

高等学校化学学报 ›› 2023, Vol. 44 ›› Issue (5): 20220722.doi: 10.7503/cjcu20220722
收稿日期:2022-11-18
出版日期:2023-05-10
发布日期:2023-01-08
通讯作者:
杜石谦,陶李
E-mail:dushiqiian@hnu.edu.cn;taoli@hnu.edu.cn
基金资助:
WANG Jun1,2, DU Shiqian2( ), TAO Li1,2(
), TAO Li1,2( )
)
Received:2022-11-18
Online:2023-05-10
Published:2023-01-08
Contact:
DU Shiqian, TAO Li
E-mail:dushiqiian@hnu.edu.cn;taoli@hnu.edu.cn
Supported by:摘要:
高温聚合物电解质膜燃料电池(HT-PEMFCs)是一类将化学能转换为电能的能量转换装置, 与传统的低温聚合物膜燃料电池相比具有诸多优势. 目前HT-PEMFCs主要是以铂作为催化剂. 铂基催化剂对于燃料电池氧还原反应(ORR)和氢氧化反应(HOR)有好的催化活性, 但在HT-PEMFCs中通常需要高载量的铂基催化剂, 以缓解磷酸在铂表面强吸附对活性表达的限制, 其存在成本高、 活性不足、 长时间运行下活性降低及载体腐蚀等问题. 本文总结了最近关于HT-PEMFCs催化剂的研究进展, 系统分析了贵金属、 非贵金属催化剂在HT-PEMFCs中的应用前景, 并对现阶段HT-PEMFCs催化剂的发展应用进行了展望.
中图分类号:
TrendMD:
王军, 杜石谦, 陶李. 高温聚合物电解质膜燃料电池催化剂的研究进展. 高等学校化学学报, 2023, 44(5): 20220722.
WANG Jun, DU Shiqian, TAO Li. Recent Progress of Catalysts in the High Temperature Polymer Electrolyte Membrane Fuel Cells. Chem. J. Chinese Universities, 2023, 44(5): 20220722.
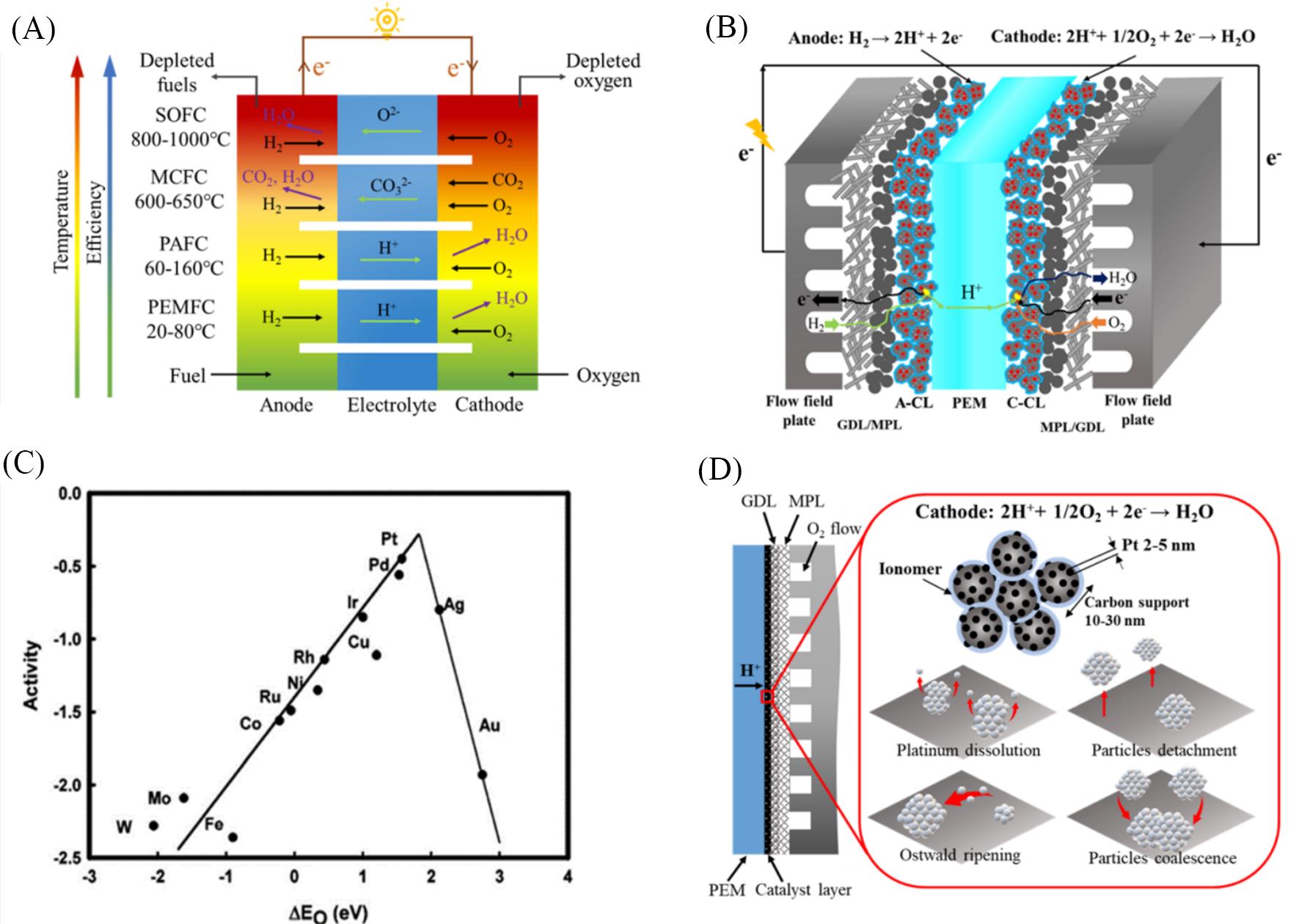
Fig.1 Structures of fuel cells and catalysts for ORR(A) Different types of fuel cells[11]; (B) a schematic of the basic structure and components for PEMFCs; (C) trends in ORR activity plotted as a function of the oxygen binding energy[20]; (D) illustration graph of Pt/C catalyst in the cathode catalyst layer and its degradation mechanism. (A) Copyright 2017, Oxford University Press; (C) Copyright 2004, America Chemistry Society.
