

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (5): 20220063.doi: 10.7503/cjcu20220063
收稿日期:2022-01-26
出版日期:2022-05-10
发布日期:2022-03-15
通讯作者:
薛玉瑞,李玉良
E-mail:yrxue@sdu.edu.cn;ylli@iccas.ac.cn
基金资助:
CHEN Zhaoyang1, XUE Yurui1( ), LI Yuliang1,2(
), LI Yuliang1,2( )
)
Received:2022-01-26
Online:2022-05-10
Published:2022-03-15
Contact:
XUE Yurui,LI Yuliang
E-mail:yrxue@sdu.edu.cn;ylli@iccas.ac.cn
Supported by:摘要:
原子催化剂是零价金属原子锚定于载体上的一种新型催化剂, 具有原子利用率高、 选择性高以及反应活性和稳定性高等优点, 一直是催化领域的研究前沿, 在催化和能量转换领域具有广阔的发展前景. 石墨炔与金属原子之间独特的不完全电荷转移性质实现了零价过渡金属原子的稳定锚定, 解决了传统单原子催化剂易迁移和聚集的问题, 被认为是新一代催化剂. 本综述从石墨炔原子催化剂的结构性质、 表征以及应用等方面出发, 综合评述了相关领域的最新研究成果, 介绍了石墨炔原子催化剂在电催化固氮制氨、 产氢、 全水解和CO2固定等方面的应用和发展前景, 为实现新概念高性能催化材料的设计合成提供了研究思路.
中图分类号:
TrendMD:
陈朝阳, 薛玉瑞, 李玉良. 石墨炔零价金属原子催化剂的合成及应用. 高等学校化学学报, 2022, 43(5): 20220063.
CHEN Zhaoyang, XUE Yurui, LI Yuliang. Synthesis and Applications of Graphdiyne Based Zerovalent Atomic Catalysts. Chem. J. Chinese Universities, 2022, 43(5): 20220063.
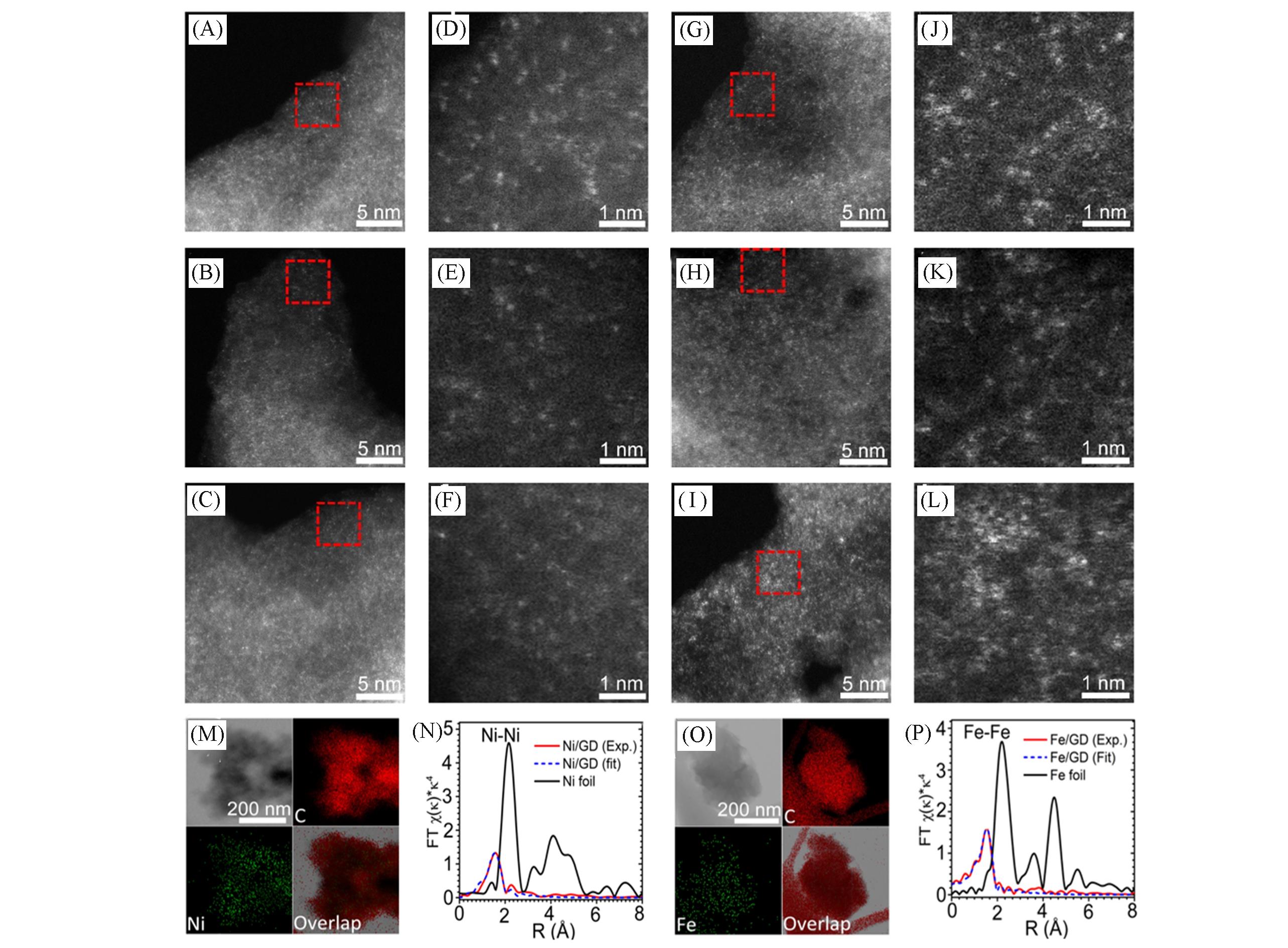
Fig.2 HAADF?STEM images of Ni/GD and Fe/GD(A—L), element distribution diagram of Ni/GD(M), off site EXAFS spectra of Ni and Ni foil at Ni K?edge(N), element distribution diagram of Fe/GD(O), off site EXAFS spectra of Fe and Fe foil at Fe K?edge(P)[3](A—C) Isolated Ni atoms(white dots) are present and uniformly dispersed on the GD; (D—F) corresponding enlargement of the marked regions in (A)—(C); (G—I) isolated Fe atoms(white dots) are present and uniformly dispersed on the GD; (J—L) corresponding enlargement of the marked regions in (G)—(I). Copyright 2018, Springer Nature.
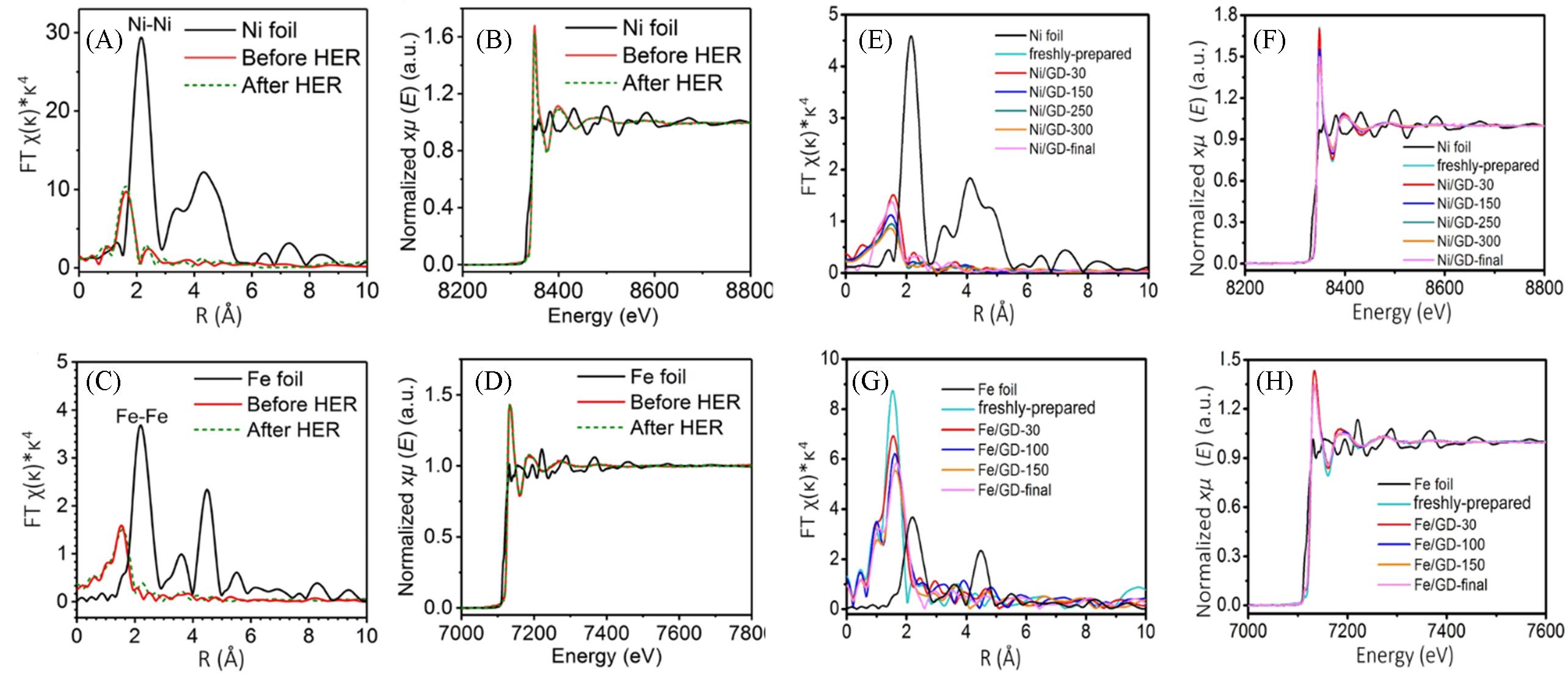
Fig.3 EXAFS(A) and XANES(B) spectra of Ni/GD and Ni foil at Ni K?edge(A, B), EXAFS(C) and XANES(D) spectra of Fe/GD and Fe foil at Fe K?edge, XAS spectra of Ni/GD at 5% H2/He and different temperatures(Ni foil as a control)(E, F), XAS spectra of Fe/GD at 5% H2/He and different temperatures(Fe foil as a control)(G, H)[3]Copyright 2018, Springer Nature.
