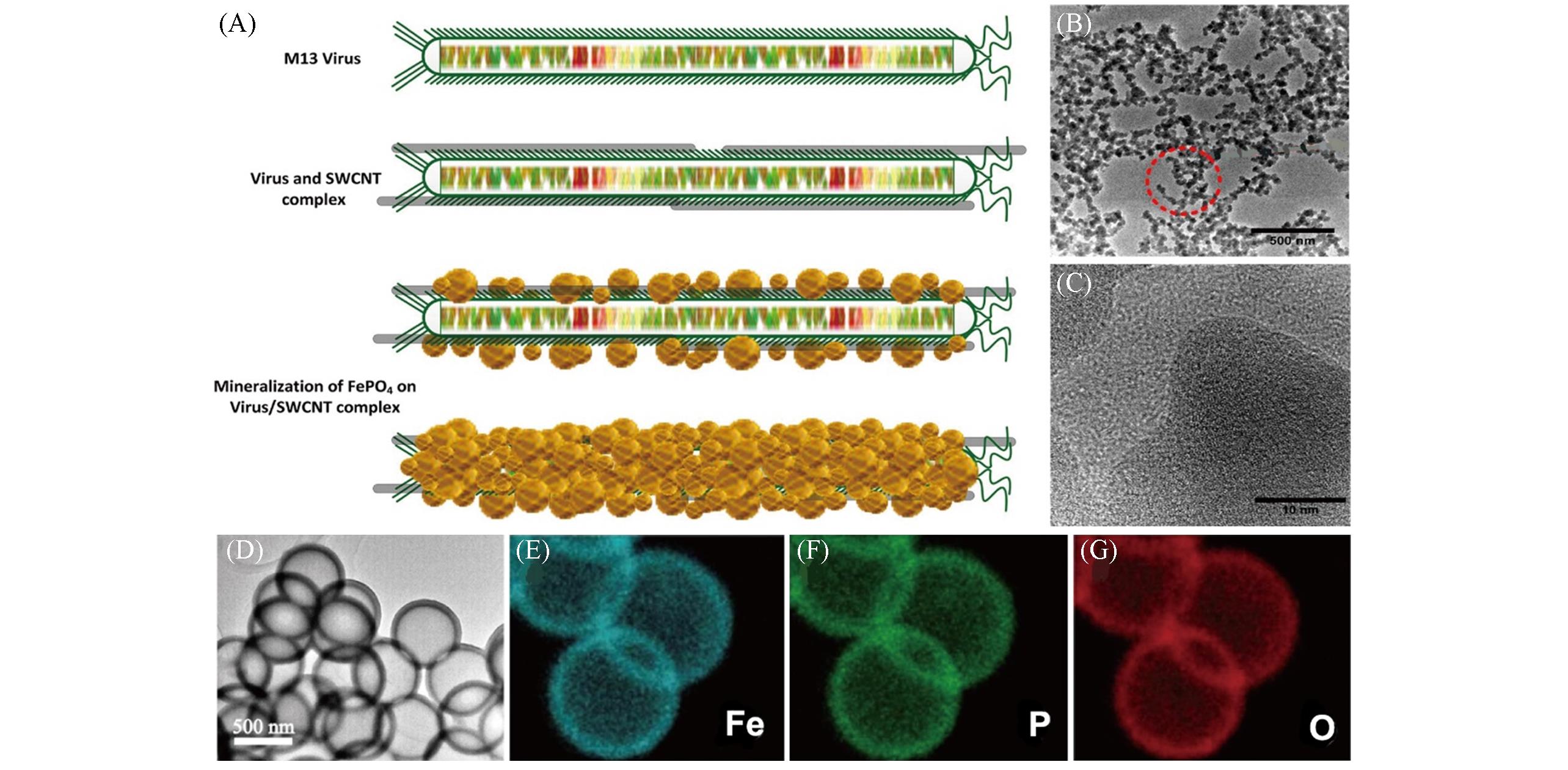
Fig.2 Schematic view of FePO4 grown on a virus/SWCNT complex showing different steps of the complexation and mineralization(A), TEM image of FePO4 grown on a virus/SWCNT complex(B), HRTEM image indicating amorphous characteristics of the grown FePO4(C)[34], TEM morphology of FePO4 hollow spheres obtained by sintering at 500 ℃ for 5 h in air(D) and elemental mappings of Fe(E), P(F) and O(G)[35](A—C) Copyright 2015, American Chemical Society; (D—G) Copyright 2017, Springer Nature.
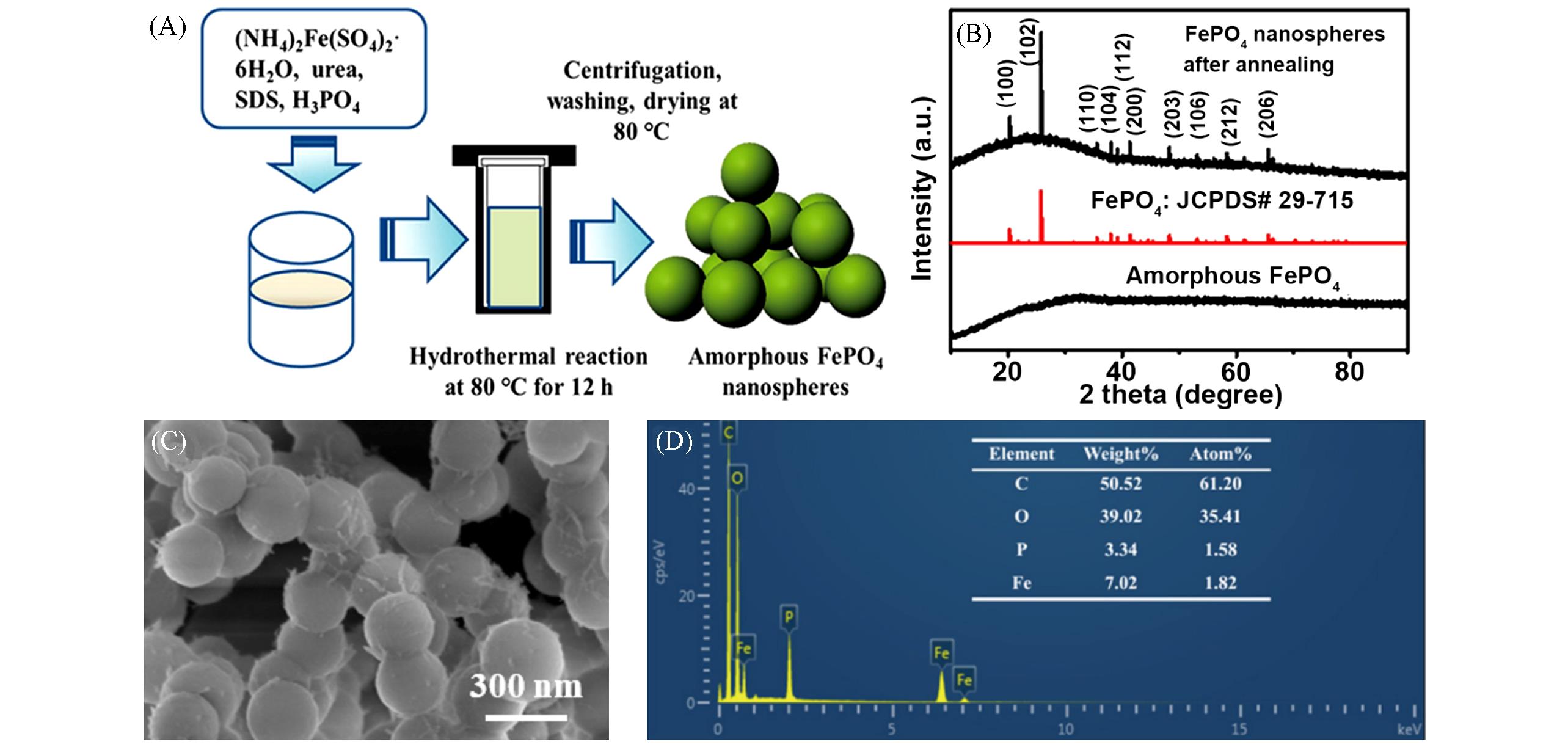
Fig.3 Schematic illustration of the preparation of amorphous FePO4 nanospheres by a simple one⁃step hydrothermal process(A) and XRD patterns of amorphous FePO4 nanospheres(below) and FePO4 nanospheres after annealing(above)(B), low⁃magnification SEM image(C), and EDS(D) of uniform amorphous FePO4 nanospheres[36]Copyright 2022, American Chemical Society.