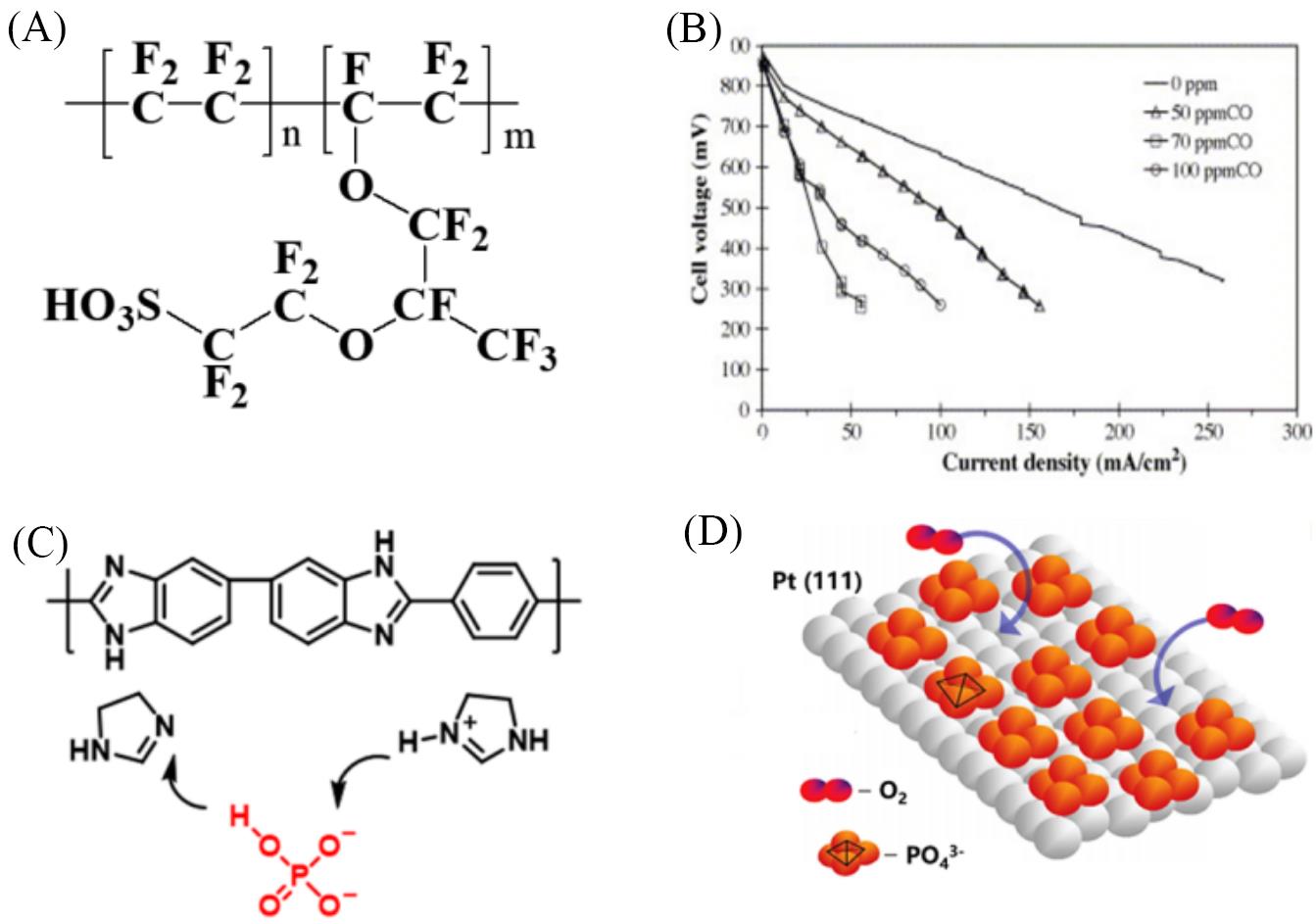
Fig.2 Structure of PEM and the negative effect of CO/phosphoric acid on Pt catalysts(A) Structure of the Nafion membrane; (B) the polarization curves after 6 h CO poisoning at different concentrations[37]; (C) the structure of PBI and mechanism of polymer transfer; (D) the absorption of phosphoric acid on the Pt surface. (B) Copyright 2005, Elsevier.
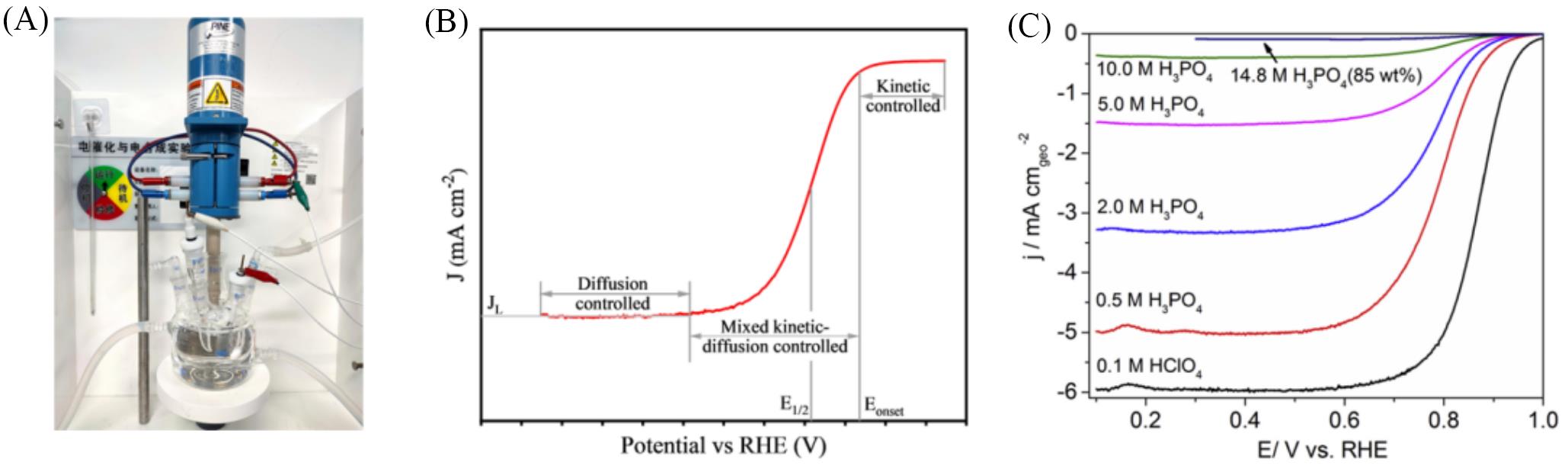
Fig.3 ORR test system and polarization curve(A) RDE test system; (B) typical ORR polarization curve; (C) ORR polarization curves of 20%(mass fraction) Pt/C by RDE testing at room temperature in 0.1 mol/L HClO4 and varying concentrations of PA[46].(C) Copyright 2018, Elsevier.
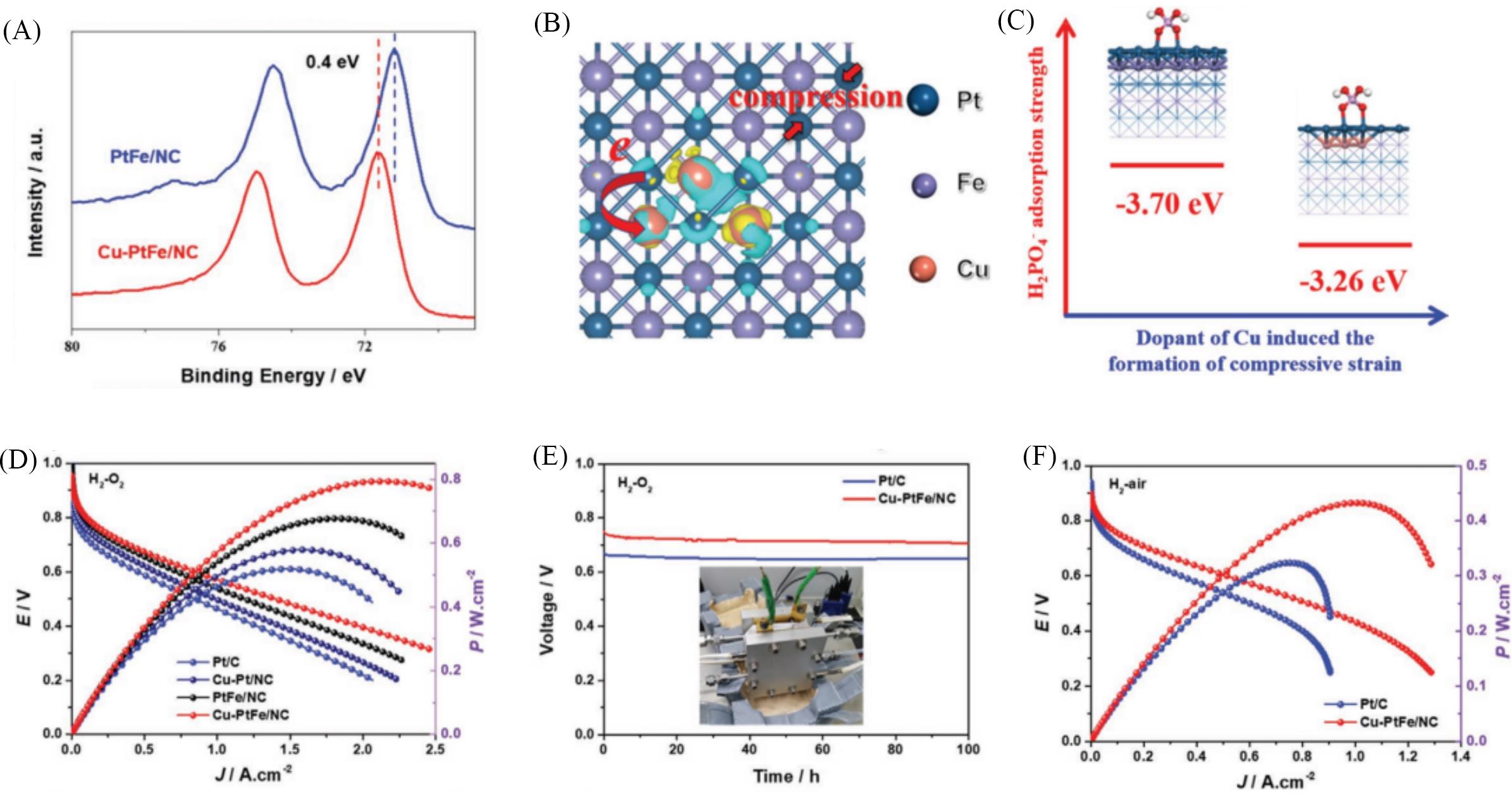
Fig.4 HT⁃PEMFCs performance and mechanism of Cu⁃PtFe/NC catalyst[64](A) High-resolution Pt4f spectra of PtFe/NC and Cu-PtFe/NC; (B) the charge density difference of the Cu-PtFe crystals; the yellow and green electron clouds correspond to an accumulation and depletion of electrons, respectively; (C) geometric structures and