

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (12): 2598.doi: 10.7503/cjcu20200505
• 庆祝《高等学校化学学报》复刊40周年专栏 • 上一篇 下一篇
收稿日期:2020-07-31
出版日期:2020-12-10
发布日期:2020-09-16
通讯作者:
白玉
E-mail:yu.bai@pku.edu.cn
基金资助:
AI Wanpeng, SONG Shiyao, BAI Yu( ), LIU Huwei
), LIU Huwei
Received:2020-07-31
Online:2020-12-10
Published:2020-09-16
Contact:
BAI Yu
E-mail:yu.bai@pku.edu.cn
Supported by:摘要:
质谱因具有分辨率高、 灵敏度好、 响应快速以及结构鉴定能力强等特点, 近年来在反应监测研究领域应用广泛. 本文介绍了基于质谱的经典在线直接采样实时监测方案; 综合评述了常压质谱离子化技术在反应监测领域的发展和应用, 主要包括基于常压质谱的快反应监测、 微滴加速在长时间反应研究中的应用, 以及其它常压质谱在反应监测中的应用; 并对质谱在反应监测研究领域面临的挑战和发展趋势进行了总结和展望.
中图分类号:
TrendMD:
艾万鹏, 宋诗瑶, 白玉, 刘虎威. 质谱技术在反应监测中的发展和应用. 高等学校化学学报, 2020, 41(12): 2598.
AI Wanpeng, SONG Shiyao, BAI Yu, LIU Huwei. Development and Applications of Mass Spectrometry in Reaction Monitoring. Chem. J. Chinese Universities, 2020, 41(12): 2598.
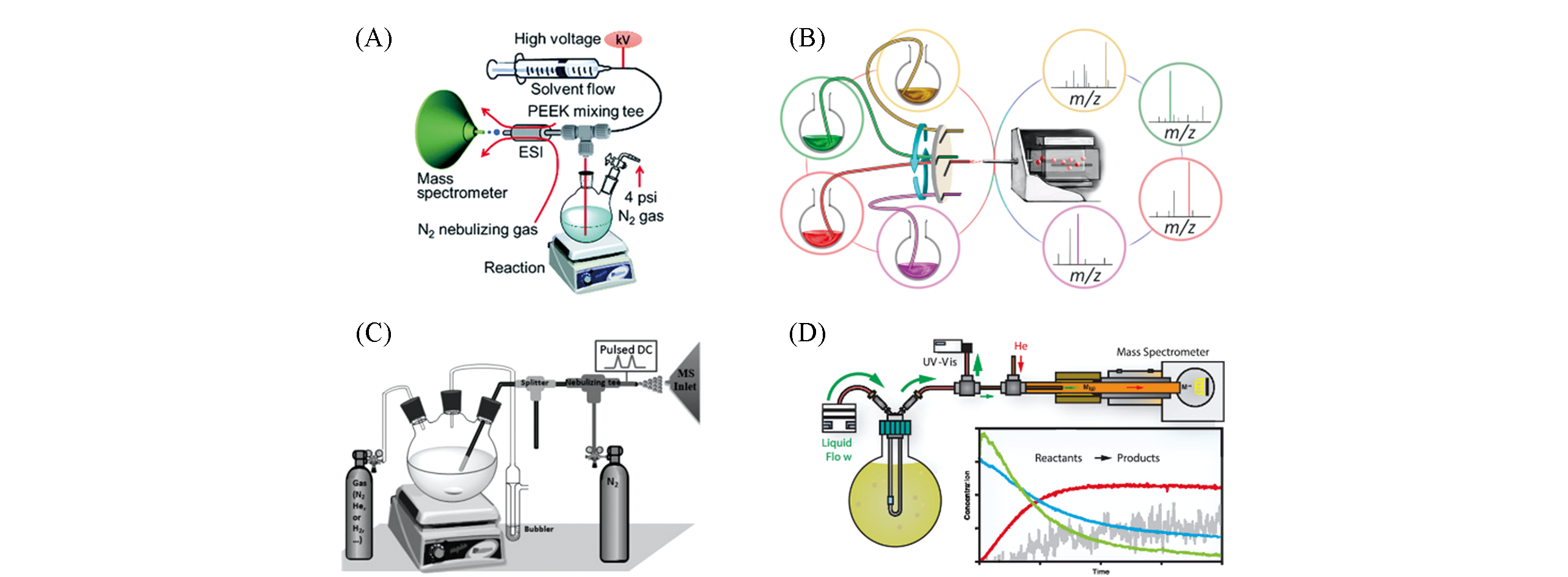
Fig.1 Real?time reaction monitoring using CSI MS online direct sampling(A) Schematic diagram of CSI MS[16]; Copyright 2017, Royal Society of Chemistry.(B) Multi-channel CSI MS device[20]; Copyright 2017, American Chemical Society. (C) Inductive ESI MS[21]; Copyright 2014, John Wiley and Sons. (D) Schematic diagram of CP-MIMS-LEI device[23]; Copyright 2019, American Chemical Society.
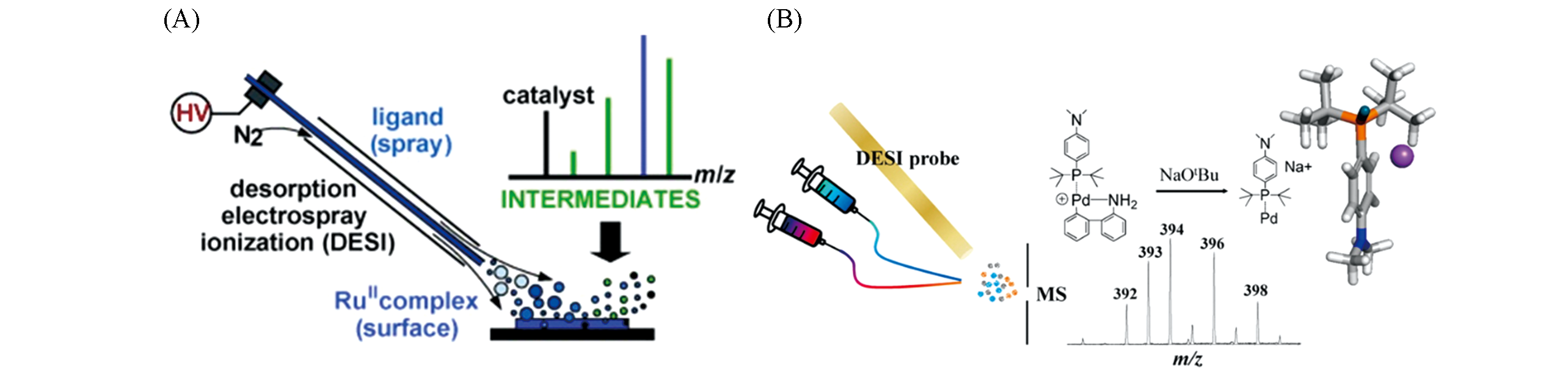
Fig.2 Application of DESI?MS in reaction monitoring(A) Detection of reaction intermediates by bombardment of surface-bound Ru(Ⅱ) complex with charged microdroplets containing ligand[29]; Copyright ? 2010, John Wiley and Sons. (B) Capture of reactive monophosphine-ligated palladium(0) intermediates using DESI-MS[33]; Copyright ? 2015, American Chemical Society.
