

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (3): 671.doi: 10.7503/cjcu20200533
收稿日期:2020-08-06
出版日期:2021-03-10
发布日期:2021-03-08
通讯作者:
徐文洲
E-mail:xuwenzhou@jlu.edu.cn;guanbuyuan@jlu.edu.cn
作者简介:关卜源, 男, 博士, 教授, 主要从事多孔储能微纳材料的设计合成研究. E-mail:
SHE Peihong1, XU Wenzhou2( ), GUAN Buyuan1,3(
), GUAN Buyuan1,3( )
)
Received:2020-08-06
Online:2021-03-10
Published:2021-03-08
Contact:
XU Wenzhou
E-mail:xuwenzhou@jlu.edu.cn;guanbuyuan@jlu.edu.cn
摘要:
由于具有较大的孔道尺寸、 丰富的化学组成以及广阔的应用前景, 大孔道介孔纳米材料近年来引起了科研工作者的广泛关注. 分别利用复合胶束和无机纳米晶作为结构基元进行可控自组装的软模板法和硬模板法是合成这类大孔道介孔纳米材料最有效的两类方法. 本文总结了一系列基于不同类型软模板或硬模板共组装形成大孔道介孔纳米材料的合成方法和策略, 并讨论了所获得的大孔道介孔纳米材料在催化、 能量转换与存储以及生物医学中的应用. 最后, 对利用新型嵌段聚合物或复杂结构纳米晶合成大孔道介孔纳米材料的前景和挑战进行了展望.
中图分类号:
TrendMD:
佘沛鸿, 徐文洲, 关卜源. 大孔道介孔氧化硅/碳基纳米材料的设计合成与应用. 高等学校化学学报, 2021, 42(3): 671.
SHE Peihong, XU Wenzhou, GUAN Buyuan. Synthesis and Application of Silica/carbon-based Large-pore Mesoporous Nanomaterials. Chem. J. Chinese Universities, 2021, 42(3): 671.
| Method | Feature | Typical example |
|---|---|---|
| Soft?templating method | Self?assembly of the amphiphilic templates and framework precursors to form mesoporous nanomaterials | Dendritic mesoporous silica nanoparticles[ Core?shell mesoporous silica nanoparticles[ N?doped carbon nanoparticles[ Macro?/Mesoporous polydopamine nanoparticles[ |
| Hard?templating method | Self?assembly of organic ligands decorated inorganic nanocrystals to form mesostructured nanomaterials | Ordered mesoporous graphene frameworks[ Inorganic nanoparticles accumulated superlattice pipeline tubes[ |
Table 1 Synthetic methods for the large-pore mesoporous nanomaterials.
| Method | Feature | Typical example |
|---|---|---|
| Soft?templating method | Self?assembly of the amphiphilic templates and framework precursors to form mesoporous nanomaterials | Dendritic mesoporous silica nanoparticles[ Core?shell mesoporous silica nanoparticles[ N?doped carbon nanoparticles[ Macro?/Mesoporous polydopamine nanoparticles[ |
| Hard?templating method | Self?assembly of organic ligands decorated inorganic nanocrystals to form mesostructured nanomaterials | Ordered mesoporous graphene frameworks[ Inorganic nanoparticles accumulated superlattice pipeline tubes[ |
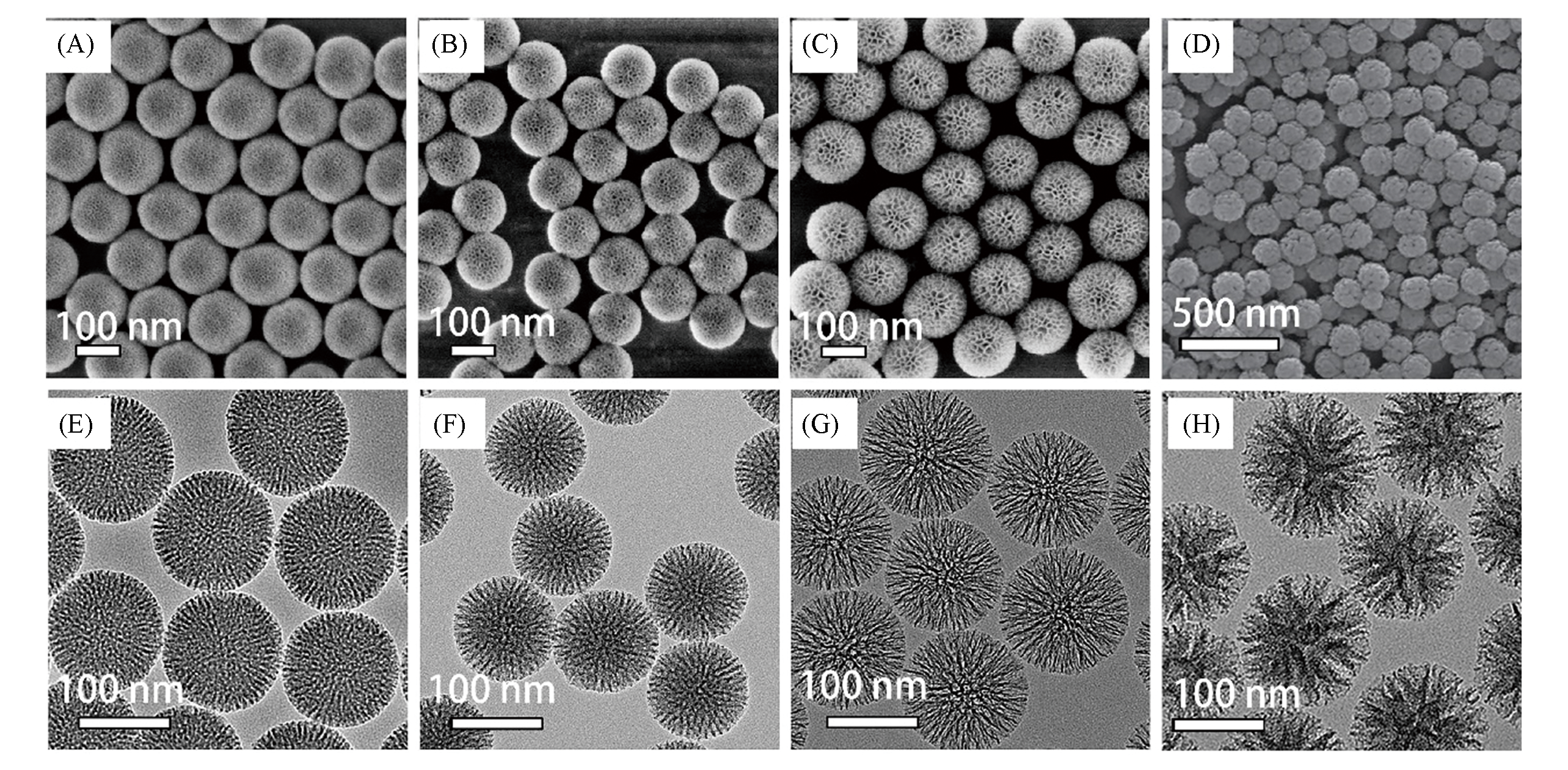
Fig.1 SEM(A—D) and TEM(E—H) images of mesoporous silica nanospheres(MSNSs) with mesopore sizes of 2.8 nm(A, E), 5.5 nm(B, F), 10 nm(C, G), and ca. 30 nm(D, H)[31,32]note:(A)—(C), (E)—(G) Copyright 2014, American Chemical Society; (D), (H) Copyright 2017, American Chemical Society.