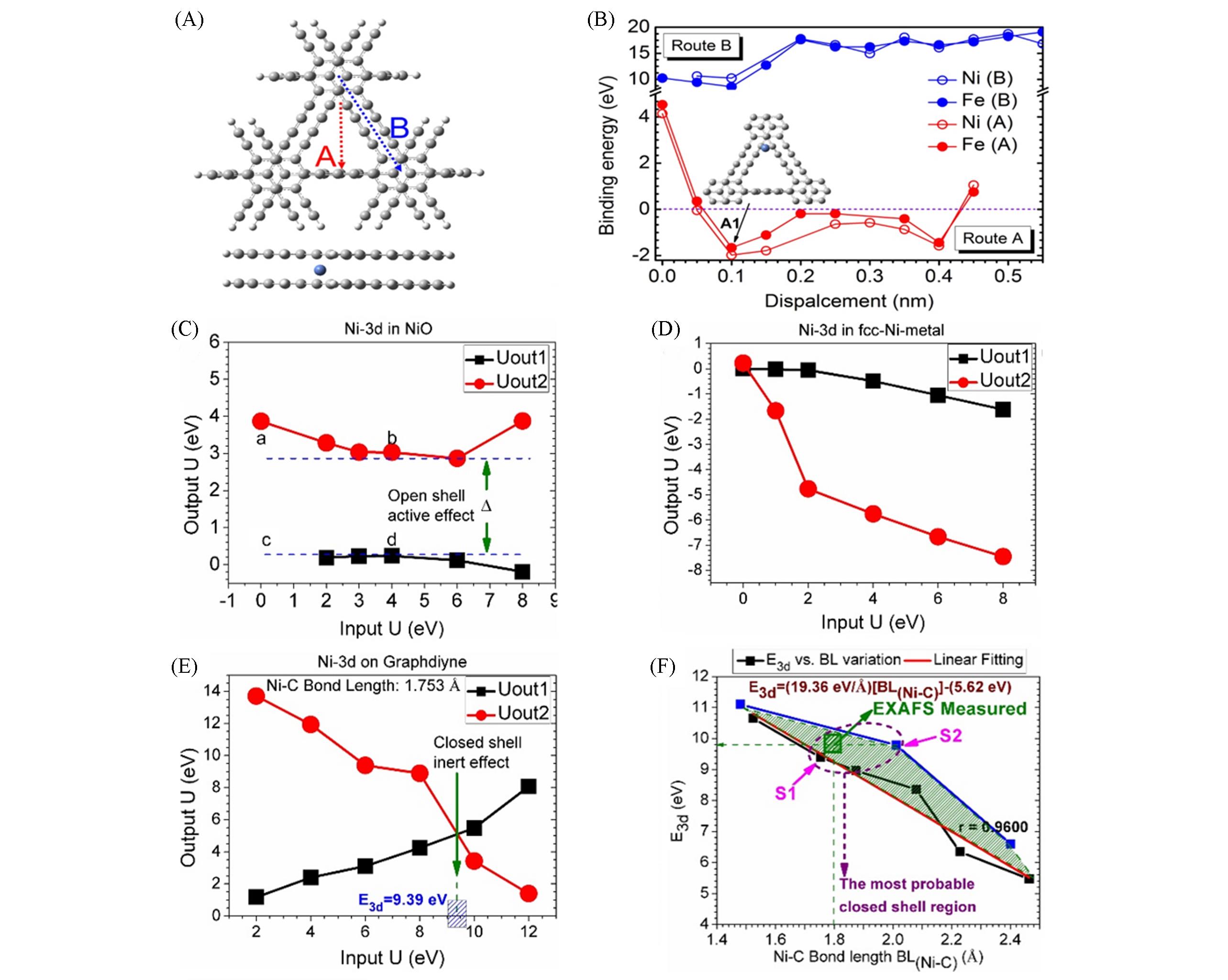
Fig.4 Possible adsorption sites of Ni/Fe atoms along route A and route B in GD layer(top: top view; bottom: side view)(A), binding energy of Ni/Fe atoms adsorbed by double?layer GD vs. the displacement along the A and B directions(B), 3D orbital binding energy of the anchored Ni position determined by the open shell charge overlap and the closed shell charge overlap[NiO(C), Ni on GD(D), and Ni-fcc(E)], and change of orbital energy related to the newly formed Ni-C(F)[3]Copyright 2018, Springer Nature.
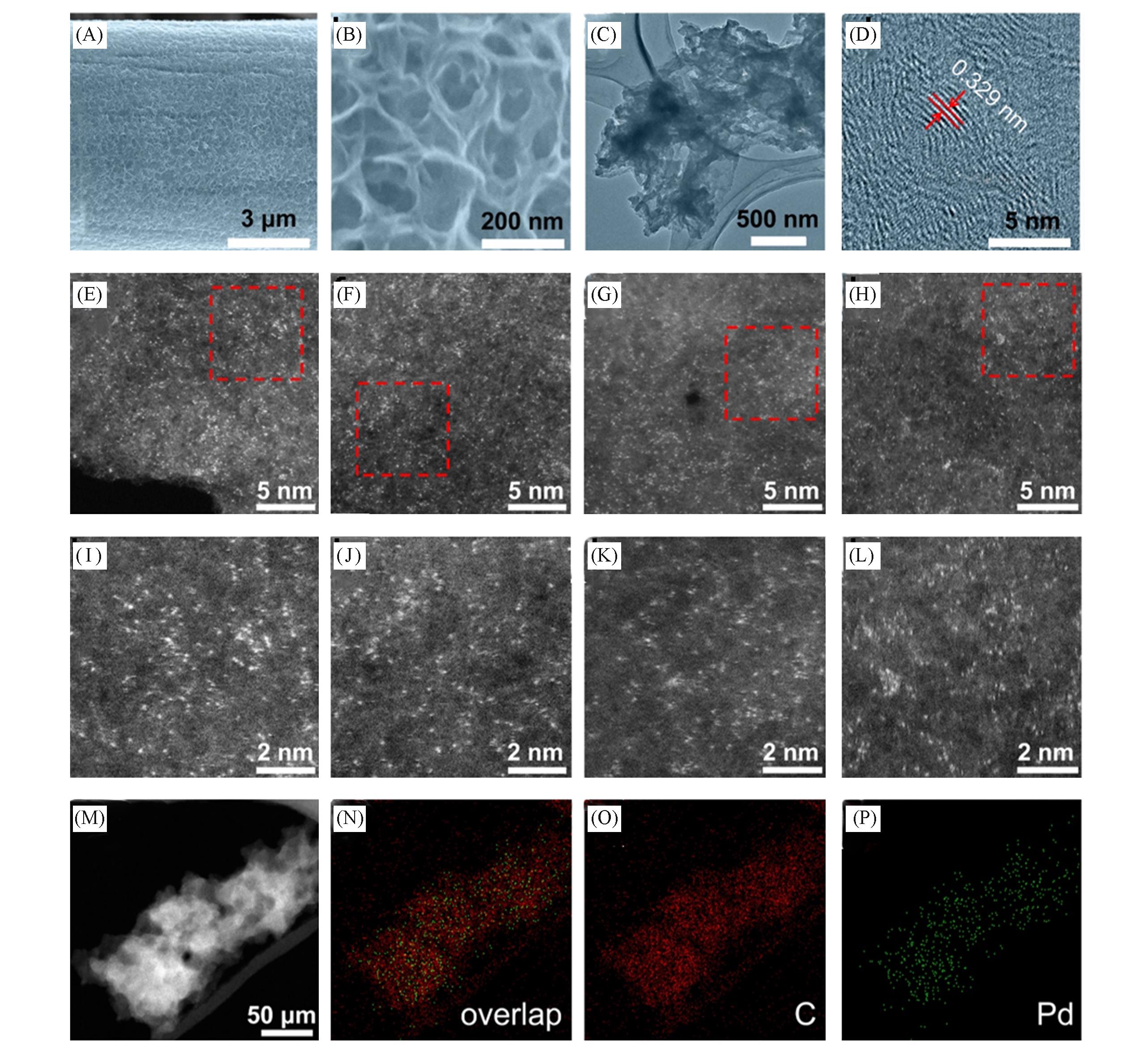
Fig.5 SEM images of Pd0/GDY(A, B), TEM(C) and high resolution TEM(D) images of Pd0/GDY, HAADF images of different regions of Pd0/GDY nanosheets(E—H), enlarged images of the selected area in (E)—(H)(I—L), STEM?HAADF images of Pd0/GDY nanosheets and corresponding element distribution diagram of Pd and C atoms(M—P)[73]Copyright 2018, Elsevier.
