

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (12): 2534.doi: 10.7503/cjcu20190392
收稿日期:2019-07-15
出版日期:2019-12-04
发布日期:2019-12-04
通讯作者:
张恒
E-mail:zhangheng@sdu.edu.cn
基金资助:
Ying MA1,Tian WANG2,Heng ZHANG1,*( )
)
Received:2019-07-15
Online:2019-12-04
Published:2019-12-04
Contact:
Heng ZHANG
E-mail:zhangheng@sdu.edu.cn
Supported by:摘要:
采用分子动力学方法研究了亚甲基蓝在不同氧化度的氧化石墨烯表面的吸附行为及其动力学性质, 从微观角度讨论了亚甲基蓝由体相到氧化石墨烯表面的吸附过程及主要作用机制, 并通过亚甲基蓝分子动力学性质解释了氧化石墨烯的氧化度和含氧官能团类型对吸附行为的影响. 结果表明, 吸附过程中, 亚甲基蓝主要受氧化石墨烯表面含氧官能团的静电作用, 以近似垂直氧化石墨烯表面的方向进入, 并以平行的方式吸附于氧化石墨烯表面; 亚甲基蓝不易脱离高氧化度氧化石墨烯的吸附位点; 吸附平衡过程中, 相对于低氧化度的氧化石墨烯, 高氧化度氧化石墨烯对亚甲基蓝的束缚性更强, 同时与亚甲基蓝间相互作用更强; 含氧官能团中的环氧基与亚甲基蓝间的作用势能更强, 且羟基能够与亚甲基蓝间形成氢键结构, 共同保障了亚甲基蓝吸附层的稳定性.
中图分类号:
TrendMD:
马莹,王恬,张恒. 氧化石墨烯吸附亚甲基蓝的分子动力学模拟. 高等学校化学学报, 2019, 40(12): 2534.
Ying MA,Tian WANG,Heng ZHANG. Molecular Dynamics Simulation of Adsorption of Methylene Blue by Graphene Oxide †. Chem. J. Chinese Universities, 2019, 40(12): 2534.
| Site | Graphene oxide | Site | Methylene blue | ||||
|---|---|---|---|---|---|---|---|
| σ/nm | ε/(kJ·mol-1) | q/e | σ/nm | ε/(kJ·mol-1) | q/e | ||
| C(graphene) | 0.355 | 0.293 | 0 | N(in N—CH3) | 0.325 | 0.711 | 0.216 |
| C(—COO-) | 0.375 | 0.439 | 0.700 | C(in N—CH3) | 0.380 | 0.209 | -0.323 |
| O(—COO-) | 0.296 | 0.879 | -0.900 | H(in N—CH3) | 0.250 | 0.125 | 0.137 |
| C(—C—O—) | 0.350 | 0.276 | 0.196 | S | 0.355 | 1.046 | -0.689 |
| O(-O-) | 0.290 | 0.586 | -0.393 | N(in middle) | 0.325 | 0.711 | -0.226 |
| O(—OH) | 0.307 | 0.711 | -0.592 | ||||
| H(—OH) | 0 | 0 | 0.329 | ||||
Table 1 Force field parameters for graphene oxide and methylene blue used in this work*
| Site | Graphene oxide | Site | Methylene blue | ||||
|---|---|---|---|---|---|---|---|
| σ/nm | ε/(kJ·mol-1) | q/e | σ/nm | ε/(kJ·mol-1) | q/e | ||
| C(graphene) | 0.355 | 0.293 | 0 | N(in N—CH3) | 0.325 | 0.711 | 0.216 |
| C(—COO-) | 0.375 | 0.439 | 0.700 | C(in N—CH3) | 0.380 | 0.209 | -0.323 |
| O(—COO-) | 0.296 | 0.879 | -0.900 | H(in N—CH3) | 0.250 | 0.125 | 0.137 |
| C(—C—O—) | 0.350 | 0.276 | 0.196 | S | 0.355 | 1.046 | -0.689 |
| O(-O-) | 0.290 | 0.586 | -0.393 | N(in middle) | 0.325 | 0.711 | -0.226 |
| O(—OH) | 0.307 | 0.711 | -0.592 | ||||
| H(—OH) | 0 | 0 | 0.329 | ||||
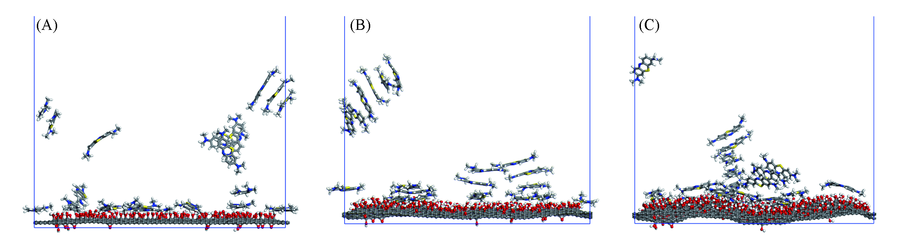
Fig.2 Adsorption of methylene blue on graphene oxide of GO10(A), GO20(B) and GO30(C) systems The water molecules in the system are removed for clarity.