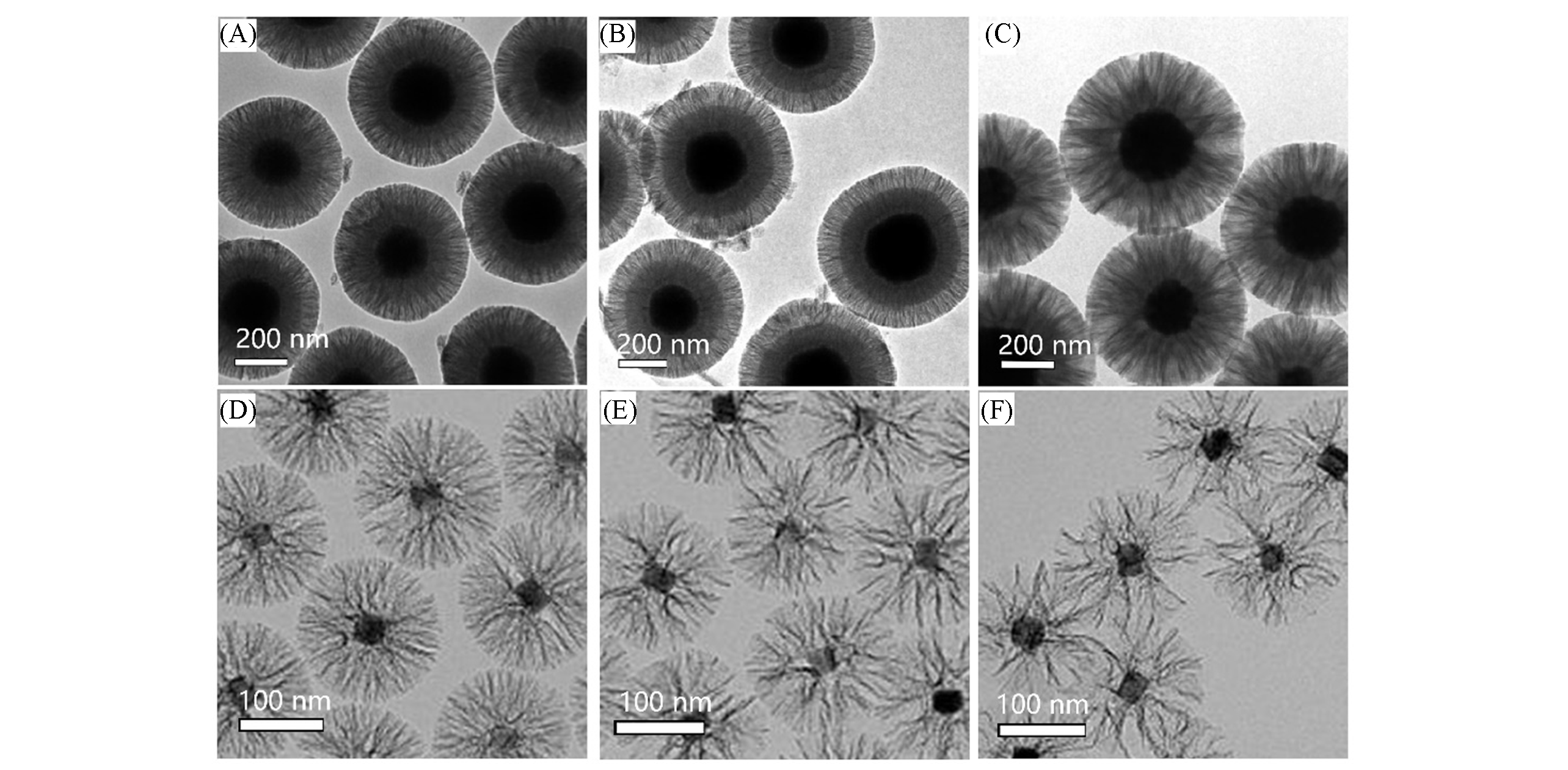
Fig.2 TEM images of core?shell magnetic mesoporous silica microspheres prepared at different stirring speed(A―C)[33] and UCNP@dMSNs prepared with different amounts of n?butanol(D―F)[35]note:Stirring speed/(r·min-1): (A) 170; (B) 250; (C) 500. V(n-Butanol)/mL: (D) 0.4; (E) 0.6; (F) 0.8. (A)―(C) Copyright 2015, American Chemical Society. (D)―(F) Copyright 2020, Royal Society of Chemistry.
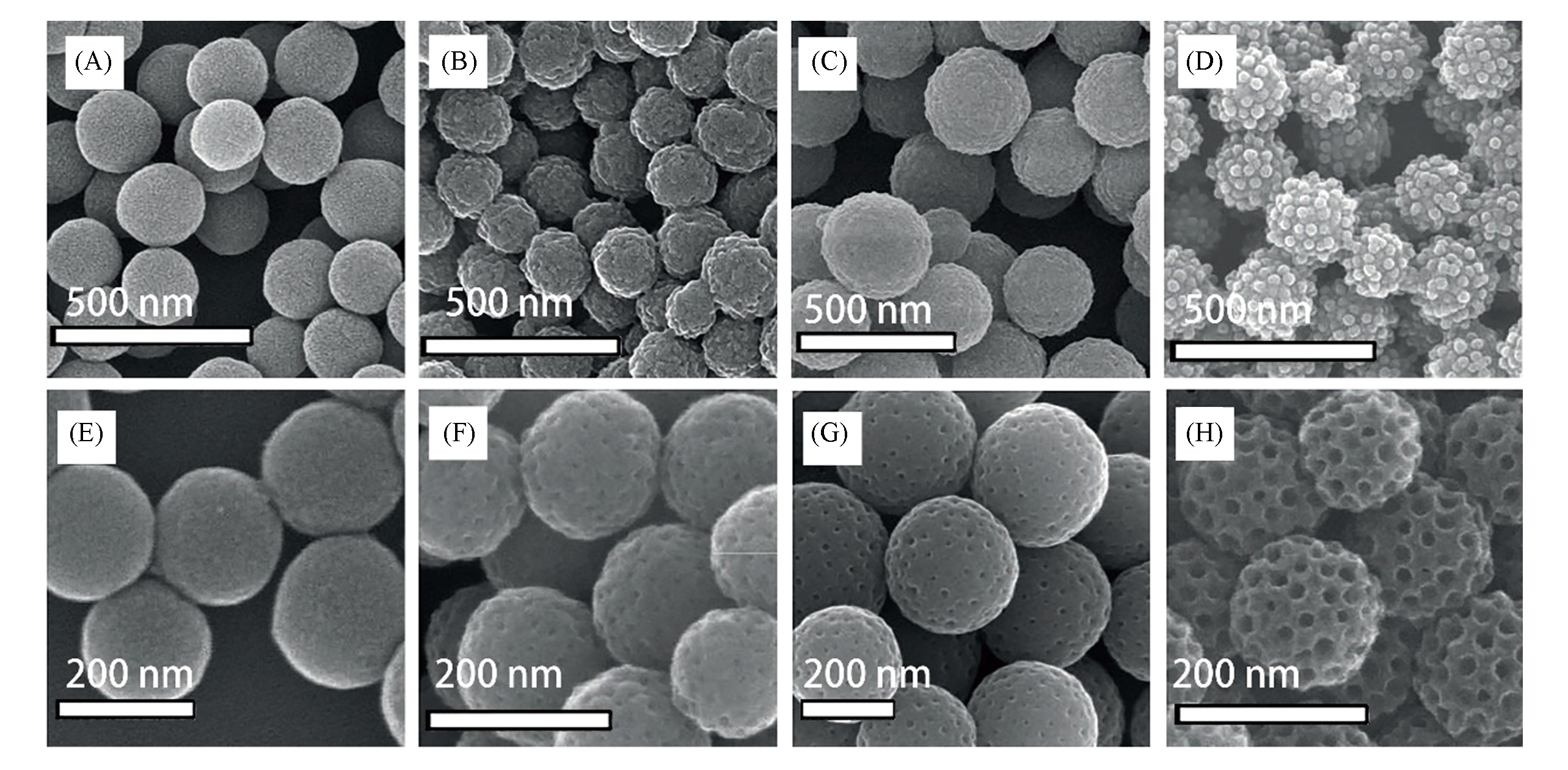
Fig.3 SEM images of polydopamine(PDA) spheres(A), PDA/PS37?b?PEO114 composite spheres(B), PDA/PS178?b?PEO886 composite spheres(C) and PDA/PS173?b?PEO170 composite spheres(D) and the corresponding N?doped carbon spheres(NCS, E) and N?doped mesoporous carbon spheres(NMCS) carbonized at 800 ℃(F―H)[36]note:Copyright 2015, John Wiley and Sons.