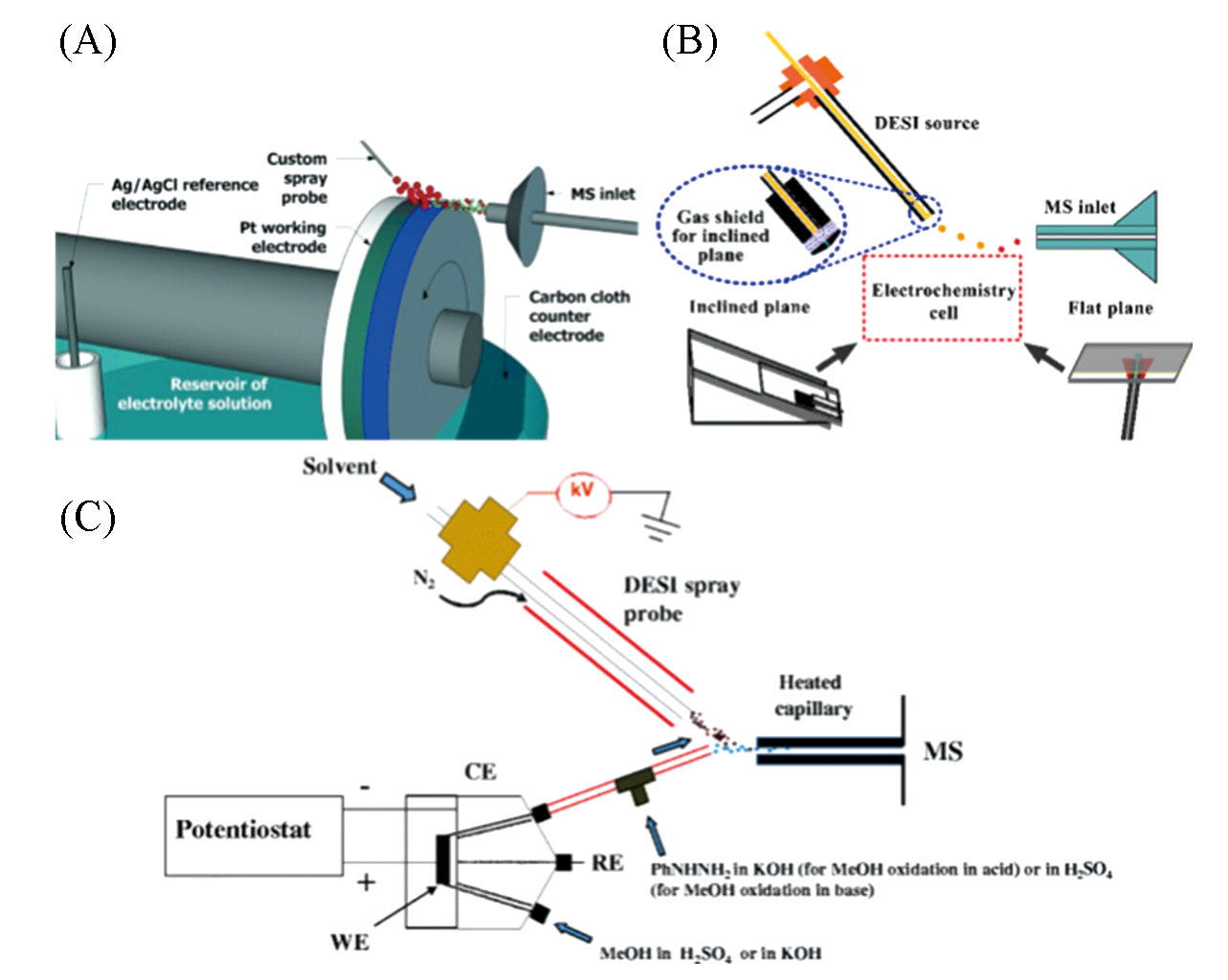
Fig.3 Direct monitoring of electrochemical reaction using DESI?MS(A) DESI integrated water wheel device[35,37]; Copyright 2015, John Wiley and Sons. (B) DESI electrochemical MS platform(bevel and plane)[38]; Copyright 2017, American Chemical Society. (C) Coupling of DESI-MS to electrochemical cell[39]; Copyright 2017, American Chemical Society.
