

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (12): 2577.doi: 10.7503/cjcu20200414
• 庆祝《高等学校化学学报》复刊40周年专栏 • 上一篇 下一篇
收稿日期:2020-07-01
出版日期:2020-12-10
发布日期:2020-12-09
通讯作者:
孔祥建
E-mail:xjkong@xmu.edu.cn
基金资助:
LI Guanjun, LONG Lasheng, KONG Xiangjian( ), ZHENG Lansun
), ZHENG Lansun
Received:2020-07-01
Online:2020-12-10
Published:2020-12-09
Contact:
KONG Xiangjian
E-mail:xjkong@xmu.edu.cn
Supported by:摘要:
稀土-钛氧簇合物作为团簇化学的一个新分支, 不但结合了稀土和钛离子的特性, 而且由于二者的协同效应而表现出优异的光、 电、 磁和催化性能.本文综合评述了不同配体稀土-钛氧簇合物的合成与结构, 介绍了稀土-钛氧簇合物的代表性成果, 并对其合成策略和发展前景进行了总结和展望.
中图分类号:
TrendMD:
李观俊, 龙腊生, 孔祥建, 郑兰荪. 稀土-钛氧簇合物的研究进展. 高等学校化学学报, 2020, 41(12): 2577.
LI Guanjun, LONG Lasheng, KONG Xiangjian, ZHENG Lansun. Recent Advances in Lanthanide-titanium-oxo Clusters. Chem. J. Chinese Universities, 2020, 41(12): 2577.
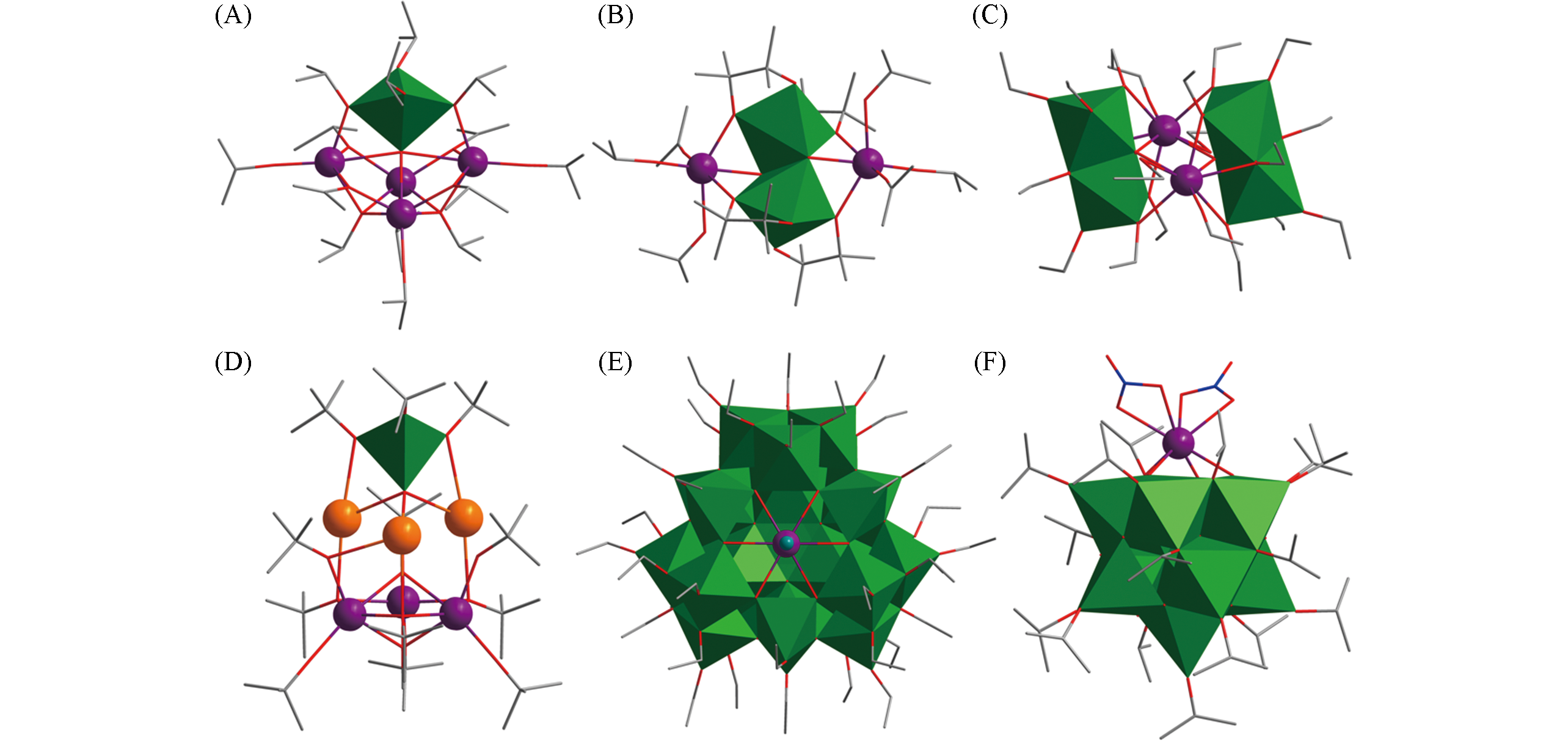
Fig.3 Structural views of Sm4Ti(μ5?O)(μ3?OiPr)2(μ?OiPr)6(OiPr)6(A), Ce2Ti2(μ3?O)2(μ,η2?pin)4(OiPr)4(iPrOH)2(B), Er2Ti4(μ4?O)2(μ3?OEt)2(μ?OEt)8(OEt)8(HOEt)2(C), Eu3K3TiO2(OtBu)11(OMe/OH)(HOtBu)(D), LnTi28O38(OEt)40H2Cl(E) and LnTi11O16(NO3)2(OiPr)17(F)Color code: Ln, violet; TiOx, green; K, orange; C, gray; O, red; N, blue; Cl, teal. H atoms are omitted for clarity.