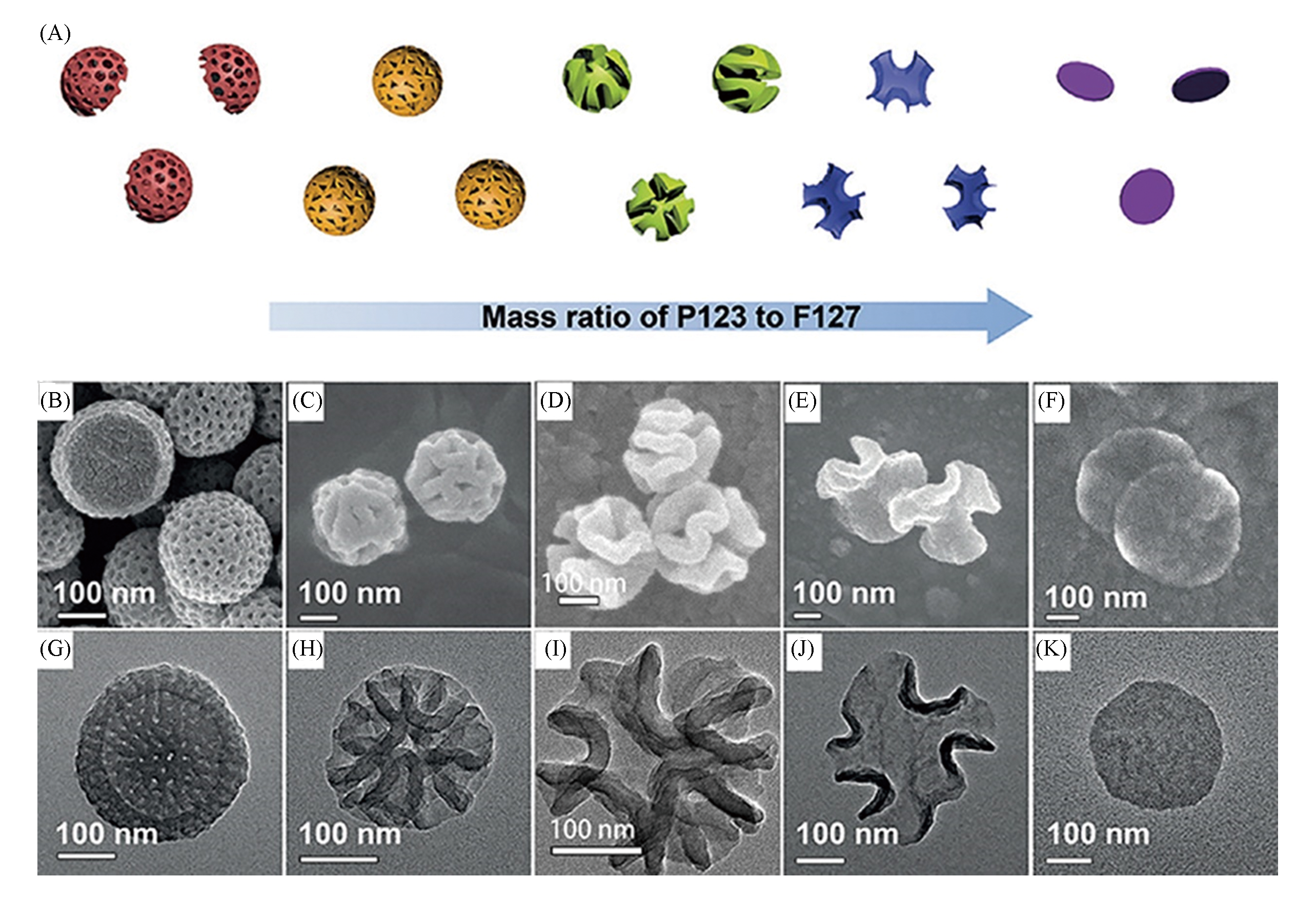
Fig.4 Schematic representation of mesophase transition of polydopamine particles prepared by increasing the P123/F127 mass ratio(A), SEM(B―F) and TEM(G―K) images of polydopamine particles prepared with different mass ratios of P123 to F127[37]note:Mass ratio of P123 to P127: (B, G) 0∶1; (C, H) 1∶15; (D, I) 1∶3; (E, J) 1∶1; (F, K) 5∶3.Copyright 2018, John Wiley and Sons.
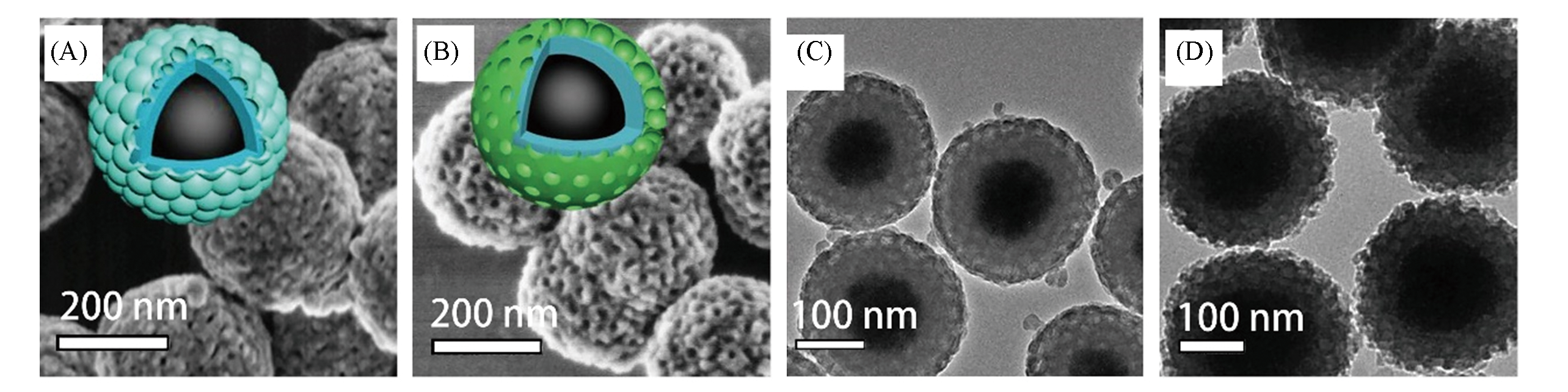
Fig.5 SEM(A, B) and TEM(C, D) images of Fe3O4@nSiO2@mSiO2(A, C) and Fe3O4@nSiO2@mZrO2/SiO2(B, D) microspheres[39]note:Copyright 2018, John Wiley and Sons.