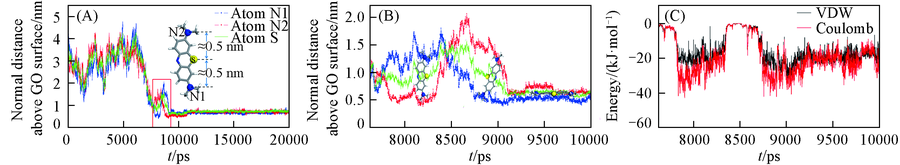
Fig.3 Trajectory displacement of methylene blue in the z direction changes with time evolution(A), local amplifications of trajectory displacement (A)(B) and the change of potential energy between amino groups of methylene blue and GO30 graphene oxide with time evolution(C)
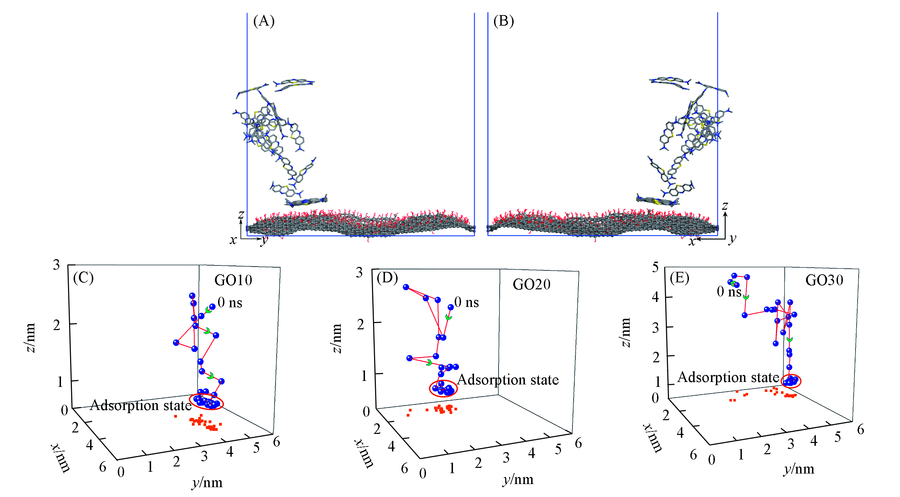
Fig.4 Side view of partial enlargements of the trajectory displacement of a methylene blue in the z direction(A), the opposite side view of a diagram(B), three-dimensional spatial coordinates of a methylene blue adsorption process change with time of GO10(C), GO20(D) and GO30(E) systems
| System | 105D/(cm2·s-1) | τr/ns |
|---|---|---|
| GO10 | 0.03±0.01 | 3.31 |
| GO20 | 0.007±0.005 | 4.94 |
| GO30 | 0.0057±0.004 | 6.16 |
Table 2 Diffusion coefficient(D) and residence time(τr) of methylene blue near graphene oxide surface
| System | 105D/(cm2·s-1) | τr/ns |
|---|---|---|
| GO10 | 0.03±0.01 | 3.31 |
| GO20 | 0.007±0.005 | 4.94 |
| GO30 | 0.0057±0.004 | 6.16 |
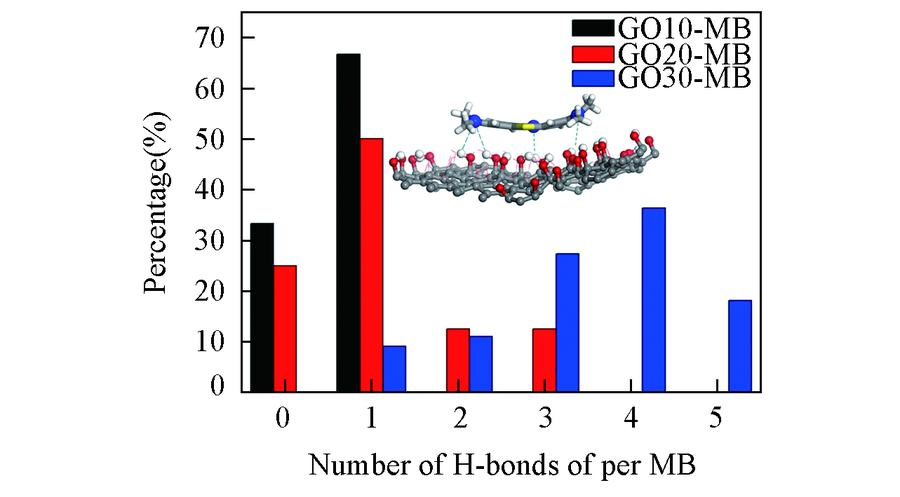
Fig.7 Hydrogen bond number distribution between methylene blue and graphene oxide in adsorption layer Inset: the microscopic structure of hydrogen bonds.