PA adsorption strength of H2PO4- adsorption on Cu-PtFe and PtFe; (D) H2-O2 HT-PEMFC polarization curves and corresponding power densities of Pt/C, Cu-Pt/NC, PtFe/NC, and Cu-PtFe/NC at 160 ℃; (E) durability test results of Pt/C and Cu-PtFe/NC under the current density of 0.2 A/cm2; (F) H2-air fuel cell of Pt/C and Cu-PtFe/NC.Copyright 2022, Wiley.
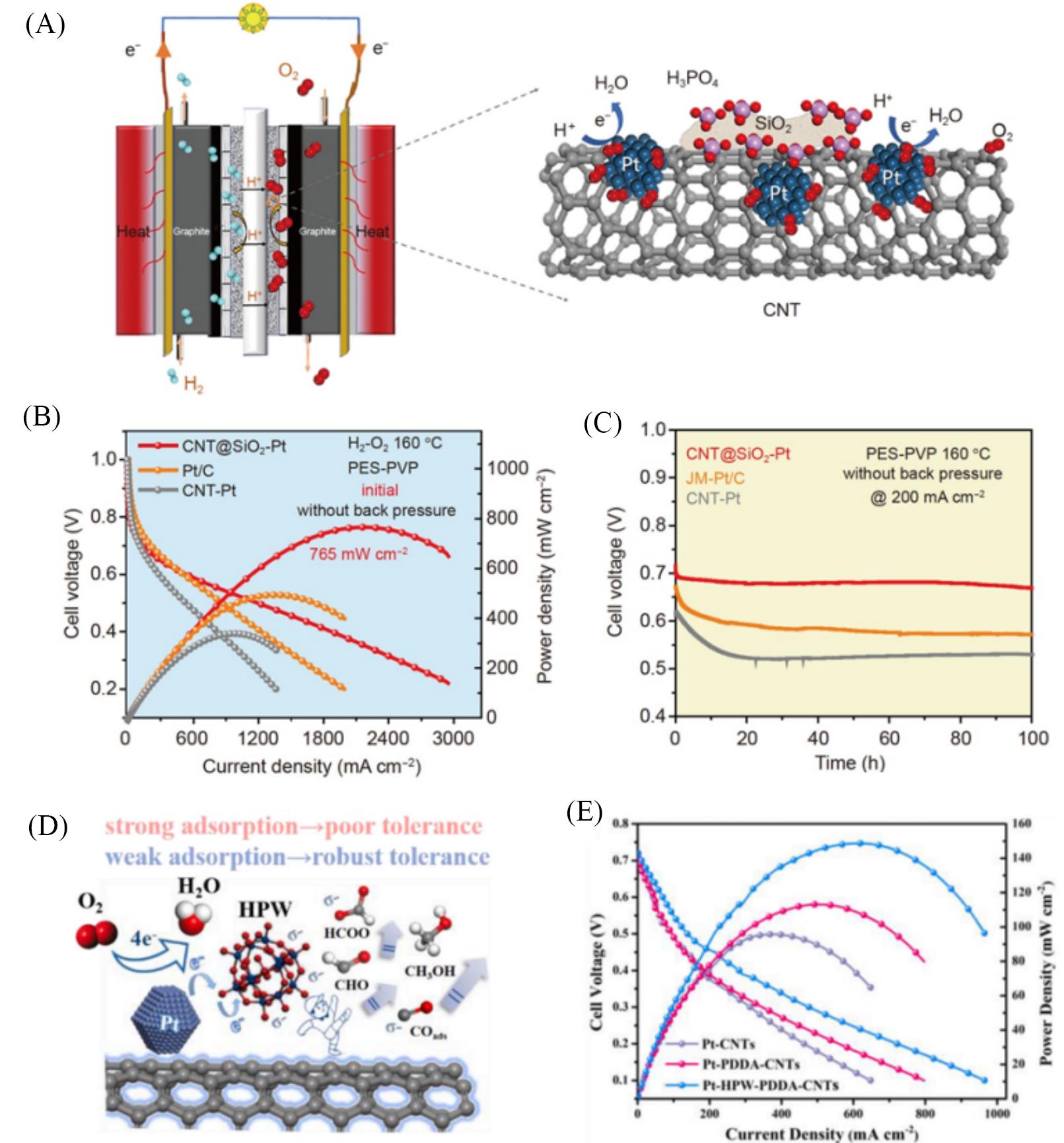
Fig.5 HT⁃PEMFCs performance of Pt⁃based catalysts with modified supports(A) Schematic representation for the HT-PEMFCs of the single cell and the CNT@SiO2-Pt in the catalytic layer of HT-PEMFCs; (B) the initial polarization and power density curves of Pt/C, CNT-Pt, CNT@SiO2-Pt at 160 ℃(H2/O2); (C) potential response of a 100 h HT-PEMFCs life test at a constant current density of 200 mA/cm2 at 160 ℃ in H2/O2[67]; (D) illustration graph for mechanism of methanol tolerance on Pt-HPW-PDDA-CNTs electrocatalyst; (E) polarization curves and powder densities for HT-DMFCs using as-designed catalysts cathodes at 240 ℃[68].(A—C) Copyright 2021, Springer; (D, E) Copyright 2022, Elsevier.