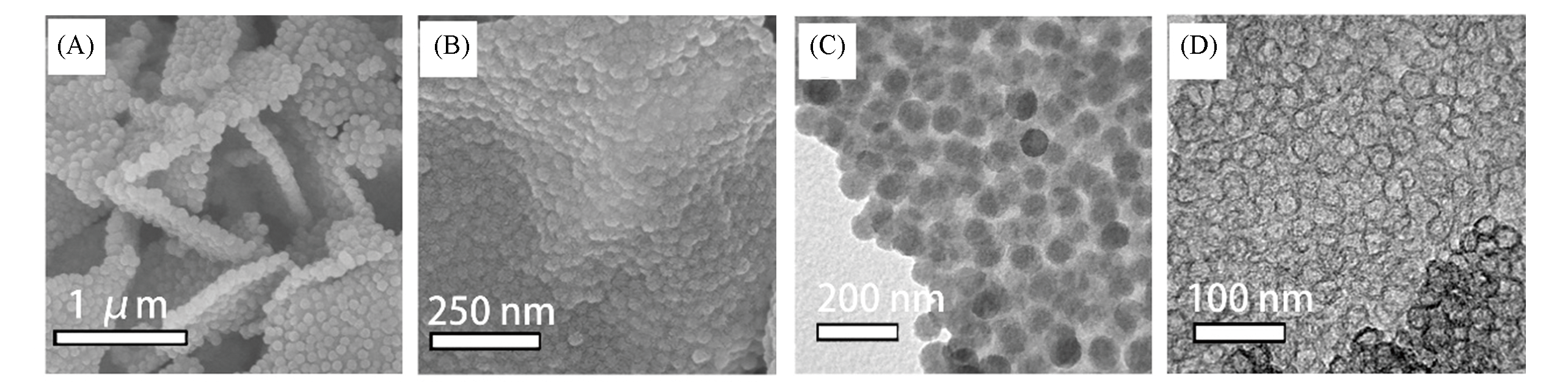
Fig.6 SEM(A, B) and TEM(C, D) images of Fe5.0?monomicelle@M?FR/GO precursor(A, C) and Fe1.6?N?HCNS/rGO?900 product(B, D)[40]note:Copyright 2018, American Chemical Society.
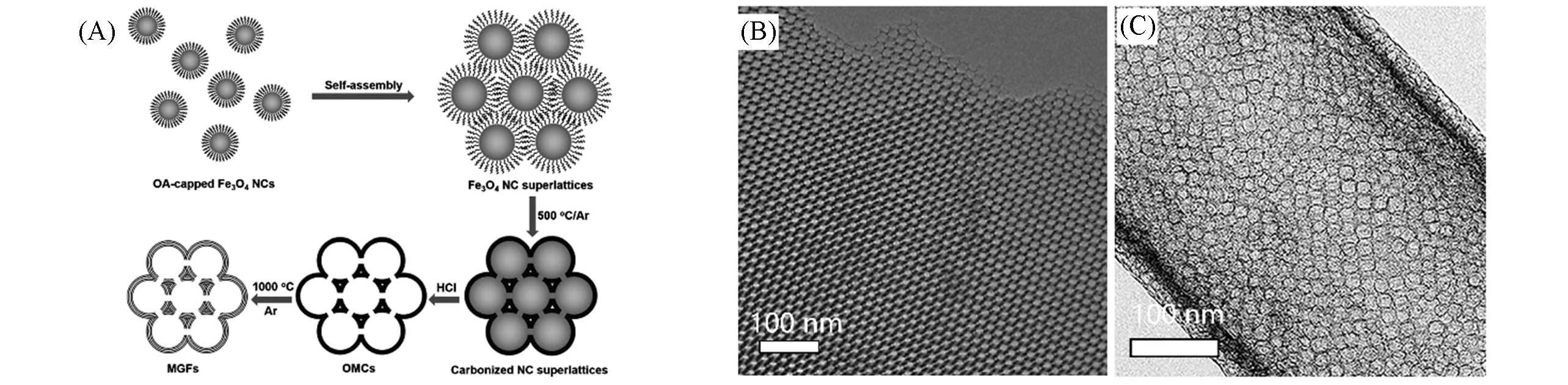
Fig.7 Schematic illustration of the fabrication of MGFs from colloidal Fe3O4 NCs(A), TEM image of Mn?Fe?N/S@mC(B)[41] and TEM image of tubular carbon frameworks resulting from the leaching of Fe3O4 NPs(C)[24]note:(A, B) Copyright 2015, John Wiley and Sons. (C) Copyright 2019, Elsevier.
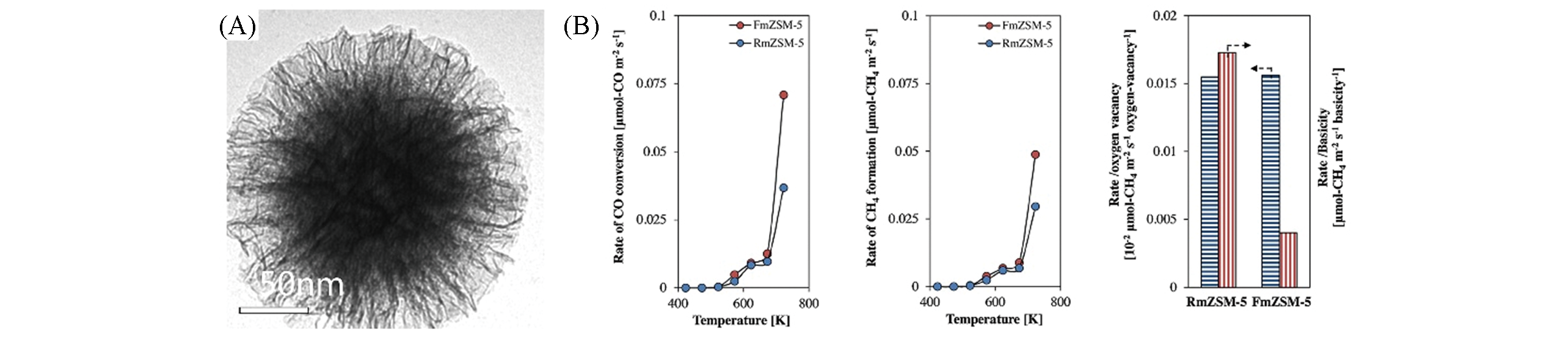
Fig.8 TEM image of FmZSM?5(A) and rate of CO conversion and rate of CH4 formation as a function of the reaction temperature at GHSV=13500 mL·g-1·h-1 and H2/CO=8/1, and oxygen vacancy and basicity dependences of the rate of CH4 formation at 723 K over RmZSM?5 and FmZSM?5(B)[19]note:Copyright 2016, Elsevier.