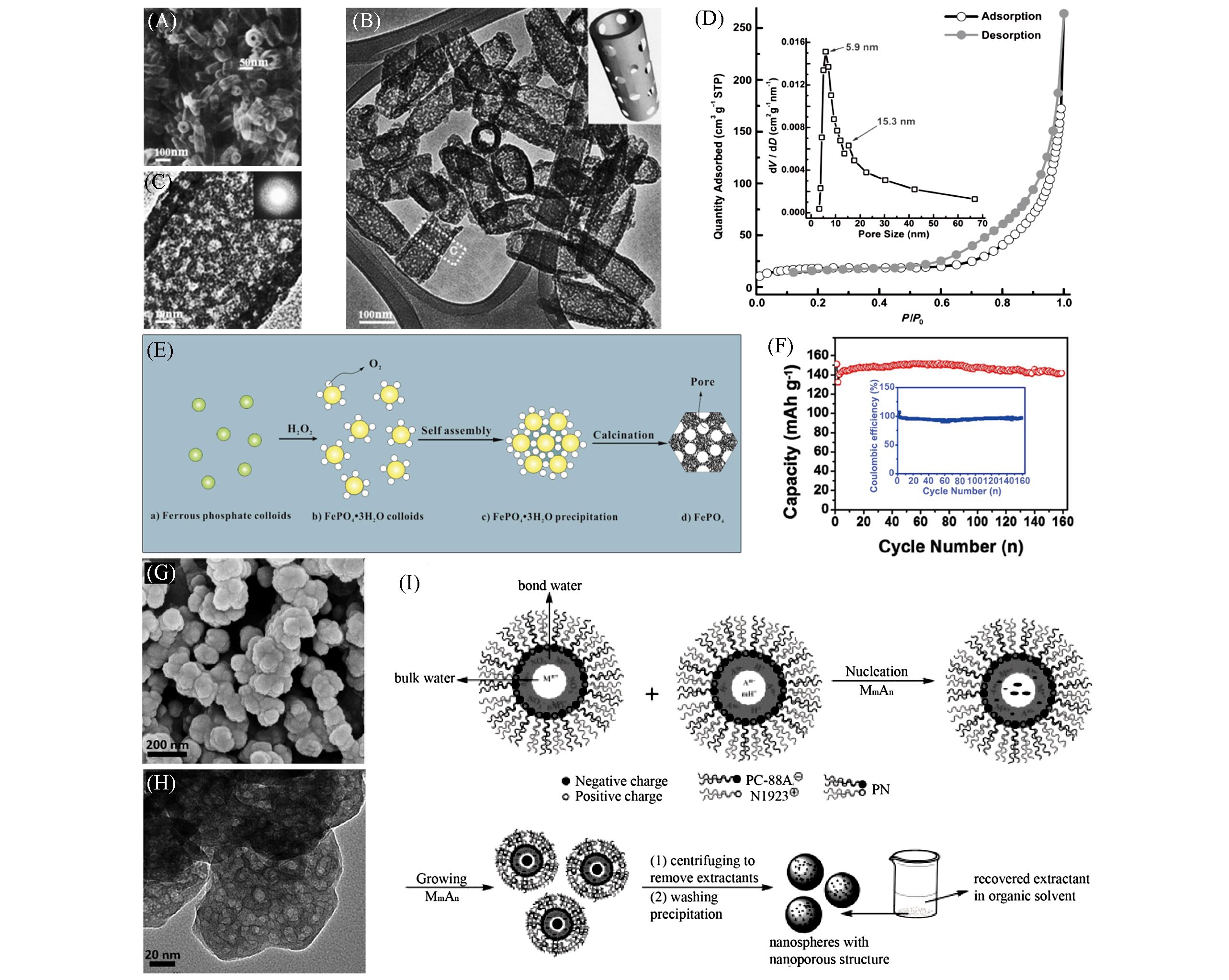
Fig.4 Low⁃magnification and high⁃magnification(inset) FESEM images of porous nanotubes(A), TEM image and illustration of a porous nanotube(inset)(B), HRTEM image and SAED pattern(inset)(C), N2 adsorption⁃ desorption isotherms and the corresponding pore⁃size distribution(inset) of porous nanotubes(D)[37], illustration of the formation mechanism of the mesoporous FePO4 nanospheres(E), cycling performance of FePO4/C cathode at a current density of 20 mA/g(F), morphological analysis of the FePO4 nanospheres and the FePO4/C composite: SEM image of the FePO4 nanospheres(G)[28], TEM image of the FePO4 nanospheres(H), general scheme for the synthesis of nanospheres by the acid⁃base⁃coupled extractant and recovery of the used extractant(I)[38](A—D) Copyright 2013, John Wiley & Sons, Inc.; (E—G) Copyright 2014, American Chemical Society; (H, I) Copyright 2012, John Wiley & Sons, Inc.