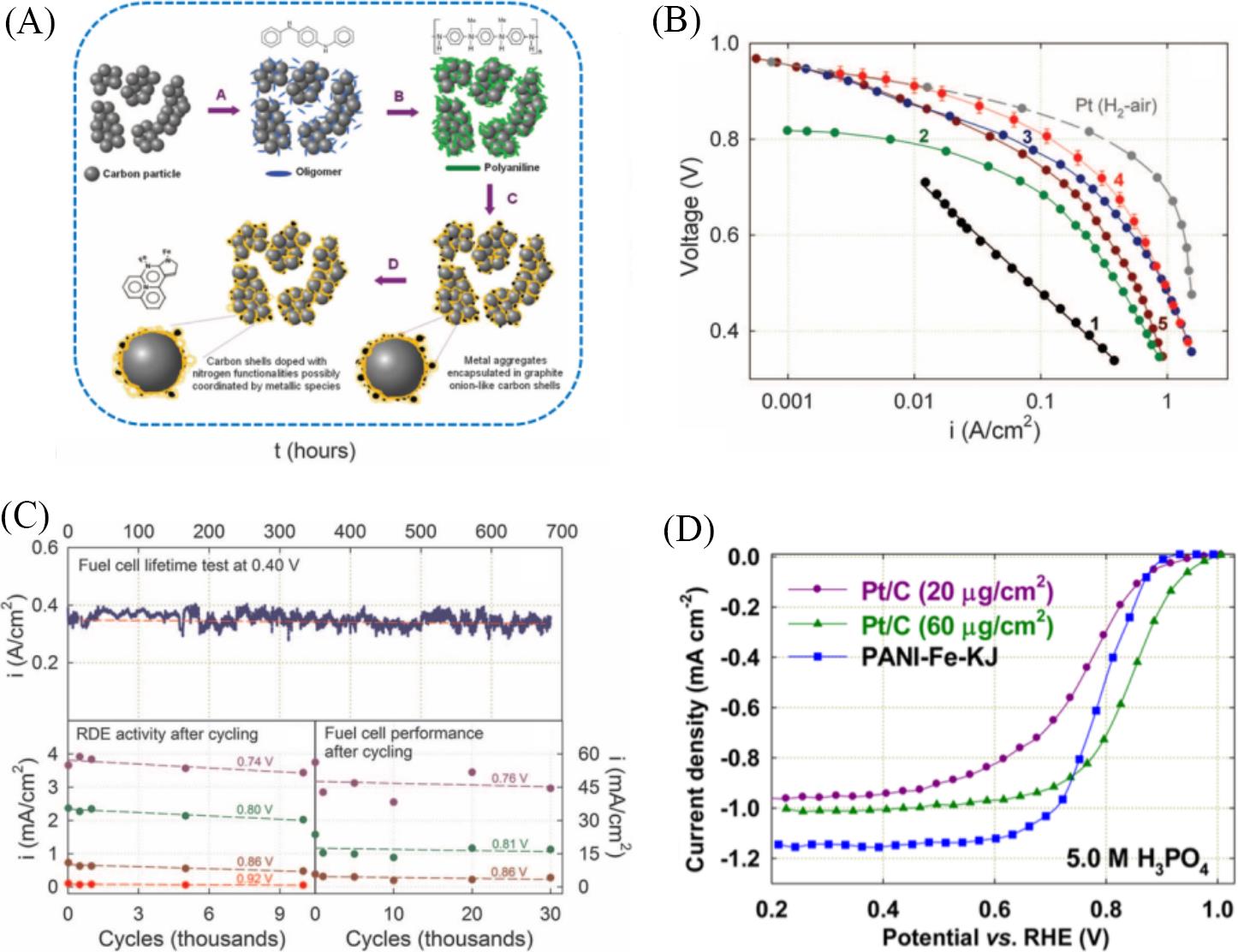
Fig.6 Performance of Fe⁃N⁃C catalysts as ORR catalysts(A) Schematic diagram of the synthesis of Fe-N-C catalysts; (B, C) fuel cell and performance durability test of the Fe-N-C catalysts[28]; (D) ORR activity comparison between Fe-N-C, 20 μgPt/cm2 and 60 μgPt/cm2 Pt/C catalysts in O2-saturated 5.0 mol/L H3PO4 electrolyte by steady-state polarization curves; rotation rate: 900 r/min; room temperature[82].(A—C) Copyright 2011, American Association for the Advancement of Science; (D) Copyright 2014, American Chemical Society.
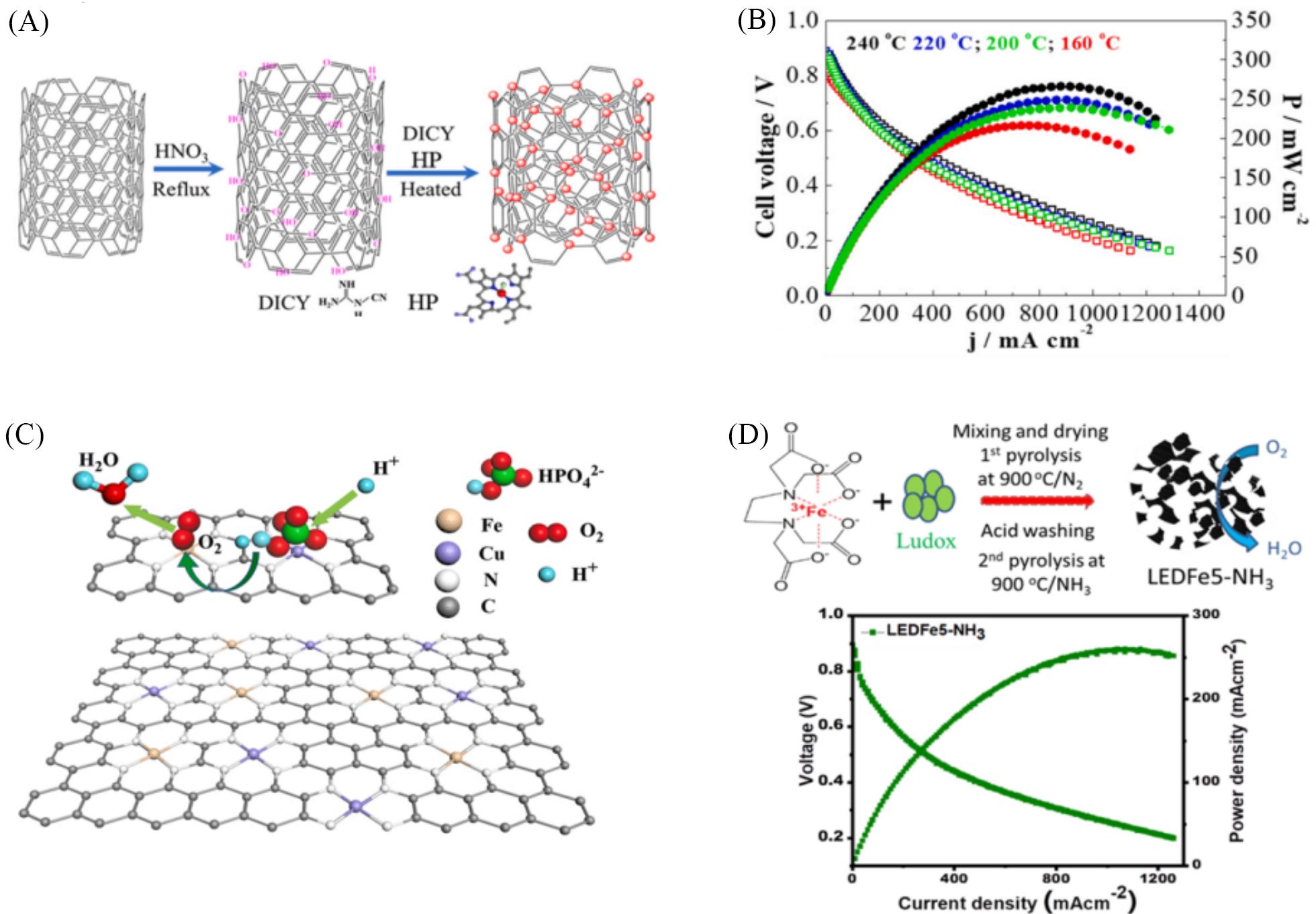
Fig.7 HT⁃PEMFCs performance of Fe⁃N⁃C catalysts(A) Scheme of the synthesis procedure of FeSA/HP; (B) I-V and power density curves of HT-PEMFCs with FeSA/HP cathode, measured at different temperatures[83]; (C) scheme of the phosphate promoted ORR, where PA adsorbed on Cu atoms provides local protons for ORR on the adjacent Fe atoms[84]; (D) schematic illustration of synthesis process for EDTA-Fe complex-based catalyst, and single-cell performance of HT-PEMFC with LEDFe5-NH3 in cathode measured at 1.5 bar(1 bar=1×105 Pa) O2 pressure and 150 ℃ cell temperature[87].(A, B) Copyright 2021, Elsevier; (C) Copyright 2021, Elsevier; (D) Copyright 2020, ACS.