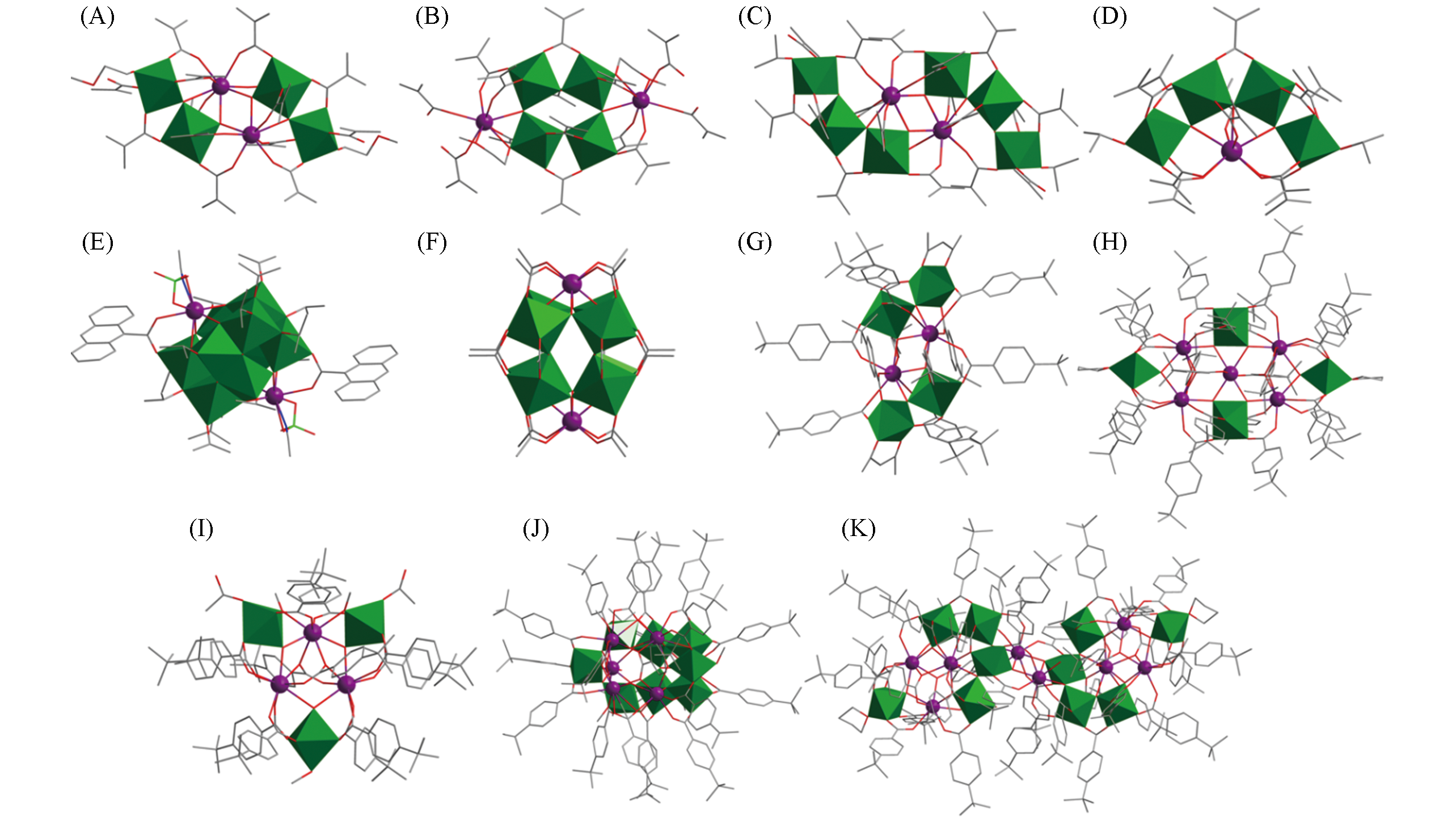
Fig.4 Structural views of Y2Ti4O4(OMc)14(MeOCH2CH2OH)2(A),Y2Ti4O4(OMc)12(OCH2CH2OMe)2(McOH)2(B), Ln2Ti6O6(OMc)18(HOiPr)2(C), LnTi4O3(OiPr)2(OMc)11(D), Ln2Ti10O14(ClO4)2(OiPr)14(9?AC)2·(CH3CN)2(E), {H2@[Ln2Ti8(μ2?O)4(μ3?O)8(Ac)16]}3(F), Eu2Ti4(μ3?O)4(tbba)12(acac)2(G), Eu5Ti4(μ3?O)6·(tbba)20(Htbba)(THF)2(H), Eu3Ti3(μ3?O)2(μ3?OH)(CH3O)2(tbba)12(Ac)2(CH3OH)(I), Eu6Ti8(μ3?O)13· (μ2?OH)?(CH3O)4(tbba)19(H2O)(CH3OH)(J) and Eu8Ti10(μ3?O)14(tbba)34(Ac)2(H2O)4(THF)2(K)Color code: Ln, violet; TiOx, green; C, gray; O, red; N, blue; Cl, teal. H atoms are omitted for clarity.