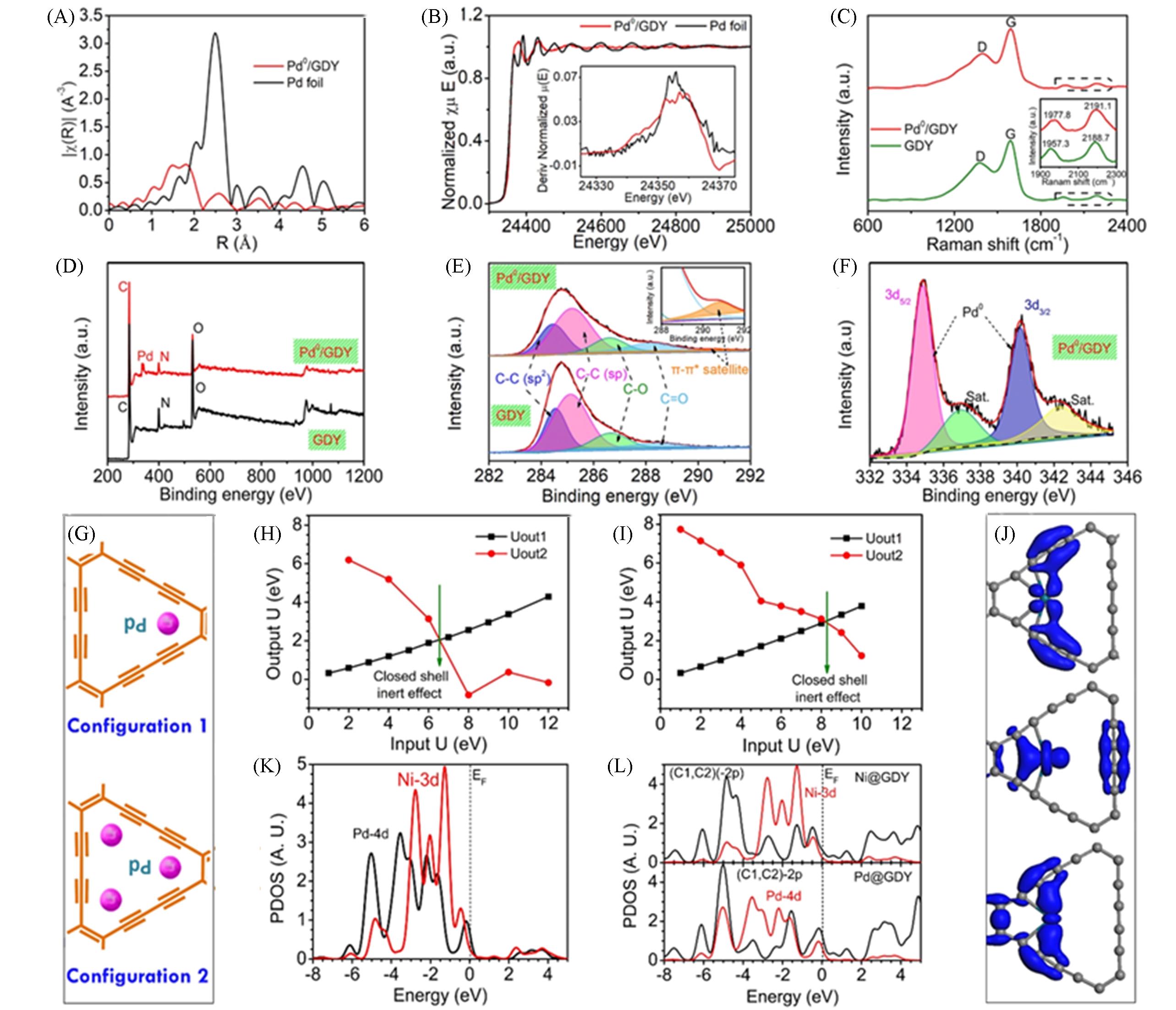
Fig.6 EXAFS spectra of Pd0/GDY and Pd foils in Pd K?band(A), normalized spectra of Pd0/GDY and Pd foil in Pd K?band(inset: first derivative curve)(B), Raman spectra of Pd0/GDY and initial GDY(inset: signal of alkyne bond structure on GDY skeleton)(C), XPS spectra of Pd0/GDY and initial GDY(D), C1s high resolution XPS spectra of Pd0/GDY and initial GDY(inset: enlarged image of 290.8 eV region)(E), XPS spectra of Pd3dof Pd0/GDY(F), in GD, the single(high) and triple(low) anchor positioning points(position a) of Pd(G), orbital potential energy projection(Uout1 and Uout2) of Pd?4d in single(H) and three anchor positioning points(I), PDOS comparison of Pd?4d and Ni?4d bands in GD?Pd and GD?Ni(J), compared with Ni?4d and (C1, C2)?2p bands, the total contribution of Pd?4d and (C1, C2)?2p bands to PDOS(K), real space 3D orbit profile of three main p?d band overlapping peaks observed from PDOS between Pd?4d and (C1, C2)?2p band(L)[73]Copyright 2018, Elsevier.
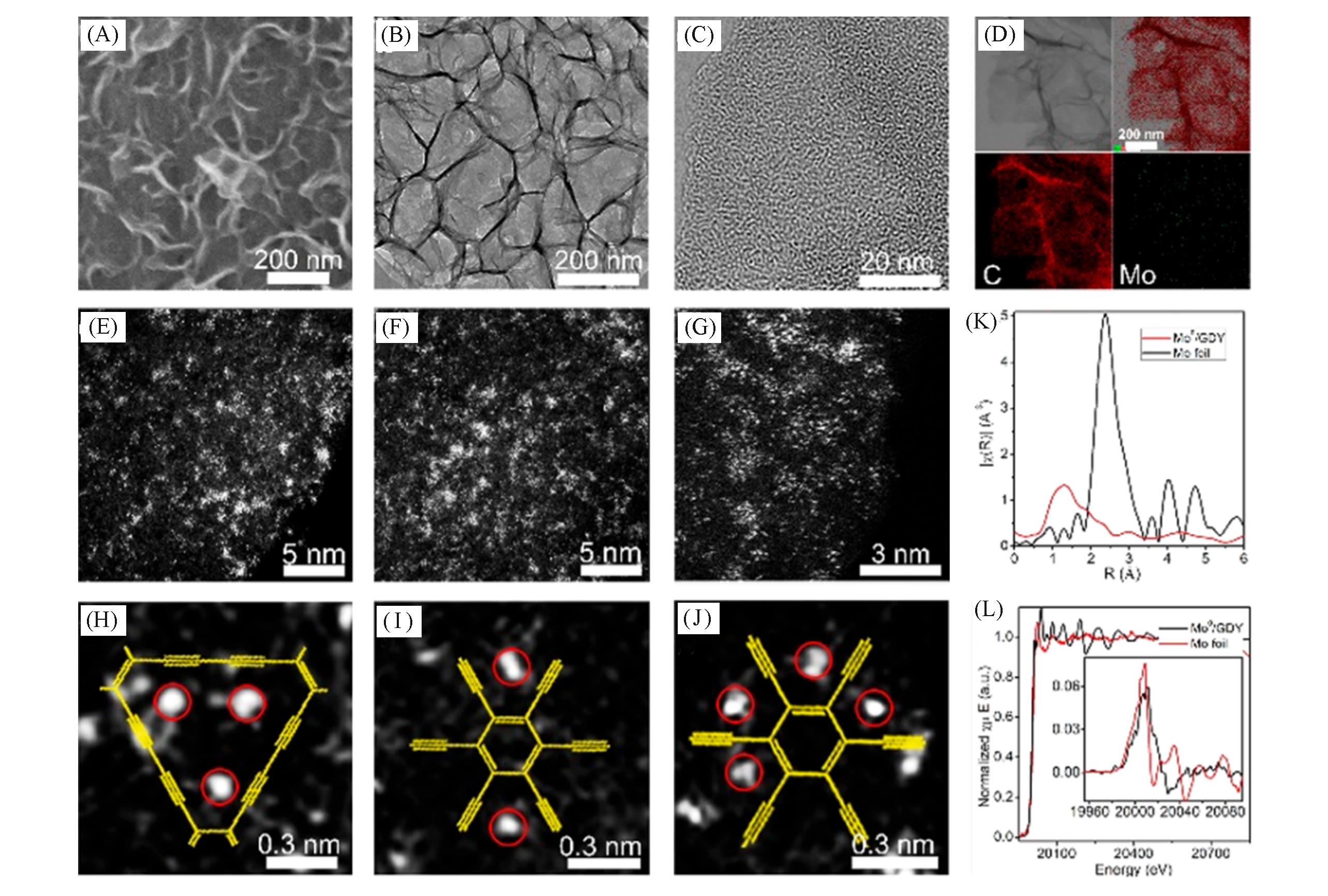
Fig.7 SEM(A), TEM(B) and HRTEM(C) images of Mo0/GDY, STEM image of Mo0/GDY and corresponding element distribution image(D), HAADF?STEM images of newly prepared Mo0/GDY samples(E—G), different configurations of each Mo atom fixed on the GDY structure(H—J), EXAFS spectra of Mo K?edge of Mo0/GDY(red line) and Mo foil(black line)(K), normalized Mo K?edge XANES spectra of Mo0/GDY and Mo foil(inset: calculated first derivative curve)(L)[5]Copyright 2019, American Chemical Society.
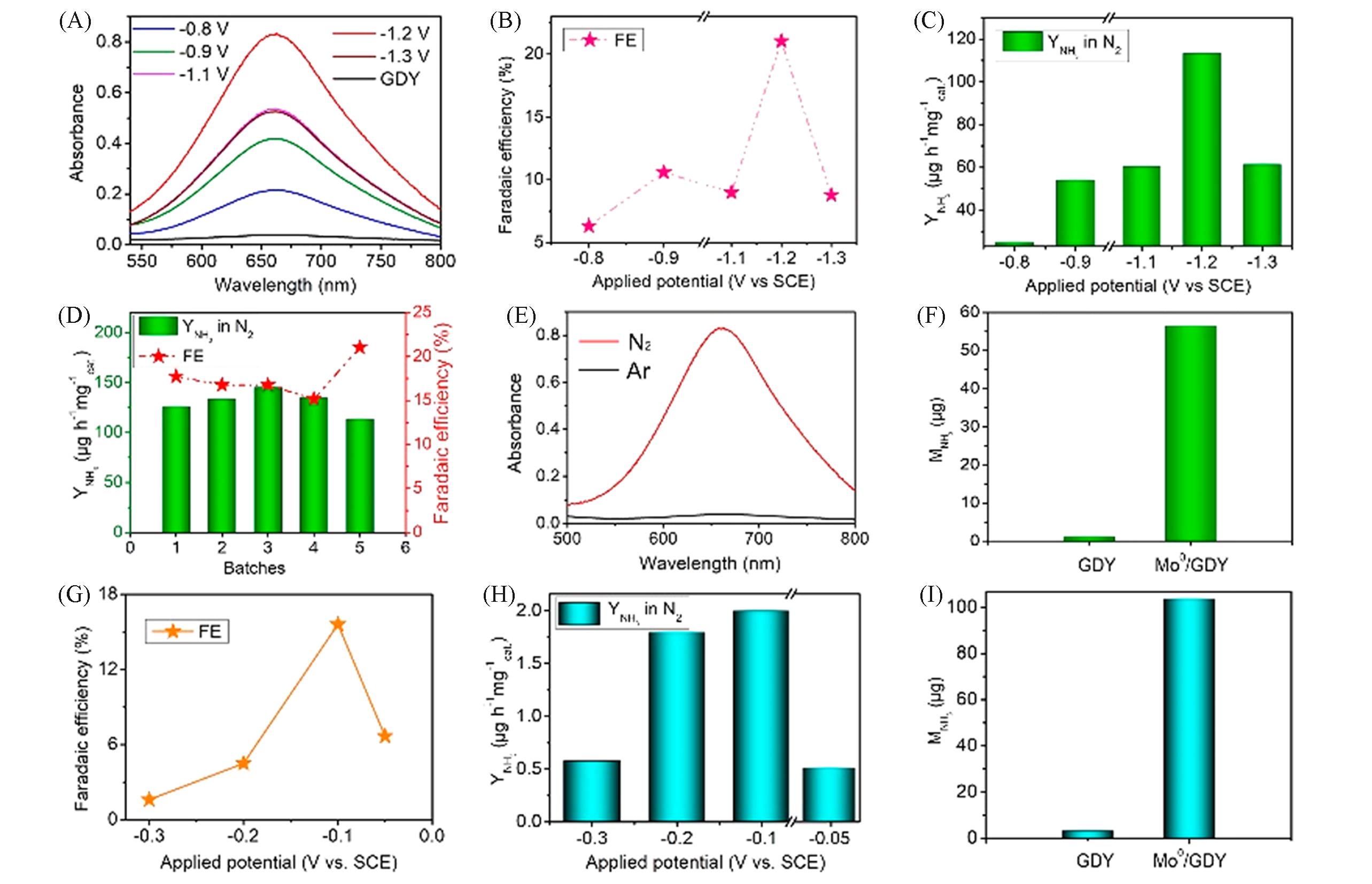
Fig.8 ECNRR performance of Mo0/GDY[5](A) UV-Vis absorption spectra of 0.1 mol/L Na2SO4 electrolyte at different potentials for 2 h after ECNRR; (B, C) Faraday efficiency(B) and ammonia yield(C) in 0.1 mol/L Na2SO4 at different applied potentials; (D) ammonia yield and Faraday efficiency produced by NH3 of different batches of Mo0/GDY samples; (E) UV-Vis absorption spectra of Mo0/GDY were measured in N2(red line) and Ar(black line) saturated electrolyte; (F) amount of NH3 produced by pure GDY and Mo0/GDY electrodes after electrolysis at room temperature -1.2 V for 2 h; (G) Faraday efficiency and (H) ammonia yield at different applied potentials in 0.1 mol/L HCl; (I) amount of NH3 produced by pure GDY and Mo0/GDY electrodes after electrolysis at room temperature -0.1 V for 2 h.Copyright 2019, American Chemical Society.