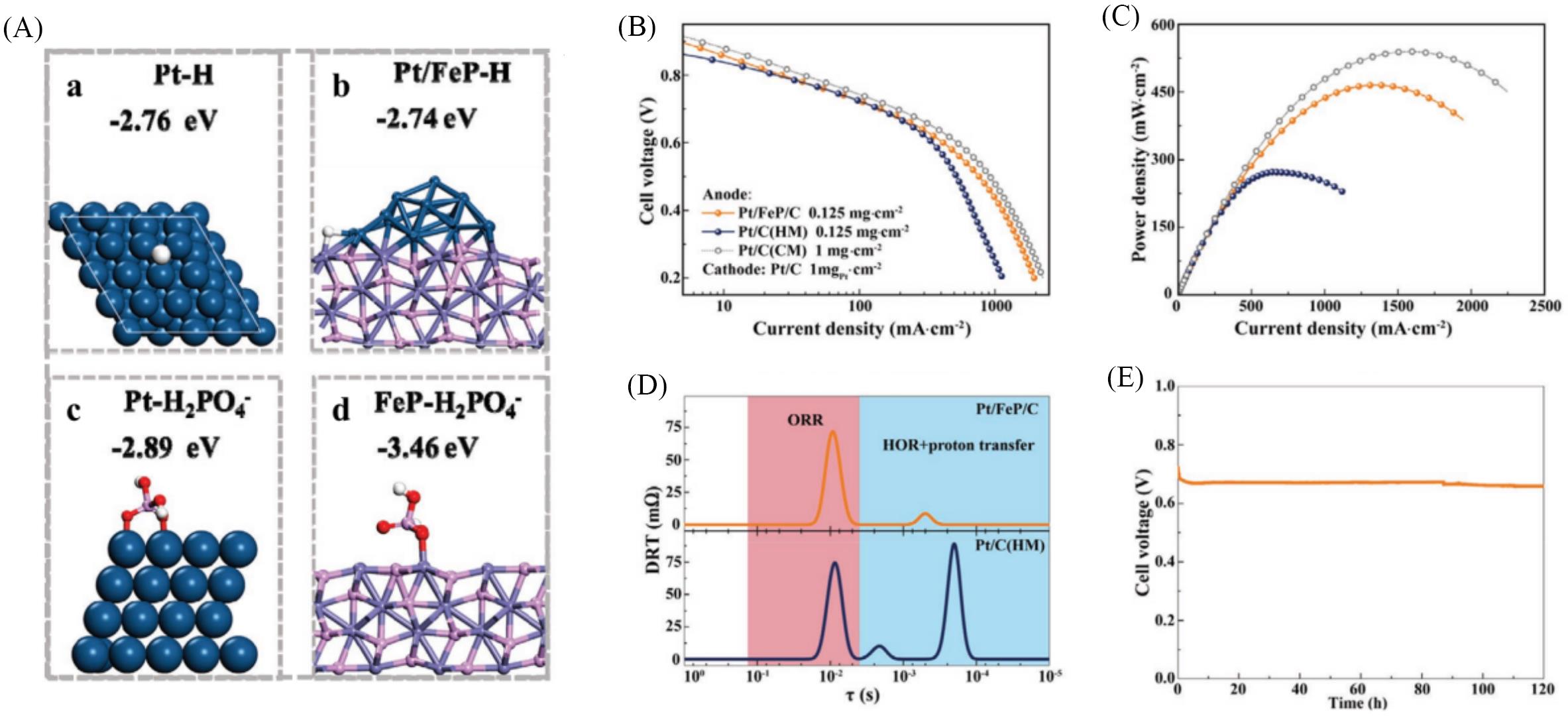
Fig.8 HT⁃PEMFCs performance using Pt/FeP/C as anode catalyst[95](A) The absorption energy of H species on Pt (111)(a) and Pt/FeP(b) models and the absorption energy of H2PO4- species on Pt(111)(c) and FeP (d) models; (B, C) fuel cell performance, I-V plots(B) and power density curves(C) of the HT-PEMFCs; (D) DRT analysis of the HT-PEMFCs with Pt/FeP/C and Pt/C(HM) anode; (E) stability test of Pt/FeP/C in HT-PEMFCs.Copyright 2022, Wiley.
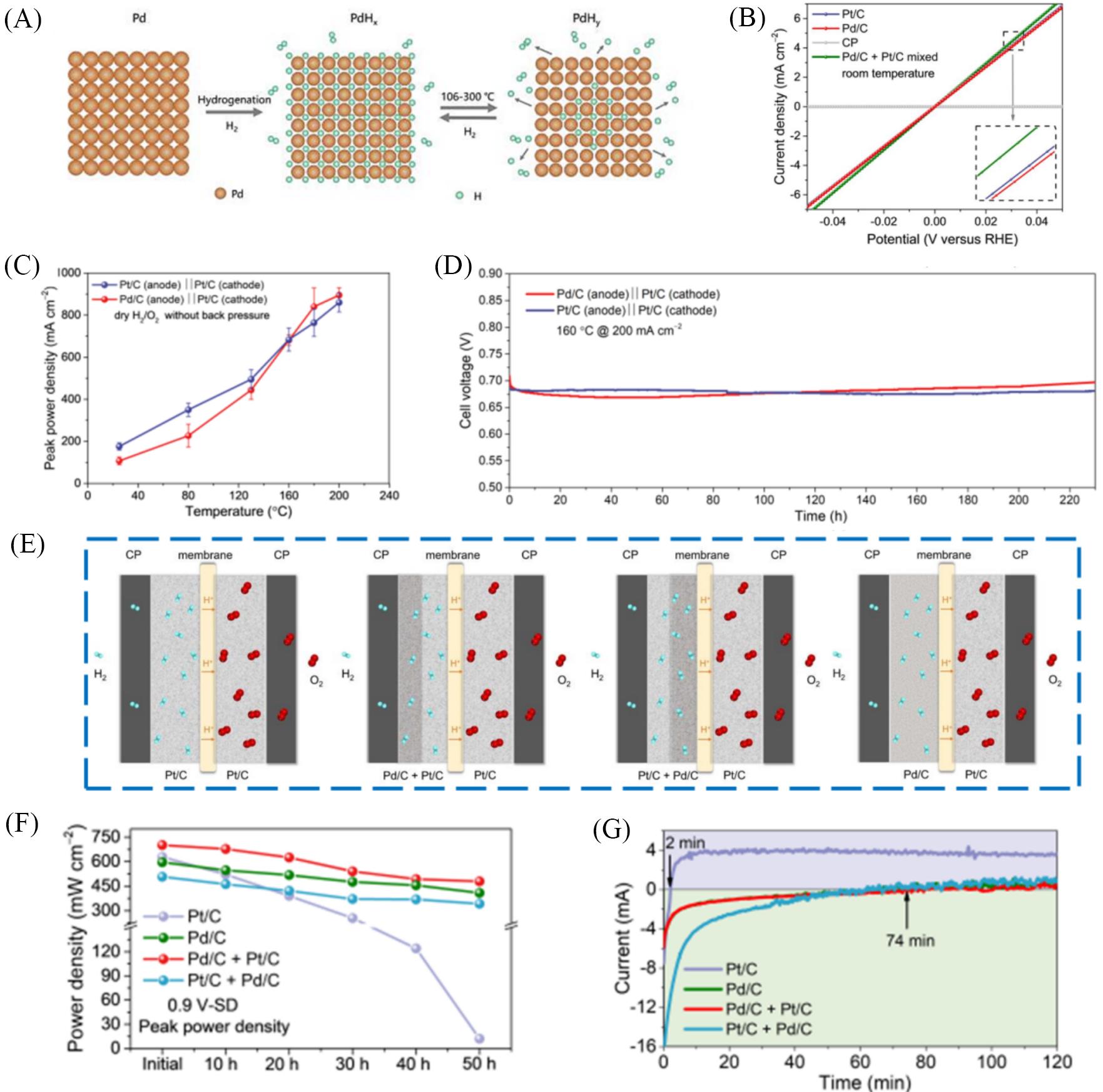
Fig.9 The application of Pd in HT⁃PEMFCs[96](A) Schematic diagram of intercalation and release of hydrogen in the Pd lattice interstitial sites with temperature changes; (B) GDE test for Pt/C, Pd/C, Pt/C+Pd/C mixed (the same total amount of precious metals: 1 mgPGM/cm2 ), and carbon paper (CP) in 0.1 mol/L HClO4 at 25 °C; (C) the plots of PPD change with increasing temperature of Pt/C and Pd/C individually for HT-PEMFCs; (D) voltage response of 230 h lifetime test for HT-PEMFCs at a constant current density of 200 mA/cm2; (E) schematic diagram of different anode catalytic layer structure designs; (F) The peak power density after the anode fuel starvation at 0.9 V; (G) the current change curve of HT-PEMFCs in the first 120 min after the shut down(SD) process of H2. Copyright 2023, Wiley.