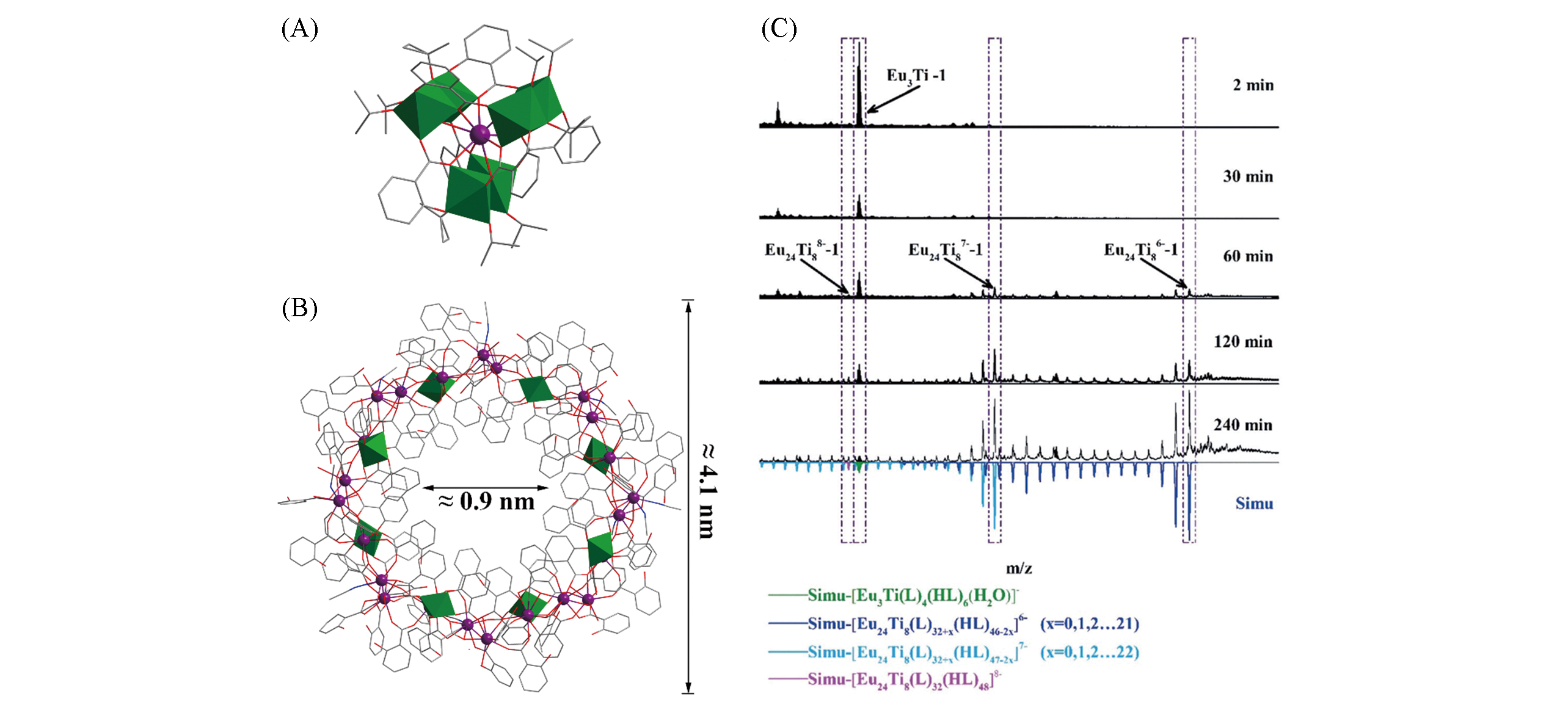
Fig.5 Structural views of LnTi6O3(OiPr)9(salicylate)6(A) and Eu24Ti8(sal)31(Hsal)42(CH3CN)11(H2O)8(B) and time?dependent HRESI?MS in range of m/z 1750—2800 on the reaction of Ti(OiPr)4, Eu(acac)3, and salicylic acid in acetonitrilesolution at 2, 30, 60, 120, and 240 min at room temperature(C)[73]Color code: Ln, violet; TiOx, green; C, gray; O, red; N, blue. H atoms are omitted for clarity.Copyright 2018, Wiley-VCH.
| Complex | Space group | Ligand | Ref. |
|---|---|---|---|
| Sm4Ti(μ5?O)(μ3?OiPr)2(μ?OiPr)6(OiPr)6 | I41cd | iso?Propanol | [ |
| Er2Ti4(μ4?O)2(μ3?OEt)2(μ?OEt)8(OEt)8(HOEt)2 | P21/n | Ethanol | [ |
| Ce2Ti2(μ3?O)2(μ,η2?OCMe2CMe2O)4(OiPr)4(iPrOH)2 | P | iso?Propanol, HOCMe2CMe2OH | [ |
| Eu3K3TiO2(OtBu)11(OMe/OH)(HOtBu) | P21/n | tert?Butanol | [ |
| LnTi28O38(OEt)38Cl(Ln=La, Ce) | P | Ethanol | [ |
| Ti4Y2O4(OMc)14(MeOCH2CH2OH)2 | P | Methacrylic acid | [ |
| Ti4Y2O4(OMc)14(McOH)2 | P | Methacrylic acid | [ |
| Ti4Y2O4(OMc)12(OCH2CH2OMe)2(McOH)2 | P | Methacrylic acid | [ |
| LnTi4O3(OiPr)2(OMc)11(Ln=La, Ce) | P | Methacrylic acid | [ |
| Ln2Ti6O6(OMc)18(HOiPr)2(Ln = La, Ce, Nd, Sm) | P21/n | Methacrylic acid | [ |
| Ln2Ti4O4(OMc)14(HOMc)2(Ln=Sm, Eu, Gd, Ho) | P | Methacrylic acid | [ |
Ln2Ti10O14(ClO4)2(OiPr)14(9?AC)2(CH3CN)2 (Ln=Nd, Eu) | P | Anthracene?9?carboxylic acid | [ |
Ln2Ti10O14(ClO4)2(OiPr)14(bza)2(HOiPr)2 (Ln=Nd, Eu) | P21/n | Benzoic acid | [ |
| [EuTi2O(OEt)8(EtOH)Cl]2 | P21/n | Ethanol | [ |
| LnTi11O16(NO3)2(OiPr)17(Ln=Sm, Eu, Gd) | P21 | iso?Propanol | [ |
| LnTi6O3(OiPr)9(sal)6(Ln=La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er) | P | Salicylic acid | [ |
Ln8Ti10(μ3?O)14(tbba)34(Ac)2(H2O)4(THF)2 (Ln=Sm, Eu, Gd) | P | 4?tert?Butylbenzoic acid, tetrahydrofuran | [ |
| Eu2Ti4(μ3?O)4(tbba)12(acac)2 | P | 4?tert?Butylbenzoic acid | [ |
| Eu5Ti4(μ3?O)6(tbba)20(Htbba)(THF)2 | C2/c | 4?tert?Butylbenzoic acid, tetrahydrofuran | [ |
| Ln2Ti6O2(C2O4)4(NO3)2(OiPr)20(Ln=La, Ce, Eu) | P | iso?Propanol, H2C2O4 | [ |
| Eu24Ti8(sal)31(Hsal)42(CH3CN)11(H2O)8 | P | Salicylic acid, CH3CN | [ |
| Ln2Ti8(μ2?O)2(μ3?O)8(μ2?OH)2(Ac)16(Ln=Eu, Tb) | Im | Acetic acid | [ |
Ln2Ti8(μ2?O)2(μ3?O)8(μ2?OH)2(p?toluic)16 (Ln=Eu,Tb) | I4/m | p?Toluic acid | [ |
Ln2Ti8(μ2?O)2(μ3?O)8(μ2?OH)2(Anthra)16 (Ln = Eu, Tb) | R | Anthracene?9?carboxylic acid | [ |
| {H2@[Ln2Ti8(μ2?O)4(μ3?O)8(Ac)16]}3(Ln=Eu, Tb, Yb) | Im | Acetic acid | [ |
| Eu3Ti3(μ3?O)2(μ3?OH)(CH3O)2(tbba)12(Ac)2(CH3OH) | Pnn2 | 4?tert?Butylbenzoic acid, CH3OH | [ |
| Eu6Ti8(μ3?O)13(μ2?OH)(CH3O)4(tbba)19(H2O)(CH3OH) | P | 4?tert?Butylbenzoic acid, CH3OH | [ |
| EuTi6(μ3?O)3(OEt)8(dtbsa)6(Hdtbsa) | P | Ethanol, 3,5?di?tert?butylsalicylic acid | [ |
| EuTi7(μ3?O)3(μ2?OH)2(OiPr)9(dtbsa)6(Hdtbsa)Cl | P21/n | iso?Propanol, 3,5?di?tert?butylsalicylic acid | [ |
| EuTi7(μ3?O)3(μ2?OH)2(OiPr)8(dtbsa)7(Hdtbsa) | P21/n | iso?Propanol, 3,5?di?tert?butylsalicylic acid | [ |
| [LaTi7(μ3?O)3(μ2?OH)2(OEt)8(dtbsa)7(Hdtbsa)]2 | P | Ethanol, 3,5?di?tert?butylsalicylic acid | [ |
表1 列出了目前为止报道的稀土-钛氧簇合物的结构和配体信息.