| Synthetic method of amorphous FePO4 | Advantage | Disadvantage |
|---|---|---|
| Template method | Easy to control the morphology of FePO4 | The prepared FePO4 has a single pore size and sacrifices the template, making it difficult to prepare on a large scale |
| Hydrothermal method | The feeding is one⁃time, and the synthesis of FePO4 can be completed in a single step | The entire growth process cannot be observed, and the size and quantity of FePO4 are limited by the volume of the autoclave container, making it difficult to achieve industrial production |
| Chemically induced precipitation method | The method is simple, and FePO4 nanoparticles with different pore sizes can be obtained | The synthesized FePO4 nanospheres are not strictly spherical structures, and the adhesion between particles is relatively serious |
| Solvent extraction method | In the organic solvent extraction system, the self⁃assembly of reverse micelles can form a unique FePO4 structure, and the extractant can be recycled | Sometimes it is difficult to find a suitable solvent, and the extraction separation is not always good |
Table 1 Comparison of different synthetic methods of amorphous FePO4
| Synthetic method of amorphous FePO4 | Advantage | Disadvantage |
|---|---|---|
| Template method | Easy to control the morphology of FePO4 | The prepared FePO4 has a single pore size and sacrifices the template, making it difficult to prepare on a large scale |
| Hydrothermal method | The feeding is one⁃time, and the synthesis of FePO4 can be completed in a single step | The entire growth process cannot be observed, and the size and quantity of FePO4 are limited by the volume of the autoclave container, making it difficult to achieve industrial production |
| Chemically induced precipitation method | The method is simple, and FePO4 nanoparticles with different pore sizes can be obtained | The synthesized FePO4 nanospheres are not strictly spherical structures, and the adhesion between particles is relatively serious |
| Solvent extraction method | In the organic solvent extraction system, the self⁃assembly of reverse micelles can form a unique FePO4 structure, and the extractant can be recycled | Sometimes it is difficult to find a suitable solvent, and the extraction separation is not always good |
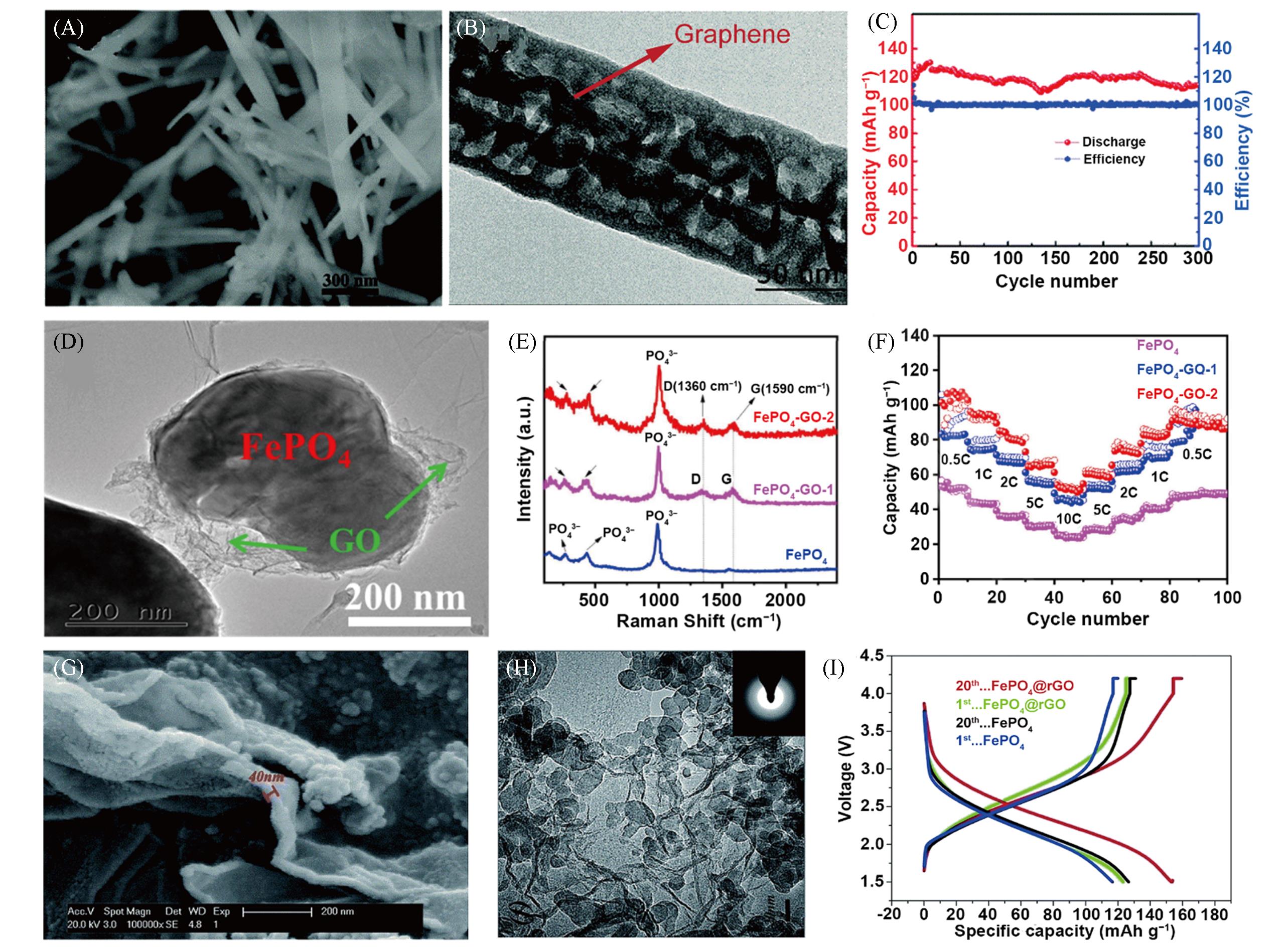
Fig.5 SEM(A) and TEM(B) images of the graphene⁃FePO4 hybrid, the cycling performance at 0.5C and Coulombic efficiency of FePO4/C cathode(C)[45], TEM image of FePO4⁃GO⁃2 sample(D), Raman spectra(E) and high⁃rate performance with current densities ranging from 0.5C to 10C(F) of three electrodes[46], SEM(G) and TEM(H) images of FePO4/rGO composite, galvanostatic discharge/ charge profiles at 1st and 20th cycles for FePO4 and FePO4/rGO composite at 0.1C(I)[47](A—C) Copyright 2016, the Royal Society of Chemistry; (D—F) Copyright 2022, Elsevier; (G—I) Copyright 2015, the Royal Society of Chemistry.