| 1 | Huang M. T., Zhai P. M., Adv. Climate Change Res., 2021, 12, 281—286 |
| 2 | Midilli A., Dincer I., Ay M., Energy Policy, 2006, 34, 3623—3633 |
| 3 | Midilli A., Dincer I., Rosen M. A., Int. J. Green Energy, 2007, 4, 65—87 |
| 4 | Ramadan M., Int. J. Hydrogen Energy, 2021, 46, 30547—30558 |
| 5 | Holladay J. D., Hu J., King D. L., Wang Y., Catal. Today, 2009, 139, 244—260 |
| 6 | Debe M. K., Nature, 2012, 486, 43—51 |
| 7 | Jiao Y., Zheng Y., Jaroniec M., Qiao S. Z., Chem. Soc. Rev., 2015, 44, 2060—2086 |
| 8 | Staffell I., Scamman D., Abad A. V., Balcombe P., Dodds P. E., Ekins P., Shah N., Ward K. R., Energy Environ. Sci., 2019, 12, 463—491 |
| 9 | Grove W. R., Philos. Mag., 1838, 13, 430—431 |
| 10 | Grove W. R., Philos. Mag., 1842, 21, 417—420 |
| 11 | Wang S., Jiang S. P., Natl. Sci. Rev., 2017, 4, 163—166 |
| 12 | Borup R., Meyers J., Pivovar B., Kim Y. S., Mukundan R., Garland N., Myers D., Wilson M., Garzon F., Wood D., Zelenay P., More K., Stroh K., Zawodzinski T., Boncella J., McGrath J. E., Inaba M., Miyatake K., Hori M., Ota K., Ogumi Z., Miyata S., Nishikata A., Siroma Z., Uchimoto Y., Yasuda K., Kimijima K., Iwashita N., Chem. Rev., 2007, 107, 3904—3951 |
| 13 | Gasteiger H. A., Kocha S. S., Sompalli B., Wagner F. T., Appl. Catal. B: Environ., 2005, 56, 9—35 |
| 14 | Lefevre M., Proietti E., Jaouen F., Dodelet J. P., Science, 2009, 324, 71—74 |
| 15 | Li Q. F., He R. H., Jensen J. O., Bjerrum N. J., Chem. Mater., 2003, 15, 4896—4915 |
| 16 | Wang Y., Chen K. S., Mishler J., Cho S. C., Adroher X. C., Appl. Energ., 2011, 88, 981—1007 |
| 17 | Freyschlag C. G., Madix R. J., Mater. Today, 2011, 14, 134—142 |
| 18 | Jin R., Nanotechnol. Rev., 2012, 1, 31—56 |
| 19 | Quinson J., Adv. Colloid Interface Sci., 2022, 303, 102643 |
| 20 | Nørskov J. K., Rossmeisl J., Logadottir A., Lindqvist L., Kitchin J. R., Bligaard T., Jonsson H., J. Phys. Chem. B, 2004, 108, 17886—17892 |
| 21 | Stamenkovic V., Mun B. S., Mayrhofer K. J. J., Ross P. N., Markovic N. M., Rossmeisl J., Greeley J., Nørskov J. K., Angew. Chem. Int. Ed., 2006, 45, 2897—2901 |
| 22 | Bu L., Zhang N., Guo S., Zhang X., Li J., Yao J., Wu T., Lu G., Ma J. Y., Su D., Huang X., Science, 2016, 354, 1410—1414 |
| 23 | Wang X., Choi S. I., Roling L. T., Luo M., Ma C., Zhang L., Chi M., Liu J., Xie Z., Herron J. A., Mavrikakis M., Xia Y., Nat. Commun., 2015, 6, 7594 |
| 24 | Xia W., Mahmood A., Liang Z., Zou R., Guo S., Angew. Chem. Int. Ed., 2016, 55, 2650—2676 |
| 25 | Greeley J., Stephens I. E. L., Bondarenko A. S., Johansson T. P., Hansen H. A., Jaramillo T. F., Rossmeisl J., Chorkendorff I., Norskov J. K., Nat. Chem., 2009, 1, 552—556 |
| 26 | Stamenkovic V. R., Mun B. S., Arenz M., Mayrhofer K. J. J., Lucas C. A., Wang G., Ross P. N., Markovic N. M., Nat. Mater., 2007, 6, 241—247 |
| 27 | Chen Y., Ji S., Chen C., Peng Q., Wang D., Li Y., Joule, 2018, 2, 1242—1264 |
| 28 | Wu G., More K. L., Johnston C. M., Zelenay P., Science, 2011, 332, 443—447 |
| 29 | Cheng X., Yang J., Yan W., Han Y., Qu X., Yin S., Chen C., Ji R., Li Y., Li G., Li G., Jiang Y., Sun S., Energy Environ. Sci., 2021, 14, 5958—5967 |
| 30 | Han Y., Yin S., Chen Y., Chen C., Yan W., Cheng X., Li Y., Zhang T., Yang J., Jiang Y., Sun S., J. Electroanal. Chem., 2022, 914, 116322 |
| 31 | Wang F., Zhou Y., Lin S., Yang L., Hu Z., Xie D., Nano Energy, 2020, 78, 105128 |
| 32 | Yin S. H., Yang J., Han Y., Li G., Wan L. Y., Chen Y. H., Chen C., Qu X. M., Jiang Y. X., Sun S. G., Angew. Chem. Int. Ed., 2020, 59, 21976—21979 |
| 33 | Zhang P. Y., Yang X. H., Jiang Q. R., Cui P. X., Zhou Z. Y., Sun S. H., Wang Y. C., Sun S. G., ACS Appl. Mater. Interfaces, 2022, 14, 30724—30734 |
| 34 | Mauritz K. A., Moore R. B., Chem. Rev., 2004, 104, 4535—4585 |
| 35 | Ehteshami S. M. M., Chan S. H., Electrochim. Acta, 2013, 93, 334—345 |
| 36 | Hassan A., Paganin V. A., Ticianelli E. A., J. Power Sources, 2016, 325, 375—382 |
| 37 | Jiménez S., Soler J., Valenzuela R. X., Daza L., J. Power Sources, 2005, 151, 69—73 |
| 38 | Lee S. J., Mukerjee S., Ticianelli E. A., McBreen J., Electrochim. Acta, 1999, 44, 3283—3293 |
| 39 | Aili D., Henkensmeier D., Martin S., Singh B., Hu Y., Jensen J. O., Cleemann L. N., Li Q., Electrochem. Energy Rev., 2020, 3, 793—845 |
| 40 | Antonio Asensio J., Sanchez E. M., Gomez⁃Romero P., Chem. Soc. Rev., 2010, 39, 3210—3239 |