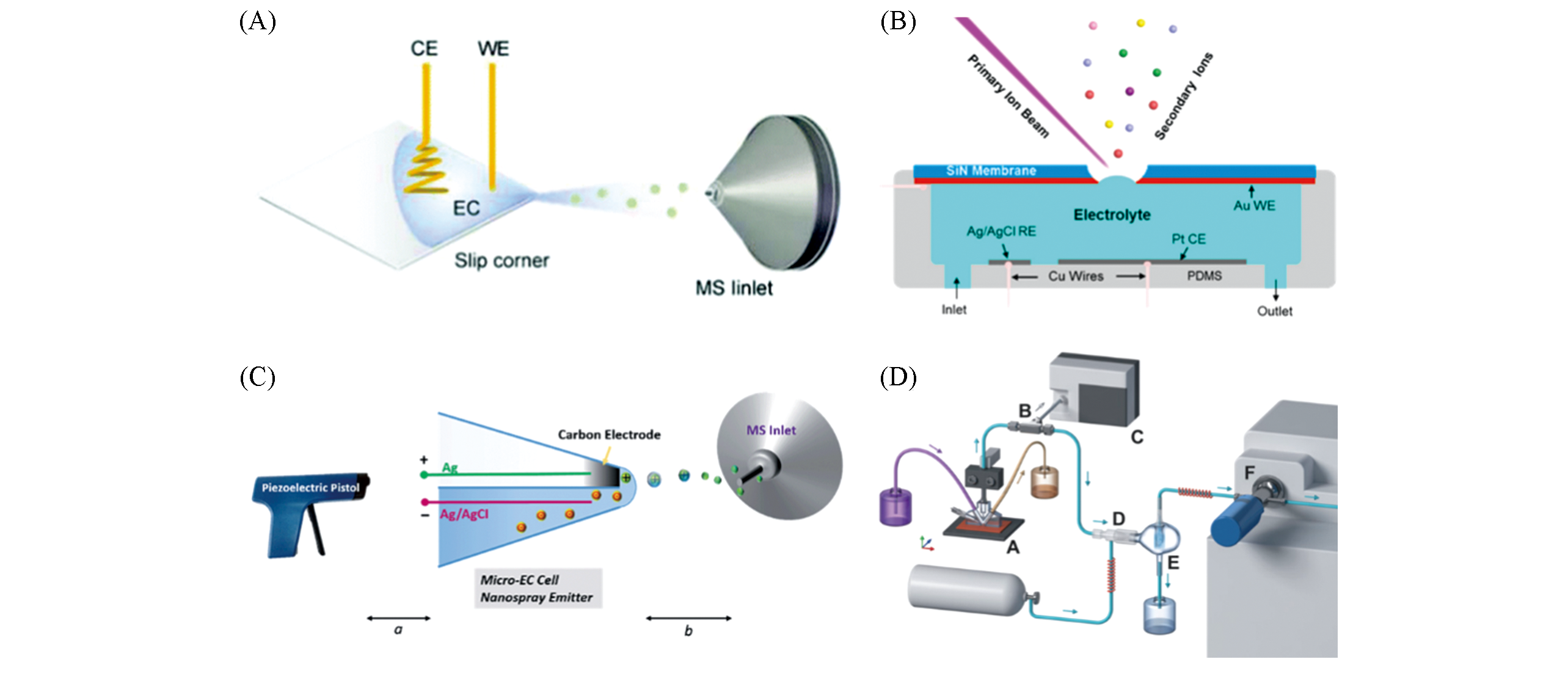
Fig.4 Other examples of electrochemical reaction monitoring(A) Droplet-scale electrochemical reaction screening setup[40]; Copyright 2018, Royal Society of Chemistry. (B) SALVI system[41]; Copyright 2017, American Chemical Society. (C) Hybrid ultramicroelectrodes[42]; Copyringht 2016, Royal Society of Chemistry. (D) Electrochemical real-time mass spectrometry for simultaneous monitoring of gaseous and liquid chemicals[44]; Copyright 2019, John Wiley and Sons.
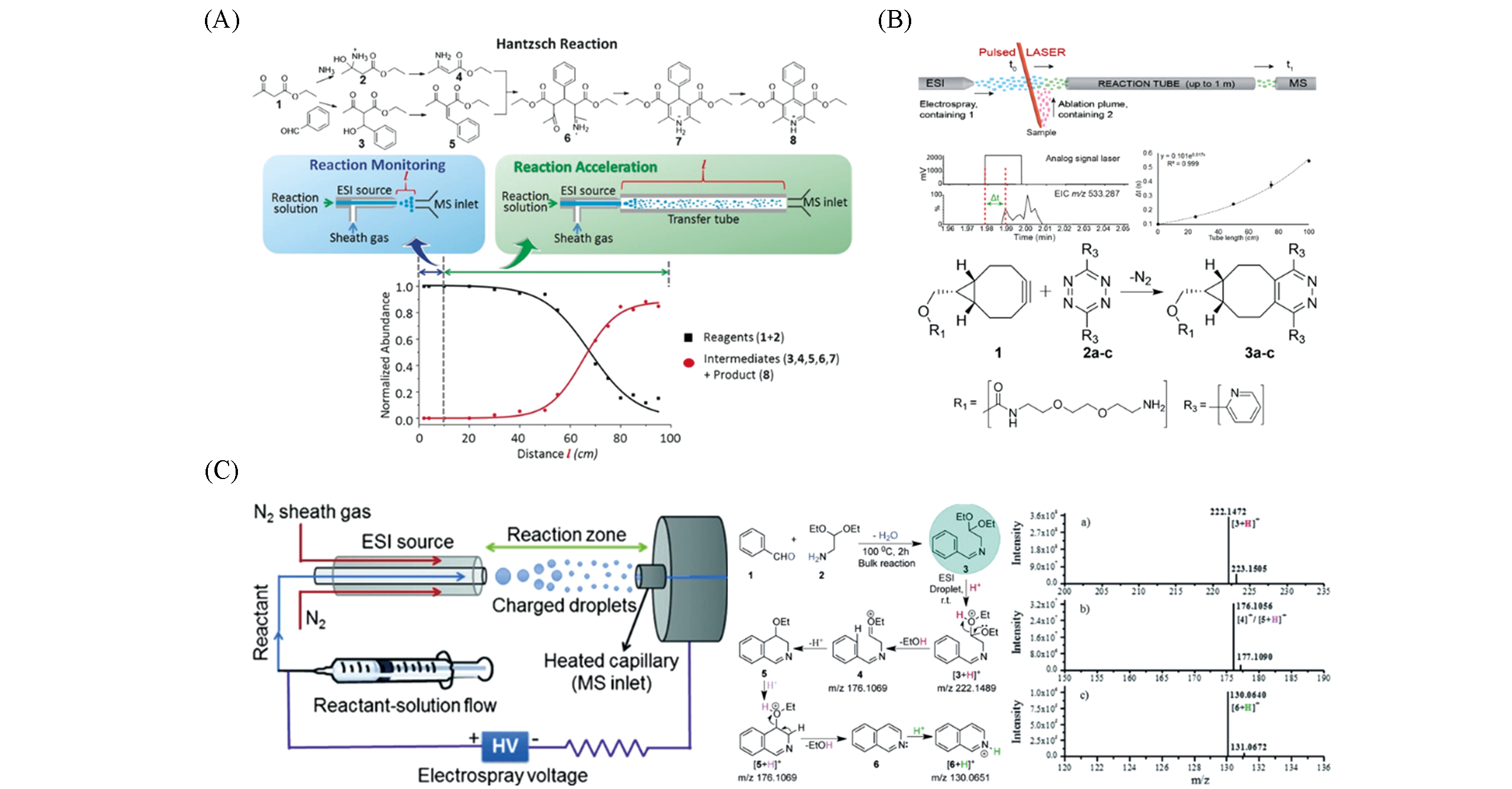
Fig.5 Application of microdroplet acceleration based on ESI?MS in reaction monitoring(A) The correlation between reaction acceleration and reaction monitoring of the Hantzsch reaction in ESI droplet and the distance between MS inlet and spray source[49]; Copyright 2016, John Wiley and Sons. (B) Monitoring of the formation of click reaction products using LAESI MS[51]; Copyright 2018, American Chemical Society. (C) Synthesis of isoquinoline and substituted quinolines in charged microdroplets[52]; Copyright 2015, John Wiley and Sons.