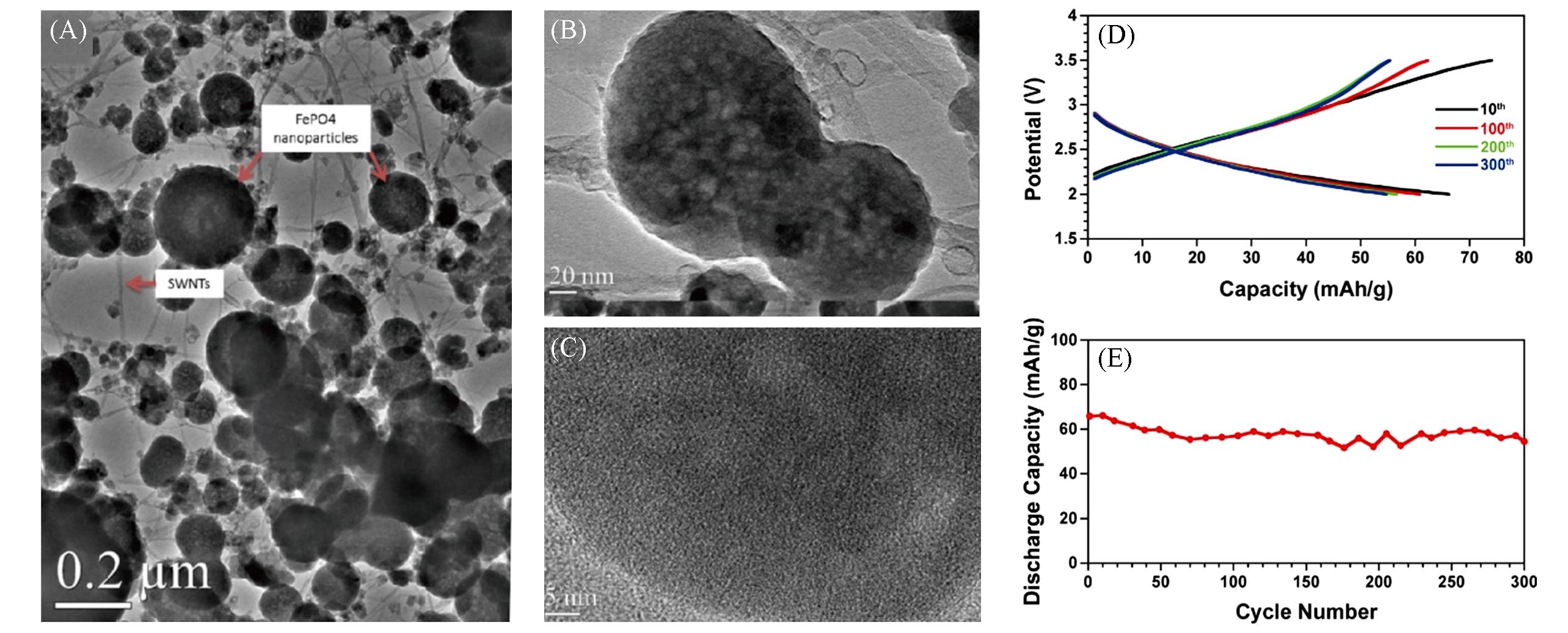
Fig.6 TEM image of the as⁃prepared networks of SWNTs⁃amorphous porous FePO4 nanoparticles(A), high⁃resolution TEM images of two FePO4 nanoparticles on SWNTs(B) and one individual nanoparticle(C), charge/discharge profiles in the 10th, 100th, 200th, and 300th cycles(D), and cycling stability at 50 mA/g(E)[48]Copyright 2012, American Chemical Society.
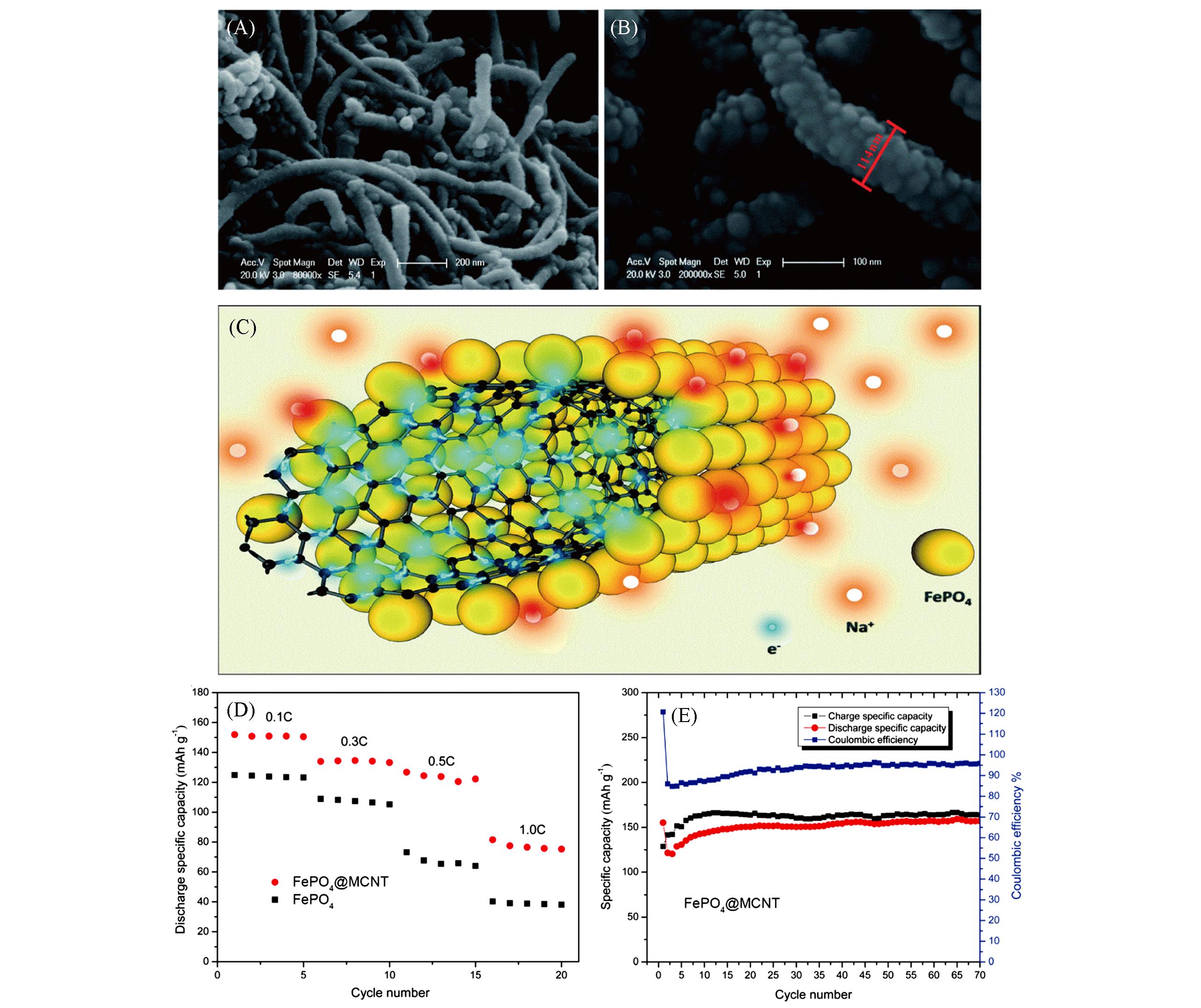
Fig.7 SEM images of the FePO4@MCNT composite(A, B), scheme of the transition processes of electrons and sodium ions in the FePO4@MCNT composite during sodium ion insertion into FePO4(C), rate capability curves of FePO4 and the FePO4@MCNT composite(D), and the cycling stability and coulombic efficiency of FePO4@MCNT at 0.1C(E)[49]Copyright 2014, the Royal Society of Chemistry.