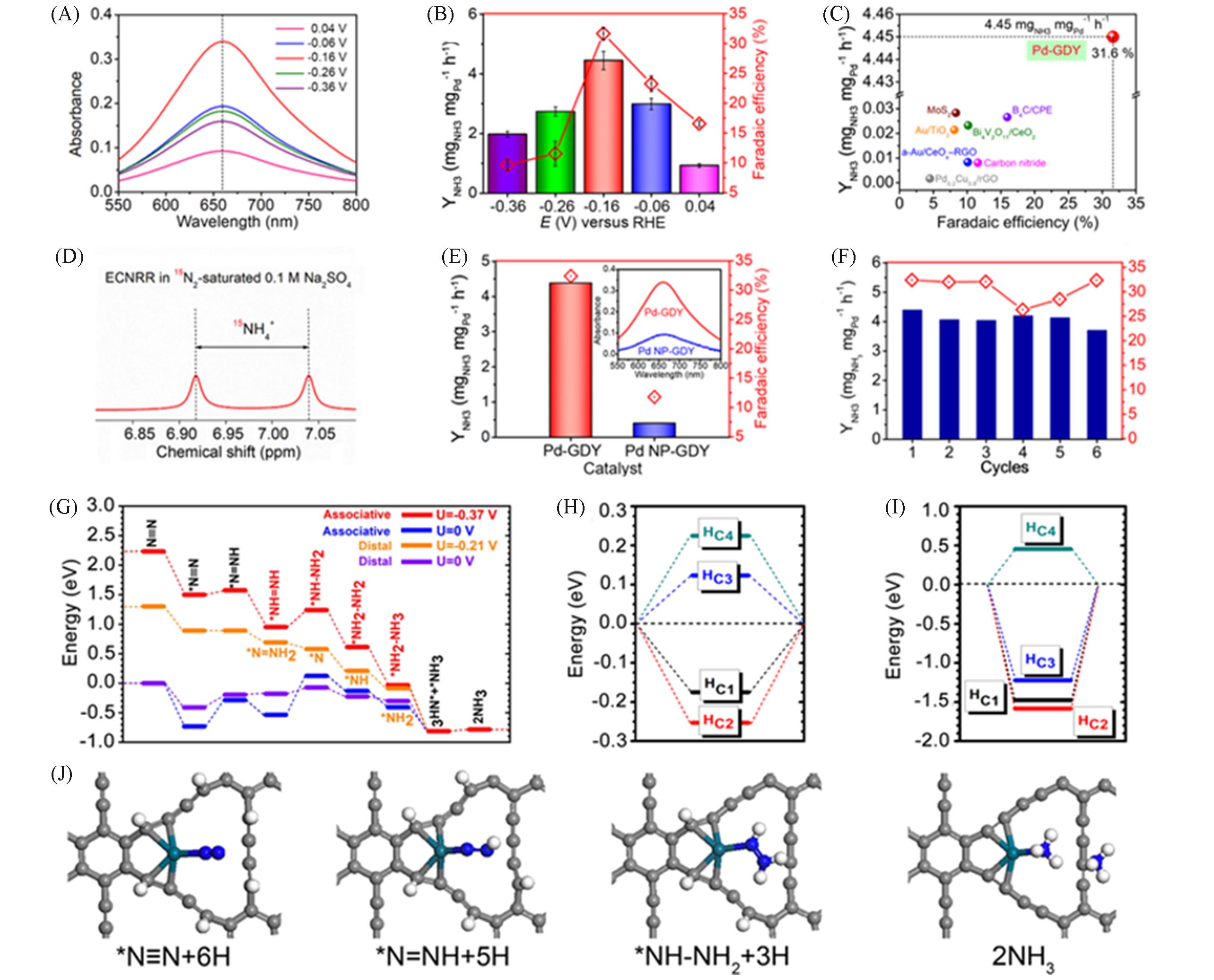
Fig.9 UV?Vis absorption spectra of 0.1 mol/LNa2SO4 electrolyte at different potentials for 2 h after ECNRR(A), ammonia yield and Faraday efficiency at applied potential in 0.1 mol/L Na2SO4(B), comparison of ECNRR performance of Pd?GDY with other catalysts(C), 1H?15N NMR spectra of 0.1 mol/L Na2SO4 after ECNRR under 15N2 with Pd?GDY as catalyst(D), ammonia yield, Faraday efficiency and corresponding UV?Vis absorption spectra of Pd?GDY and Pd?NP/GDY catalysts at -0.40 V after electrolysis for 2 h at room temperature(illustration)(E), stability test of Pd?GDY under the following conditions?under ambient conditions(the voltage: 0.4 V in 0.1 mol/L Na2SO4)(F), ECNRR kinetic steps of Pd?GDY(G), H formation energy of Pd?GDY on the C side(H), H chemisorption energy of Pd?GDY on the C side(I), and structural evolution of ECNRR catalytic process(J)[76](B) Error bar represents the standard deviation calculated from independent experiments(at least three times);(C) error bar represents the standard deviation calculated from independent experiments(at least three times).Copyright 2020, Oxford University Press.
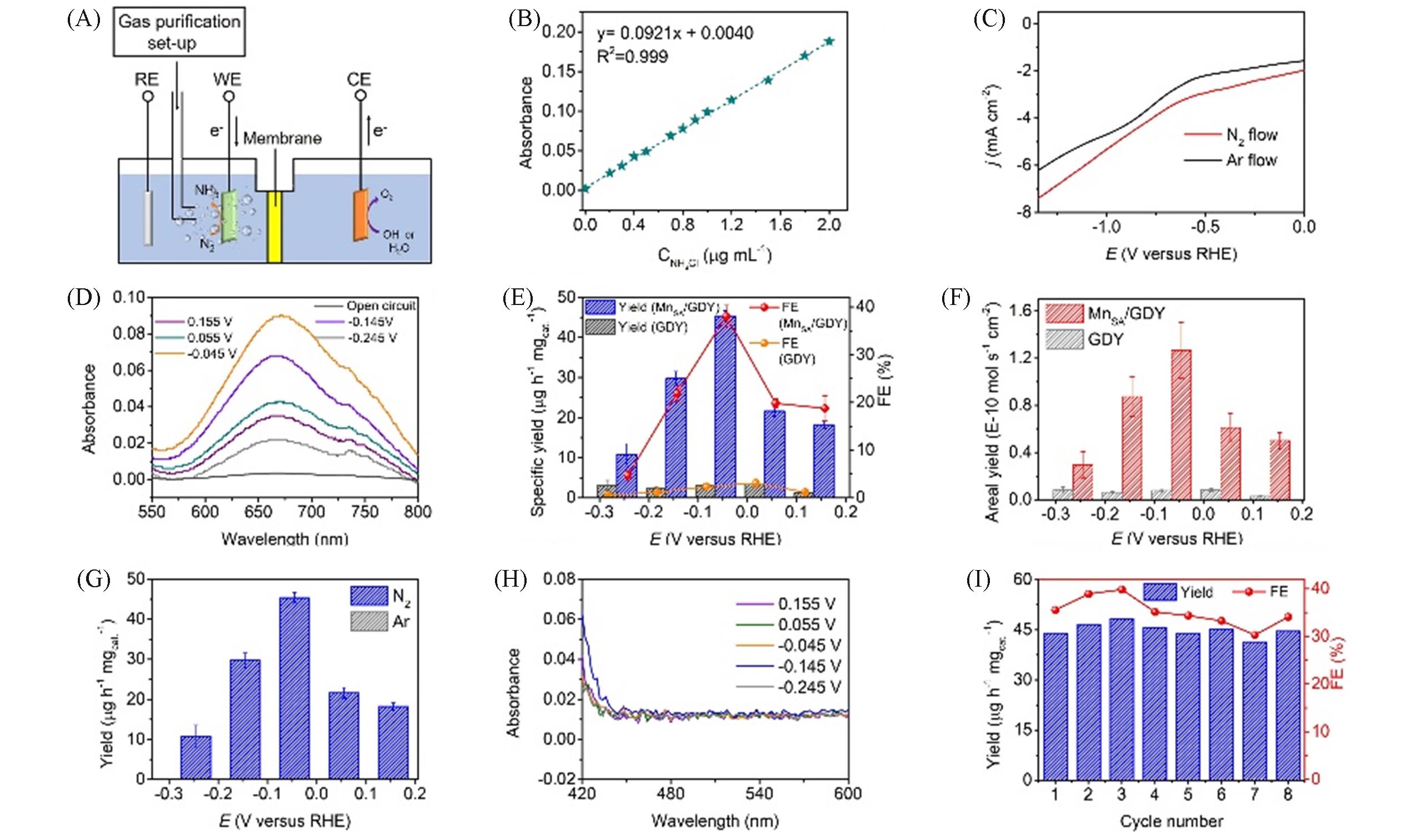
Fig.10 Schematic diagram of NRR battery configuration at room temperature(A), a calibration curve used to quantify the ammonia produced(B), polarization curves tested in nitrogen and argon atmosphere(C), UV?Vis spectra of electrolyte after NRR for 1 h at different potentials at room temperature(D), specific ammonia yield and Faraday efficiency of MnSA/GDY and pure GDY at different potentials in saturated N2 0.1 mol/L Na2SO4(E), area ammonia yield and Faraday efficiency of MnSA/GDY and pure GDY at different potentials in N2 saturated 0.1 mol/L Na2SO4(F), ammonia yield of MnSA/GDY obtained at different potentials in 0.1 mol/L Na2SO4 saturated with Ar and N2, respectively(the error bar represents the standard deviation)(G), in N2 saturated 0.1 mol/L Na2SO4, the UV?Vis spectrum of N2H4 in MnSA/GDY detected under applied potential(H), stability test results of MnSA/GDY in 0.1 mol/L Na2SO4 at -0.045 V(vs. RHE)(I)[77](E) Error bar represents standard deviation; (F) Bar graph shows standard deviation.Copyright 2022, Royal Society of Chemistry.