| 41 | Zhang J., Xie Z., Zhang J., Tang Y., Song C., Navessin T., Shi Z., Song D., Wang H., Wilkinson D. P., Liu Z. S., Holdcroft S., J. Power Sources, 2006, 160, 872—891 |
| 42 | Shao Y., Yin G., Wang Z., Gao Y., J. Power Sources, 2007, 167, 235—242 |
| 43 | Nie Y., Li L., Wei Z., Chem. Soc. Rev., 2015, 44, 2168—2201 |
| 44 | Haider R., Wen Y., Ma Z. F., Wilkinson D. P., Zhang L., Yuan X., Song S., Zhang J., Chem. Soc. Rev., 2021, 50, 1138—1187 |
| 45 | Araya S. S., Zhou F., Liso V., Sahlin S. L., Vang J. R., Thomas S., Gao X., Jeppesen C., Kær S. K., Int. J. Hydrogen Energy, 2016, 41, 21310—21344 |
| 46 | Hu Y., Jiang Y., Jensen J. O., Cleemann L. N., Li Q., J. Power Sources, 2018, 375, 77—81 |
| 47 | Stephens I. E. L., Bondarenko A. S., Gronbjerg U., Rossmeisl J., Chorkendorff I., Energy Environ. Sci., 2012, 5, 6744—6762 |
| 48 | Escudero E. M., Malacrida P., Hansen M. H., Vej⁃Hansen U. G., Velazquez P. A., Tripkovic V., Schiotz J., Rossmeisl J., Stephens I. E. L., Chorkendorff I., Science, 2016, 352, 73—76 |
| 49 | Li W., Zhao L., Jiang X., Chen Z., Zhang Y., Wang S., Adv. Funct. Mater., 2022, 32, 2207727 |
| 50 | Paulus U. A., Wokaun A., Scherer G. G., Schmidt T. J., Stamenkovic V., Markovic N. M., Ross P. N., Electrochim. Acta, 2002, 47, 3787—3798 |
| 51 | Paulus U. A., Wokaun A., Scherer G. G., Schmidt T. J., Stamenkovic V., Radmilovic V., Markovic N. M., Ross P. N., J. Phys. Chem. B, 2002, 106, 4181—4191 |
| 52 | Stamenkovic V., Schmidt T. J., Ross P. N., Markovic N. M., J. Phys. Chem. B, 2002, 106, 11970—11979 |
| 53 | Stonehart P., Ber. Bunsenges. Phys. Chem., 1990, 94, 913—921 |
| 54 | Lim B., Jiang M., Camargo P. H. C., Cho E. C., Tao J., Lu X., Zhu Y., Xia Y., Science, 2009, 324, 1302—1305 |
| 55 | Wang D., Yu Y., Xin H. L., Hovden R., Ercius P., Mundy J. A., Chen H., Richard J. H., Muller D. A., DiSalvo F. J., Abruna H. D., Nano Lett., 2012, 12, 5230—5238 |
| 56 | Wang D., Xin H. L., Hovden R., Wang H., Yu Y., Muller D. A., DiSalvo F. J., Abruna H. D., Nat. Mater., 2013, 12, 81—87 |
| 57 | Du X. X., He Y., Wang X. X., Wang J. N., Energy Environ. Sci., 2016, 9, 2623—2632 |
| 58 | Qin Y., Luo M., Sun Y., Li C., Huang B., Yang Y., Li Y., Wang L., Guo S., ACS Catal., 2018, 8, 5581—5590 |
| 59 | Liu M., Zhao Z., Duan X., Huang Y., Adv. Mater., 2019, 31, 1802234 |
| 60 | Choi S. I., Xie S., Shao M., Odell J. H., Lu N., Peng H. C., Protsailo L., Guerrero S., Park J., Xia X., Wang J., Kim M. J., Xia Y., Nano Lett., 2013, 13, 3420—3425 |
| 61 | Wu J., Gross A., Yang H., Nano Lett., 2011, 11, 798—802 |
| 62 | Li M., Zhao Z., Cheng T., Fortunelli A., Chen C. Y., Yu R., Zhang Q., Gu L., Merinov B. V., Lin Z., Zhu E., Yu T., Jia Q., Guo J., Zhang L., Goddard W. A., III, Huang Y., Duan X., Science, 2016, 354, 1414—1419 |
| 63 | Wang C., Daimon H., Onodera T., Koda T., Sun S., Angew. Chem. Int. Ed., 2008, 47, 3588—3591 |
| 64 | Li W., Wang D., Liu T., Tao L., Zhang Y., Huang Y. C., Du S., Dong C. L., Kong Z., Li Y. F., Lu S., Wang S., Adv. Funct. Mater., 2022, 32, 2109244 |
| 65 | Lim S. Y., Martin S., Gao G., Dou Y., Simonsen S. B., Jensen J. O., Li Q., Norrman K., Jing S., Zhang W., Adv. Funct. Mater., 2020, 31, 2006771 |
| 66 | Long P., Du S., Liu Q., Tao L., Peng C., Wang T., Gu K., Xie C., Zhang Y., Chen R., Lu S., Cheng Y., Feng W., Wang S., Sci. China Mater., 2022, 65, 904—912 |
| 67 | Huang G., Li Y., Du S., Wu Y., Chen R., Zhang J., Cheng Y., Lu S., Tao L., Wang S., Sci. China Chem., 2021, 64, 2203—2211 |
| 68 | Wu Y., Wang J., Huang G., Du S., Lin J., Zhou B., Lu Y., Wang D., Li M., Tao L., Wang S., J. Power Sources, 2022, 541, 231643 |
| 69 | Parrondo J., Mijangos F., Rambabu B., J. Power Sources, 2010, 195, 3977—3983 |
| 70 | Xiao L., Zhuang L., Liu Y., Lu J., Abruna H. D., J. Am. Chem. Soc., 2009, 131, 602—608 |
| 71 | Antolini E., Energy Environ. Sci., 2009, 2, 915—931 |
| 72 | Luo M., Zhao Z., Zhang Y., Sun Y., Xing Y., Lv F., Yang Y., Zhang X., Hwang S., Qin Y., Ma J. Y., Lin F., Su D., Lu G., Guo S., Nature, 2019, 574, 81—85 |
| 73 | Shao M. H., Sasaki K., Adzic R. R., J. Am. Chem. Soc., 2006, 128, 3526—3527 |
| 74 | Shao M. H., Huang T., Liu P., Zhang J., Sasaki K., Vukmirovic M. B., Adzic R. R., Langmuir, 2006, 22, 10409—10415 |
| 75 | You D. J., Pak C., Jin S. A., Lee K. H., Kwon K., Choi K. H., Heo P. W., Jang H., Kim J. Y., Kim J. M., J. Nanosci. Nanotechno., 2016, 16, 4357—4361 |