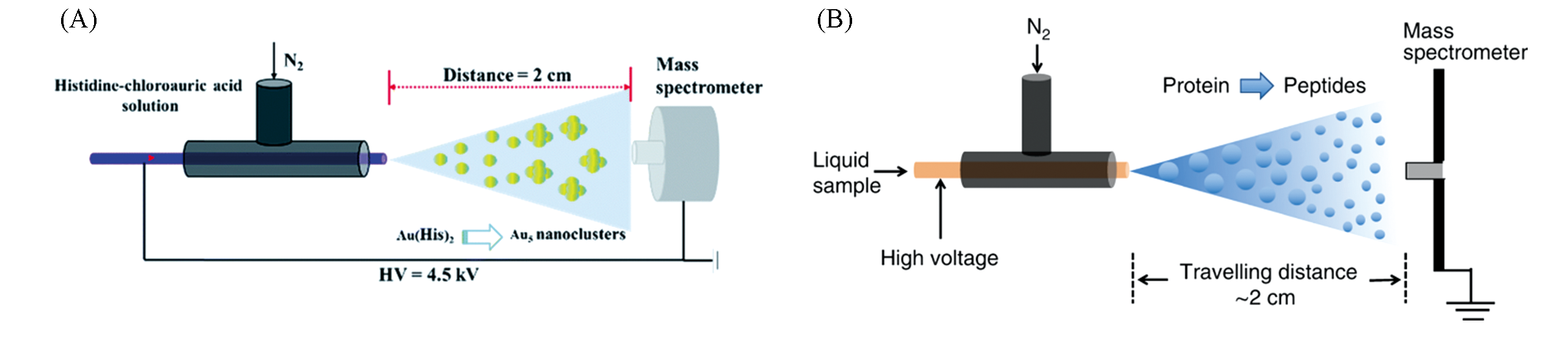
Fig.6 Application of ESSI?MS?based microdroplet acceleration in reaction monitoring(A) Accelerated synthesis Au-(His)2 complex catalyst using ESSI-MS[58]; Copyright 2020, Royal Society of Chemistry.(B) accelerated proteolysis using ESSI-MS[59]; Copyright 2020, Springer Nature.
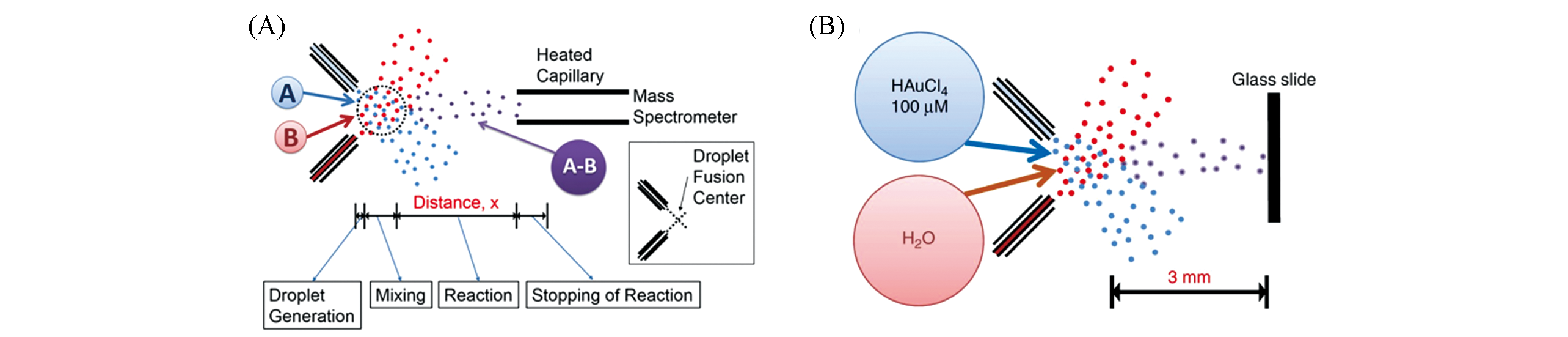
Fig.7 Application of microdroplet acceleration based on EESI?MS in reaction monitoring(A) Experimental schematic diagram of EESI-MS for the study of microdroplet reaction kinetics[61]; Copyright 2015, Proceedings of the National Academy of Sciences. (B) Gold nanoparticle synthesis using EESI-MS[63]; Copyright 2018, Springer Nature.
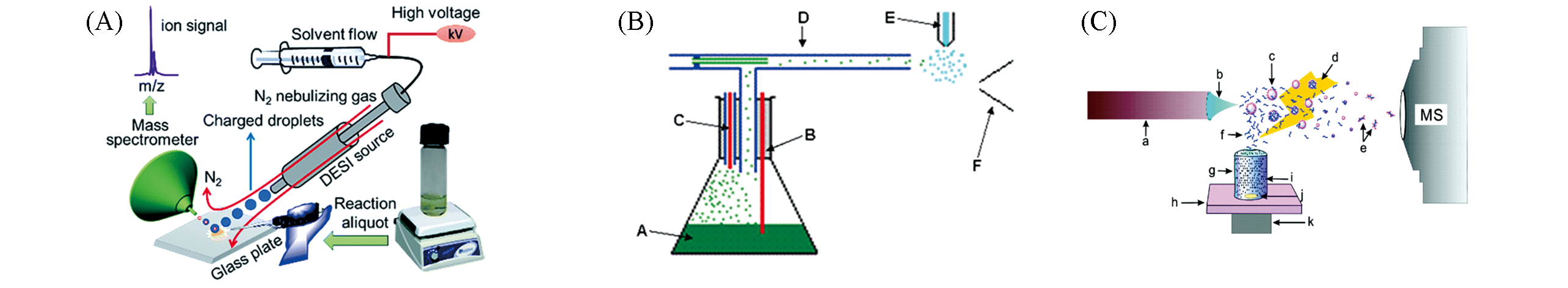
Fig.8 Ambient mass spectrometry used for online direct sampling real?time reaction monitoring(A) DESI online direct sampling[16]; Copyright 2017, Royal Society of Chemistry. (B) EESI sampling[70]; Copyright 2011, John Wiley and Sons. (C) ELDI matrix-assisted sampling[73]; Copyright 2008, American Chemical Society.
