

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (2): 254.doi: 10.7503/cjcu20180573
收稿日期:2018-08-14
出版日期:2019-02-10
发布日期:2018-10-08
作者简介:联系人简介: 方 浩, 男, 博士, 教授, 博士生导师, 主要从事抗肿瘤药物方面的研究. E-mail:
基金资助:
LÜ Mingjun, LI Wen, YANG Xinying, FANG Hao*( )
)
Received:2018-08-14
Online:2019-02-10
Published:2018-10-08
Contact:
FANG Hao
E-mail:haofangcn@sdu.edu.cn
Supported by:摘要:
合成了一系列N9位芳基取代嘌呤-8-酮类衍生物, 利用核磁共振氢谱(1H NMR)、 核磁共振碳谱(13C NMR)和高分辨质谱(HRMS)进行了结构确证. 采用四甲基偶氮唑盐(MTT)法测定了目标化合物的体外抗肿瘤细胞增殖活性. 结果表明, 嘌呤酮环的C2位及N9位的取代对活性有较大影响, C2位引入对位由含氮六元环取代的苯胺, N9位引入对三氟甲基苯均有利于提高抗肿瘤活性. 化合物12c对人白血病细胞(K562)、 人前列腺癌细胞(PC-3)、 人乳腺癌细胞(MDA-MB-231)及人结肠癌细胞(HCT116)的抑制效果明显优于阳性对照药R-Roscovitine.
中图分类号:
TrendMD:
吕明君, 李雯, 杨新颖, 方浩. N9位芳基取代嘌呤-8-酮类衍生物的合成及抗肿瘤活性. 高等学校化学学报, 2019, 40(2): 254.
LÜ Mingjun,LI Wen,YANG Xinying,FANG Hao. Synthesis and Antitumor Activity of N9 Position Aromatic Substituted Purine-8-one Derivatives†. Chem. J. Chinese Universities, 2019, 40(2): 254.
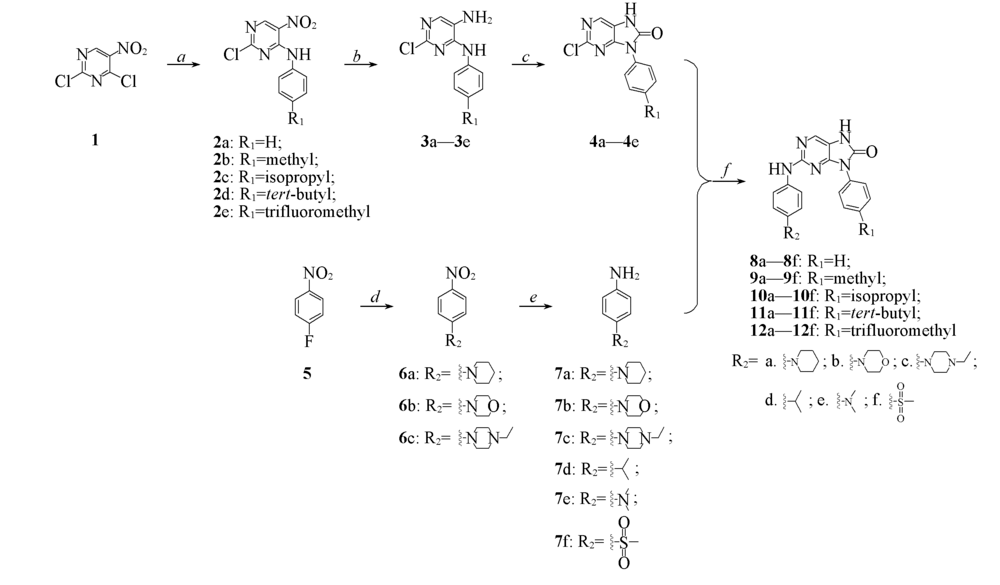
Scheme 1 Synthetic routes of target compounds 8—12a. Benzyl amine, DIPEA, dichloromethane, 0—25 ℃, 30 min; b. SnCl2·2H2O, ethyl acetate, 60 ℃, 4 h; c. phenyl carbonochloridate, NaHCO3, ethyl acetate and H2O, 0—60 ℃, 8 h; d. nitrogen heterocycle, triethylamine, DMSO, 110 ℃, 6 h; e. Pd/C, methanol, r. t., overnight; f. TsOH, n-butanol, 110 ℃, 6 h. Compounds 7d—7f were commercially available reagents.
| Compd. | ESI-MS(calcd.), m/z[M+H]+ | Compd. | ESI-MS(calcd.), m/z[M+H]+ |
|---|---|---|---|
| 2a | 251.05(251.03) | 3d | 277.11(277.12) |
| 2b | 265.06(265.04) | 3e | 289.07(289.05) |
| 2c | 293.13(293.08) | 6a | 207.05(207.11) |
| 2d | 307.11(307.10) | 6b | 209.05(209.09) |
| 2e | 319.01(319.02) | 6c | 236.12(236.14) |
| 3a | 221.03(221.06) | 7a | 177.05(177.14) |
| 3b | 235.02(235.07) | 7b | 179.02(179.12) |
| 3c | 263.07(263.10) | 7c | 206.10(206.16) |
Table 1 ESI-MS data of compounds 2a—2e, 3a—3e, 6a—6c and 7a—7c
| Compd. | ESI-MS(calcd.), m/z[M+H]+ | Compd. | ESI-MS(calcd.), m/z[M+H]+ |
|---|---|---|---|
| 2a | 251.05(251.03) | 3d | 277.11(277.12) |
| 2b | 265.06(265.04) | 3e | 289.07(289.05) |
| 2c | 293.13(293.08) | 6a | 207.05(207.11) |
| 2d | 307.11(307.10) | 6b | 209.05(209.09) |
| 2e | 319.01(319.02) | 6c | 236.12(236.14) |
| 3a | 221.03(221.06) | 7a | 177.05(177.14) |
| 3b | 235.02(235.07) | 7b | 179.02(179.12) |
| 3c | 263.07(263.10) | 7c | 206.10(206.16) |
| Compd. | Appearance | Yield(%) | m.p./℃ | ESI-MS(calcd.), m/z[M-H]- | 1H NMR(400 MHz), δ |
|---|---|---|---|---|---|
| 4a | Yellow solid | 92 | >280 | 245.09(245.02) | 8.20(s, 1H, pyrimidine H), 7.49(d, J=7.3 Hz, 2H, PhH), 7.36(d, J=7.3 Hz, 2H, PhH), 2.43(s, 3H, CH3) |
| 4b | Yellow solid | 98 | >280 | 259.14(259.04) | 8.20(s, 1H, pyrimidine H), 7.49(d, J=7.3 Hz, 2H, PhH), 7.36(d, J=7.3 Hz, 2H, PhH), 2.43(s, 3H, CH3) |
| 4c | Yellow solid | 90 | 267—269 | 287.20(287.07) | 11.83(s, 1H, NH), 8.24(s, 1H, pyrimidine H), 7.54—7.40(m, 4H, PhH), 2.99(Hept, J=6.8 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2] |
| 4d | Gray solid | 94 | 266—268 | 301.14(301.08) | 10.37(s, 1H, NH), 8.25(s, 1H, pyrimidine H), 7.60(d, J=8.6 Hz, 2H, PhH), 7.54(d, J=8.7 Hz, 2H, PhH), 1.38[s, 9H, C(CH3)3] |
| 4e | Gray solid | 90 | 218—220 | 313.11(313.01) | 9.64(s, 1H, NH), 8.29(s, 1H, pyrimidine H), 7.87(m, 4H, PhH) |
Table 2 Appearance, yields, melting points, ESI-MS and 1H NMR data of compounds 4a—4e*
| Compd. | Appearance | Yield(%) | m.p./℃ | ESI-MS(calcd.), m/z[M-H]- | 1H NMR(400 MHz), δ |
|---|---|---|---|---|---|
| 4a | Yellow solid | 92 | >280 | 245.09(245.02) | 8.20(s, 1H, pyrimidine H), 7.49(d, J=7.3 Hz, 2H, PhH), 7.36(d, J=7.3 Hz, 2H, PhH), 2.43(s, 3H, CH3) |
| 4b | Yellow solid | 98 | >280 | 259.14(259.04) | 8.20(s, 1H, pyrimidine H), 7.49(d, J=7.3 Hz, 2H, PhH), 7.36(d, J=7.3 Hz, 2H, PhH), 2.43(s, 3H, CH3) |
| 4c | Yellow solid | 90 | 267—269 | 287.20(287.07) | 11.83(s, 1H, NH), 8.24(s, 1H, pyrimidine H), 7.54—7.40(m, 4H, PhH), 2.99(Hept, J=6.8 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2] |
| 4d | Gray solid | 94 | 266—268 | 301.14(301.08) | 10.37(s, 1H, NH), 8.25(s, 1H, pyrimidine H), 7.60(d, J=8.6 Hz, 2H, PhH), 7.54(d, J=8.7 Hz, 2H, PhH), 1.38[s, 9H, C(CH3)3] |
| 4e | Gray solid | 90 | 218—220 | 313.11(313.01) | 9.64(s, 1H, NH), 8.29(s, 1H, pyrimidine H), 7.87(m, 4H, PhH) |