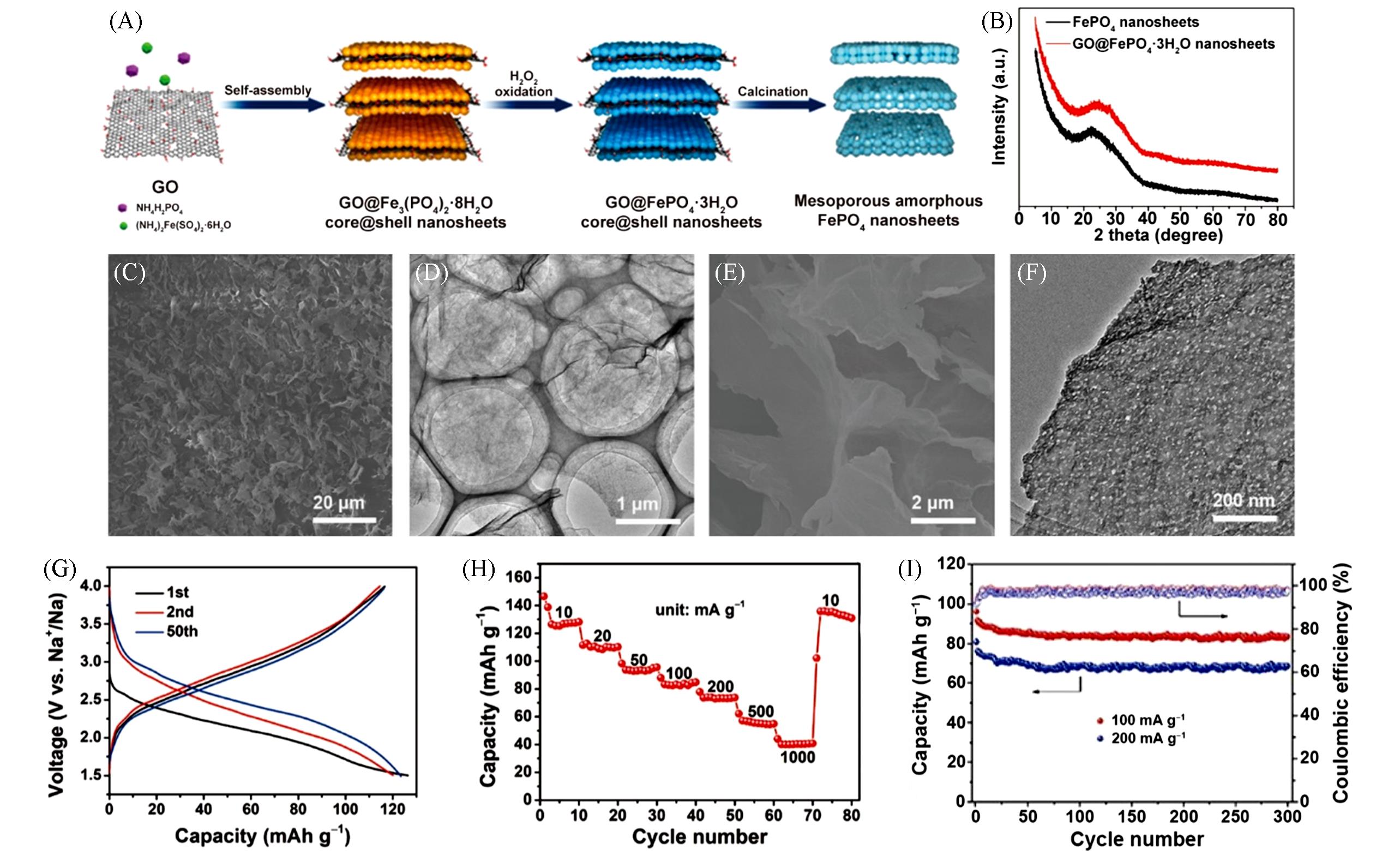
Fig.8 Schematic representation of the fabrication process for the mesoporous amorphous FePO4 nanosheets(A), XRD patterns of the GO@FePO4·3H2O nanosheets and the amorphous FePO4 nanosheets(B), SEM images(C, E), TEM images(D, F) of the GO@FePO4·3H2O nanosheets(C, D) and the amorphous FePO4 nanosheets(E, F), respectively, galvanostatic charge and discharge profiles conducted at 20 mA/g(G), rate performance(H) and corresponding Coulombic efficiency(I) of the amorphous FePO4 nanosheets as a cathode material in SIBs[50]Copyright 2018, American Chemical Society.
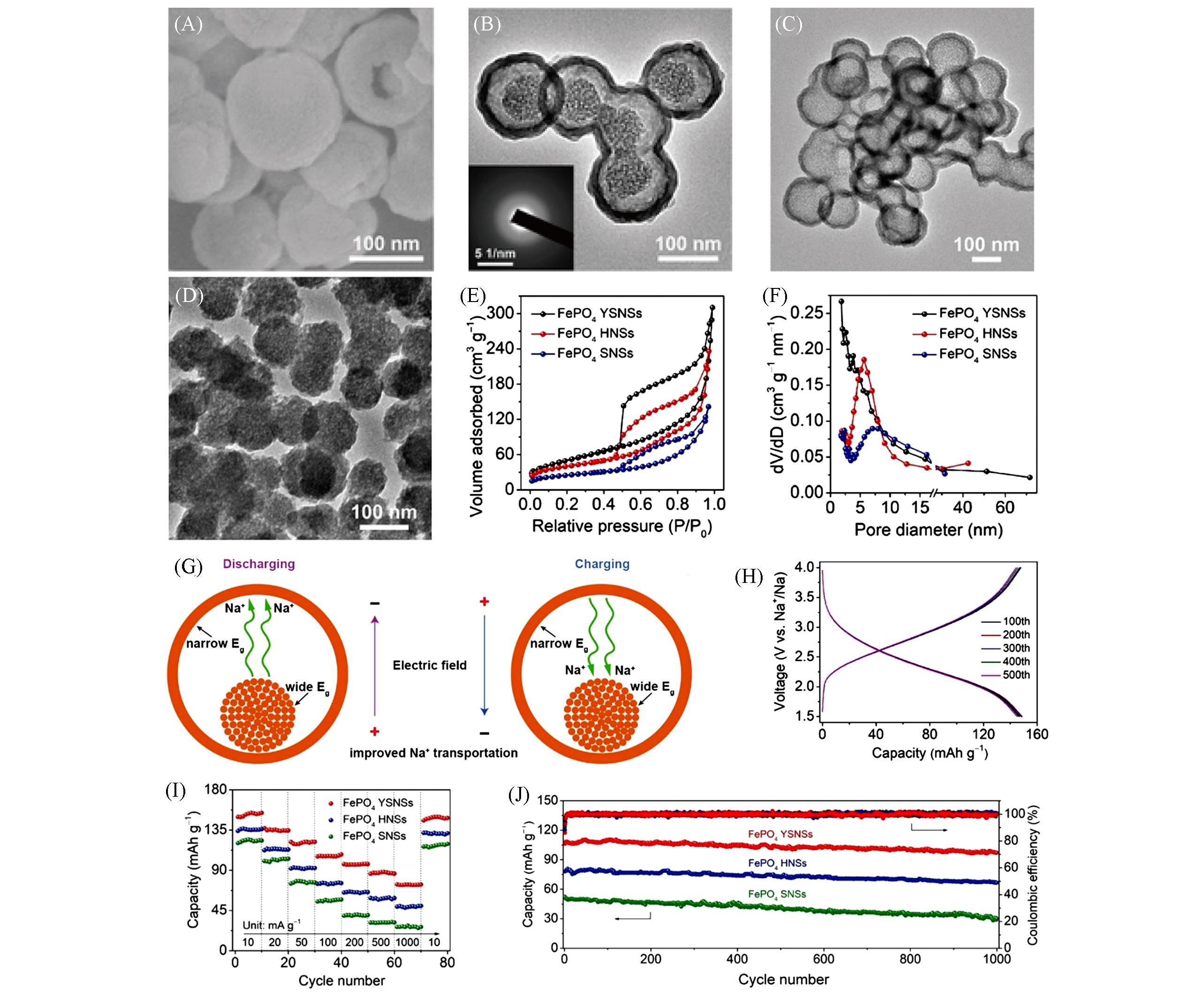
Fig.9 SEM(A) and TEM(B) images of FePO4 YSNSs, TEM images of FePO4 HNSs(C) and FePO4 SNSs(D), N2 adsorption⁃desorption isotherms(E) and the relevant pore size distribution(F) of FePO4 YSNSs, FePO4 HNSs, and FePO4 SNSs, schematic diagram of the built⁃in electric field formed in FePO4 YSNSs(G), discharge/charge profiles from the chosen cycles of 100th to 500th of FePO4 YSNSs(H), rate properties(I) and long⁃term cyclic performance at 100 mA/g(J) of FePO4 YSNSs, FePO4 HNSs and FePO4 SNSs[57]Copyright 2020, John Wiley & Sons, Inc.