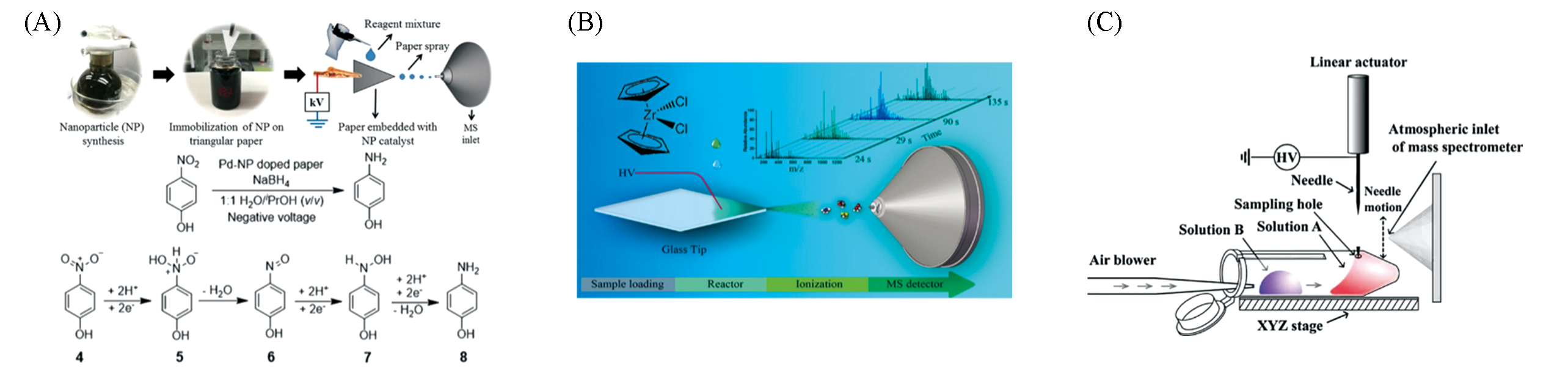
Fig.9 Application of substrate spray in reaction monitoring(A) Heterogeneous catalytic reaction study using nano-particles coated PSI(the reaction shows the 4-nitrophenol reduction)[75]; Copyright 2016, John Wiley and Sons. (B) Real-time monitoring of ethylene polymerization reactions by DSI MS[77]; Copyright 2015, American Chemical Society. (C) PESI MS real-time reaction monitoring device[79]; Copyright 2010, John Wiley and Sons.
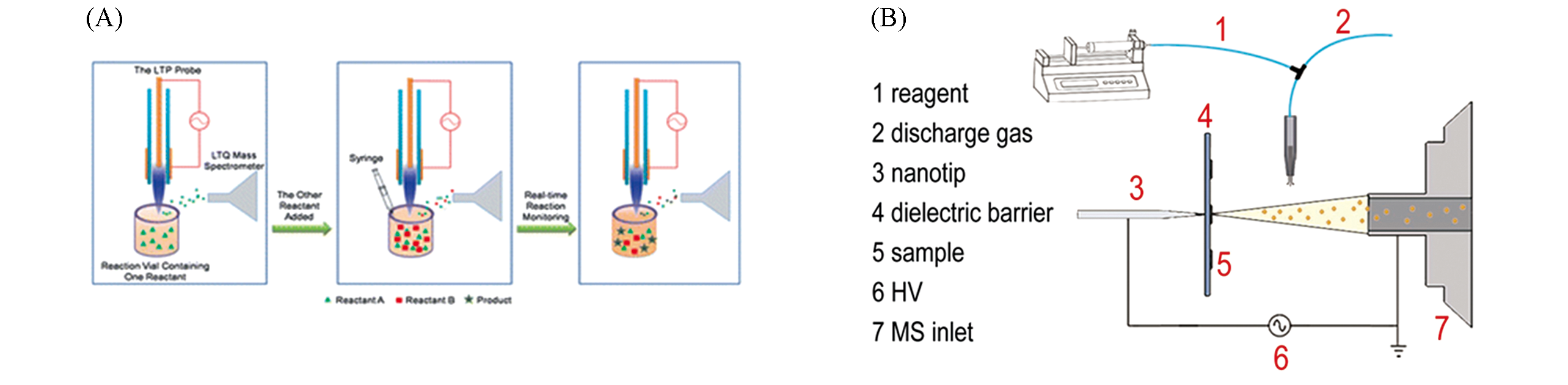
Fig.10 Application of plasma?based ambient ionization in reaction monitoring(A) The procedure of reaction monitoring using LTP MS [81]; Copyright 2009, Royal Society of Chemistry.(B) Schematic diagram of SDDBDI device[82]; Copyright 2018, John Wiley and Sons.
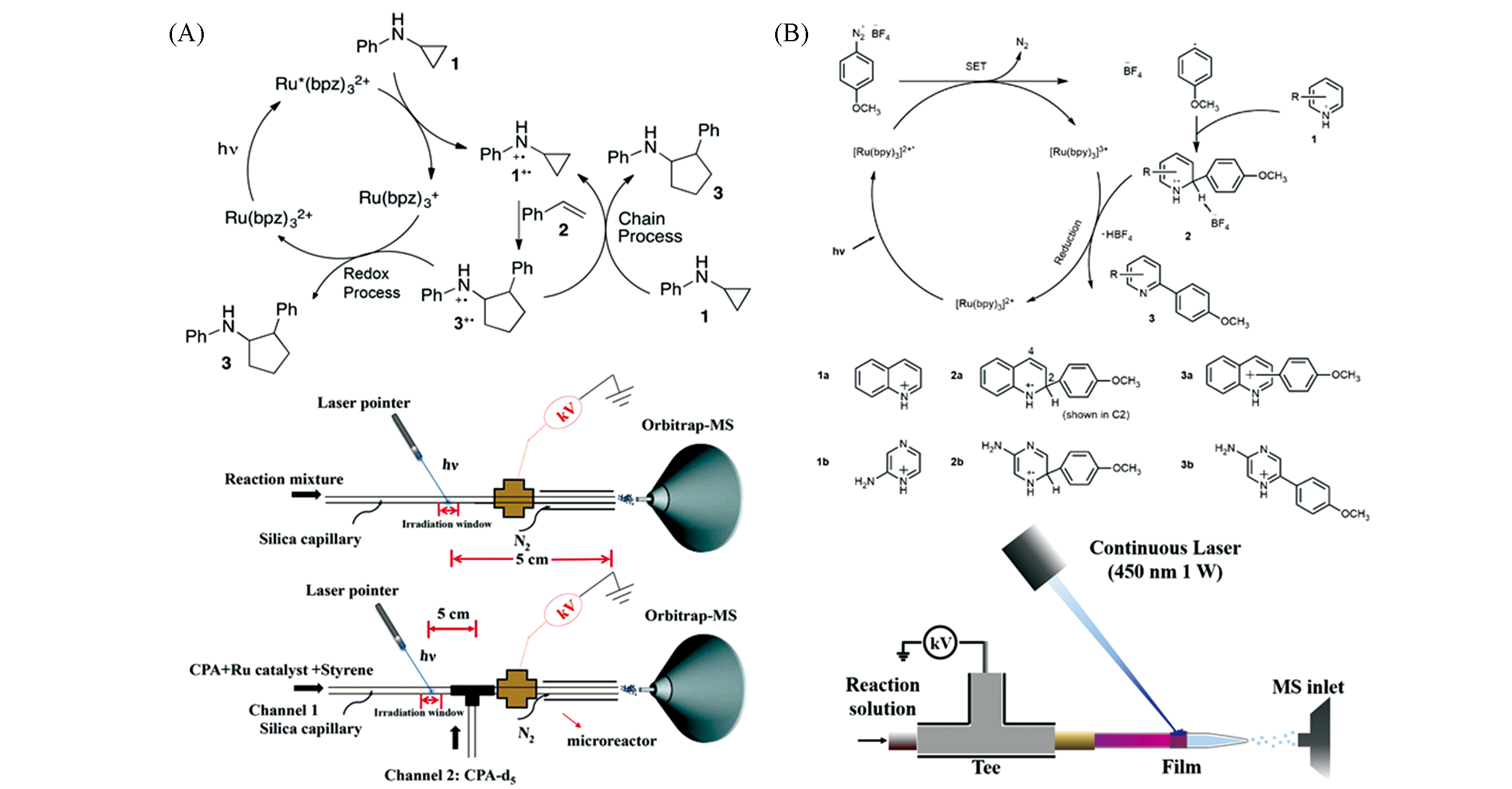
Fig.11 Application of laser?based ambient ionization in reaction monitoring(A) Detection of fleeting amine radical cations and elucidation of chain processes in visible-light-mediated [3+2] annulation by direct laser irradiation[85]; Copyright 2017, American Chemical Society. (B) The in?situ LS ESI-MS and its application in the mechanism of photo-induced direct C—H arylation of heteroarenes[87]; Copyright 2020, American Chemical Society.
| 1 | Schroder D., Acc. Chem. Res., 2012, 45(9), 1521—1532 |
| 2 | Arceo E., Jurberg I. D., Álvarez⁃Fernández A., Melchiorre P., Nat. Chem., 2013, 5(9), 750—756 |
| 3 | Brimioulle R., Bach T., Science, 2013, 342(6160), 840 |
| 4 | Zhong J. J., Meng Q. Y., Liu B., Li X. B., Gao X. W., Lei T., Wu C. J., Li Z. J., Tung C. H., Wu L. Z., Org. Lett., 2014, 16(7), 1988—1991 |
| 5 | Zhong J. J., Wu C. J., Meng Q. Y., Gao X. W., Lei T., Tung C. H., Wu L. Z., Adv. Synth. Catal., 2014, 356(13), 2846—2852 |
| 6 | Wang T., Schrempp M., Berndhauser A., Schiemann O., Menche D., Org. Lett., 2015, 17(16), 3982—3985 |
| 7 | de Carvalho G. S. G., Granato Á. S., de Castro P. P., Amarante G. W., Curr. Organocatal., 2019, 7(1), 7—22 |
| 8 | Ingold K. U., Pratt D. A., Chem. Rev., 2014, 114(18), 9022—9046 |
| 9 | Farr E. P., Quintana J. C., Reynoso V., Ruberry J. D., Shin W. R., Swartz K. R, J. Chem. Educ., 2018, 95(5), 864—871 |
| 10 | Tsang A. S. K., Sanhueza I A., Schoenebeck F., Chem. Eur. J., 2014, 20(50), 16432—16441 |
| 11 | Sperger T., Sanhueza I. A., Kalvet I., Schoenebeck F., Chem. Rev., 2015, 115(17), 9532—9586 |
| 12 | Cooks R. G., Ouyang Z., Takats Z., Wiseman J. M., Science, 2006, 311(5767), 1566 |
| 13 | Harris G. A., Galhena A. S., Fernández F. M., Anal. Chem., 2011, 83(12), 4508—4538 |
| 14 | Vikse K. L., Woods M. P., McIndoe J. S., Organometallics, 2010, 29(23), 6615—6618 |
| 15 | Yunker L. P., Stoddard R. L., McIndoe J. S., J. Mass Spectrom., 2014, 49(1), 1—8 |
| 16 | Banerjee S., Sathyamoorthi S., Du Bois J., Zare R. N., Chem. Sci., 2017, 8(10), 7003—7008 |
| 17 | Ingram A. J., Walker K. L., Zare R. N., Waymouth R. M., J. Am. Chem. Soc., 2015, 137(42), 13632—13646 |
| 18 | Sathyamoorthi S., Lai Y. H., Bain R. M., Zare R. N., J. Org. Chem., 2018, 83(10), 5681—5687 |