Table 1 Structural information of lanthanide-titanium-oxo clusters
| Complex | Space group | Ligand | Ref. |
|---|---|---|---|
| Sm4Ti(μ5?O)(μ3?OiPr)2(μ?OiPr)6(OiPr)6 | I41cd | iso?Propanol | [ |
| Er2Ti4(μ4?O)2(μ3?OEt)2(μ?OEt)8(OEt)8(HOEt)2 | P21/n | Ethanol | [ |
| Ce2Ti2(μ3?O)2(μ,η2?OCMe2CMe2O)4(OiPr)4(iPrOH)2 | P | iso?Propanol, HOCMe2CMe2OH | [ |
| Eu3K3TiO2(OtBu)11(OMe/OH)(HOtBu) | P21/n | tert?Butanol | [ |
| LnTi28O38(OEt)38Cl(Ln=La, Ce) | P | Ethanol | [ |
| Ti4Y2O4(OMc)14(MeOCH2CH2OH)2 | P | Methacrylic acid | [ |
| Ti4Y2O4(OMc)14(McOH)2 | P | Methacrylic acid | [ |
| Ti4Y2O4(OMc)12(OCH2CH2OMe)2(McOH)2 | P | Methacrylic acid | [ |
| LnTi4O3(OiPr)2(OMc)11(Ln=La, Ce) | P | Methacrylic acid | [ |
| Ln2Ti6O6(OMc)18(HOiPr)2(Ln = La, Ce, Nd, Sm) | P21/n | Methacrylic acid | [ |
| Ln2Ti4O4(OMc)14(HOMc)2(Ln=Sm, Eu, Gd, Ho) | P | Methacrylic acid | [ |
Ln2Ti10O14(ClO4)2(OiPr)14(9?AC)2(CH3CN)2 (Ln=Nd, Eu) | P | Anthracene?9?carboxylic acid | [ |
Ln2Ti10O14(ClO4)2(OiPr)14(bza)2(HOiPr)2 (Ln=Nd, Eu) | P21/n | Benzoic acid | [ |
| [EuTi2O(OEt)8(EtOH)Cl]2 | P21/n | Ethanol | [ |
| LnTi11O16(NO3)2(OiPr)17(Ln=Sm, Eu, Gd) | P21 | iso?Propanol | [ |
| LnTi6O3(OiPr)9(sal)6(Ln=La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er) | P | Salicylic acid | [ |
Ln8Ti10(μ3?O)14(tbba)34(Ac)2(H2O)4(THF)2 (Ln=Sm, Eu, Gd) | P | 4?tert?Butylbenzoic acid, tetrahydrofuran | [ |
| Eu2Ti4(μ3?O)4(tbba)12(acac)2 | P | 4?tert?Butylbenzoic acid | [ |
| Eu5Ti4(μ3?O)6(tbba)20(Htbba)(THF)2 | C2/c | 4?tert?Butylbenzoic acid, tetrahydrofuran | [ |
| Ln2Ti6O2(C2O4)4(NO3)2(OiPr)20(Ln=La, Ce, Eu) | P | iso?Propanol, H2C2O4 | [ |
| Eu24Ti8(sal)31(Hsal)42(CH3CN)11(H2O)8 | P | Salicylic acid, CH3CN | [ |