| Compd. | Appearance | Yield(%) | m.p./℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 8a | Gray solid | 54 | >280 | 387.1941(387.1928) |
| 8b | Gray solid | 58 | >280 | 389.1730(389.1721) |
| 8c | Gray solid | 46 | 244—246 | 416.2205(416.2193) |
| 8d | Yellowsolid | 51 | >280 | 346.1675(346.1662) |
| 8f | Yellow solid | 37 | >280 | 382.0984(382.0968) |
| 9a | Gray solid | 40 | 245—247 | 401.2095(401.2084) |
| 9b | Yellow solid | 58 | 270—272 | 403.1889(403.1877) |
| 9c | Gray solid | 28 | 246—248 | 430.2364(430.2350) |
| 9d | White solid | 53 | 276—278 | 360.1836(360.1819) |
| 9e | Gray solid | 40 | 264—266 | 361.1769(361.1771) |
| 9f | White solid | 33 | >280 | 396.1148(396.1125) |
| 10a | Gray solid | 42 | 252—254 | 429.2401(429.2397) |
| 10b | White solid | 63 | 259—261 | 431.2209(431.2190) |
| 10c | Gray solid | 51 | >280 | 458.2681(458.2663) |
| 10d | White solid | 43 | 260—262 | 388.2145(388.2132) |
| 10e | Gray solid | 51 | 242—244 | 389.2086(389.2084) |
| 10f | White solid | 28 | >280 | 424.1456(424.1438) |
| 11a | Gray solid | 59 | >280 | 443.2556(443.2554) |
| 11b | White solid | 54 | >280 | 445.2369(445.2347) |
| 11c | Gray solid | 41 | 260—262 | 472.2836(472.2819) |
| 11d | White solid | 50 | 254—256 | 402.2301(402.2288) |
| 11e | Gray solid | 53 | 264—266 | 403.2241(403.2241) |
| 11f | White solid | 15 | >280 | 438.1616(438.1594) |
| 12a | Gray solid | 40 | >280 | 455.1811(455.1802) |
| 12b | Gray solid | 45 | >280 | 457.1604(457.1594) |
| 12c | Gray solid | 29 | >280 | 484.2090(484.2067) |
| 12d | White solid | 36 | >280 | 414.1535(414.1536) |
| 12e | Gray solid | 48 | >280 | 415.1490(415.1489) |
| 12f | White solid | 56 | >280 | 450.0863(450.0842) |
Table 3 Appearance, yields, melting points and HRMS data of compounds 8—12
| Compd. | Appearance | Yield(%) | m.p./℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 8a | Gray solid | 54 | >280 | 387.1941(387.1928) |
| 8b | Gray solid | 58 | >280 | 389.1730(389.1721) |
| 8c | Gray solid | 46 | 244—246 | 416.2205(416.2193) |
| 8d | Yellowsolid | 51 | >280 | 346.1675(346.1662) |
| 8f | Yellow solid | 37 | >280 | 382.0984(382.0968) |
| 9a | Gray solid | 40 | 245—247 | 401.2095(401.2084) |
| 9b | Yellow solid | 58 | 270—272 | 403.1889(403.1877) |
| 9c | Gray solid | 28 | 246—248 | 430.2364(430.2350) |
| 9d | White solid | 53 | 276—278 | 360.1836(360.1819) |
| 9e | Gray solid | 40 | 264—266 | 361.1769(361.1771) |
| 9f | White solid | 33 | >280 | 396.1148(396.1125) |
| 10a | Gray solid | 42 | 252—254 | 429.2401(429.2397) |
| 10b | White solid | 63 | 259—261 | 431.2209(431.2190) |
| 10c | Gray solid | 51 | >280 | 458.2681(458.2663) |
| 10d | White solid | 43 | 260—262 | 388.2145(388.2132) |
| 10e | Gray solid | 51 | 242—244 | 389.2086(389.2084) |
| 10f | White solid | 28 | >280 | 424.1456(424.1438) |
| 11a | Gray solid | 59 | >280 | 443.2556(443.2554) |
| 11b | White solid | 54 | >280 | 445.2369(445.2347) |
| 11c | Gray solid | 41 | 260—262 | 472.2836(472.2819) |
| 11d | White solid | 50 | 254—256 | 402.2301(402.2288) |
| 11e | Gray solid | 53 | 264—266 | 403.2241(403.2241) |
| 11f | White solid | 15 | >280 | 438.1616(438.1594) |
| 12a | Gray solid | 40 | >280 | 455.1811(455.1802) |
| 12b | Gray solid | 45 | >280 | 457.1604(457.1594) |
| 12c | Gray solid | 29 | >280 | 484.2090(484.2067) |
| 12d | White solid | 36 | >280 | 414.1535(414.1536) |
| 12e | Gray solid | 48 | >280 | 415.1490(415.1489) |
| 12f | White solid | 56 | >280 | 450.0863(450.0842) |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
|---|---|---|
| 8a | 11.14(s, 1H, NH), 9.02(s, 1H, NH), 8.01(s, 1H, pyrimidine H), 7.65(d, J=7.5 Hz, 2H, PhH), 7.55(q, 4H, PhH), 7.44(t, 1H, PhH), 6.80(d, J=7.4 Hz, 2H, PhH), 3.08—2.92[m, 4H, N(CH2)2], 1.61[m, 4H, CH2], 1.49(m, 2H, CH2) | 155.78, 152.78, 151.37, 147.00, 134.82, 133.76, 133.43, 129.35, 128.16, 127.00, 119.88, 117.06, 115.03, 51.22, 25.98, 24.34 |
| 8b | 11.16(s, 1H, NH), 9.07(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.65(d, J=7.9 Hz, 2H, PhH), 7.62—7.51(m, 4H, PhH), 7.44(t, 1H, PhH), 6.82(d, J=7.9 Hz, 2H, PhH), 3.80—3.67[m, 4H, N(CH2)2], 3.07—2.93[m, 4H, O(CH2)2] | 155.73, 152.78, 151.37, 146.02, 134.80, 134.23, 129.36, 128.18, 127.01, 119.84, 116.14, 115.10, 66.65, 49.94 |