| 19 | Mack J. B. C., Walker K. L., Robinson S. G., Zare R. N., Sigman M. S., Waymouth R. M., Du Bois J., J. Am. Chem. Soc., 2019, 141(2), 972—980 |
| 20 | Pulliam C. J., Bain R. M., Osswald H. L., Snyder D. T., Fedick P. W., Ayrton S. T., Flick T. G., Cooks R. G., Anal. Chem., 2017, 89(13), 6969—6975 |
| 21 | Yan X., Sokol E., Li X., Li G., Xu S., Cooks R. G., Angew. Chem. Int. Ed., 2014, 53(23), 5931—5935 |
| 22 | Yan X., Bain R. M., Li Y., Qiu R., Flick T. G., Cooks R. G., Org. Process Res. Dev., 2016, 20(5), 940—947 |
| 23 | Termopoli V., Torrisi E., Famiglini G., Palma P., Zappia G., Cappiello A., Vandergrift G. W., Zvekic M., Krogh E. T., Gill C. G., Anal. Chem., 2019, 91(18), 11916—11922 |
| 24 | Takáts Z., Wiseman J. M., Gologan B., Cooks R. G., Science, 2004, 306(5695), 471—473 |
| 25 | Cody R. B., Laramee J. A., Durst H. D., Anal. Chem., 2005, 77(8), 2297—2302 |
| 26 | Green F., Salter T., Gilmore I., Stokes P., O’Connor G., Analyst, 2010, 135(4), 731—737 |
| 27 | Badu-Tawiah A., Cooks R. G., J. Am. Soc. Mass. Spectrom., 2010, 21(8), 1423—1431 |
| 28 | Badu-Tawiah A., Bland C., Campbell D. I., Cooks R. G., J. Am. Soc. Mass. Spectrom., 2010, 21(4), 572—579 |
| 29 | Perry R. H., Splendore M., Chien A., Davis N. K., Zare R. N., Angew. Chem. Int. Ed., 2011, 50(1), 250—254 |
| 30 | Perry R. H., Brownell K. R., Chingin K., Cahill T J, 3rd., Waymouth R. M., Zare R. N., Proc. Natl. Acad. Sci. USA, 2012, 109(7), 2246—2250 |
| 31 | Perry R. H., Cahill T. J., Roizen J. L., Du Bois J., Zare R. N., Proc. Natl. Acad. Sci. USA, 2012, 109(45), 18295—18299 |
| 32 | Xu G., Chen B., Guo B., He D., Yao S., Analyst, 2011, 136(11), 2385—2390 |
| 33 | Zheng Q., Liu Y., Chen Q., Hu M., Helmy R., Sherer E. C., Welch C. J., Chen H., J. Am. Chem. Soc., 2015, 137(44), 14035—14038 |
| 34 | Lu M., Su Y., Zhao P., Ye X., Cai Y., Shi X., Masson E., Li F., Campbell J. L., Chen H., Chem. Eur. J., 2018, 24(9), 2144—2150 |
| 35 | Brown T. A., Chen H., Zare R. N., J. Am. Chem. Soc., 2015, 137(23), 7274—7277 |
| 36 | Brown T. A., Hosseini⁃Nassab N., Chen H., Zare R. N., Chem. Sci., 2016, 7(1), 329—332 |
| 37 | Brown T. A., Chen H., Zare R. N., Angew. Chem. Int. Ed., 2015, 54(38), 11183—11185 |
| 38 | Cheng H., Yan X., Zare R. N., Anal. Chem., 2017, 89(5), 3191—3198 |
| 39 | Cheng S., Wu Q., Dewald H. D., Chen H., J. Am. Soc. Mass. Spectrom., 2017, 28(6), 1005—1012 |
| 40 | Zhang H., Yu K., Li N., He J., Qiao L., Li M., Wang Y., Zhang D., Jiang J., Zare R. N., Analyst, 2018, 143(18), 4247—4250 |
| 41 | Wang Z., Zhang Y., Liu B., Wu K., Thevuthasan S., Baer D. R., Zhu Z., Yu X. Y., Wang F., Anal. Chem., 2017, 89(1), 960—965 |
| 42 | Qiu R., Zhang X., Luo H., Shao Y., Chem. Sci., 2016, 7(11), 6684—6688 |
| 43 | Gu C., Nie X., Jiang J., Chen Z., Dong Y., Zhang X., Liu J., Yu Z., Zhu Z., Liu J., Liu X., Shao Y., J. Am. Chem. Soc., 2019, 141(33), 13212—13221 |
| 44 | Khanipour P., Loffler M., Reichert A. M., Haase F. T., Mayrhofer K. J. J., Katsounaros I., Angew. Chem. Int. Ed., 2019, 58(22), 7273—7277 |