| Ln2Ti8(μ2?O)2(μ3?O)8(μ2?OH)2(Ac)16(Ln=Eu, Tb) | Im | Acetic acid | [ |
Ln2Ti8(μ2?O)2(μ3?O)8(μ2?OH)2(p?toluic)16 (Ln=Eu,Tb) | I4/m | p?Toluic acid | [ |
Ln2Ti8(μ2?O)2(μ3?O)8(μ2?OH)2(Anthra)16 (Ln = Eu, Tb) | R | Anthracene?9?carboxylic acid | [ |
| {H2@[Ln2Ti8(μ2?O)4(μ3?O)8(Ac)16]}3(Ln=Eu, Tb, Yb) | Im | Acetic acid | [ |
| Eu3Ti3(μ3?O)2(μ3?OH)(CH3O)2(tbba)12(Ac)2(CH3OH) | Pnn2 | 4?tert?Butylbenzoic acid, CH3OH | [ |
| Eu6Ti8(μ3?O)13(μ2?OH)(CH3O)4(tbba)19(H2O)(CH3OH) | P | 4?tert?Butylbenzoic acid, CH3OH | [ |
| EuTi6(μ3?O)3(OEt)8(dtbsa)6(Hdtbsa) | P | Ethanol, 3,5?di?tert?butylsalicylic acid | [ |
| EuTi7(μ3?O)3(μ2?OH)2(OiPr)9(dtbsa)6(Hdtbsa)Cl | P21/n | iso?Propanol, 3,5?di?tert?butylsalicylic acid | [ |
| EuTi7(μ3?O)3(μ2?OH)2(OiPr)8(dtbsa)7(Hdtbsa) | P21/n | iso?Propanol, 3,5?di?tert?butylsalicylic acid | [ |
| [LaTi7(μ3?O)3(μ2?OH)2(OEt)8(dtbsa)7(Hdtbsa)]2 | P | Ethanol, 3,5?di?tert?butylsalicylic acid | [ |
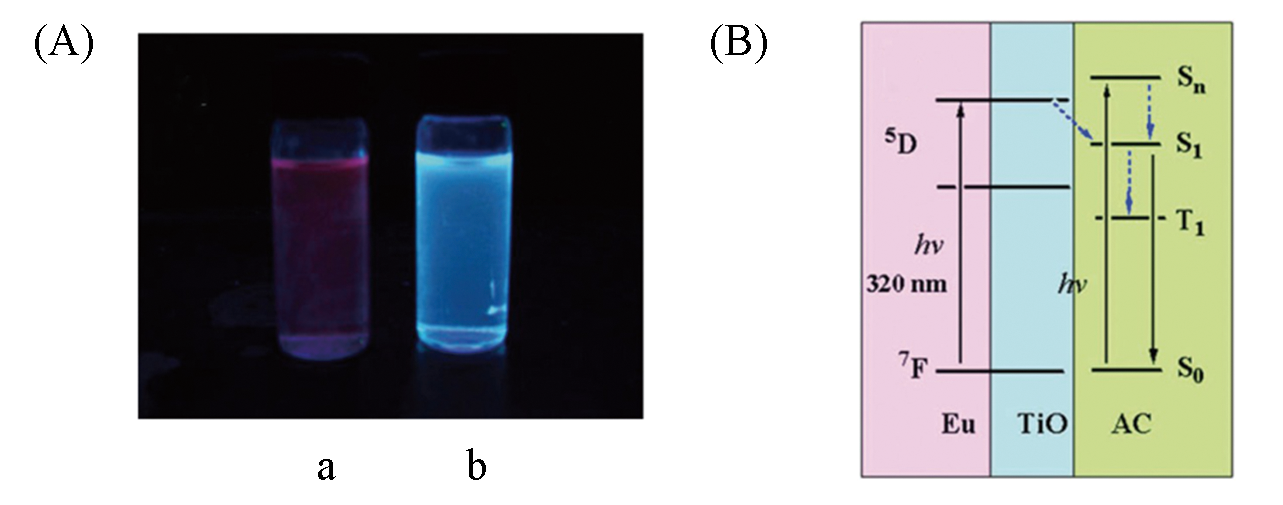
Fig.6 Photos of the fluorescence emission of Eu2Ti10?bza(a) and Eu2Ti10?9?AC(b)(A) and proposed energy transfer processes in cluster Eu2Ti10?9?AC[48](B)Copyright 2015, Royal Society of Chemistry.
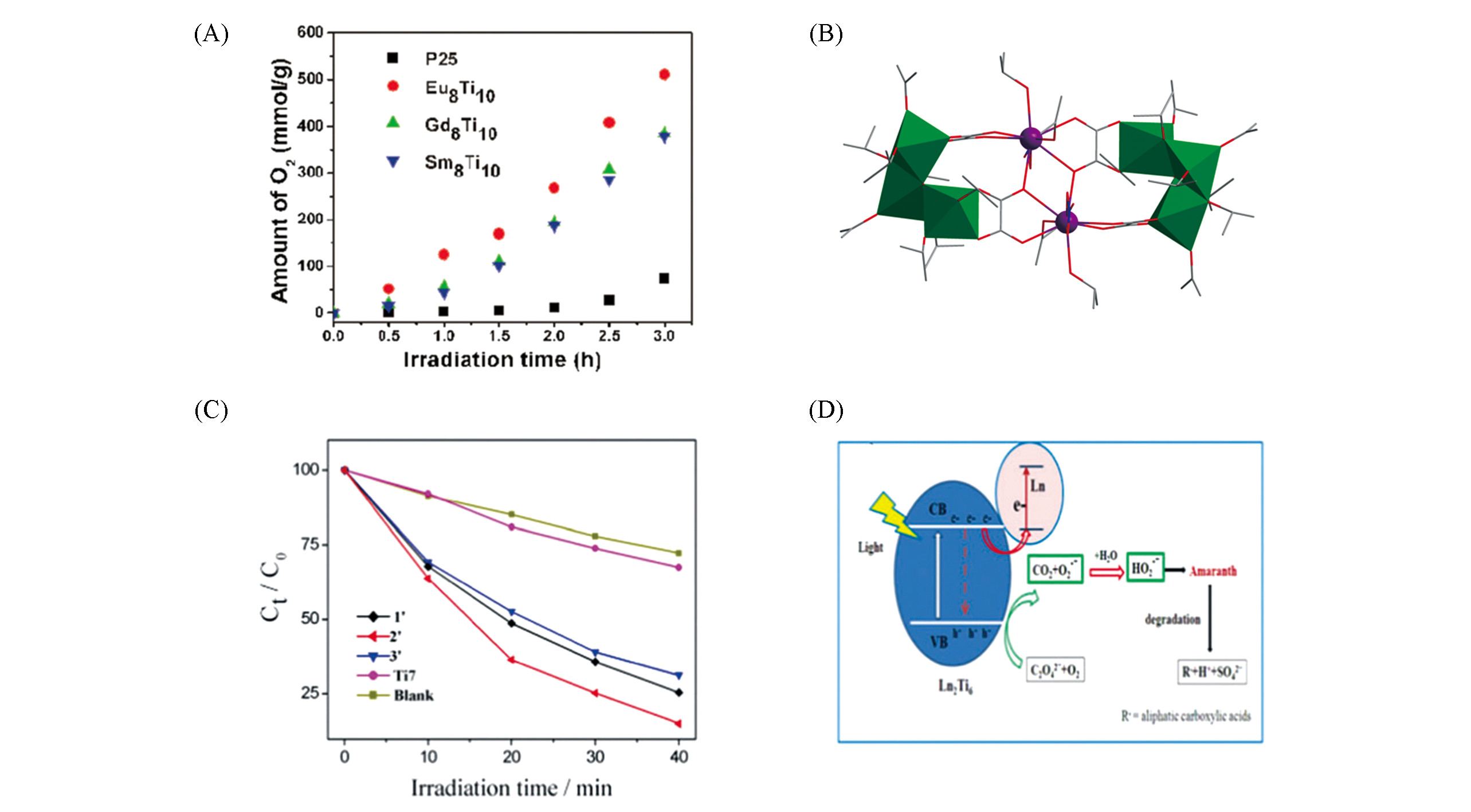
Fig.9 Photoelectrocatalytic amount of O2 of Ln8Ti10(A)[47], structural view of Ln2Ti6O2(C2O4)4(NO3)2·(OiPr)20(B), remaining concentrations using the degradation crystals of Ln2Ti6 ?after 40 min illumination(C)[46] and a proposed cooperative effect of Ln and oxalate promoting the photocatalytic degradation of the dye(D)[46]Color code: Ln, violet; TiOx, green; C, gray; O, red; N, blue. H atoms are omitted for clarity.(A) Copyright 2017, American Chemical Society;(C, D) Copyright 2018, Royal Society of Chemistry.