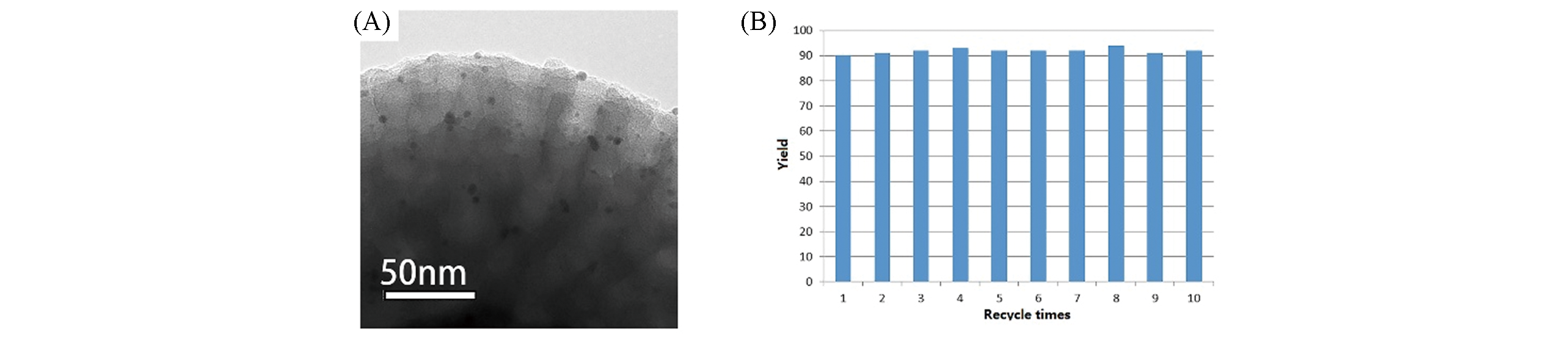
Fig.9 TEM image of CS?MMAS?Au microspheres showing that the Au NPs were distributed in the mesopores(A) and catalytic performance of CS?MMAS?Au catalyst for the N?alkylation of aniline with benzyl alcohol after recycling 1—10 times(B)[39]note:Copyright 2018, John Wiley and Sons.
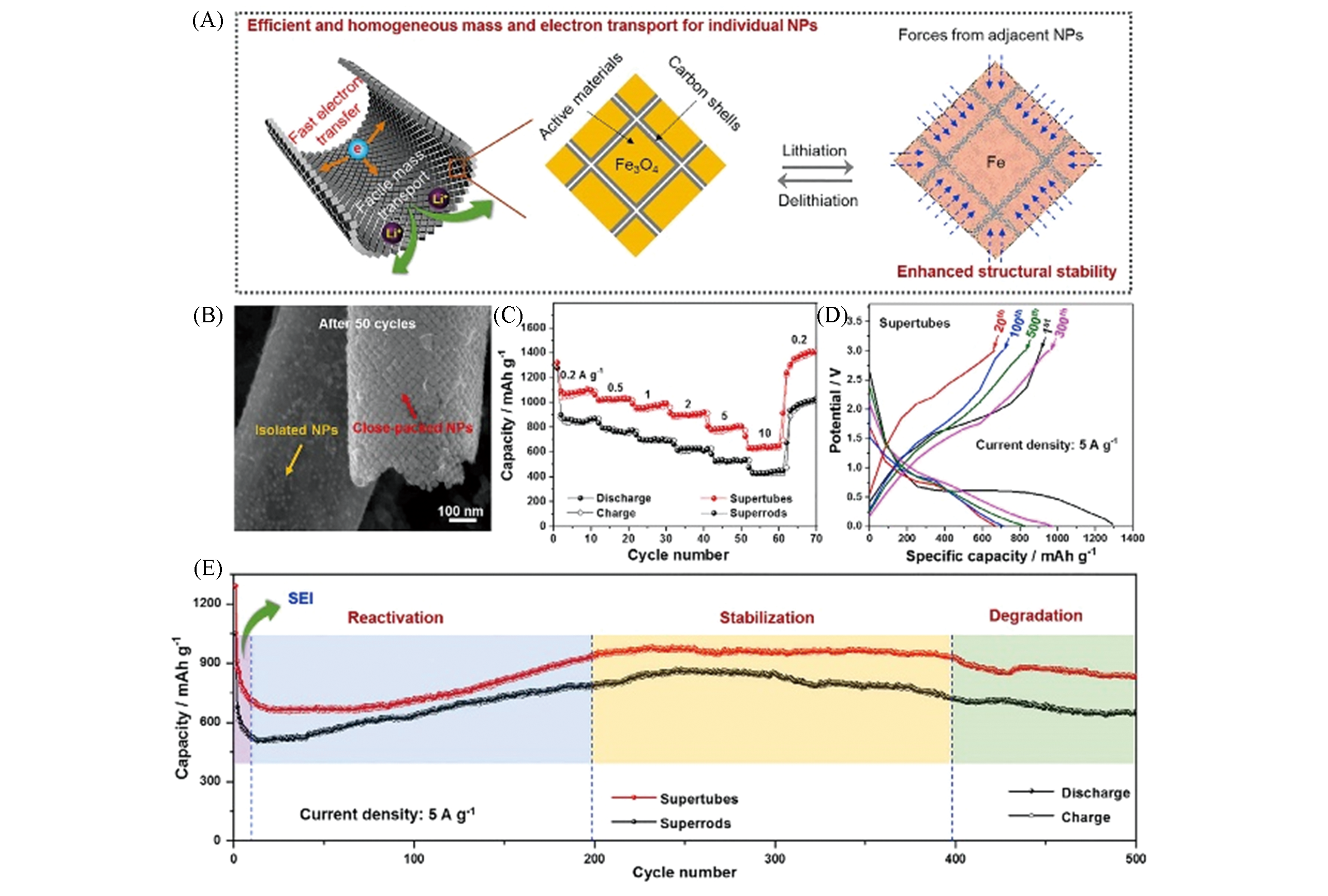
Fig.10 Schematic of the lithiation/delithiation of Fe3O4 supertubes, showing the enhanced electrochemical performance and structural stability enabled by the tubular monolayer superlattice geometry(A), SEM image of the controlled Fe3O4 supertube sample after 50 cycles at 0.2 A/g(B), rate performance of Fe3O4 supertubes and super?rods(C), galvanostatic charge/discharge voltage profifiles of Fe3O4 supertubes at different cycles(D) and cycling performance of Fe3O4supertubes and super?rods at 5 A/g(E)[24]note:Copyright 2019, Elsevier.