| 76 | Wu G., Santandreu A., Kellogg W., Gupta S., Ogoke O., Zhang H., Wang H. L., Dai L., Nano Energy, 2016, 29, 83—110 |
| 77 | Li J. C., Zhong H., Xu M., Li T., Wang L., Shi Q., Peng S., Lyu Z., Liu D., Du D., Beckman S. P., Pan X., Lin Y., Shao M., Sci. China Mater., 2020, 63, 965—971 |
| 78 | Wen X., Qi H., Cheng Y., Zhang Q., Hou C., Guan J., Chin. J. Chem., 2020, 38, 941—946 |
| 79 | Wang R., Zhang P., Wang Y., Wang Y., Zaghib K., Zhou Z., Prog. Nat. Sci.: Mater. Int., 2020, 30, 855—860 |
| 80 | Wang H. F., Chen L., Pang H., Kaskel S., Xu Q., Chem. Soc. Rev., 2020, 49, 1414—1448 |
| 81 | Chen Y., Gao R., Ji S., Li H., Tang K., Jiang P., Hu H., Zhang Z., Hao H., Qu Q., Liang X., Chen W., Dong J., Wang D., Li Y., Angew. Chem. Int. Ed., 2021, 60, 3212—3221 |
| 82 | Li Q., Wu G., Cullen D. A., More K. L., Mack N. H., Chung H. T., Zelenay P., ACS Catal., 2014, 4, 3193—3200 |
| 83 | Cheng Y., Zhang J., Wu X., Tang C., Yang S. Z., Su P., Thomsen L., Zhao F., Lu S., Liu J., Jiang S. P., Nano Energy, 2021, 80, 105534 |
| 84 | Cheng Y., Wang M., Lu S., Tang C., Wu X., Veder J. P., Johannessen B., Thomsen L., Zhang J., Yang S. Z., Wang S., Jiang S. P., Appl. Catal. B: Environ, 2021, 284, 119717 |
| 85 | Eren E. O., Ozkan N., Devrim Y., Int. J. Hydrogen Energy, 2020, 45, 33957—33967 |
| 86 | Gokhale R., Asset T., Qian G., Serov A., Artyushkova K., Benicewicz B. C., Atanassov P., Electrochem. Commun., 2018, 93, 91—94 |
| 87 | Razmjooei F., Yu J. H., Lee H. Y., Lee B. J., Singh K. P., Kang T. H., Kim H. J., Yu J. S., ACS Appl. Energy Mater., 2020, 3, 11164—11176 |
| 88 | Mittermeier T., Madkikar P., Wang X., Gasteiger H. A., Piana M., Materials, 2017, 10, 661 |
| 89 | Zhang W., Shironita S., Umeda M., Catal. Lett., 2014, 144, 112—116 |
| 90 | Das R. K., Wang Y., Vasilyeva S. V., Donoghue E., Pucher I., Kamenov G., Cheng H. P., Rinzler A. G., ACS Nano, 2014, 8, 8447—8456 |
| 91 | Park H. Y., Park I. S., Choi B., Lee K. S., Jeon T. Y., Sung Y. E., Yoo S. J., Phys. Chem. Chem. Phys., 2013, 15, 2125—2130 |
| 92 | Elezovic N. R., Babic B. M., Vracar L. M., Radmilovic V. R., Krstajic N. V., Phys. Chem. Chem. Phys., 2009, 11, 5192—5197 |
| 93 | Sheng W., Bivens A. P., Myint M., Zhuang Z., Forest R. V., Fang Q., Chen J. G., Yan Y., Energy Environ. Sci., 2014, 7, 1719—1724 |
| 94 | Davydova E. S., Mukerjee S., Jaouen F., Dekel D. R., ACS Catal., 2018, 8, 6665—6690 |
| 95 | Du S., Li Y., Wu X., Huang G., Wu Y., Zhang J., Zhang J., Lu S., Cheng Y., Tao L., Wang S., Adv. Funct. Mater., 2022, 32, 2106758 |
| 96 | Huang G., Li Y., Tao L., Huang Z., Kong Z., Xie C., Du S., Wang T., Wu Y., Liu Q., Zhang D., Lin J., Li M., Wang J., Zhang J., Lu S., Cheng Y., Wang S., Angew. Chem. Int. Ed., 2023, 62, e202215177 |
| 97 | Li Q., Hjuler H. A., Bjerrum N. J., J. Appl. Electrochem., 2001, 31, 773—779 |
| 98 | Oettel C., Rihko⁃Struckmann L., Sundmacher K., J. Fuel Cell Sci. Tech., 2012, 9, 031009 |
| [1] | 李轩, 亓帅, 周伟良, 李小杰, 景玲胭, 冯超, 蒋兴星, 杨恒攀, 胡琪, 何传新. 纤维基氧化还原电催化剂的研究进展[J]. 高等学校化学学报, 2023, 44(5): 316. |
| [2] | 鲍春竹, 向中华. 非热解共价有机聚合物基氧还原电催化材料[J]. 高等学校化学学报, 2023, 44(5): 20220715. |
| [3] | 李瑞松, 苗政培, 李静, 田新龙. 中空贵金属纳米材料氧还原催化的研究进展[J]. 高等学校化学学报, 2023, 44(5): 190. |
| [4] | 李姿若, 张红娟, 朱国勋, 夏伟, 汤静. 负载酞菁铁的氮掺杂中空碳球的电催化氧还原性能[J]. 高等学校化学学报, 2023, 44(1): 20220677. |
| [5] | 程前, 杨博龙, 吴文依, 向中华. S掺杂Fe-N-C高活性氧还原反应催化剂[J]. 高等学校化学学报, 2022, 43(9): 20220341. |
| [6] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [7] | 谷雨, 奚宝娟, 李江潇, 熊胜林. 单原子催化剂在氧还原反应中的分子级调控[J]. 高等学校化学学报, 2022, 43(5): 20220036. |
| [8] | 胡慧敏, 崔静, 刘丹丹, 宋佳欣, 张宁, 范晓强, 赵震, 孔莲, 肖霞, 解则安. 过渡金属修饰对Pt/M-DMSN催化剂丙烷脱氢性能的影响[J]. 高等学校化学学报, 2022, 43(4): 20210815. |
| [9] | 张小玉, 薛冬萍, 杜宇, 蒋粟, 魏一帆, 闫文付, 夏会聪, 张佳楠. MOF衍生碳基电催化剂限域催化O2还原和CO2还原反应[J]. 高等学校化学学报, 2022, 43(3): 20210689. |
| [10] | 何宇婧, 李佳乐, 王东洋, 王福玲, 肖作旭, 陈艳丽. 锌活化Fe/Co/N掺杂的生物质碳基高效氧还原催化剂[J]. 高等学校化学学报, 2022, 43(11): 20220475. |
| [11] | 马骏, 钟洋, 张珊珊, 黄仪珺, 张利鹏, 李亚平, 孙晓明, 夏振海. 高效催化氧还原及氧析出反应的掺杂石墨炔的设计与理论计算[J]. 高等学校化学学报, 2021, 42(2): 624. |
| [12] | 殷雯婧, 刘啸, 钱汇东, 邹志青. 高活性位点密度Fe-N共掺杂碳纳米片的制备及氧还原性能[J]. 高等学校化学学报, 2019, 40(7): 1480. |
| [13] | 徐朝权, 马俊红, 石旻慧, 冯超, 谢亚红, 米红宇. 基于天然产物的新型铁氮共掺杂碳电催化剂的制备及氧还原性能[J]. 高等学校化学学报, 2018, 39(7): 1532. |
| [14] | 王秀利, 何兴权. 氮/硫双掺多孔碳负载Fe9S10纳米粒子的氧还原电催化性能[J]. 高等学校化学学报, 2018, 39(7): 1524. |
| [15] | 黄骥培, 李毅, 杨申辉, 周亚洲, 程晓农, 朱佳, 杨娟. 三维多孔结构Pt-Ag气凝胶的制备及电催化氧还原反应性能[J]. 高等学校化学学报, 2018, 39(5): 1063. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||