| 1 | Fujishima A., Honda K., Nature, 1972, 238, 37—38 |
| 2 | Linsebigler A. L., Lu G. Q., Yates J. T., Chem. Rev., 1995, 95, 735—758 |
| 3 | Carp O., Huisman C. L., Reller A., Prog. Solid State Chem., 2004, 32, 33—177 |
| 4 | Thompson T. L., Yates J. T. Jr., Chem. Rev., 2006, 106, 4428—4453 |
| 5 | Henderson M. A., Lyubinetsky I., Chem. Rev., 2013, 113, 4428—4455 |
| 6 | Matsumoto Y., Murakami M., Shono T., Hasegawa T., Fukumura T., Kawasaki M., Ahmet P., Chikyow T., Koshihara S., Koinuma H., Science, 2001, 291, 854—856 |
| 7 | Iwasaki M., Hara M., Kawada H., Tada H., Ito S., J. Colloid Interface Sci., 2000, 224, 202—204 |
| 8 | Griffin K. A., Pakhomov A. B., Wang C. M., Heald S. M., Krishnan K. M., Phys. Rev. Lett., 2005, 94, 157204 |
| 9 | Coppens P., Chen Y., Trzop E., Chem. Rev., 2014, 114, 9645—9661 |
| 10 | In S., Orlov A., Garcia F., Tikhov M., Wright D. S., Lambert R. M., Chem. Commun., 2006, 4236—4238 |
| 11 | In S., Orlov A., Berg R., García F., Pedrosa⁃Jimenez S., Tikhov M. S., Wright D. S., Lambert R. M., J. Am. Chem. Soc., 2007, 129, 13790—13791 |
| 12 | Lin W. Q., Liao X. F., Jia J. H., Leng J. D., Liu J. L., Guo F. S., Tong M. L., Chem. Eur. J., 2013, 19, 12254—12258 |
| 13 | Romanelli M., Kumar G. A., Emge T. J., Riman R. E., Brennan J. G., Angew. Chem. Int. Ed., 2008, 47, 6049—6051 |
| 14 | Feng D., Gu Z. Y., Li J. R., Jiang H. L., Wei Z., Zhou H. C., Angew. Chem. Int. Ed., 2012, 51, 10307—10310 |
| 15 | Nwe K., Andolina C. M., Morrow J. R., J. Am. Chem. Soc., 2008, 130, 14861—14871 |
| 16 | Wu P., He C., Wang J., Peng X., Li X., An Y., Duan C., J. Am. Chem. Soc., 2012, 134, 14991—14999 |
| 17 | Woodruff D. N., Winpenny R. E. P., Layfield R. A., Chem. Rev., 2013, 113, 5110—5148 |
| 18 | Zheng Y. Z., Xue W., Zheng S. L., Tong M. L., Chen X. M., Adv. Mater., 2008, 20, 1534—1538 |
| 19 | Song L., Chen C., Zhang S., Wei Q., Ultrason. Sonochem., 2011, 18, 1057—1061 |
| 20 | Sidheswaran M., Tavlarides L. L., Ind. Eng. Chem. Res., 2009, 48, 10292—10306 |
| 21 | Kumaresan L., Prabhu A., Palanichamy M., Arumugam E., Murugesan V., J. Hazard. Mater., 2011, 186, 1183—1192 |
| 22 | Iwaszuk A., Nolan M., J. Phys. Chem. C, 2011, 115, 12995—13007 |
| 23 | Vahrenkamp H., Angew. Chem. Int. Ed., 1978, 17, 379—392 |
| 24 | Andruh M., Chem. Commun., 2007, 2565—2577 |
| 25 | Cooke M. W., Hanan G. S., Chem. Soc. Rev., 2007, 36, 1466—1476 |
| 26 | Kong X. J., Ren Y. P., Long L. S., Zheng Z., Huang R. B., Zheng L. S., J. Am. Chem. Soc., 2007, 129, 7016—7017 |
| 27 | Kong X. J., Ren Y. P., Chen W. X., Long L. S., Zheng Z., Huang R. B., Zheng L. S., Angew. Chem. Int. Ed., 2008, 47, 2398—2401 |
| 28 | Kong X. J., Long L. S., Huang R. B., Zheng L. S., Harris T. D., Zheng Z., Chem. Commun., 2009, 4354—4356 |
| 29 | Zhang Z. M., Yao S., Li Y. G., Clerac R., Lu Y., Su Z. M., Wang E. B., J. Am. Chem. Soc., 2009, 131, 14600—14601 |
| 30 | Zeng Y. F., Xu G. C., Hu X., Chen Z., Bu X. H., Gao S., Sanudo E. C., Inorg. Chem., 2010, 49, 9734—9736 |
| 31 | Evangelisti F., More R., Hodel F., Luber S., Patzke G. R., J. Am. Chem. Soc., 2015, 137, 11076—11084 |
| 32 | Papatriantafyllopoulou C., Moushi E. E., Christou G., Tasiopoulos A. J., Chem. Soc. Rev., 2016, 45, 1597—1628 |
| 33 | Yang X., Wang S., King T. L., Kerr C. J., Blanchet C., Svergun D., Pal R., Beeby A., Vadivelu J., Brown K. A., Jones R. A., Zhang L., Huang S., Faraday Discuss., 2016, 191, 465—479 |
| 34 | Yang X., Wang S., Schipper D., Zhang L., Li Z., Huang S., Yuan D., Chen Z., Gnanam A. J., Hall J. W., King T. L., Que E., Dieye Y., Vadivelu J., Brown K. A., Jones R. A., Nanoscale, 2016, 8, 11123—11129 |
| 35 | Rozes L., Sanchez C., Chem. Soc. Rev., 2011, 40, 1006—1030 |