| 8c | 11.16(s, 1H, NH), 9.04(s, 1H, NH), 8.01(s, 1H, pyrimidine H), 7.65(d, J=7.9 Hz, 2H, PhH), 7.56(q, 4H, PhH), 7.45(t, 1H, PhH), 6.81(d, J=8.0 Hz, 2H, PhH), 3.02[m, 4H, N(CH2)2], 2.50[m, 4H, N(CH2)2], 2.37(q, J=7.1 Hz, 2H, CH2), 1.03(t, J=7.2 Hz, 3H, CH3) | 155.76, 152.90, 151.36, 146.08, 135.08, 134.81, 129.53, 129.36, 128.17, 126.97, 119.87, 116.35, 115.06, 52.88, 52.09, 49.60, 12.42 |
| 8d | 11.19(s, 1H, NH), 9.20(s, 1H, NH), 8.04(s, 1H, pyrimidine H), 7.73—7.52(m, 6H, PhH), 7.45(t, J=7.4 Hz, 1H, PhH), 7.06(d, 2H, J=8.4 Hz, PhH), 2.79(Hept, J=6.9 Hz, 1H, CH), 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.80, 151.36, 141.03, 139.29, 134.72, 133.39, 129.37, 128.23, 127.06, 126.45, 118.74, 115.40, 33.21, 24.56 |
| 8f | 11.37(s, 1H, NH), 9.94(s, 1H, NH), 8.15(s, 1H, pyrimidine H), 7.97(d, J=8.9 Hz, 2H, PhH), 7.73(d, J=8.9 Hz, 2H, PhH), 7.66(d, J=7.9 Hz, 2H, PhH), 7.59(t, J=7.7 Hz, 2H, PhH), 7.47(t, J=7.3 Hz, 1H, PhH), 3.12(s, 3H, CH3) | 154.40, 152.85, 151.42, 146.24, 134.33, 133.17, 131.75, 129.50, 128.49, 128.38, 127.21, 117.50, 116.70, 44.53 |
| 9a | 11.11(s, 1H, NH), 9.01(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.60—7.45(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.04—2.94[m, 4H, N(CH2)2], 2.39(s, 3H, CH3), 1.61(m, 4H, CH2), 1.49(m, 2H, CH2) | 155.78, 152.88, 151.50, 146.97, 137.72, 134.63, 133.82, 130.80, 129.82, 126.91, 119.82, 117.10, 115.05, 51.24, 25.98, 24.34, 21.22 |
| 9b | 11.12(s, 1H, NH), 9.05(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.56(d, J=9.0 Hz, 2H, PhH), 7.50(d, J=8.2 Hz, 2H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.83—3.63[m, 4H, N(CH2)2], 3.08—2.93[m, 4H, O(CH2)2], 2.39(s, 3H, CH3) | 155.72, 152.88, 151.51, 146.00, 137.74, 134.62, 134.28, 130.79, 129.83, 126.92, 119.77, 116.16, 115.11, 66.66, 49.95, 21.23 |
| 9c | 11.12(s, 1H, NH), 9.02(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.52(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.07—2.92[m, 4H, N(CH2)2], 2.50[m, 4H, N(CH2)2], 2.42—2.32(m, 5H, CH3, CH2), 1.02(t, J=7.2 Hz, 3H, CH3) | 155.76, 152.88, 151.50, 146.09, 137.72, 134.63, 133.95, 130.80, 129.82, 126.90, 119.82, 116.36, 115.07, 52.93, 52.12, 49.67, 21.23, 12.49 |
| 9d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.61(d, J=8.5 Hz, 2H, PhH), 7.51(d, J=8.2 Hz, 2H, PhH), 7.36(d, J=8.2 Hz, 2H, PhH), 7.07(d, J=8.5 Hz, 2H, PhH), 2.79(Hept, J=6.9 Hz, 1H, CH), 2.39(s, 3H, CH3), 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.90, 151.49, 140.99, 139.32, 137.79, 134.55, 130.76, 129.83, 126.95, 126.46, 118.69, 115.40, 33.21, 24.57, 21.22 |
| 9e | 11.08(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.50(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.65(d, J=9.0 Hz, 2H, PhH), 2.80[s, 6H, N(CH3)2], 2.39(s, 3H, CH3) | 155.98, 152.87, 151.51, 146.17, 137.67, 134.70, 131.86, 130.83, 129.80, 126.88, 120.42, 114.88, 113.53, 41.37, 21.22 |
| 9f | 11.33(s, 1H, NH), 9.92(s, 1H, NH), 8.13(s, 1H, pyrimidine H), 7.96(d, J=8.9 Hz, 2H, PhH), 7.73(d, J=8.9 Hz, 2H, PhH), 7.51(d, J=8.2 Hz, 2H, PhH), 7.38(d, J=8.2 Hz, 2H, PhH), 3.12(s, 3H, CH3), 2.40(s, 3H, CH3) | 154.41, 152.94, 151.53, 146.27, 138.07, 134.19, 131.72, 130.56, 129.94, 128.38, 127.06, 117.46, 116.70, 44.55, 21.24 |
| 10a | 11.09(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.52(dd, J=9.0, 8.4 Hz, 4H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.02—2.97[m, 5H, N(CH2)2, CH], 1.61(m, 4H, CH2), 1.50(m, 2H, CH2), 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 155.79, 152.92, 151.59, 148.57, 146.98, 134.54, 133.79, 131.04, 127.23, 127.14, 119.94, 117.06, 115.11, 51.23, 33.68, 25.96, 24.34 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
| 10b | 11.11(s, 1H, NH), 9.04(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.56(d, J=9.0 Hz, 2H, PhH), 7.52(d, J=8.4 Hz, 2H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.78—3.67[m, 4H, N(CH2)2], 3.04—2.94[m, 5H, O(CH2)2, CH], 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 155.73, 152.93, 151.60, 148.58, 146.03, 134.50, 134.28, 127.24, 127.14, 119.88, 116.15, 115.18, 66.65, 49.96, 33.69, 24.35 |