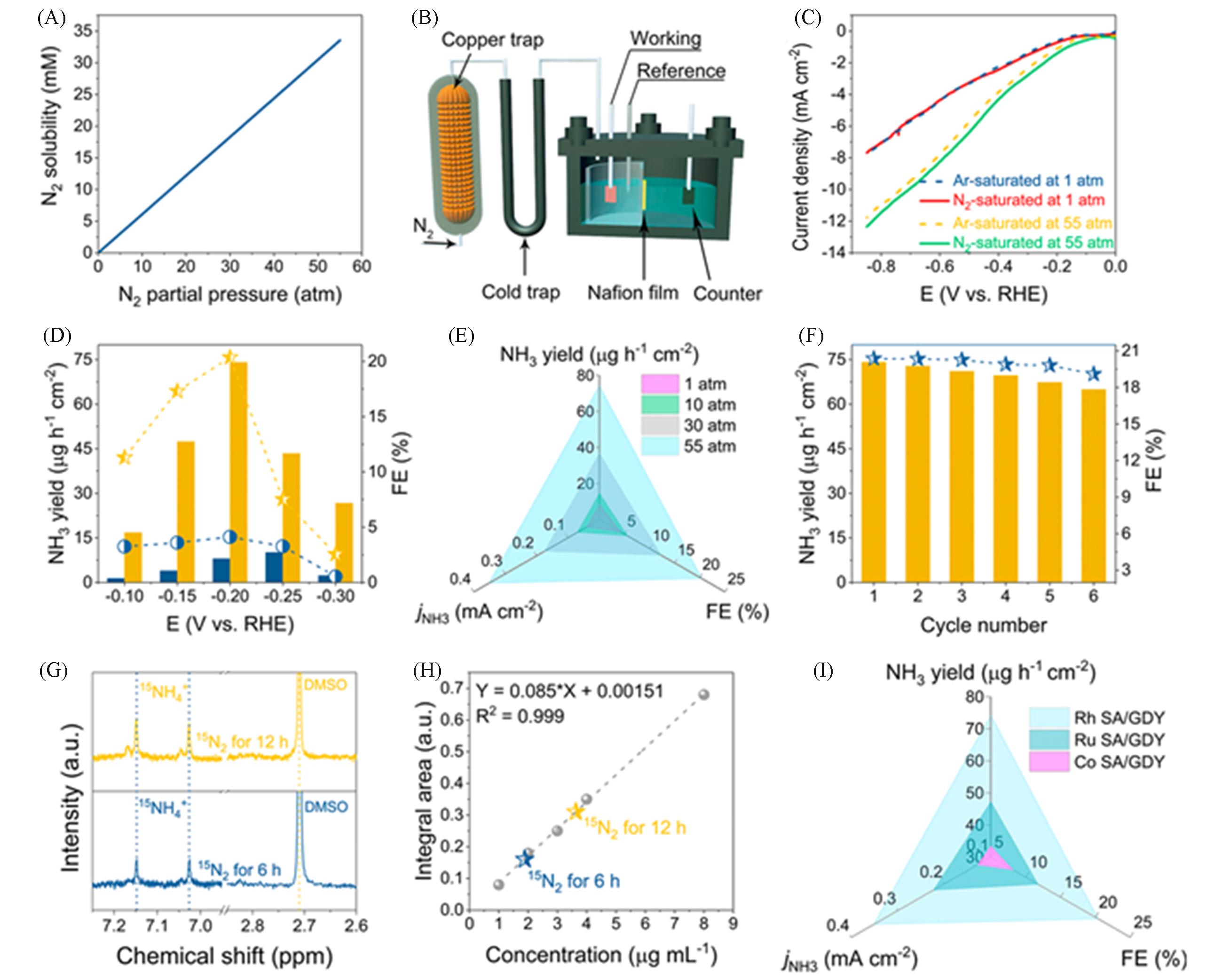
Fig.11 Pressurized ENRR test[78](A) Relationship between solubility and partial pressure of nitrogen; (B) schematic diagram of self-made pressurized ENRR device; (C) Ar and N2 saturated LSV curves of Rh/GDY at room temperature and 55 atm, respectively; (D) NH3 yield and its corresponding Faraday efficiency of Rh/GDY at room temperature and 55 atm; (E) relationship between NH3 yield, Faraday efficiency and jNH3 of Rh/GDY and applied N2 pressure; (F) cyclic stability of Rh/GDY at room temperature, -0.2 V; (G, H) 1H NMR spectra and corresponding 15NH4+ production, and 6 and 12 h electroreduced 15N2; (I) optimum NH3 yield, Faraday efficiency and jNH3 of Rh, Ru and Co/GDY.Copyright 2020, Proceedings of the National Academy of Sciences.
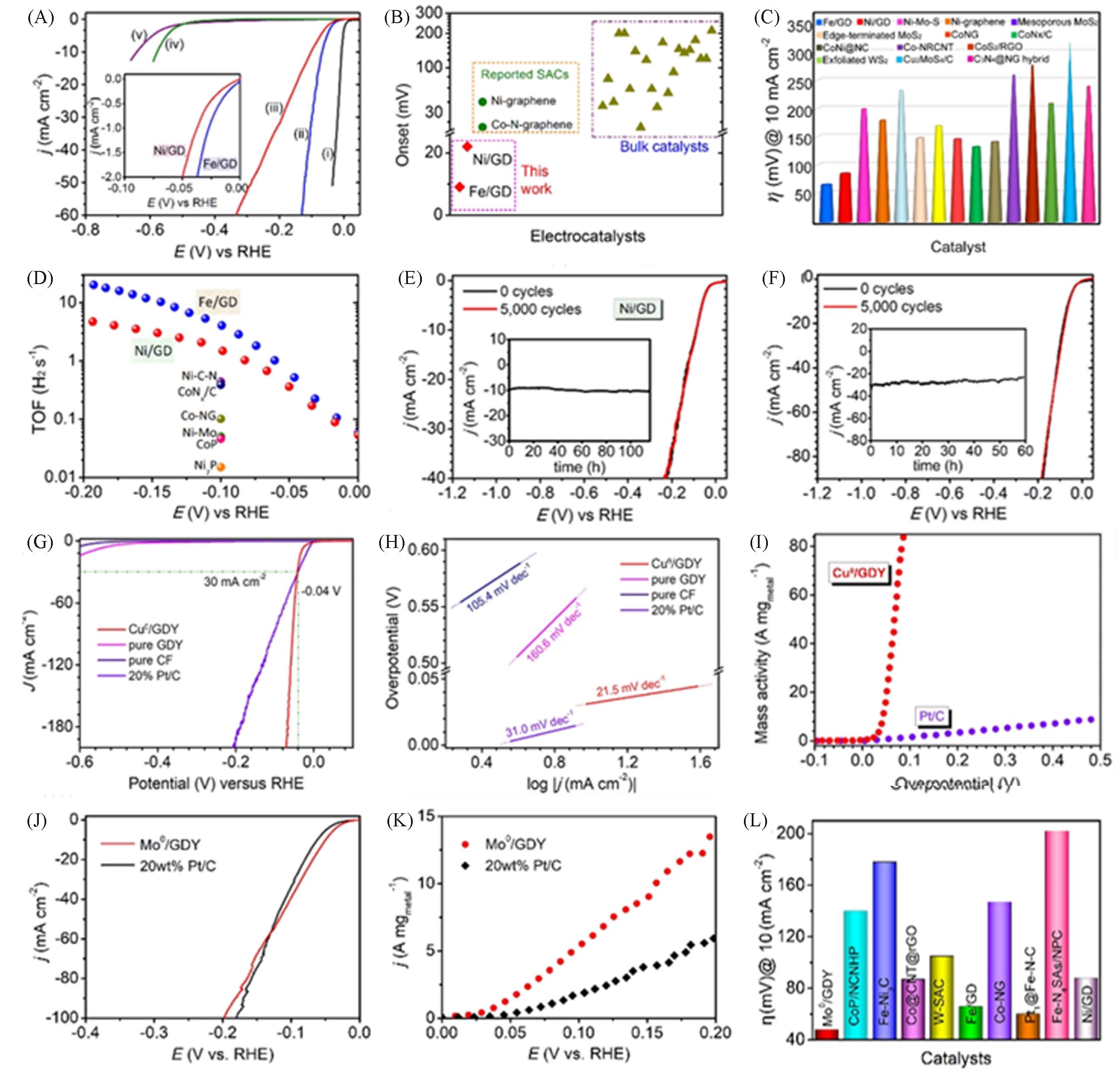
Fig.12 Polarization curves of Pt/C(I), Fe/GD(II), Ni/GD(III), GDF(IV) and CC(V) in 0.5 mol/L H2SO4 solution(inset: enlarged LSV curves of Fe/GD and Ni/GD near the starting region)(A), Ni/GD and Fe/GD(red region) and other non?noble metal monatomic HER catalysts(green region) and several bulk catalysts(olivine triangle) at 10 mA/cm2(B) and overpotential(C), TOF values of Fe/GD (blue dot) and Ni/GD(red dot) and several state?of?the?art her electrocatalysts(D), Ni/GD(E) and Fe/GD(F)[3], LSV curve(G) and corresponding Tafel slope(H), mass activity of Cu0/GDY and Pt/C(I)[4], polarization curve(J) and mass activity(K) of Mo0/GDY and 20% Pt/C, over potential of various catalysts at 10 mA/cm2(L)[5](A—F) Copyright 2018, Springer Nature; (G—I) Copyright 2020, Wiley‐VCH GmbH; (J—L) Copyright 2019, American Chemical Society.