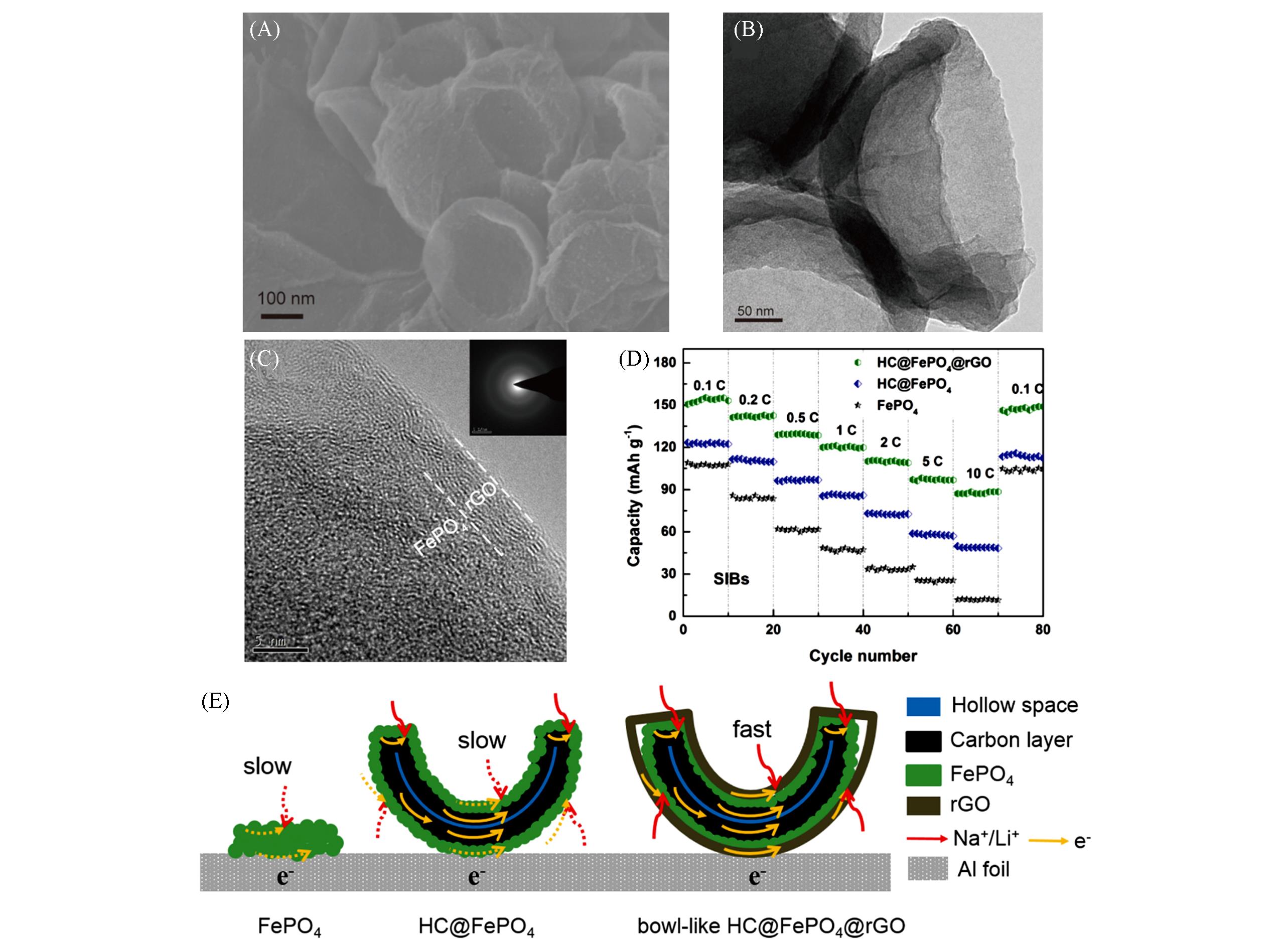
Fig.10 SEM image(A) and TEM images(B, C)(insert: corresponding SAED image) of bowl⁃like HC@FePO4@rGO, rates capabilities of bowl⁃like HC@FePO4@rGO, HC@FePO4, FePO4(D), a schematic model of the transport pathways of ion and electron in FePO4, HC@FePO4 and bowl⁃like HC@FePO4@rGO during electrochemical reaction process(E)[61]Copyright 2019, Elsevier.