| 10c | 11.09(s, 1H, NH), 9.01(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.62—7.47(dd, J=9.0, 8.4 Hz, 4H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.10—2.89[m, 5H, N(CH2)2, CH], 2.47[m, 4H, N(CH2)2], 2.35(q, J=7.2 Hz, 2H, CH2), 1.26[d, J=6.9 Hz, 6H, (CH3)2], 1.02(t, J=7.2 Hz, 3H, CH3) | 155.77, 152.92, 151.59, 148.57, 146.12, 134.53, 133.94, 131.03, 127.23, 127.13, 119.93, 116.35, 115.13, 52.92, 52.11, 49.68, 33.68, 24.35, 12.49 |
| 10d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.60(d, J=8.5 Hz, 2H, PhH), 7.53(d, J=8.3 Hz, 2H, PhH), 7.42(d, J=8.4 Hz, 2H, PhH), 7.06(d, J=8.5 Hz, 2H, PhH), 2.98(Hept, J=6.6 Hz, 1H, CH), 2.80(Hept, J=6.8 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2], 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.93, 151.57, 148.63, 141.02, 139.31, 134.46, 130.99, 127.24, 127.17, 126.43, 118.81, 115.45, 33.69, 33.21, 24.56, 24.34 |
| 10e | 11.07(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.59—7.46(dd, J=9.0, 8.3 Hz, 4H, PhH), 7.41(d, J=8.3 Hz, 2H, PhH), 6.64(d, J=9.0 Hz, 2H, PhH), 2.98(Hept, J=6.8 Hz, 1H, CH), 2.80[s, 6H, N(CH3)2], 1.25[d, J=6.9 Hz, 6H, (CH3)2] | 155.98, 152.91, 151.58, 148.52, 146.18, 134.62, 131.87, 131.06, 127.21, 127.10, 120.50, 114.92, 113.50, 41.35, 33.68, 24.34 |
| 10f | 11.29(s, 1H, NH), 9.92(s, 1H, NH), 8.14(s, 1H, pyrimidine H), 7.97(d, J=8.8 Hz, 2H, PhH), 7.73(d, J=8.8 Hz, 2H, PhH), 7.53(d, J=8.2 Hz, 2H, PhH), 7.44(d, J=8.4 Hz, 2H, PhH), 3.12(s, 3H, CH3), 3.02—2.95(Hept, J=6.9 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 154.40, 153.00, 151.64, 148.89, 146.28, 134.04, 131.73, 130.80, 128.37, 127.36, 127.29, 117.51, 116.77, 44.55, 33.71, 24.35 |
| 11a | 11.10(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.54(m, 6H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.03—2.95[m, 4H, N(CH2)2], 1.61(m, 4H, CH2), 1.50(m, 2H, CH2), 1.34[s, 9H, C(CH3)3] | 155.79, 152.92, 151.61, 150.75, 146.98, 134.51, 133.78, 130.75, 126.82, 126.16, 119.99, 117.04, 115.13, 51.22, 34.94, 31.58, 25.95, 24.35 |
| 11b | 11.11(s, 1H, NH), 9.04(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.54(m, 6H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.78—3.66[m, 4H, N(CH2)2], 3.06—2.92[m, 4H, O(CH2)2], 1.34[s, 9H, C(CH3)3] | 155.73, 152.93, 151.62, 150.77, 146.03, 134.47, 134.28, 130.74, 126.83, 126.18, 119.92, 116.15, 115.21, 66.65, 49.96, 34.94, 31.58 |
| 11c | 11.08(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.53(m, 6H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.05—2.99[m, 4H, N(CH2)2], 2.47[m, 4H, N(CH2)2], 2.35(q, J=7.1 Hz, 2H, CH2), 1.34[s, 9H, C(CH3)3], 1.02(t, J=7.2 Hz, 3H, CH3) | 155.77, 152.92, 151.60, 150.75, 146.12, 134.50, 133.93, 130.74, 126.81, 126.17, 119.97, 116.34, 115.15, 52.92, 52.11, 49.67, 34.94, 31.58, 12.49 |
| 11d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.71—7.47(m, 6H, PhH), 7.06(d, J=8.5 Hz, 2H, PhH), 2.80(Hept, J=7.1 Hz, 1H, CH), 1.34[s, 9H, C(CH3)3], 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.93, 151.58, 150.81, 141.03, 139.31, 134.44, 130.70, 126.83, 126.42, 126.18, 118.86, 115.47, 34.94, 33.21, 31.58, 24.56 |
| 11e | 11.07(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.64—7.43(m, 6H, PhH), 6.64(d, J=9.0 Hz, 2H, PhH), 2.80[s, 6H, N(CH3)2], 1.34[s, 9H, C(CH3)3] | 155.98, 152.90, 151.58, 150.69, 146.19, 134.61, 131.85, 130.78, 126.76, 126.14, 120.54, 114.94, 113.49, 41.34, 34.93, 31.58 |
| 11f | 11.22(s, 1H, NH), 9.92(s, 1H, NH), 8.14(s, 1H, pyrimidine H), 7.97(d, J=8.8 Hz, 2H, PhH), 7.73(d, J=8.8 Hz, 2H, PhH), 7.59(d, J=8.6 Hz, 2H, PhH), 7.54(d, J=8.8 Hz, 2H, PhH), 3.12(s, 3H, CH3), 1.35[s, 9H, C(CH3)3] | 154.39, 153.01, 151.68, 151.08, 146.29, 133.99, 131.73, 130.53, 128.37, 126.98, 126.30, 117.53, 116.83, 44.55, 34.98, 31.58 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
| 12a | 11.27(s, 1H, NH), 9.05(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.97(dd, J=8.7 Hz, 4H, PhH), 7.52(d, J=8.9 Hz, 2H, PhH), 6.84(d, J=8.9 Hz, 2H, PhH), 3.11—2.92[m, 4H, N(CH2)2], 1.73—1.57(m, 4H, CH2), 1.57—1.40(m, 2H, CH2) | 155.76, 152.36, 150.90, 147.12, 137.20, 135.32, 133.55, 128.18, 127.86, 127.06, 126.42, 126.38, 125.87, 123.17, 120.06, 117.07, 115.04, 51.18, 25.95, 24.35 |