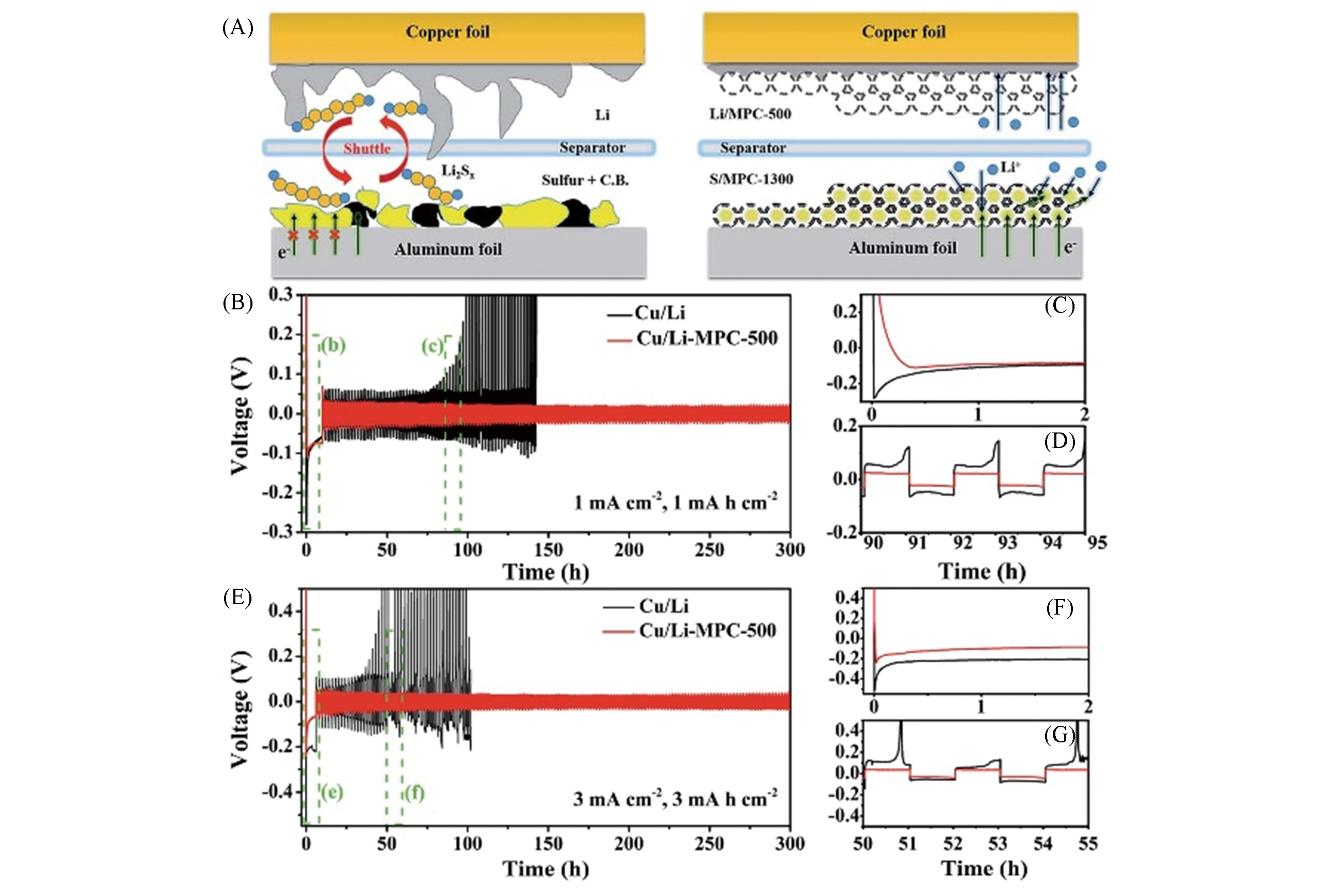
Fig.11 Control Li?S battery assembled with bare Li on Cu as the anode, and sulfur composited with carbon black and binder as the cathode(A), comparison of the cycling performance of Cu/Li and Cu/Li?MPC?500 anodes at a current density of 1 mA/cm2 and an areal charge of 1 mA·h·cm-2(B—D) and at a current density of 3 mA/cm2 and an areal charge of 3 mA·h·cm-2(E—G)[43]note:(C) and (D) Plating/stripping curves in the initial stage(C) and between 90 to 95 h(D); (F) and (G) the plating/stripping curves in the initial stage(F), between 50 to 55 h(G). Copyright 2019, Royal Society of Chemistry.
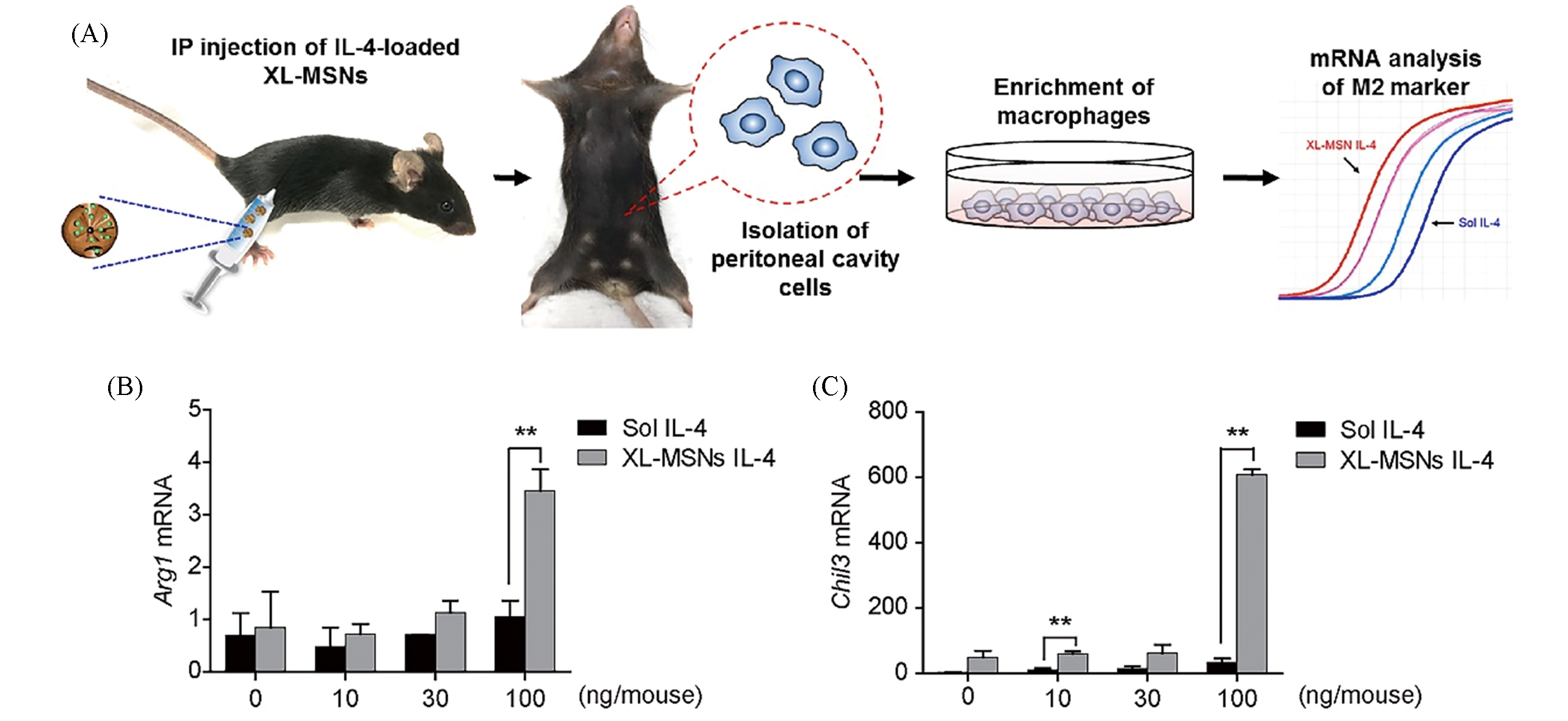
Fig.12 Scheme for analysis of M2 polarization by XL?MSNs IL?4 or Sol IL?4 invivo(A) and the results(B, C)[28]note:Mice were administered intraperitoneally with soluble IL-4(Sol IL-4) or 180 nm XL-MSNs(200 μg/mouse) loaded with IL-4(XL-MSNs IL-4) at the indicated doses of IL-4(0, 10, 30, 100 ng/mouse). Cells in the peritoneal cavity were harvested 3 d later, and peritoneal macrophages were enriched by attaching to the culture plates and used for measuring M2 gene expression, the mRNA levels(B, C) were quantifified by RT?qPCR and are presented as the relative amount normalized to the mRNA level of F4/80.Copyright 2017, American Chemical Society.