| 45 | Chen H., Venter A., Cooks R. G., Chem. Commun., 2006, 19, 2042—2044 |
| 46 | Huang G., Li G., Ducan J., Ouyang Z., Cooks R. G., Angew. Chem. Int. Ed., 2011, 50(11), 2503—2506 |
| 47 | Huang G. M., Li G. T., Cooks R. G., Angew. Chem. Int. Ed., 2011, 50(42), 9907—9910 |
| 48 | Alvim H. G. O., Bataglion G. A., Ramos L. M., de Oliveira A. L., de Oliveira H. C. B., Eberlin M. N., de Macedo J. L., da Silva W. A., Neto B. A. D., Tetrahedron, 2014, 70(20), 3306—3313 |
| 49 | Bain R. M., Pulliam C. J., Cooks R. G., Chem. Sci., 2015, 6(1), 397—401 |
| 50 | Yan X., Bain R. M., Cooks R. G., Angew. Chem. Int. Ed., 2016, 55(42), 12960—12972 |
| 51 | van Geenen F., Franssen M. C. R., Zuilhof H., Nielen M. W. F., Anal. Chem., 2018, 90(17), 10409—10416 |
| 52 | Banerjee S., Zare R. N., Angew. Chem. Int. Ed., 2015, 127(49), 15008—15012 |
| 53 | Gao D., Jin F., Yan X., Zare R. N., Chem. Eur. J., 2019, 25(6), 1466—1471 |
| 54 | Muller T., Badu-Tawiah A., Cooks R. G., Angew. Chem. Int. Ed., 2012, 51(47), 11832—11835 |
| 55 | Takats Z., Nanita S. C., Cooks R. G., Schlosser G., Vekey K., Anal. Chem., 2003, 75(6), 1514—1523 |
| 56 | Takats Z., Wiseman J. M., Gologan B., Cooks R. G., Anal. Chem., 2004, 76(14), 4050—4058 |
| 57 | Bain R. M., Pulliam C. J., Ayrton S. T., Bain K., Cooks R. G., Rapid Commun. Mass Spectrom., 2016, 30(16), 1875—1878 |
| 58 | Luo K., Li J., Cao Y., Liu C., Ge J., Chen H., Zare R. N., Chem. Sci., 2020, 11(9), 2558—2565 |
| 59 | Zhong X., Chen H., Zare R. N., Nat. Commun., 2020, 11(1), 1049 |
| 60 | Girod M., Moyano E., Campbell D. I., Cooks R. G., Chem. Sci., 2011, 2(3), 501—510 |
| 61 | Lee J. K., Kim S., Nam H. G., Zare R. N., Proc. Natl. Acad. Sci. USA, 2015, 112(13), 3898—3903 |
| 62 | Lee J. K., Nam H. G., Zare R. N., Q. Rev. Biophys., 2017, 50, e2, 1—7 |
| 63 | Lee J. K., Samanta D., Nam H. G., Zare R. N., Nat. Commun., 2018, 9(1), 1562 |
| 64 | Marquez C. A., Wang H., Fabbretti F., Metzger J. O., J. Am. Chem. Soc., 2008, 130(51), 17208—17209 |
| 65 | Marsh B. M., Iyer K., Cooks R. G., J. Am. Soc. Mass. Spectrom., 2019, 30(10), 2022—2030 |
| 66 | Mondal S., Acharya S., Biswas R., Bagchi B., Zare R. N., J. Chem. Phys., 2018, 148(24), 1—10 |
| 67 | Banerjee S., Gnanamani E., Yan X., Zare R. N., Analyst, 2017, 142(9), 1399—1402 |
| 68 | Boeser C. L., Holder J. C., Taylor B. L., Houk K. N., Stoltz B. M., Zare R. N., Chem. Sci., 2015, 6(3), 1917—1922 |
| 69 | Banerjee S., Yang Y. F., Jenkins I. D., Liang Y., Toutov A. A., Liu W. B., Schuman D. P., Grubbs R. H., Stoltz B. M., Krenske E. H., Houk K. N., Zare R. N., J. Am. Chem. Soc., 2017, 139(20), 6880—6887 |
| 70 | McCullough B. J., Bristow T., O’Connor G., Hopley C., Rapid Commun. Mass Spectrom., 2011, 25(10), 1445—1451 |
| 71 | Wang Y., Sun M., Qiao J., Ouyang J., Na N., Chem. Sci., 2018, 9(3), 594—599 |
| 72 | Lin S. Y., Huang M. Z., Chang H. C., Shiea J., Anal. Chem., 2007, 79(22), 8789—8795 |
| 73 | Cheng C. Y., Yuan C. H., Cheng S. C., Huang M. Z., Chang H. C., Cheng T. L., Yeh C. S., Shiea J., Anal. Chem., 2008, 80(20), 7699—7705 |