| 12b | 11.28(s, 1H, NH), 9.09(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.98(dd, J=8.7 Hz, 4H, PhH), 7.56(d, J=9.0 Hz, 2H, PhH), 6.85(d, J=8.9 Hz, 2H, PhH), 3.75—3.71[m, 4H, N(CH2)2], 3.02—2.96[m, 4H, O(CH2)2] | 155.70, 152.37, 150.91, 146.17, 137.19, 135.29, 134.05, 127.06, 126.43, 126.39, 119.99, 116.17, 116.01, 115.11, 66.65, 49.91 |
| 12c | 11.27(s, 1H, NH), 9.06(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.98(dd, J=8.6 Hz, 4H, PhH), 7.53(d, J=8.8 Hz, 2H, PhH), 6.83(d, J=8.7 Hz, 2H, PhH), 3.06—3.01[m, 4H, N(CH2)2], 2.40—2.34(q, J=7.2 Hz, 2H, CH2), 1.03(t, J=7.2 Hz, 3H, CH3) | 155.74, 152.37, 150.91, 146.25, 135.31, 133.71, 127.05, 126.42, 126.39, 121.17, 120.05, 116.37, 116.20, 115.08, 52.90, 52.11, 49.62, 12.46 |
| 12d | 11.32(s, 1H, NH), 9.22(s, 1H, pyrimidineH), 8.08(s, 1H, pyrimidine H), 7.97(dd, J=8.9 Hz, 4H, PhH), 7.60(d, J=8.5 Hz, 2H, PhH), 7.09(d, J=8.5 Hz, 2H, PhH), 2.81(Hept, J=6.9 Hz, 1H, CH), 1.17[d, J=6.9 Hz, 6H, (CH3)2] | 155.50, 152.39, 150.91, 141.24, 139.13, 137.13, 135.21, 127.16, 126.51, 126.44, 126.40, 118.89, 115.42, 33.22, 24.55 |
| 12e | 11.24(s, 1H, NH), 8.95(s, 1H, NH), 8.03(s, 1H, pyrimidine H), 8.01—7.92(dd, J=8.7 Hz, 4H, PhH, NH), 7.48(d, J=9.0 Hz, 2H, PhH), 6.67(d, J=9.0 Hz, 2H, PhH), 2.81[s, 6H, N(CH3)2] | 155.96, 152.36, 150.90, 146.34, 137.22, 135.41, 131.57, 127.02, 126.40, 120.68, 114.86, 113.51, 99.99, 41.32 |
| 12f | 11.51(s, 1H, NH), 9.96(s, 1H, NH), 8.19(s, 1H, pyrimidine H), 7.97(m, 6H, PhH), 7.76(d, J=8.8 Hz, 2H, PhH), 3.13(s, 3H, CH3) | 154.34, 152.47, 150.98, 146.12, 136.94, 134.81, 131.89, 128.46, 127.34, 126.58, 117.55, 116.78, 44.53 |
Table 4 1H NMR and 13C NMR data of compounds 8—12
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
|---|---|---|
| 8a | 11.14(s, 1H, NH), 9.02(s, 1H, NH), 8.01(s, 1H, pyrimidine H), 7.65(d, J=7.5 Hz, 2H, PhH), 7.55(q, 4H, PhH), 7.44(t, 1H, PhH), 6.80(d, J=7.4 Hz, 2H, PhH), 3.08—2.92[m, 4H, N(CH2)2], 1.61[m, 4H, CH2], 1.49(m, 2H, CH2) | 155.78, 152.78, 151.37, 147.00, 134.82, 133.76, 133.43, 129.35, 128.16, 127.00, 119.88, 117.06, 115.03, 51.22, 25.98, 24.34 |
| 8b | 11.16(s, 1H, NH), 9.07(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.65(d, J=7.9 Hz, 2H, PhH), 7.62—7.51(m, 4H, PhH), 7.44(t, 1H, PhH), 6.82(d, J=7.9 Hz, 2H, PhH), 3.80—3.67[m, 4H, N(CH2)2], 3.07—2.93[m, 4H, O(CH2)2] | 155.73, 152.78, 151.37, 146.02, 134.80, 134.23, 129.36, 128.18, 127.01, 119.84, 116.14, 115.10, 66.65, 49.94 |
| 8c | 11.16(s, 1H, NH), 9.04(s, 1H, NH), 8.01(s, 1H, pyrimidine H), 7.65(d, J=7.9 Hz, 2H, PhH), 7.56(q, 4H, PhH), 7.45(t, 1H, PhH), 6.81(d, J=8.0 Hz, 2H, PhH), 3.02[m, 4H, N(CH2)2], 2.50[m, 4H, N(CH2)2], 2.37(q, J=7.1 Hz, 2H, CH2), 1.03(t, J=7.2 Hz, 3H, CH3) | 155.76, 152.90, 151.36, 146.08, 135.08, 134.81, 129.53, 129.36, 128.17, 126.97, 119.87, 116.35, 115.06, 52.88, 52.09, 49.60, 12.42 |
| 8d | 11.19(s, 1H, NH), 9.20(s, 1H, NH), 8.04(s, 1H, pyrimidine H), 7.73—7.52(m, 6H, PhH), 7.45(t, J=7.4 Hz, 1H, PhH), 7.06(d, 2H, J=8.4 Hz, PhH), 2.79(Hept, J=6.9 Hz, 1H, CH), 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.80, 151.36, 141.03, 139.29, 134.72, 133.39, 129.37, 128.23, 127.06, 126.45, 118.74, 115.40, 33.21, 24.56 |
| 8f | 11.37(s, 1H, NH), 9.94(s, 1H, NH), 8.15(s, 1H, pyrimidine H), 7.97(d, J=8.9 Hz, 2H, PhH), 7.73(d, J=8.9 Hz, 2H, PhH), 7.66(d, J=7.9 Hz, 2H, PhH), 7.59(t, J=7.7 Hz, 2H, PhH), 7.47(t, J=7.3 Hz, 1H, PhH), 3.12(s, 3H, CH3) | 154.40, 152.85, 151.42, 146.24, 134.33, 133.17, 131.75, 129.50, 128.49, 128.38, 127.21, 117.50, 116.70, 44.53 |
| 9a | 11.11(s, 1H, NH), 9.01(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.60—7.45(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.04—2.94[m, 4H, N(CH2)2], 2.39(s, 3H, CH3), 1.61(m, 4H, CH2), 1.49(m, 2H, CH2) | 155.78, 152.88, 151.50, 146.97, 137.72, 134.63, 133.82, 130.80, 129.82, 126.91, 119.82, 117.10, 115.05, 51.24, 25.98, 24.34, 21.22 |
| 9b | 11.12(s, 1H, NH), 9.05(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.56(d, J=9.0 Hz, 2H, PhH), 7.50(d, J=8.2 Hz, 2H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.83—3.63[m, 4H, N(CH2)2], 3.08—2.93[m, 4H, O(CH2)2], 2.39(s, 3H, CH3) | 155.72, 152.88, 151.51, 146.00, 137.74, 134.62, 134.28, 130.79, 129.83, 126.92, 119.77, 116.16, 115.11, 66.66, 49.95, 21.23 |