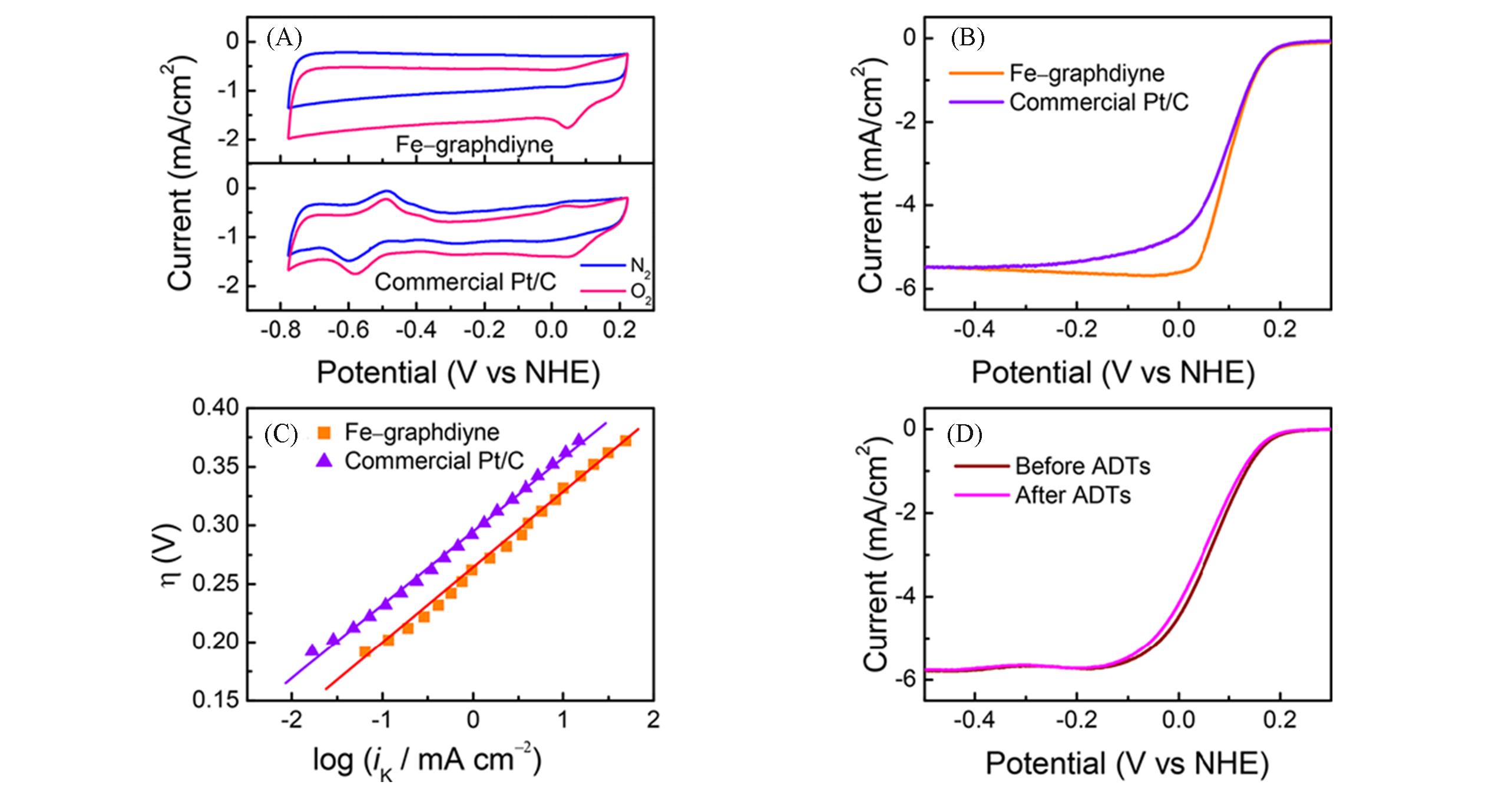
Fig.13 CV curves of Fe?GDY and commercial Pt/C catalysts in N2(blue line) and O2 saturated(red line) in 0.1 mol/L KOH solution at room temperature(A), RDE test of Fe?GDY and 20% Pt/C catalyst in 0.1 mol/L KOH solution(B), Tafel slope(C), ORR stability(D) of Fe?GDY[83]Copyright 2018, American Chemical Society.
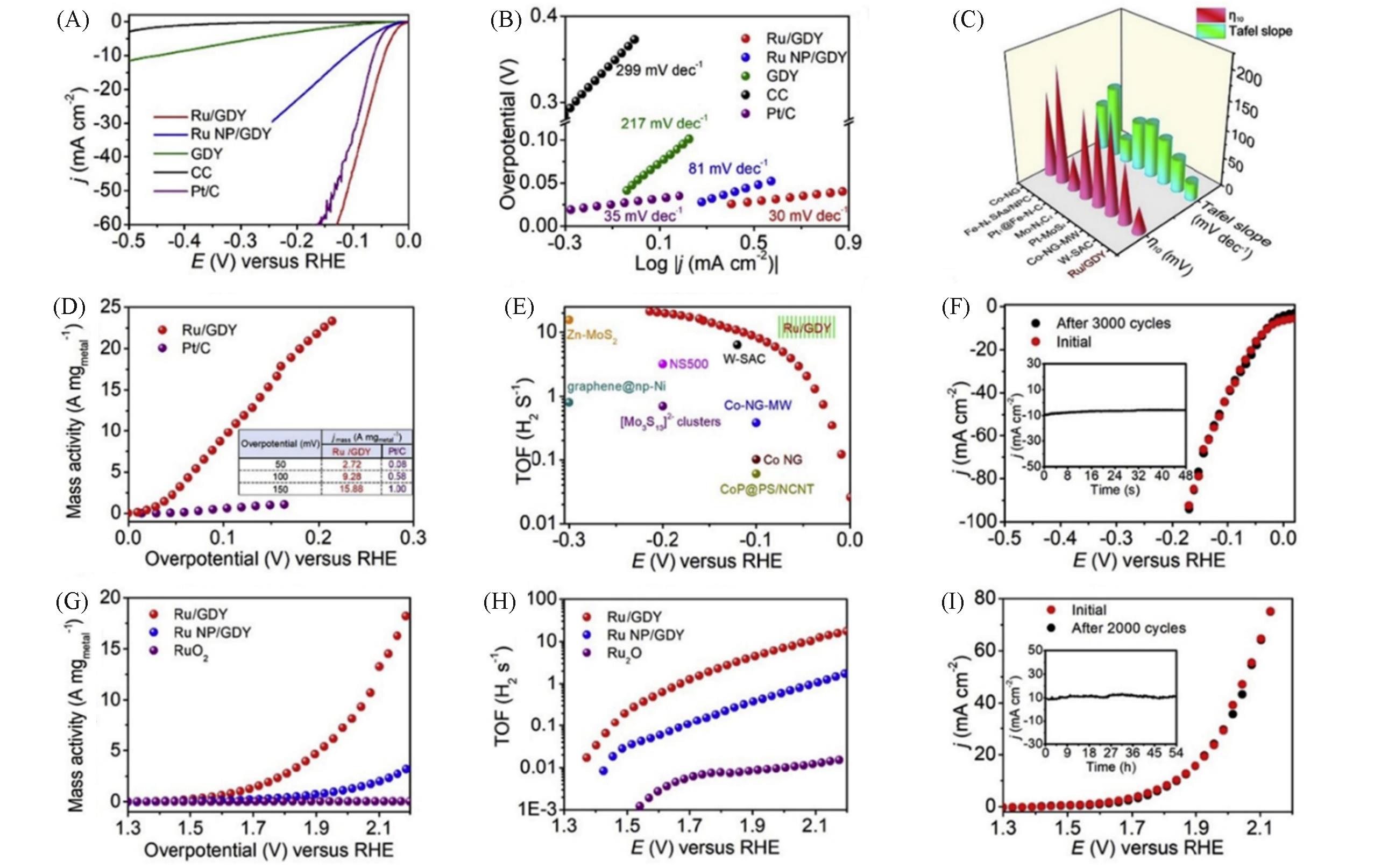
Fig.14 Polarization curve(A), Tafel slope of HER of corresponding sample(B), comparison of Tafel slopes(C), HER mass activity of Ru/GDY and Pt/C(D), comparison of TOF values between Ru/GDY and other catalysts(E) polarization curve of Ru/GDY after 3000 HER cycles(F), OER mass activity of Ru/GDY and Ru NP/GDY and RuO2(G), TOF values of OER for Ru/GDY and Ru NP/GDY and RuO2(H), polarization curve of Ru/GDY before and after 2000 OER cycles(I)[80]Copyright 2020, Elsevier.