| 74 | Wang H., Liu J., Cooks R G., Ouyang Z., Angew. Chem. Int. Ed., 2010, 122(5), 889—892 |
| 75 | Banerjee S., Basheer C., Zare R. N., Angew. Chem. Int. Ed., 2016, 55(41), 12807—12811 |
| 76 | Sarkar D., Som A., Pradeep T., Anal. Chem., 2017, 89(21), 11378—11382 |
| 77 | Jiang J., Zhang H., Li M., Dulay M. T., Ingram A. J., Li N., You H., Zare R. N., Anal. Chem., 2015, 87(16), 8057—8062 |
| 78 | Hiraoka K., Nishidate K., Mori K., Asakawa D., Suzuki S., Rapid Commun. Mass Spectrom., 2007, 21(18), 3139—3144 |
| 79 | Yu Z., Chen L. C., Erra⁃Balsells R., Nonami H., Hiraoka K., Rapid Commun. Mass Spectrom., 2010, 24(11), 1507—1513 |
| 80 | Harper J. D., Charipar N. A., Mulligan C. C., Zhang X., Cooks R. G., Ouyang Z., Anal. Chem., 2008, 80(23), 9097—9104 |
| 81 | Ma X., Zhang S., Lin Z., Liu Y., Xing Z., Yang C., Zhang X., Analyst, 2009, 134(9), 1863—1867 |
| 82 | Bi C., Liang Y., Shen L., Tian S., Zhang K., Li Y., He X., Chen L., Zhang Y., ACS Omega, 2018, 3(2), 1572—1580 |
| 83 | Na N., Zhao M., Zhang S., Yang C., Zhang X., J. Am. Soc. Mass. Spectrom., 2007, 18(10), 1859—1862 |
| 84 | Chen S., Wan Q., Badu-Tawiah A. K., Angew. Chem. Int. Ed., 2016, 55(32), 9345—9349 |
| 85 | Cai Y., Wang J., Zhang Y., Li Z., Hu D., Zheng N., Chen H., J. Am. Chem. Soc., 2017, 139(35), 12259—12266 |
| 86 | Ai W., Gao Y., Xue J., Liu X., Liu H., Wang J., Bai Y., Chem. Commun., 2020, 56(14), 2163—2166 |
| 87 | Ai W., Yang Q., Gao Y., Liu X., Liu H., Bai Y., Anal. Chem., 2020, 92(17), 11967—11972 |
| [1] | 周雷雷, 程海洋, 赵凤玉. Pd基多相催化剂上CO2加氢反应的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220279. |
| [2] | 周紫璇, 杨海艳, 孙予罕, 高鹏. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220235. |
| [3] | 杨丹, 刘旭, 戴翼虎, 祝艳, 杨艳辉. 金团簇电催化二氧化碳还原反应的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220198. |
| [4] | 任娜娜, 薛洁, 王治钒, 姚晓霞, 王繁. 热力学数据对1, 3-丁二烯燃烧特性的影响[J]. 高等学校化学学报, 2022, 43(6): 20220151. |
| [5] | 朱凯, 利婕, 武潇逸, 胡薇薇, 吴冬梅, 虞诚潇, 葛志伟, 叶兴乾, 陈士国. 基于多孔石墨化碳柱-四极杆-飞行时间质谱解析甜菜果胶精细结构[J]. 高等学校化学学报, 2022, 43(6): 20220023. |
| [6] | 常云飞, 廖明义, 温佳明. NaBH4/MCl x 体系对液体端羧基氟橡胶的还原及还原机理[J]. 高等学校化学学报, 2022, 43(6): 20210835. |
| [7] | 张诗昱, 何润合, 李永兵, 魏士俊, 张兴祥. 辐照交联制备低分子量聚丙烯腈纤维锂硫电池正极材料及其储硫机理[J]. 高等学校化学学报, 2022, 43(3): 20210632. |
| [8] | 闭格宁, 肖小华, 李攻科. 微波辅助萃取多物理场耦合模型的构建及验证[J]. 高等学校化学学报, 2022, 43(3): 20210739. |
| [9] | 孙翠红, 吕立强, 刘迎, 王妍, 杨静, 张绍文. 硝酸异丙酯与Cl原子、 OH和NO3自由基反应的机理及动力学[J]. 高等学校化学学报, 2022, 43(2): 20210591. |
| [10] | 常斯惠, 陈涛, 赵黎明, 邱勇隽. 离子液体增塑生物基聚丁内酰胺的热分解机理[J]. 高等学校化学学报, 2022, 43(11): 20220353. |
| [11] | 温志国, 谯在银, 田冲, Borzov Maxim, 聂万丽. FLPs催化烯胺还原活性研究及反应机理探讨[J]. 高等学校化学学报, 2022, 43(11): 20220555. |
| [12] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [13] | 孟繁伟, 高琦, 叶青, 李晨曦. Cu-SAPO-18催化剂氨选择性催化还原NOx钾中毒机理的研究[J]. 高等学校化学学报, 2021, 42(9): 2832. |
| [14] | 杨一莹, 朱荣秀, 张冬菊, 刘成卜. 金催化炔基苯并二𫫇英环化合成8-羟基异香豆素的理论研究[J]. 高等学校化学学报, 2021, 42(7): 2299. |
| [15] | 李心怡, 刘永军. 人工设计逆醛缩酶RA95.5-8F催化β-羟基酮C—C裂解的理论研究[J]. 高等学校化学学报, 2021, 42(7): 2306. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||