| 9c | 11.12(s, 1H, NH), 9.02(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.52(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.07—2.92[m, 4H, N(CH2)2], 2.50[m, 4H, N(CH2)2], 2.42—2.32(m, 5H, CH3, CH2), 1.02(t, J=7.2 Hz, 3H, CH3) | 155.76, 152.88, 151.50, 146.09, 137.72, 134.63, 133.95, 130.80, 129.82, 126.90, 119.82, 116.36, 115.07, 52.93, 52.12, 49.67, 21.23, 12.49 |
| 9d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.61(d, J=8.5 Hz, 2H, PhH), 7.51(d, J=8.2 Hz, 2H, PhH), 7.36(d, J=8.2 Hz, 2H, PhH), 7.07(d, J=8.5 Hz, 2H, PhH), 2.79(Hept, J=6.9 Hz, 1H, CH), 2.39(s, 3H, CH3), 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.90, 151.49, 140.99, 139.32, 137.79, 134.55, 130.76, 129.83, 126.95, 126.46, 118.69, 115.40, 33.21, 24.57, 21.22 |
| 9e | 11.08(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.50(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.65(d, J=9.0 Hz, 2H, PhH), 2.80[s, 6H, N(CH3)2], 2.39(s, 3H, CH3) | 155.98, 152.87, 151.51, 146.17, 137.67, 134.70, 131.86, 130.83, 129.80, 126.88, 120.42, 114.88, 113.53, 41.37, 21.22 |
| 9f | 11.33(s, 1H, NH), 9.92(s, 1H, NH), 8.13(s, 1H, pyrimidine H), 7.96(d, J=8.9 Hz, 2H, PhH), 7.73(d, J=8.9 Hz, 2H, PhH), 7.51(d, J=8.2 Hz, 2H, PhH), 7.38(d, J=8.2 Hz, 2H, PhH), 3.12(s, 3H, CH3), 2.40(s, 3H, CH3) | 154.41, 152.94, 151.53, 146.27, 138.07, 134.19, 131.72, 130.56, 129.94, 128.38, 127.06, 117.46, 116.70, 44.55, 21.24 |
| 10a | 11.09(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.52(dd, J=9.0, 8.4 Hz, 4H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.02—2.97[m, 5H, N(CH2)2, CH], 1.61(m, 4H, CH2), 1.50(m, 2H, CH2), 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 155.79, 152.92, 151.59, 148.57, 146.98, 134.54, 133.79, 131.04, 127.23, 127.14, 119.94, 117.06, 115.11, 51.23, 33.68, 25.96, 24.34 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
| 10b | 11.11(s, 1H, NH), 9.04(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.56(d, J=9.0 Hz, 2H, PhH), 7.52(d, J=8.4 Hz, 2H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.78—3.67[m, 4H, N(CH2)2], 3.04—2.94[m, 5H, O(CH2)2, CH], 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 155.73, 152.93, 151.60, 148.58, 146.03, 134.50, 134.28, 127.24, 127.14, 119.88, 116.15, 115.18, 66.65, 49.96, 33.69, 24.35 |
| 10c | 11.09(s, 1H, NH), 9.01(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.62—7.47(dd, J=9.0, 8.4 Hz, 4H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.10—2.89[m, 5H, N(CH2)2, CH], 2.47[m, 4H, N(CH2)2], 2.35(q, J=7.2 Hz, 2H, CH2), 1.26[d, J=6.9 Hz, 6H, (CH3)2], 1.02(t, J=7.2 Hz, 3H, CH3) | 155.77, 152.92, 151.59, 148.57, 146.12, 134.53, 133.94, 131.03, 127.23, 127.13, 119.93, 116.35, 115.13, 52.92, 52.11, 49.68, 33.68, 24.35, 12.49 |
| 10d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.60(d, J=8.5 Hz, 2H, PhH), 7.53(d, J=8.3 Hz, 2H, PhH), 7.42(d, J=8.4 Hz, 2H, PhH), 7.06(d, J=8.5 Hz, 2H, PhH), 2.98(Hept, J=6.6 Hz, 1H, CH), 2.80(Hept, J=6.8 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2], 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.93, 151.57, 148.63, 141.02, 139.31, 134.46, 130.99, 127.24, 127.17, 126.43, 118.81, 115.45, 33.69, 33.21, 24.56, 24.34 |
| 10e | 11.07(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.59—7.46(dd, J=9.0, 8.3 Hz, 4H, PhH), 7.41(d, J=8.3 Hz, 2H, PhH), 6.64(d, J=9.0 Hz, 2H, PhH), 2.98(Hept, J=6.8 Hz, 1H, CH), 2.80[s, 6H, N(CH3)2], 1.25[d, J=6.9 Hz, 6H, (CH3)2] | 155.98, 152.91, 151.58, 148.52, 146.18, 134.62, 131.87, 131.06, 127.21, 127.10, 120.50, 114.92, 113.50, 41.35, 33.68, 24.34 |
| 10f | 11.29(s, 1H, NH), 9.92(s, 1H, NH), 8.14(s, 1H, pyrimidine H), 7.97(d, J=8.8 Hz, 2H, PhH), 7.73(d, J=8.8 Hz, 2H, PhH), 7.53(d, J=8.2 Hz, 2H, PhH), 7.44(d, J=8.4 Hz, 2H, PhH), 3.12(s, 3H, CH3), 3.02—2.95(Hept, J=6.9 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 154.40, 153.00, 151.64, 148.89, 146.28, 134.04, 131.73, 130.80, 128.37, 127.36, 127.29, 117.51, 116.77, 44.55, 33.71, 24.35 |
| 11a | 11.10(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.54(m, 6H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.03—2.95[m, 4H, N(CH2)2], 1.61(m, 4H, CH2), 1.50(m, 2H, CH2), 1.34[s, 9H, C(CH3)3] | 155.79, 152.92, 151.61, 150.75, 146.98, 134.51, 133.78, 130.75, 126.82, 126.16, 119.99, 117.04, 115.13, 51.22, 34.94, 31.58, 25.95, 24.35 |