| 1 | Thackeray M. M., Wolverton C., Isaacs E. D., Energy Environ. Sci., 2012, 5(7), 7854—7863 |
| 2 | Zhao J. L., Yang M., Yang N. L., Wang J. Y., Wang D., Chem. Res. Chinese Universities, 2020, 36(3), 313—319 |
| 3 | Manthiram A., Nat. Commun., 2020, 11(1), 1550 |
| 4 | Wang H. F., Wang X. X., Li M. L., Zheng L. J., Guan D. H., Huang X. L., Xu J. J., Yu J. H., Adv. Mater., 2020, 32(44), 2002559 |
| 5 | Xiao J., Li Q. Y., Bi Y. J., Cai M., Dunn B., Glossmann T., Liu J., Osaka T., Sugiura R., Wu B. B., Yang J. H., Zhang J. G., Whittingham M. S., Nat. Energy, 2020, 5(8), 561—568 |
| 6 | Tian Y., Zeng G. B., Rutt A., Shi T., Kim H., Wang J. Y., Koettgen J., Sun Y. Z., Ouyang B., Chen T. N., Lun Z., Rong Z. Q., Persson K., Ceder G., Chem. Rev., 2021, 121(3), 1623—1669 |
| 7 | Palomares V., Serras P., Villaluenga I., Hueso K. B., Carretero-González J., Rojo T., Energy Environ. Sci., 2012, 5(3), 5884—5901 |
| 8 | Nayak P. K., Yang L., Brehm W., Adelhelm P., Angew. Chem. Int. Ed., 2018, 57(1), 102—120 |
| 9 | Deng J. Q., Luo W. B., Chou S. L., Liu H. K., Dou S. X., Adv. Energy Mater., 2018, 8(4), 1701428 |
| 10 | Zhao C. L., Liu L. L., Qi X. G., Lu Y. X., Wu F. X., Zhao J. M., Yu Y., Hu Y. S., Chen L. Q., Adv. Energy Mater., 2018, 8(17), 1703012 |
| 11 | Ong S. P., Chevrier V. L., Hautier G., Jain A., Moore C., Kim S., Ma X. H., Ceder G., Energ. Environ. Sci., 2011, 4(9), 3680—3688 |
| 12 | Slater M. D., Kim D., Lee E., Johnson C. S., Adv. Funct. Mater., 2013, 23(8), 947—958 |
| 13 | Park Y. U., Seo D. H., Kwon H. S., Kim B., Kim J., Kim H., Kim I., Yoo H. I., Kang K., J. Am. Chem. Soc., 2013, 135(37), 13870—13878 |
| 14 | Gong D. C., Wang B., Zhu J. Y., Podila R., Rao A. M., Yu X. Z., Xu Z., Lu B. A., Adv. Energy Mater., 2017, 7(3), 1601885 |
| 15 | Fan L., Liu Q., Chen S. H., Xu Z., Lu B. A., Adv. Energy Mater., 2017, 7(14), 1602778 |
| 16 | Moreau P., Guyomard D., Gaubicher J., Boucher F., Chem. Mater., 2010, 22(14), 4126—4128 |
| 17 | Mathew V., Kim S., Kang J. W., Gim J., Song J. J., Baboo J. P., Park W., Ahn D., Han J., Gu L., Wang Y. S., Hu Y. S., Sun Y., Kim J., NPG Asia Mater., 2014, 6(10), e138 |
| 18 | Zhu Y. J., Xu Y. H., Liu Y. H., Luo C., Wang C. S., Nanoscale, 2013, 5(2), 780—787 |
| 19 | Oh S. M., Myung S. T., Hassoun J., Scrosati B., Sun Y. K., Electrochem. Commun., 2012, 22, 149—152 |
| 20 | Zaghib K., Trottier J., Hovington P., Brochu F., Guerfi A., Mauger A., Julien C. M., J. Power Sources, 2011, 196(22), 9612—9617 |
| 21 | Wang J. J., Sun X. L., Energ. Environ. Sci., 2015, 8(4), 1110—1138 |
| 22 | Hwang J., Matsumoto K., Orikasa Y., Katayama M., Inada Y., Nohira T., Hagiwara R., J. Power Sources, 2018, 377, 80—86 |
| 23 | Kim J., Seo D. H., Kim H., Park I., Yoo J. K., Jung S. K., Park Y. U., Goddard III W. A., Kang K., Energ. Environ. Sci., 2015, 8(2), 540—545 |
| 24 | Nakayama M., Yamada S., Jalem R., Kasuga T., Solid State Ionics, 2016, 286, 40—44 |
| 25 | Jiang D. P., Zhang X. J., Zhao T., Liu B. X., Yang R., Zhang H. K., Fan T. F., Wang F., Bull. Mater. Sci., 2020, 43(1), 50 |
| 26 | Yin Y. J., Wu P., Zhang H., Cai C. X., Electrochem. Commun., 2012, 18, 1—3 |
| 27 | Wang Y. S., Yang S. Z., You Y., Feng Z. M., Zhu W., Gariépy V., Xia J. X., Commarieu B., Darwiche A., Guerfi A., Zaghib K., ACS Appl. Mater. Interfaces, 2018, 10(8), 7061—7068 |
| 28 | Fang Y. J., Xiao L. F., Qian J. F., Ai X. P., Yang H. X., Cao Y. L., Nano Lett., 2014, 14(6), 3539—3543 |
| 29 | Hong S. Y., Kim Y., Park Y., Choi A., Choi N. S., Lee K. T., Energ. Environ. Sci., 2013, 6(7), 2067—2081 |
| 30 | Baggetto L., Ganesh P., Meisner R. P., Unocic R. R., Jumas J. C., Bridges C. A., Veith G. M., J. Power Sources, 2013, 234, 48—59 |
| 31 | Ellis B. L., Makahnouk W. R. M., Makimura Y., Toghill K., Nazar L. F., Nature Mater., 2007, 6(10), 749—753 |
| 32 | Hwang T. H., Jung D. S., Kim J. S., Kim B. G., Choi J. W., Nano Lett., 2013, 13(9), 4532—4538 |
| 33 | Liu Y. D., Goebl J., Yin Y. D., Chem. Soc. Rev., 2013, 42(7), 2610—2653 |
| 34 | Moradi M., Li Z., Qi J. F., Xing W., Xiang K., Chiang Y. M., Belcher A. M., Nano Lett., 2015, 15(5), 2917—2921 |
| 35 | Duan S. Y., Piao J. Y., Zhang T. Q., Sun Y. G., Liu X. C., Cao A. M., Wan L. J., NPG Asia Mater., 2017, 9(7), e414 |
| 36 | Zhang L. G., Yu L. T., Li O. L., Choi S. Y., Saeed G., Lee D., Kim K. H., ACS Appl. Energy Mater., 2022, 5(5), 5954—5963 |
| 37 | Cai R., Du Y. P., Zhang W. Y., Tan H. T., Zeng T., Huang X., Yang H. F., Chen C. P., Liu H., Zhu J. X., Peng S. J., Chen J., Zhao Y. L., Wu H. C., Huang Y. Z., Xu R., Lim T. M., Zhang Q. C., Zhang H., Yan Q. Y., Chemistry, 2013, 19(5), 1568—1572 |