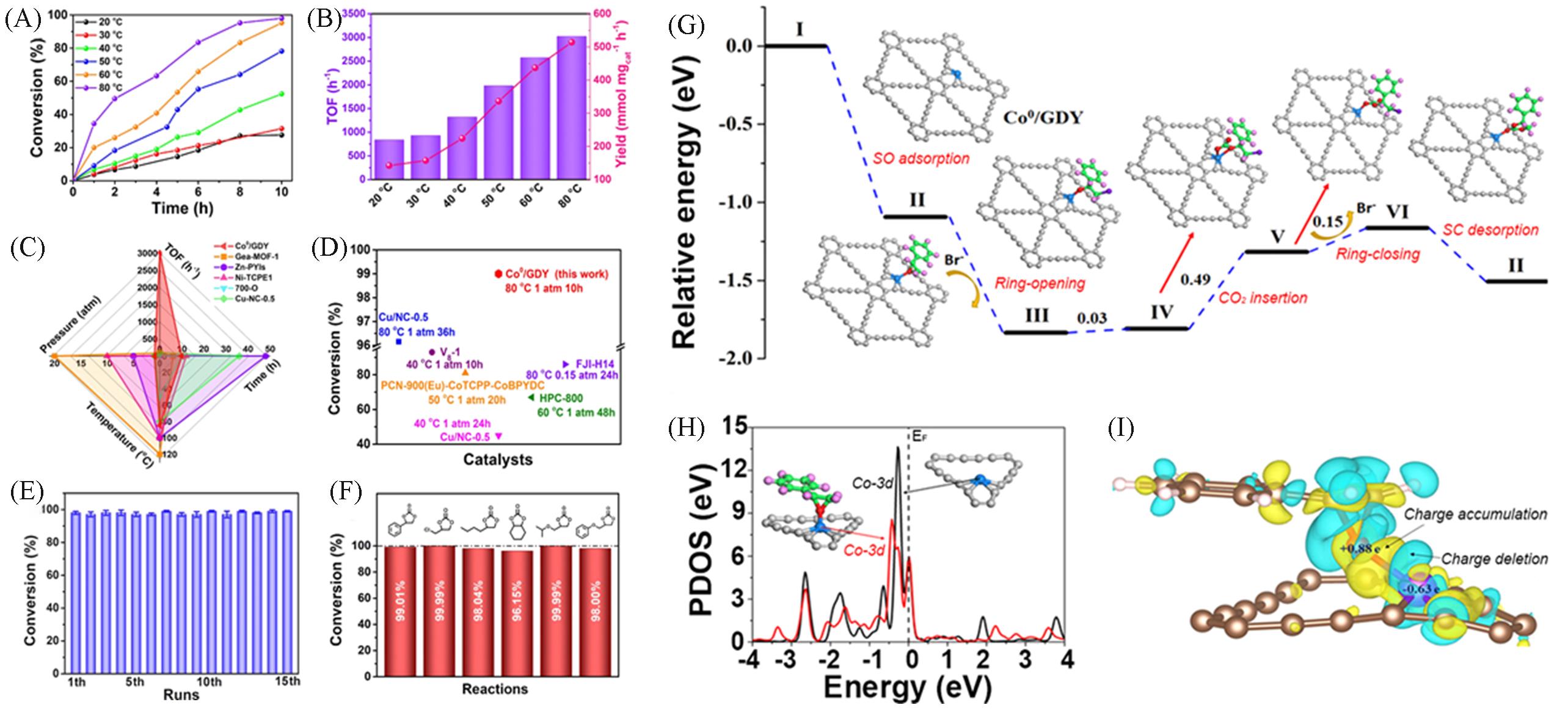
Fig.15 Time course of catalytic conversion at different temperatures(A), TOF value and yield of Co0/GDY at different temperatures(B), compared with the reported catalysts(C), comparison of SO conversion between Co0/GDY and reported catalysts(D), stability test of catalyst(E), under the same conditions, carbonates with different substituents generated from the corresponding epoxy compounds under the catalysis of Co0/GDY(F), free energy distribution of key intermediates(G), Co0/GDY and SO adsorbed Co0/GDY Co?3d PDOS(H), differential charge density diagram of SO adsorbed Co0/GDY(I)[85]Copyright 2021, American Chemical Society.
| 1 | Chen Z., Vorobyeva E., Mitchell S., Fako E., Ortuño M. A., López N., Collins S. M., Midgley P. A., Richard S., Vilé G., Pérez⁃Ramírez J., Nat. Nanotechnol., 2018, 13, 702—707 |
| 2 | Xu H., Cheng D., Cao D., Zeng X. C., Nat. Catal., 2018, 1, 339—348 |
| 3 | Xue Y., Huang B., Yi Y., Guo Y., Zuo Z., Li Y., Jia Z., Liu H., Li Y., Nat. Commun., 2018, 9, 1460 |
| 4 | Hui L., Xue Y., YuH., Zhang C., Huang B., Li, Y., Chemphyschem, 2020, 21, 2145—2149 |
| 5 | Hui L., Xue Y., Yu H., Liu Y., Fang Y., Xing C., Huang B., Li Y., J. Am. Chem. Soc., 2019, 141, 10677—10683 |
| 6 | Huang C., Li Y., Wang N., Xue Y., Zuo Z., Liu H., Li Y., Chem. Rev., 2018, 118, 7744—7803 |
| 7 | Qiao B., Wang A., Yang X., Allard L. F., Jiang Z., Cui Y., Liu J., Li J., Zhang T., Nature Chem., 2011, 3, 634—641 |
| 8 | Zhang H., Watanabe T., Okumura M., Haruta M., Toshima N., Nature Mater., 2012, 11, 49—52 |
| 9 | Kyriakou G., Boucher M. B., Jewell A. D., Lewis E. A., Lawton T. J., Baber A. E., Tierney H. L., Flytzani⁃Stephanopoulos M., Sykes E. C. H., Science, 2012, 335, 1209 |
| 10 | Huang Z., Gu X., Cao Q., Hu P., Hao J., Li J., Tang X., Angew. Chem. Int. Ed., 2012, 51, 4198—4203 |
| 11 | Moses⁃DeBusk M., Yoon M., Allard L. F., Mullins D. R., Wu Z., Yang X., Veith G., Stocks G. M., Narula C. K., J. Am. Chem. Soc., 2013, 135, 12634—12645 |
| 12 | Song W., Hensen E. J. M., J. Phys. Chem. C, 2013, 117, 7721—7726 |
| 13 | Wei H., Liu X., Wang A., Zhang L., Qiao B., Yang X., Huang Y., Miao S., Liu J., Zhang T., Nature Commun., 2014, 5, 5634 |
| 14 | Zhao Y.X., Li Z.Y., Yuan Z., Li X.N., He S.G., Angew. Chem. Int. Ed., 2014, 53, 9482—9486 |
| 15 | Shi Y., Zhao C., Wei H., Guo J., Liang S., Wang A., Zhang T., Liu J., Ma T., Adv. Mater., 2014, 26, 8147—8153 |
| 16 | Li Z.Y., Yuan Z., Li X.N., Zhao Y.X., He S.G., J. Am. Chem. Soc., 2014, 136, 14307—14313 |
| 17 | Li X.N., Yuan Z., He S.G., J. Am. Chem. Soc., 2014, 136, 3617—3623 |
| 18 | Hu P., Huang Z., Amghouz Z., Makkee M., Xu F., Kapteijn F., Dikhtiarenko A., Chen Y., Gu X., Tang X., Angew. Chem. Int. Ed., 2014, 53, 3418—3421 |
| 19 | Guo X., Fang G., Li G., Ma H., Fan H., Yu L., Ma C., Wu X., Deng D., Wei M., Tan D., Si R., Zhang S., Li J., Sun L., Tang Z., Pan X., Bao X., Science, 2014, 344, 616 |
| 20 | Deng D., Chen X., Yu L., Wu X., Liu Q., Liu Y., Yang H., Tian H., Hu Y., Du P., Si R., Wang J., Cui X., Li H., Xiao J., Xu T., Deng J., Yang F., Duchesne P. N., Zhang P., Zhou J., Sun L., Li J., Pan X., Bao X., Sci. Adv., 2015, 1, e1500462 |
| 21 | Fei H., Dong J., Arellano⁃Jiménez M. J., Ye G., Dong Kim N., Samuel E. L. G., Peng Z., Zhu Z., Qin F., Bao J., Yacaman M. J., Ajayan P. M., Chen D., Tour J. M., Nature Commun., 2015, 6, 8668 |
| 22 | Yan H., Cheng H., Yi H., Lin Y., Yao T., Wang C., Li J., Wei S., Lu J., J. Am. Chem. Soc., 2015, 137, 10484—10487 |
| 23 | Pei G. X., Liu X. Y., Wang A., Lee A. F., Isaacs M. A., Li L., Pan X., Yang X., Wang X., Tai Z., Wilson K., Zhang T., ACS Catal., 2015, 5, 3717—3725 |
| 24 | Liu P., Zhao Y., Qin R., Mo S., Chen G., Gu L., Chevrier D. M., Zhang P., Guo Q., Zang D., Wu B., Fu G., Zheng N., Science, 2016, 352, 797 |
| 25 | Qiao B., Liu J., Wang Y.G., Lin Q., Liu X., Wang A., Li J., Zhang T., Liu J., ACS Catal., 2015, 5, 6249—6254 |
| 26 | Thomas J. M., Nature, 2015, 525, 325—326 |
| 27 | Zhang Z., Zhu Y., Asakura H., Zhang B., Zhang J., Zhou M., Han Y., Tanaka T., Wang A., Zhang T., Yan N., Nature Commun., 2017, 8, 16100 |
| 28 | Fang X., Shang Q., Wang Y., Jiao L., Yao T., Li Y., Zhang Q., Luo Y., Jiang H. L., Adv. Mater., 2018, 30, 1705112 |
| 29 | Kuo C.T., Lu Y., Kovarik L., Engelhard M., Karim A. M., ACS Catal., 2019, 9, 11030—11041 |
| 30 | Zhang J., Zhao Y., Guo X., Chen C., Dong C. L., Liu R. S., Han C. P., Li Y., Gogotsi, Y., Wang, G., Nature Catal., 2018, 1, 985—992 |
| 31 | Narula C. K., Allard L. F., Wu Z., Sci. Rep., 2017, 7, 6231 |
| 32 | Xi W., Wang K., Shen Y., Ge M., Deng Z., Zhao Y., Cao Q., Ding Y., Hu G., Luo J., Nature Commun., 2020, 11, 1919 |
| 33 | Jiao L., Wan G., Zhang R., Zhou H., Yu S. H., Jiang H. L., Angew. Chem. Int. Ed., 2018, 57, 8525—8529 |
| 34 | Zhu C., Shi Q., Xu B. Z., Fu S., Wan G., Yang C., Yao S., Song J., Zhou H., Du D., Beckman S. P., Su D., Lin Y., Adv. Energy Mater., 2018, 8, 1801956 |
| 35 | Liu P., Qin R., Fu G., Zheng N., J. Am. Chem. Soc., 2017, 139, 2122—2131 |
| 36 | Wang L., Zhang S., Zhu Y., Patlolla A., Shan J., Yoshida H., Takeda S., Frenkel A. I., Tao F., ACS Catal., 2013, 3, 1011—1019 |
| 37 | Yardimci D., Serna P., Gates B. C., Chem. Eur. J., 2013, 19, 1235—1245 |
| 38 | Lin J., Wang A., Qiao B., Liu X., Yang X., Wang X., Liang J., Li J., Liu J., Zhang T., J. Am. Chem. Soc., 2013, 135, 15314—15317 |
| 39 | Qu Y., Li Z., Chen W., Lin Y., Yuan T., Yang Z., Zhao C., Wang J., Zhao C., Wang X., Zhou F., Zhuang Z., Wu Y., Li Y., Nature Catal., 2018, 1, 781—786 |
| 40 | Liu M. J., Lee J. Y., Yang T. C., Zheng F. Y., Zhao J., Yang C. M., Lee L. Y. S., Small Methods, 2021, 5, 10 |
| 41 | Wang X. N., Zhou H. Y., Yan Z., Zhang X. Y., Jia J. F., Wu H. S., Theor. Chem. Acc., 2017, 136, 6 |
| 42 | Xu Y. S., Zhu L. P., Cui X. X., Zhao M. Y., Li Y. L., Chen L. L., Jiang W. C., Jiang T., Yang S. G., Wang Y., Nano Res., 2020, 13, 752—758 |
| 43 | Shi J. L., Chem, 2017, 2, 468—469 |
| 44 | Chen M. T., Wang N., Zhu L. H., Catal. Today, 2020, 348, 187—193 |
| 45 | Vile G., Sharma P., Nachtegaal M., Tollini F., Moscatelli D., Sroka⁃Bartnicka A., Tomanec O., Petr M., Filip J., Pieta I. S., Zboril R., Gawande M. B., Sol. RRL, 2021, 5, 12 |