| 11b | 11.11(s, 1H, NH), 9.04(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.54(m, 6H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.78—3.66[m, 4H, N(CH2)2], 3.06—2.92[m, 4H, O(CH2)2], 1.34[s, 9H, C(CH3)3] | 155.73, 152.93, 151.62, 150.77, 146.03, 134.47, 134.28, 130.74, 126.83, 126.18, 119.92, 116.15, 115.21, 66.65, 49.96, 34.94, 31.58 |
| 11c | 11.08(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.53(m, 6H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.05—2.99[m, 4H, N(CH2)2], 2.47[m, 4H, N(CH2)2], 2.35(q, J=7.1 Hz, 2H, CH2), 1.34[s, 9H, C(CH3)3], 1.02(t, J=7.2 Hz, 3H, CH3) | 155.77, 152.92, 151.60, 150.75, 146.12, 134.50, 133.93, 130.74, 126.81, 126.17, 119.97, 116.34, 115.15, 52.92, 52.11, 49.67, 34.94, 31.58, 12.49 |
| 11d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.71—7.47(m, 6H, PhH), 7.06(d, J=8.5 Hz, 2H, PhH), 2.80(Hept, J=7.1 Hz, 1H, CH), 1.34[s, 9H, C(CH3)3], 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.93, 151.58, 150.81, 141.03, 139.31, 134.44, 130.70, 126.83, 126.42, 126.18, 118.86, 115.47, 34.94, 33.21, 31.58, 24.56 |
| 11e | 11.07(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.64—7.43(m, 6H, PhH), 6.64(d, J=9.0 Hz, 2H, PhH), 2.80[s, 6H, N(CH3)2], 1.34[s, 9H, C(CH3)3] | 155.98, 152.90, 151.58, 150.69, 146.19, 134.61, 131.85, 130.78, 126.76, 126.14, 120.54, 114.94, 113.49, 41.34, 34.93, 31.58 |
| 11f | 11.22(s, 1H, NH), 9.92(s, 1H, NH), 8.14(s, 1H, pyrimidine H), 7.97(d, J=8.8 Hz, 2H, PhH), 7.73(d, J=8.8 Hz, 2H, PhH), 7.59(d, J=8.6 Hz, 2H, PhH), 7.54(d, J=8.8 Hz, 2H, PhH), 3.12(s, 3H, CH3), 1.35[s, 9H, C(CH3)3] | 154.39, 153.01, 151.68, 151.08, 146.29, 133.99, 131.73, 130.53, 128.37, 126.98, 126.30, 117.53, 116.83, 44.55, 34.98, 31.58 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
| 12a | 11.27(s, 1H, NH), 9.05(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.97(dd, J=8.7 Hz, 4H, PhH), 7.52(d, J=8.9 Hz, 2H, PhH), 6.84(d, J=8.9 Hz, 2H, PhH), 3.11—2.92[m, 4H, N(CH2)2], 1.73—1.57(m, 4H, CH2), 1.57—1.40(m, 2H, CH2) | 155.76, 152.36, 150.90, 147.12, 137.20, 135.32, 133.55, 128.18, 127.86, 127.06, 126.42, 126.38, 125.87, 123.17, 120.06, 117.07, 115.04, 51.18, 25.95, 24.35 |
| 12b | 11.28(s, 1H, NH), 9.09(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.98(dd, J=8.7 Hz, 4H, PhH), 7.56(d, J=9.0 Hz, 2H, PhH), 6.85(d, J=8.9 Hz, 2H, PhH), 3.75—3.71[m, 4H, N(CH2)2], 3.02—2.96[m, 4H, O(CH2)2] | 155.70, 152.37, 150.91, 146.17, 137.19, 135.29, 134.05, 127.06, 126.43, 126.39, 119.99, 116.17, 116.01, 115.11, 66.65, 49.91 |
| 12c | 11.27(s, 1H, NH), 9.06(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.98(dd, J=8.6 Hz, 4H, PhH), 7.53(d, J=8.8 Hz, 2H, PhH), 6.83(d, J=8.7 Hz, 2H, PhH), 3.06—3.01[m, 4H, N(CH2)2], 2.40—2.34(q, J=7.2 Hz, 2H, CH2), 1.03(t, J=7.2 Hz, 3H, CH3) | 155.74, 152.37, 150.91, 146.25, 135.31, 133.71, 127.05, 126.42, 126.39, 121.17, 120.05, 116.37, 116.20, 115.08, 52.90, 52.11, 49.62, 12.46 |
| 12d | 11.32(s, 1H, NH), 9.22(s, 1H, pyrimidineH), 8.08(s, 1H, pyrimidine H), 7.97(dd, J=8.9 Hz, 4H, PhH), 7.60(d, J=8.5 Hz, 2H, PhH), 7.09(d, J=8.5 Hz, 2H, PhH), 2.81(Hept, J=6.9 Hz, 1H, CH), 1.17[d, J=6.9 Hz, 6H, (CH3)2] | 155.50, 152.39, 150.91, 141.24, 139.13, 137.13, 135.21, 127.16, 126.51, 126.44, 126.40, 118.89, 115.42, 33.22, 24.55 |
| 12e | 11.24(s, 1H, NH), 8.95(s, 1H, NH), 8.03(s, 1H, pyrimidine H), 8.01—7.92(dd, J=8.7 Hz, 4H, PhH, NH), 7.48(d, J=9.0 Hz, 2H, PhH), 6.67(d, J=9.0 Hz, 2H, PhH), 2.81[s, 6H, N(CH3)2] | 155.96, 152.36, 150.90, 146.34, 137.22, 135.41, 131.57, 127.02, 126.40, 120.68, 114.86, 113.51, 99.99, 41.32 |
| 12f | 11.51(s, 1H, NH), 9.96(s, 1H, NH), 8.19(s, 1H, pyrimidine H), 7.97(m, 6H, PhH), 7.76(d, J=8.8 Hz, 2H, PhH), 3.13(s, 3H, CH3) | 154.34, 152.47, 150.98, 146.12, 136.94, 134.81, 131.89, 128.46, 127.34, 126.58, 117.55, 116.78, 44.53 |
| Compd. | IC50/(μmol·L-1) | |||
|---|---|---|---|---|
| K562 | PC-3 | MDA-MB-231 | HCT116 | |
| 8c | 3.68±0.08 | 11.46±0.67 | 18.16±1.64 | 4.48±0.12 |
| 10b | 9.13±2.35 | 4.43±0.19 | 11.79±0.08 | 4.41±0.12 |
| 10c | 2.23±0.26 | 6.17±1.49 | 11.67±4.02 | 12.24±2.50 |
| 12c | 3.25±0.85 | 5.07±0.34 | 3.35±0.27 | 1.15±0.09 |
| R-Roscovitine | 35.34±0.51 | 17.20±0.63 | 28.61±4.63 | 12.04±1.75 |
Table 5 Cytotoxicity of representative compounds in vitro*