| 36 | Sokolow J. D., Trzop E., Chen Y., Tang J., Allen L. J., Crabtree R. H., Benedict J. B., Coppens P., J. Am. Chem. Soc., 2012, 134, 11695—11700 |
| 37 | Fang W. H., Zhang L., Zhang J., J. Am. Chem. Soc., 2016, 138, 7480—7483 |
| 38 | Gao M. Y., Wang F., Gu Z. G., Zhang D. X., Zhang L., Zhang J., J. Am. Chem. Soc., 2016, 138, 2556—2559 |
| 39 | Li N., Matthews P. D., Luo H. K., Wright D. S., Chem. Commun., 2016, 52, 11180—11190 |
| 40 | Zhang G., Liu C., Long D. L., Cronin L., Tung C. H., Wang Y., J. Am. Chem. Soc., 2016, 138, 11097—11100 |
| 41 | Jiang Z., Liu J., Gao M., Fan X., Zhang L., Zhang J., Adv. Mater., 2017, 29, 1—5 |
| 42 | Fang W. H., Zhang L., Zhang J., Chem. Soc. Rev., 2018, 47, 404—421 |
| 43 | Hong Z. F., Xu S. H., Yan Z. H., Lu D. F., Kong X. J., Long L. S., Zheng L. S., Cryst. Growth Des., 2018, 18, 4864—4868 |
| 44 | Zhang G., Li W., Liu C., Jia J., Tung C. H., Wang Y., J. Am. Chem. Soc., 2018, 140, 66—69 |
| 45 | Lv Y., Yao M., Holgado J. P., Roth T., Steiner A., Gan L., Lambert R. M., Wright D. S., RSC Adv., 2013, 3, 1—5 |
| 46 | Luo W., Hou J.L., Zou D.H., Cui L.N., Zhu Q.Y., Dai J., New J. Chem., 2018, 42, 11629—11634 |
| 47 | Lu D. F., Kong X. J., Lu T. B., Long L. S., Zheng L. S., Inorg. Chem., 2017, 56, 1057—1060 |
| 48 | Wang S., Su H. C., Yu L., Zhao X. W., Qian L. W., Zhu Q. Y., Dai J., Dalton Trans., 2015, 44, 1882—1888 |
| 49 | Lv Y., Cai Z., Yan D., Su C., Li W., Chen W., Ren Z., Wei Y., Mi O., Zhang C., Wright D. S., RSC Adv., 2016, 6, 57—60 |
| 50 | Zhang G. L., Wang S., Hou J. L., Mo C. J., Que C. J., Zhu Q. Y., Dai J., Dalton Trans., 2016, 45, 17681—17686 |
| 51 | Lu D. F., Hong Z. F., Xie J., Kong X. J., Long L. S., Zheng L. S., Inorg. Chem., 2017, 56, 12186—12192 |
| 52 | Li N., Subramanian G. S., Matthews P. D., Xiao J., Chellappan V., Rosser T. E., Reisner E., Luo H. K., Wright D. S., Dalton Trans., 2018, 47, 5679—5686 |
| 53 | Chen R., Hong Z. F., Zhao Y. R., Zheng H., Li G. J., Zhang Q. C., Kong X. J., Long L. S., Zheng L. S., Inorg. Chem., 2019, 58, 15008—15012 |
| 54 | Luo W., Zou D. H., Yang S., Cui L. N., Liu P. Y., Zhu Q. Y., Dai J., Inorg. Chem., 2019, 58, 14617—14625 |
| 55 | Zhao Y. R., Zheng H., Chen L. Q., Chen H. J., Kong X. J., Long L. S., Zheng L. S., Inorg. Chem., 2019, 58, 10078—10083 |
| 56 | Adams R. D., Captain B., Acc. Chem. Res., 2009, 42, 409—418 |
| 57 | Zhou J., Coord. Chem. Rev., 2016, 315, 112—134 |
| 58 | Nesterov D. S., Nesterova O. V., Pombeiro A. J. L., Coord. Chem. Rev., 2018, 355, 199—222 |
| 59 | Wolpher H., Sinha S., Pan J., Johansson A., Lundqvist M. J., Persson P., Lomoth R., Bergquist J., Sun L., Sundstrom V., Akermark B., Polivka T., Inorg. Chem., 2007, 46, 638—651 |
| 60 | Zheng X. Y., Zhang H., Wang Z., Liu P., Du M. H., Han Y. Z., Wei R. J., Ouyang Z. W., Kong X. J., Zhuang G. L., Long L. S., Zheng L. S., Angew. Chem. Int. Ed., 2017, 56, 11475—11479 |
| 61 | Yan Z. H., Du M. H., Liu J., Jin S., Wang C., Zhuang G. L., Kong X. J., Long L. S., Zheng L. S., Nat. Commun.,2018, 9, 3353 |
| 62 | Daniele S., Hubert⁃Pfalzgraf L. G., Daran J. C., Halut S., Polyhedron, 1994, 13, 927—932 |
| 63 | Hubert⁃Pfalzgraf L. G., Abada V., Vaissermann J., Polyhedron, 1999, 18, 3497—3504 |
| 64 | Westin G., Norreatam R., Nygren M., Wijk M., J. Solid State Chem., 1998, 135, 149—158 |
| 65 | Berger E., Westin G., J. Sol-Gel Sci. Technol., 2010, 53, 681—688 |
| 66 | Lv Y., Willkomm J., Leskes M., Steiner A., King T. C., Gan L., Reisner E., Wood P. T., Wright D. S., Chem. Eur. J., 2012, 18, 11867—11870 |
| 67 | Jupa M., Kickelbick G., Schubert U., Eur. J. Inorg. Chem., 2004, 2004, 1835—1839 |
| 68 | Artner C., Kronister S., Czakler M., Schubert U., Eur. J. Inorg. Chem., 2014, 32, 5596—5602 |