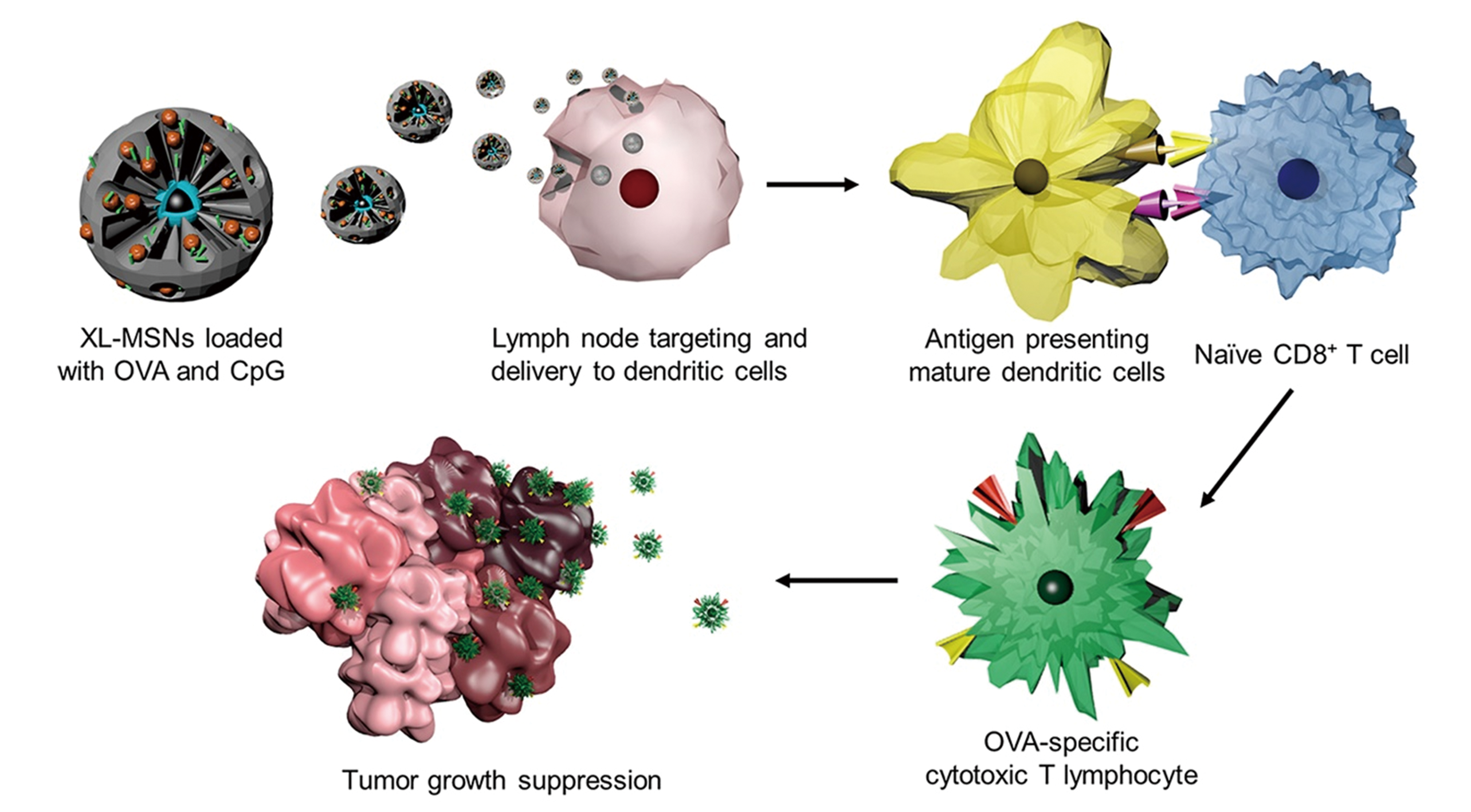
Fig.13 Extra?large pore mesoporous silica nanoparticles(XL?MSNs) loaded with antigen and TLR9 agonist to invoke antigen?specific cytotoxic T lymphocytes(CTLs) to suppress tumor growth[44]note:Copyright 2018, John Wiley and Sons.
| 1 | Liu J., Wickramaratne N. P., Qiao S. Z. , Jaroniec M., Nat. Mater., 2015, 14,763—774 |
| 2 | Wan Y. , Zhao D., Chem. Rev., 2007, 107,2821—2860 |
| 3 | Giordano F., Abate A., Baena J. P. C., Saliba M., Matsui T., Im S. H., Zakeeruddin S. M., Nazeeruddin M. K., Hagfeldt A. , Graetzel M., Nat. Commun., 2016, 7,10379 |
| 4 | Li W., Liu J. , Zhao D., Nat. Rev. Mater., 2016, 1,16023 |
| 5 | Cui Z., Zu C., Zhou W., Manthiram A. , Goodenough J. B., Adv. Mater., 2016, 28,6926—6931 |
| 6 | Zhao D., Feng J., Huo Q., Melosh N., Fredrickson G. H., Chmelka B. F., Stucky G., Science, 1998, 279,548—552 |
| 7 | Che S., Liu Z., Ohsuna T., Sakamoto K., Terasaki O. , Tatsumi T., Nature, 2004, 429,281—284 |
| 8 | Gao X., Li G., Xu Y., Hong Z., Liang C. , Lin Z., Angew. Chem., Int. Ed., 2015, 54,14331—14335 |
| 9 | Naushad M., Ahamad T., Al-Maswari B. M., Alqadami A. A. , Alshehri S. M., Chem. Eng. J., 2017, 330,1351—1360 |
| 10 | Le Z., Liu F., Nie P., Li X., Liu X., Bian Z., Chen G., Wu H. B. , Lu Y., ACS Nano, 2017, 11,2952—2960 |
| 11 | Benzigar M. R., Talapaneni S. N., Joseph S., Ramadass K., Singh G., Scaranto J., Ravon U., Al-Bahily K., Vinu A., Chem. Soc. Rev., 2018, 47,2680—2721 |
| 12 | Cook J. B., Kim H. S., Yan Y., Ko J. S., Robbennolt S., Dunn B. , Tolbert S. H., Adv. Energy Mater., 2016, 6, 1501937 |
| 13 | Kuang M., Wang Q., Han P. , Zheng G., Adv. Energy Mater., 2017, 7, 1700193 |
| 14 | Lakhi K. S., Park D. H., Al⁃Bahily K., Cha W., Viswanathan B., Choy J. H., Vinu A., Chem. Soc. Rev., 2017, 46,72—101 |
| 15 | Liu J., Zheng M., Shi X., Zeng H. , Xia H., Adv. Funct. Mater., 2016, 26,919—930 |
| 16 | Singh R., Belgamwar R., Dhiman M. , Polshettiwar V., J. Mater. Chem. B, 2018, 6,1600—1604 |
| 17 | Abbaraju P. L., Meka A. K., Song H., Yang Y., Jambhrunkar M., Zhang J., Xu C., Yu M. , Yu C., J. Am. Chem. Soc., 2017, 139,6321—6328 |
| 18 | Fan J., Yu C. Z., Lei J., Zhang Q., Li T. C., Tu B., Zhou W. Z. , Zhao D. Y., J. Am. Chem. Soc., 2005, 127,10794—10795 |
| 19 | Teh L. P., Triwahyono S., Jalil A. A., Firmansyah M. L., Mamat C. R. , Majid Z. A., Appl. Catal. A, 2016, 523,200—208 |
| 20 | Wang B., Zou J., Shen X., Yang Y., Hu G., Li W., Peng Z., Banham D., Dong A. , Zhao D., Nano Energy, 2019, 63, 103851 |
| 21 | de Vos D. E., Dams M., Sels B. F. , Jacobs P. A., Chem. Rev., 2002, 102,3615—3640 |
| 22 | Huang H. S., Chang K. H., Suzuki N., Yamauchi Y., Hu C. C. , Wu K. C., Small, 2013, 9,2520—2526 |
| 23 | Su L., Han D., Zhu G., Xu H., Luo W., Wang L., Jiang W., Dong A. , Yang J., Nano Lett., 2019, 19,5423—5430 |
| 24 | Li T., Wang B., Ning J., Li W., Guo G., Han D., Xue B., Zou J., Wu G., Yang Y., Dong A. , Zhao D., Matter, 2019, 1,976—987 |
| 25 | Su Y. S., Manthiram A., Nat. Commun., 2012, 3,1166 |
| 26 | Xing W., Qiao S. Z., Ding R. G., Li F., Lu G. Q., Yan Z. F. , Cheng H. M., Carbon, 2006, 44,216—224 |
| 27 | Gai S., Yang P., Ma P., Wang L., Li C., Zhang M., Jun L., Dalton Trans., 2012, 41,4511—4516 |
| 28 | Kwon D., Cha B. G., Cho Y., Min J., Park E. B., Kang S. J., Kim J., Nano Lett., 2017, 17,2747—2756 |