| Compd. | IC50/(μmol·L-1) | |||
|---|---|---|---|---|
| K562 | PC-3 | MDA-MB-231 | HCT116 | |
| 8c | 3.68±0.08 | 11.46±0.67 | 18.16±1.64 | 4.48±0.12 |
| 10b | 9.13±2.35 | 4.43±0.19 | 11.79±0.08 | 4.41±0.12 |
| 10c | 2.23±0.26 | 6.17±1.49 | 11.67±4.02 | 12.24±2.50 |
| 12c | 3.25±0.85 | 5.07±0.34 | 3.35±0.27 | 1.15±0.09 |
| R-Roscovitine | 35.34±0.51 | 17.20±0.63 | 28.61±4.63 | 12.04±1.75 |
| [1] | Di V. F., Adinolfi E., Oncogene,2017, 36(3), 293-303 |
| [2] | Di V. F., Cancer Res., 2012, 72(21), 5441-5447 |
| [3] | Legraverend M., Grierson D. S., Bioorg. Med. Chem.,2006, 14(12), 3987-4006 |
| [4] | Sun L., Vasilevich N. I., Fuselier J. A., Hocart S. J., Coy D. H., Bioorg. Med. Chem. Lett.,2004, 14(9), 2041-2046 |
| [5] | Mulamoottil V. A., Nayak A., Jeong L. S., Asian J. Org. Chem.,2015, 45(37), 748-761 |
| [6] | Trávnícek Z., Štarha P., Vanco J., Šilha T., Hošek J., Pavel Suchý J., Pražanová G., J. Med. Chem.,2012, 55(10), 4568-4579 |
| [7] | Nivsarkar M., Thavaselvam D., Prasanna S., Sharma M., Kaushik M. P., Bioorg. Med. Chem. Lett.,2005, 15(5), 1371-1373 |
| [8] | Wu F., Li P., Hu D., Song B., Res. Chem. Intermediat.,2016, 42(9), 1-16 |
| [9] | Niu H. Y., Bai S. X., Wu S., Qu G. R., Guo H. M., Asian J. Org. Chem.,2012, 1(3), 238-244 |
| [10] | Guo J., Tan B., Ye Q., Liang G., Yi M., Jiang R., Chem. Res. Chinese Universities,2017, 33(4), 581-586 |
| [11] | Pan Y., Wang K., Liu Y., Qin R., Cao L., Wang J., Zhou G., Zhang A., Chem. Res. Chinese Universities,2017, 33(3), 388-391 |
| [12] | Pui C. H., Jeha S., Kirkpatrick P., Nat. Rev. Drug Discov.,2005, 4(5), S12-S13 |
| [13] | Gandhi V., Keating M. J., Bate G., Kirkpatrick P., Nat. Rev. Drug Discov.,2006, 5(1), 17-18 |
| [14] | Keating M. J., Kantarjian H., Talpaz M., Redman J., Koller C., Barlogie B., Velasquez W., Plunkett W., Freireich E. J., Mccredie K. B., Blood,1989, 74(1), 19-25 |
| [15] | Newlands E. S., Stevens M. F., Wedge S. R., Wheelhouse R. T., Brock C., Cancer Treat. Rev.,1997, 23(1), 35-61 |
| [16] | Sanford D. S., Wierda W. G., Burger J. A., Keating M. J., O'Brien S. M., Cl. Lymph. Myelom. Leuk.,2015, 15(7), 385-391 |
| [17] | Asghar U., Witkiewicz A. K., Turner N. C., Knudsen E. S., Nat. Rev. Drug Discov.,2015, 14(2), 130-146 |
| [18] | Zhang Z. P., Yang X. Y., Fang H., Chinese J. Org. Chem.,2015, 37(6), 1479-1486 |
| (张自鹏, 杨新颖, 方浩. 有机化学, 2017, 37(6), 1479-1486) | |
| [19] | Yang J., Wang L. J., Liu J. J., Zhong L., Zheng R. L., Xu Y., Ji P., Zhang C. H., Wang W. J., Lin X. D., J. Med. Chem.,2012, 55(23), 10685-10699 |
| [20] | Lu X., Li X., Yang J., Huang B., Kang D., Zhao F., Zhou Z., Clercq E. D., Daelemans D., Pannecouque C., Bioorg. Med. Chem.,2016, 24(18), 4424-4433 |
| [21] | Liu Z., Tang L., Zhu H., Xu T., Qiu C., Zheng S., Gu Y., Feng J., Zhang Y., Liang G., J. Med. Chem.,2016, 59(10), 4637-4650 |
| [1] | 曹舒杰, 李泓君, 管文丽, 任梦田, 周传政. 硫代磷酸酯寡聚核苷酸的立体控制合成研究进展[J]. 高等学校化学学报, 2022, 43(Album-4): 20220304. |
| [2] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [3] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| [4] | 韦春洪, 蒋倩, 王盼盼, 江成发, 刘岳峰. 贵金属Pt促进Co基费托合成催化剂的原子尺度结构分析[J]. 高等学校化学学报, 2022, 43(8): 20220074. |
| [5] | 金睿明, 穆晓清, 徐岩. 生物-化学法合成黑色素前体5, 6-二羟基吲哚[J]. 高等学校化学学报, 2022, 43(8): 20220134. |
| [6] | 张昕昕, 许狄, 王艳秋, 洪昕林, 刘国亮, 杨恒权. CO2加氢制低碳醇CuFe基催化剂中的Mn助剂效应[J]. 高等学校化学学报, 2022, 43(7): 20220187. |
| [7] | 周紫璇, 杨海艳, 孙予罕, 高鹏. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220235. |
| [8] | 鲁聪, 李振华, 刘金露, 华佳, 李光华, 施展, 冯守华. 一种新的镧系金属有机骨架材料的合成、 结构及荧光检测性质[J]. 高等学校化学学报, 2022, 43(6): 20220037. |
| [9] | 李祎頔, 田晓春, 李俊鹏, 陈立香, 赵峰. 半导体-微生物界面电子传递及其在环境领域的应用[J]. 高等学校化学学报, 2022, 43(6): 20220089. |
| [10] | 冯丽, 邵兰兴, 李思骏, 全文选, 庄金亮. 超薄Sm-MOF纳米片的合成及可见光催化降解芥子气模拟剂性能[J]. 高等学校化学学报, 2022, 43(4): 20210867. |
| [11] | 张志男, 程海明, 滕士勇, 张颖. RbPb2Cl5的合成及光学性质[J]. 高等学校化学学报, 2022, 43(11): 20220418. |
| [12] | 邢珮琪, 陆通, 李光华, 王力彦. 两个镉(II)金属有机骨架的可控合成与结构相关性[J]. 高等学校化学学报, 2022, 43(10): 20220218. |
| [13] | 谭玉玲, 杨玲, 虞虹, 倪春燕, 郎建平. 高核Ag/S纳米团簇合成的研究进展[J]. 高等学校化学学报, 2022, 43(1): 20210476. |
| [14] | 陈崇安, 杨国昱. 两种基于B5On(n=11, 12)簇构筑的具有深紫外吸收的硼酸盐[J]. 高等学校化学学报, 2022, 43(1): 20210711. |
| [15] | 王婕, 霍海燕, 王洋, 张仲, 刘术侠. 铜箔上原位合成NENU-n系列多酸基MOFs的通用策略[J]. 高等学校化学学报, 2022, 43(1): 20210557. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||