| 38 | Zhao J. M., Jian Z. L., Ma J., Wang F. C., Hu Y. S., Chen W., Chen L. Q., Liu H. Z., Dai S., ChemSusChem, 2012, 5(8), 1495—1500 |
| 39 | Ren X. L., Turcheniuk K., Lewis D., Fu W. B., Magasinski A., Schauer M. W., Yushin G., Small, 2018, 14(43), 1703425 |
| 40 | Ren X. L., Turcheniuk K., Lewis D., Fu W. B., Magasinski A., Schauer M. W., Yushin G., Adv. Mater. Interfaces, 2016, 3(21), 1600468 |
| 41 | Wang Z. Y., Lu Y. C., ACS Appl. Mater. Interfaces, 2019, 11(14), 13225—13233 |
| 42 | Guo L., Huang Y. X., Ding M., Leong Z. Y., Vafakhah S., Yang H. Y., J. Mater. Chem. A, 2018, 6(19), 8901—8908 |
| 43 | Liu T. C., Duan Y. D., Zhang G. X., Li M. F., Feng Y. C., Hu J. T., Zheng J. X., Chen J., Pan F., J. Mater. Chem. A, 2016, 4(12), 4479—4484 |
| 44 | Berger C., Song Z., Li X., Wu X, Brown N., Naud C., Mayou D., Li T., Hass J., Marchenkov A. N., Conrad E. H., First P. N., de Heer W. A., Science, 2006, 312(5777), 1191—1196 |
| 45 | Yang G. L., Ding B., Wang J., Nie P., Dou H., Zhang X. G., Nanoscale, 2016, 8(16), 8495—8499 |
| 46 | Zeng S. H., Xu Q. X., Jin H. J., Zeng L. X., Wang Y. Y., Lai W. B., Yao Q., Zhang J. X., Chen Q. H., Qian Q. R., J. Electroanal. Chem., 2022, 913, 116287 |
| 47 | Liu Y., Xu S. J., Zhang S. M., Zhang J. X., Fan J. C., Zhou Y. R., J. Mater. Chem. A, 2015, 3(10), 5501—5508 |
| 48 | Liu Y. L., Xu Y., Han X. G., Pellegrinelli C., Zhu Y. J., Zhu H. J., Wan J. Y., Chung A. C., Vaaland O., Wang C. S., Hu Li. B., Nano Lett., 2012, 12(11), 5664—5668 |
| 49 | Xu S. J., Zhang S. M., Zhang J. X., Tan T., Liu Y., J. Mater. Chem. A, 2014, 2(20), 7221—7228 |
| 50 | Zhang Z. Z., Han Y., Xu J. M., Ma J. H., Zhou X. S., Bao J. C., ACS Appl. Energy Mater., 2018, 1(8), 4395—4402 |
| 51 | Hummers W. S., Offeman R. E., J. Am. Chem. Soc., 1958, 80(6), 1339—1339 |
| 52 | Wang Y. X., Yang J. P., Chou S. L., Liu H. K., Zhang W. X., Zhao D., Dou S. X., Nat. Commun., 2015, 6(1), 8689 |
| 53 | Ge P., Hou H. S., Li S. J., Yang L., Ji X. B., Adv. Funct. Mater., 2018, 28(30), 1801765 |
| 54 | Lin H. Z., Li M. L., Yang X., Yu D. X., Zeng Y., Wang C. Z., Chen G., Du F., Adv. Energy Mater., 2019, 9(20), 1900323 |
| 55 | Wang J. J., Luo C., Mao J. F., Zhu Y. J., Fan X. L., Gao T., Mignerey A. C., Wang C. S., ACS Appl. Mater. Interfaces, 2015, 7(21), 11476—11481 |
| 56 | Liu Y. H., Yu X. Y., Fang Y. J., Zhu X. S., Bao J. C., Zhou X. S., Lou X. W., Joule, 2018, 2(4), 725—735 |
| 57 | Zhang Z. Z., Du Y. C., Wang Q. C., Xu J. Y., Zhou Y. N., Bao J. C., Shen J., Zhou X. S., Angew. Chem. Int. Ed., 2020, 59(40), 17504—17510 |
| 58 | Zhou Y. L., Zhang M., Wang Q., Yang J., Luo X. Y., Li Y. L., Du R., Yan X. S., Sun X. Q., Dong C. F., Zhang X. Y., Jiang F. Y., Nano Res., 2020, 13(3), 691—700 |
| 59 | Sun Y. G., Piao J. Y., Hu L. L., Bin D. S., Lin X. J., Duan S. Y., Cao A. M., Wan L. J., J. Am. Chem. Soc., 2018, 140(29), 9070—9073 |
| 60 | Zheng Y., Zhou T. F., Zhang C. F., Mao J. F., Liu H. K., Guo Z. P., Angew. Chem. Int. Ed., 2016, 55(10), 3408—3413 |
| 61 | Zhang Y. Y., Huang C., Min H., Shu H. B., Gao P., Liang Q. Q., Yang X. K., Liu L., Wang X. Y., J. Alloys Compd., 2019, 795, 34—44 |
| [1] | 杨翠云, 杨成浩. 钠离子电池硬炭负极材料的研究进展[J]. 高等学校化学学报, 2023, 44(5): 152. |
| [2] | 任思远, 郭玮, 付永柱. 有机硫化物在可充电电池中的研究进展[J]. 高等学校化学学报, 2023, 44(5): 168. |
| [3] | 薛转, 穆钟林, 何润合, 李永兵, 张兴祥. 不同氨基含量碳纳米管对锂硫电池正极比容量的影响[J]. 高等学校化学学报, 2023, 44(4): 20220709. |
| [4] | 赵霄朗, 杨梅, 王江艳, 王丹. 富锂正极材料结构设计和表面调控的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220263. |
| [5] | 张玲玲, 董欢欢, 何祥喜, 李丽, 李林, 吴星樵, 侴术雷. 中空碳材料用于钠离子电池负极的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220620. |
| [6] | 张诗昱, 何润合, 李永兵, 魏士俊, 张兴祥. 辐照交联制备低分子量聚丙烯腈纤维锂硫电池正极材料及其储硫机理[J]. 高等学校化学学报, 2022, 43(3): 20210632. |
| [7] | 李晓辉, 魏爱佳, 穆金萍, 何蕊, 张利辉, 王军, 刘振法. 磷酸钐包覆对高电压镍锰酸锂正极材料电化学性能的影响[J]. 高等学校化学学报, 2022, 43(2): 20210546. |
| [8] | 卢美如, 张宏宇, 石百媚, 孙茂忠, 徐丽广, 胥传来, 匡华. 手性纳米材料: 生物成像、 生物传感与治疗[J]. 高等学校化学学报, 2022, 43(12): 20220683. |
| [9] | 鲍俊全, 郑仕兵, 苑旭明, 史金强, 孙田将, 梁静. 有机盐PTO(KPD)2作为高性能锂离子电池正极材料的研究[J]. 高等学校化学学报, 2021, 42(9): 2911. |
| [10] | 孙浩, 宫杰, 杨燕, 王新庆, 陈慧东. 三维有序In2O3纳米线阵列的合成及纳米结构有序度对气敏性能的影响[J]. 高等学校化学学报, 2021, 42(6): 1730. |
| [11] | 樊小勇, 毋妍, 孙瑞波, 苟蕾, 李东林. 三维多孔MnOx@In2O3立方盒子的构筑及储锌性能[J]. 高等学校化学学报, 2021, 42(6): 1816. |
| [12] | 刘冬生. 超分子作用构筑具有高光学不对称性的表面等离子纳米粒子手性组装体[J]. 高等学校化学学报, 2021, 42(6): 1619. |
| [13] | 王弈艨, 刘凯, 王保国. 高镍三元正极材料的表面包覆策略[J]. 高等学校化学学报, 2021, 42(5): 1514. |
| [14] | 高娟, 孙全虎, 黄长水. 石墨炔纳米材料的制备及在电化学能源中的应用[J]. 高等学校化学学报, 2021, 42(5): 1501. |
| [15] | 窦树珍, 王中舜, 吕男. 硅纳米结构对表面辅助激光解吸/电离质谱检测性能的提高[J]. 高等学校化学学报, 2021, 42(4): 1156. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||