| 46 | Millet M. M., Algara⁃Siller G., Wrabetz S., Mazheika A., Girgsdies F., Teschner D., Seitz F., Tarasov A., Leychenko S. V., Schlogl R., Frei E., J. Am. Chem. Soc., 2019, 141, 2451—2461 |
| 47 | Ren Y. J., Tang Y., Zhang L. L., Liu X. Y., Li L., Miao S., Su D. S., Wang A. Q., Li J., Zhang T., Nature Commun., 2019, 10, 9 |
| 48 | Liu K. R., Badamdorj B., Yang F., Janik M. J., Antonietti M., Angew. Chem. Int. Ed., 2021, 60, 24220—24226 |
| 49 | Marcinkowski M. D., Yuk S. F., Doudin N., Smith R. S., Nguyen M. T., Kay B. D., Glezakou V. A., Rousseau R., Dohnalek Z., ACS Catal., 2019, 9, 10977—10982 |
| 50 | Tao X. Q., Long R., Wu D., Hu Y. G., Qiu G. H., Qi Z. M., Li B. X., Jiang R. B., Xiong Y. J., Small, 2020, 16, 11 |
| 51 | Bai S. X., Liu F. F., Huang B. L., Li F., Lin H. P., Wu T., Sun M. Z., Wu J. B., Shao Q., Xu Y., Huang X. Q., Nature Commun., 2020, 11, 9 |
| 52 | Zhao W., Wang J., Yin R., Li B. Y., Huang X. S., Zhao L. L., Qian L., J. Colloid Interface Sci., 2020, 564, 28—36 |
| 53 | Song Z. X., Zhang L., Doyle⁃Davis K., Fu X. Z., Luo J. L., Sun X. L., Adv. Energy Mater., 2020, 10, 42 |
| 54 | Chen Y. J., Zhuo H. Y., Pan Y., Liang J. X., Liu C. G., Li J., Sci. China Mater., 2021, 64, 1939—1951 |
| 55 | Zhang Q., Zhang X. X., Wang J. Z., Wang C. W., Nanotechnology, 2021, 32, 23 |
| 56 | Ge J. M., Zhang D. B., Qin Y., Dou T., Jiang M. H., Zhang F. Z., Lei X. D., Appl. Catal. B, 2021, 298, 8 |
| 57 | Zhang J., Wu X., Cheong W. C., Chen W., Lin R., Li J., Zheng L., Yan W., Gu L., Chen C., Peng Q., Wang D., Li Y., Nat. Commun., 2018, 9, 1002 |
| 58 | Liu J.C., Wang Y.G., Li J., J. Am. Chem. Soc., 2017, 139, 6190—6199 |
| 59 | Qiao B., Wang A., Yang X., Allard L. F., Jiang Z., Cui Y., Liu J., Li J., Zhang T., Nat. Chem., 2011, 3, 634—641 |
| 60 | Wei H., Liu X., Wang A., Zhang L., Qiao B., Yang X., Huang Y., Miao S., Liu J., Zhang T., Nat. Commun., 2014, 5, 5634 |
| 61 | Gong X.Q., Selloni A., Dulub O., Jacobson P., Diebold U., J. Am. Chem. Soc., 2008, 130, 370—381 |
| 62 | Liu L. C., Corma A., Nat. Rev. Mater., 2021, 6, 244—263 |
| 63 | Li G. X., Li Y. L., Liu H. B., Guo Y. B., Li Y. J., Zhu D. B., Chem. Commun., 2010, 46, 3256—3258 |
| 64 | Jia Z., Li Y., Zuo Z., Liu H., Huang C., Li Y., Acc. Chem. Res., 2017, 50, 2470—2478 |
| 65 | Zuo Z., Wang D., Zhang J., Lu F., Li Y., Adv. Mater., 2019, 31, 1803762 |
| 66 | Lu C., Yang Y., Wang J., Fu R., Zhao X., Zhao L., Ming Y., Hu Y., Lin H., Tao X., Li Y., Chen W., Nat. Commun., 2018, 9, 752 |
| 67 | Gao X., Liu H., Wang D., Zhang J., Chem. Soc. Rev., 2019, 48, 908—936 |
| 68 | Li Y. J., Xu L., Liu H. B., Li Y. L., Chem. Soc. Rev., 2014, 43, 2572—2586 |
| 69 | Wang X., Sci. China Chem., 2015, 58, 347—348 |
| 70 | Xue Y., Li Y., Zhang J., Liu Z., Zhao Y., Sci. China Chem., 2018, 61, 765—786 |
| 71 | Li Y., Sci. Sin. Chim., 2017, 47, 1045—1056 |
| 72 | Wang F., Zuo Z., Li L., He F., Lu F., Li Y., Adv. Mater., 2019, 31, 1806272 |
| 73 | Yu H., Xue Y., Huang B., Hui L., Zhang C., Fang Y., Liu Y., Zhao Y., Li Y., Liu H., Li Y., iScience, 2018, 11, 31—41 |
| 74 | Sun M., Wu T., Xue Y., Dougherty A. W., Huang B., Li Y., Yan C.H., Nano Energy, 2019, 62, 754—763 |
| 75 | He T., Matta S. K., Will G., Du A., Small Methods, 2019, 3, 1800419 |
| 76 | Yu H., Xue Y., Hui L., Zhang C., Fang Y., Liu Y., Chen X., Zhang D., Huang B., Li Y., Natl. Sci. Rev., 2021, 8, nwaa213 |
| 77 | Fang Y., Xue Y., Hui L., Chen X., Li Y., J. Mater. Chem. A, 2022, 10, 6073—6077 |
| 78 | Zou H. Y., Rong W. F., Wei S. T., Ji Y. F., Duan L. L., Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 29462—29468 |
| 79 | Yin X. P., Wang H. J., Tang S. F., Lu X. L., Shu M., Si R., Lu T. B., Angew. Chem. Int. Ed., 2018, 57, 9382—9386 |
| 80 | Yu H., Hui L., Xue Y., Liu Y., Fang Y., Xing C., Zhang C., Zhang D., Chen X., Du Y., Wang Z., Gao Y., Huang B., Li Y., Nano Energy, 2020, 72, 104667 |
| 81 | Qi S., Wang J., Song X., Fan Y., Li W., Du A., Zhao M., Sci. Bull., 2020, 65, 995—1002 |
| 82 | Feng Z., Li R., Ma Y., Li Y., Wei D., Tang Y., Dai X., Phys. Chem. Chem. Phys., 2019, 21, 19651—19659 |
| 83 | Gao Y., Cai Z. W., Wu X. C., Lv Z. L., Wu P., Cai C. X., ACS Catal., 2018, 8, 10364—10374 |
| 84 | He T., Matta S. K., Will G., Du A., Small Methods, 2019, 3, n/a1800419 |
| 85 | Zheng Z., Wang Z., Xue Y., He F., Li Y., ACS Mater. Au, 2021, 1, 107—115 |
| 86 | Liu T., Wang G., Bao X., J. Phys. Chem. C, 2021, 125, 26013—26020 |
| 87 | He T. W., Zhang L., Kour G., Du A. J., J. CO2 Util., 2020, 37, 272—277 |
| 88 | Yin X. P., Tang S. F., Zhang C., Wang H. J., Si R., Lu X. L., Lu T. B., J. Mater. Chem. A, 2020, 8, 20925—20930 |
| 89 | Ali S., Lian Z., Li B., ACS Appl. Nano Mater., 2021, 4, 6152—6159 |
| 90 | Xu G. L., Liu F. X., Lu Z. S., Talib S. H., Ma D. W., Yang Z. X., Physica E, 2021, 130, 7 |
| 91 | Sun C. N., Huang S. M., Huang M. R., Zhang X. R., Xu S. S., Wang H., Chen Y. N., Shi X. R., Dalton Trans., 2021, 50, 10867—10879 |
| 92 | Xu G. L., Wang R., Ding Y. C., Lu Z. S., Ma D. W., Yang Z. X., J. Phys. Chem. C, 2018, 122, 23481—23492 |
| 93 | Li J. A., Zhong L. X., Tong L. M., Yu Y., Liu Q., Zhang S. C., Yin C., Qiao L., Li S. Z., Si R., Zhang J., Adv. Funct. Mater., 2019, 29, 9 |
| [1] | 杨静怡, 李庆贺, 乔波涛. 铱单原子和纳米粒子在N2O分解反应中的协同催化[J]. 高等学校化学学报, 2022, 43(9): 20220388. |
| [2] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [3] | 任诗杰, 谯思聪, 刘崇静, 张文华, 宋礼. 铂单原子催化剂同步辐射X射线吸收谱的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220466. |
| [4] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [5] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [6] | 杨静怡, 施思齐, 彭怀涛, 杨其浩, 陈亮. Ga-C3N4单原子催化剂高效光驱动CO2环加成[J]. 高等学校化学学报, 2022, 43(9): 20220349. |
| [7] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| [8] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [9] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [10] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [11] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [12] | 唐全骏, 刘颖馨, 孟蓉炜, 张若天, 凌国维, 张辰. 单原子催化在海洋能源领域的应用[J]. 高等学校化学学报, 2022, 43(9): 20220324. |
| [13] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [14] | 韩付超, 李福进, 陈良, 贺磊义, 姜玉南, 徐守冬, 张鼎, 其鲁. CoSe2/C复合电催化材料修饰隔膜对高载量锂硫电池性能的影响[J]. 高等学校化学学报, 2022, 43(8): 20220163. |
| [15] | 赵润瑶, 纪桂鹏, 刘志敏. 吡咯氮配位单原子铜催化剂的电催化二氧化碳还原性能[J]. 高等学校化学学报, 2022, 43(7): 20220272. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||