| 29 | Li Z., Barnes J. C., Bosoy A., Stoddart J. F. , Zink J. I., Chem. Soc. Rev., 2012, 41,2590—2605 |
| 30 | Tang F., Li L. , Chen D., Adv. Mater., 2012, 24,1504—1534 |
| 31 | Shen D., Yang J., Li X., Zhou L., Zhang R., Li W., Chen L., Wang R., Zhang F. , Zhao D., Nano Lett., 2014, 14,923—932 |
| 32 | Huang M., Liu L., Wang S., Zhu H., Wu D., Yu Z., Zhou S., Langmuir, 2017, 33,519—526 |
| 33 | Yue Q., Li J., Luo W., Zhang Y., Elzatahry A. A., Wang X., Wang C., Li W., Cheng X., Alghamdi A., Abdullah A. M., Deng Y., Zhao D., J. Am. Chem. Soc., 2015, 137,13282—13289 |
| 34 | Qu Q., Min Y., Zhang L., Xu Q., Yin Y., Anal. Chem., 2015, 87,9631—9638 |
| 35 | Dai Y., Yang D., Yu D., Xie S., Wang B., Bu J., Shen B., Feng W., Li F., Nanoscale, 2020, 12,5075—5083 |
| 36 | Tang J., Liu J., Li C., Li Y., Tade M. O., Dai S. , Yamauchi Y., Angew. Chem. Int. Ed., 2015, 54,588—593 |
| 37 | Guan B. Y., Zhang S. L. , Lou X. W., Angew. Chem. Int. Ed., 2018, 57,6176—6180 |
| 38 | Li C., Iqbal M., Jiang B., Wang Z., Kim J., Nanjundan A. K., Whitten A. E., Wood K. , Yamauchi Y., Chem. Sci., 2019, 10,4054—4061 |
| 39 | Zhang Y., Yue Q., Yu L., Yang X., Hou X. F., Zhao D., Cheng X. , Deng Y., Adv. Mater., 2018, 30,e1800345 |
| 40 | Tan H., Tang J., Henzie J., Li Y., Xu X., Chen T., Wang Z., Wang J., Ide Y., Bando Y., Yamauchi Y., ACS Nano, 2018, 12,5674—5683 |
| 41 | Jiao Y., Han D., Liu L., Ji L., Guo G., Hu J., Yang D., Dong A., Angew. Chem. Int. Ed., 2015, 54,5727—5731 |
| 42 | Fihri A., Cha D., Bouhrara M., Almana N. , Polshettiwar V., ChemSusChem, 2012, 5,85—89 |
| 43 | Cao D., Jiao Y., Cai Q., Han D., Zhang Q., Ma Y., Dong A. , Zhu H., J. Mater. Chem. A, 2019, 7,3289—3297 |
| 44 | Ding B., Shao S., Yu C., Teng B., Wang M., Cheng Z., Wong K. L., Ma P. , Lin J., Adv. Mater., 2018, 30,e1802479 |
| 45 | Cha B. G., Jeong J. H. , Kim J., ACS Cent. Sci., 2018, 4,484—492 |
| [1] | 郭程, 张威, 唐云. 有序介孔材料: 历史、 现状与发展趋势[J]. 高等学校化学学报, 2022, 43(8): 20220167. |
| [2] | 章丽玲, 刘浏, 郑明秋, 方文凯, 刘达, 唐宏武. 基于上转换发光共振能量转移的CRISPR/Cas12a生物传感系统用于HPV16 DNA双信号检测[J]. 高等学校化学学报, 2022, 43(11): 20220412. |
| [3] | 丁中振, 李天, 李长明, 赵宇飞, 宋宇飞. 水滑石基催化剂催化合成碳纳米材料的研究进展[J]. 高等学校化学学报, 2021, 42(6): 1622. |
| [4] | 王常耀, 王帅, 段林林, 朱晓航, 张兴淼, 李伟. 原位限域生长策略制备有序介孔碳负载的超小MoO3纳米颗粒[J]. 高等学校化学学报, 2021, 42(5): 1589. |
| [5] | 杨瑞琪, 于欣, 刘宏. 四氧化三锡基光催化纳米材料的研究进展[J]. 高等学校化学学报, 2021, 42(5): 1340. |
| [6] | 葛浩英, 杜健军, 龙飒然, 孙文, 樊江莉, 彭孝军. 功能化金纳米材料在肿瘤诊疗中的研究与应用[J]. 高等学校化学学报, 2021, 42(4): 1202. |
| [7] | 郑海娇, 姜丽艳, 贾琼. 精氨酸功能化磁性纳米材料的制备及在磷酸化肽富集中的应用[J]. 高等学校化学学报, 2021, 42(3): 717. |
| [8] | 万月, 宋美娜, 赵美廷. 二维金属有机框架纳米片的合成及在超电容和电催化领域的应用[J]. 高等学校化学学报, 2021, 42(2): 575. |
| [9] | 史江维, 孟楠楠, 郭亚梅, 于一夫, 张兵 . 二维材料用于电催化析氢的研究进展[J]. 高等学校化学学报, 2021, 42(2): 492. |
| [10] | 皮业灿, 张应, 成子方, 黄小青. 二维金属纳米材料的合成及电催化应用的研究进展[J]. 高等学校化学学报, 2021, 42(2): 456. |
| [11] | 王军, 王铁. 基于自组装技术的纳米功能材料研究进展[J]. 高等学校化学学报, 2020, 41(3): 377. |
| [12] | 盛炳琛, 李从, 刘颖雅, 王安杰, 王瑶, 张箭, 刘伟旭. 微通道连续合成UiO-66系列改性MOF材料[J]. 高等学校化学学报, 2019, 40(7): 1365. |
| [13] | 陈艳, 董雪娇, 单桂晔. 脂质体@Ag/Au中空纳米壳层材料的制备及与H2O2的作用[J]. 高等学校化学学报, 2019, 40(4): 639. |
| [14] | 田龙,龙艳,宋术岩,王成. 花状结构Mn/CuO-CeO2的合成及对CO氧化反应的催化性能[J]. 高等学校化学学报, 2019, 40(12): 2549. |
| [15] | 孙琳, 张涵, 杜一平. 基于SBA-15的表面增强拉曼基底的制备及对鸡肉和鸡饲料中恩诺沙星的检测[J]. 高等学校化学学报, 2018, 39(3): 455. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||