| 69 | Day V. W., Eberspacher T. A., Klemperer W. G., Park C. W., J. Am. Chem. Soc., 1993, 115, 8469—8470 |
| 70 | Steunou N., Robert F., Boubekeur K., Ribot F., Sanchez C., Inorg. Chim. Acta, 1998, 279, 144—151 |
| 71 | Steunou N., Ribot F., Boubekeur K., Maquet J., Sanchez C., New J. Chem., 1999, 23, 1079—1086 |
| 72 | Li N., Garcia⁃Rodriguez R., Matthews P. D., Luo H. K., Wright D. S., Dalton Trans., 2017, 46, 4287—4295 |
| 73 | Zheng H., Du M. H., Lin S. C., Tang Z. C., Kong X. J., Long L. S., Zheng L. S., Angew. Chem. Int. Ed., 2018, 57, 10976—10979 |
| 74 | Yang Y. M., Lun H. J., Long L. S., Kong X. J., Zheng L. S., Acta Phys. Chim. Sin., 2020, 36, 1—8(杨亚梅, 伦会洁, 龙腊生, 孔祥建, 郑兰荪. 物理化学学报, 2020, 36, 1—8) |
| 75 | Crosby G. A., Whan R. E., Alire R. M., J. Chem. Phys., 1961, 34, 743—748 |
| 76 | Binnemans K., Chem. Rev., 2009, 109, 4283—4374 |
| 77 | Moore E. G., Samuel A. P. S., Raymond K. N., Acc. Chem. Res., 2009, 42, 542—552 |
| 78 | Bunzli J. C., Chem. Rev., 2010, 110, 2729—2755 |
| 79 | Accorsi G., Listorti A., Yoosaf K., Armaroli N., Chem. Soc. Rev., 2009, 38, 1690—1700 |
| 80 | Kong X. J., Ren Y. P., Long L. S., Zheng Z., Nichol G., Huang R. B., Zheng L. S., Inorg. Chem., 2008, 47, 2728—2739 |
| 81 | Peng J. B., Zhang Q. C., Kong X. J., Ren Y. P., Long L. S., Huang R. B., Zheng L. S., Zheng Z., Angew. Chem. Int. Ed., 2011, 50, 10649—10652 |
| 82 | Leng J. D., Liu J. L., Tong M. L., Chem. Commun., 2012, 48, 5286—5288 |
| 83 | Zhang Z. M., Pan L. Y., Lin W. Q., Leng J. D., Guo F. S., Chen Y. C., Liu J. L., Tong M. L., Chem. Commun., 2013, 49, 8081—8083 |
| 84 | Peng J. B., Kong X. J., Zhang Q. C., Orendac M., Prokleska J., Ren Y. P., Long L. S., Zheng Z., Zheng L. S., J. Am. Chem. Soc., 2014, 136, 17938—17941 |
| 85 | Liu D. P., Lin X. P., Zhang H., Zheng X. Y., Zhuang G. L., Kong X. J., Long L. S., Zheng L. S., Angew. Chem. Int. Ed., 2016, 55, 4532—4536 |
| 86 | Qin L., Yu Y. Z., Liao P. Q., Xue W., Zheng Z., Chen X. M., Zheng Y. Z., Adv. Mater., 2016, 28, 10772—10779 |
| 87 | Lin Q., Zhang Y., Cheng W., Liu Y., Xu Y., Dalton Trans., 2017, 46, 643—646 |
| 88 | Zheng X. Y., Jiang Y. H., Zhuang G. L., Liu D. P., Liao H. G., Kong X. J., Long L. S., Zheng L. S., J. Am. Chem. Soc., 2017, 139, 18178—18181 |
| 89 | Zheng X. Y., Xie J., Kong X. J., Long L. S., Zheng L. S., Coord. Chem. Rev., 2019, 378, 222—236 |
| [1] | 曹舒杰, 李泓君, 管文丽, 任梦田, 周传政. 硫代磷酸酯寡聚核苷酸的立体控制合成研究进展[J]. 高等学校化学学报, 2022, 43(Album-4): 20220304. |
| [2] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [3] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| [4] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [5] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [6] | 江博文, 陈敬轩, 成永华, 桑微, 寇宗魁. 单原子材料在电化学生物传感中的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220334. |
| [7] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [8] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [9] | 金睿明, 穆晓清, 徐岩. 生物-化学法合成黑色素前体5, 6-二羟基吲哚[J]. 高等学校化学学报, 2022, 43(8): 20220134. |
| [10] | 仵宇帅, 尚颖旭, 蒋乔, 丁宝全. 可控自组装DNA折纸结构作为药物载体的研究进展[J]. 高等学校化学学报, 2022, 43(8): 20220179. |
| [11] | 李琳, 齐丰莲, 邱丽莉, 孟子晖. 基于六边形磁纳米片构建动态非晶态光学结构图案[J]. 高等学校化学学报, 2022, 43(8): 20220123. |
| [12] | 贾洋刚, 邵霞, 程婕, 王朋朋, 冒爱琴. 赝电容控制型钙钛矿高熵氧化物La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3负极材料的制备及储锂性能[J]. 高等学校化学学报, 2022, 43(8): 20220157. |
| [13] | 韦春洪, 蒋倩, 王盼盼, 江成发, 刘岳峰. 贵金属Pt促进Co基费托合成催化剂的原子尺度结构分析[J]. 高等学校化学学报, 2022, 43(8): 20220074. |
| [14] | 张昕昕, 许狄, 王艳秋, 洪昕林, 刘国亮, 杨恒权. CO2加氢制低碳醇CuFe基催化剂中的Mn助剂效应[J]. 高等学校化学学报, 2022, 43(7): 20220187. |
| [15] | 周紫璇, 杨海艳, 孙予罕, 高鹏. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220235. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||