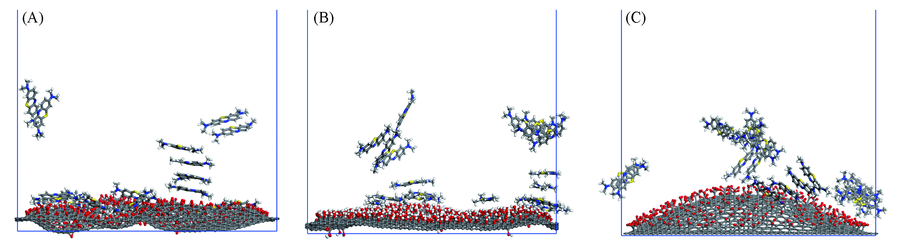
Fig.8 Adsorption of methylene blue on graphene oxide of GO20-O(A), GO20-OH(B) and GO20-COO system(C) The water molecules in the system are removed for clarity.
| [1] |
Wang C., Shi Z. H., Peng L., He W. M., Li B. L., Li K. Z ., Surfaces & Interfaces, 2017,7, 116— 124
doi: 10.1016/j.jcis.2019.11.096 URL pmid: 31830629 |
| [2] |
Krika F., Benlahbib O. E. F ., Desalination & Water Treatment, 2015,53(13), 3711— 3723
doi: 10.1021/acs.est.9b04823 URL pmid: 31829572 |
| [3] |
Isloor A. M., Shenvi S. S., Ismail A. F., Shilton S. J., Al-Ahmad A ., Industrial & Engineering Chemistry Research, 2015,54(18), 4965— 4975
doi: 10.1016/j.socscimed.2019.112710 URL pmid: 31829531 |
| [4] |
Mouzdahir Y. E., Elmchaouri A., Mahboub R., Gil A., Korili S. A ., J. Chem. Engin. Data, 2007,52(5), 1621— 1625
doi: 10.1021/je700008g URL |
| [5] |
Cheah W., Hosseini S., Khan M. A., Chuah T. G., Choong T. S. Y ., Chem. Engin. J., 2013,215/216(3), 747— 754
doi: 10.1016/j.cej.2012.07.004 URL |
| [6] |
And C. D., Israelachvili J ., Macromolecules, 2000,33(13), 4910— 4920
doi: 10.1021/ma9919918 URL |
| [7] |
Sheng J., Xie Y., Yan Z ., Applied Clay Science, 2009,46(4), 422— 424
doi: 10.1016/j.clay.2009.10.006 URL |
| [8] |
Senthilkumaar S., Varadarajan P. R., Porkodi K., Subbhuraam C. V ., J. Colloid & Interface Science, 2005,284(1), 78— 82
doi: 10.1016/j.jcis.2019.11.122 URL pmid: 31830737 |
| [9] |
Wang S., Zhu Z. H ., J. Hazardous Mater., 2006,136(3), 946— 952
doi: 10.1016/j.jhazmat.2006.01.038 URL pmid: 16504394 |
| [10] |
Da C., Hongbin F., Jinghong L ., Chem. Rev., 2012,112(11), 6027— 6053
doi: 10.1021/cr300115g URL pmid: 22889102 |
| [11] |
Ramesha G. K., Kumara A. V., Muralidhara H. B., Sampath S ., J. Colloid & Interface Science, 2011,361(1), 270— 277
doi: 10.1016/j.jcis.2019.11.122 URL pmid: 31830737 |
| [12] |
Sun Y., Wang Q., Chen C., Tan X., Wang X ., Environmental Science & Technology, 2012,46(11), 6020— 6027
doi: 10.1038/s41586-019-1875-y URL pmid: 31830756 |
| [13] |
Bradder P., Ling S. K., Wang S., Liu S ., J. Chem. Eng. Data, 2011,56(1), 138— 141
doi: 10.1021/je101049g URL |
| [14] |
Zhang W., Zhou C., Zhou W., Lei A., Zhang Q., Wan Q., Zou B ., Bull. Environ. Contam. Toxicol., 2011,87(1), 86— 90
doi: 10.1007/s00128-011-0304-1 URL pmid: 21567134 |
| [15] |
Pei Z., Li L., Sun L., Zhang S., Shan X. Q., Shuang Y., Bei W ., Carbon, 2013,51(1), 156— 163
doi: 10.1016/j.carbon.2012.08.024 URL |
| [16] |
Han Y., Xue T., Zhen Y., Li K., Hu Y., Li A., Cheng R ., J. Hazardous Mater., 2014,268(268), 191— 198
doi: 10.1016/j.jhazmat.2014.01.015 URL pmid: 24491443 |
| [17] | Liu S. S., Zheng H., Wang H., Yuan S. L., Hou S. F ., Chem. J. Chinese Universities, 2017,38(1), 63— 71 |
| ( 刘沙沙, 张恒, 王华, 苑世领, 侯士峰 . 高等学校化学学报, 2017,38(1), 63— 71) | |
| [18] |
Jorgensen W. L., Maxwell D. S., Tirado-Rives J ., J. Am. Chem. Soc., 1996,118(45), 11225— 11236
doi: 10.1021/ja9621760 URL |
| [19] |
Van Der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A. E., Berendsen H. J ., J. Comput. Chem., 2005,26(16), 1701— 1718
doi: 10.1002/jcc.20291 URL pmid: 16211538 |
| [20] |
Guo K., Zheng H., Sun J. C., Yuan S. L., Liu C. B ., Chem. J. Chinese Universities, 2015,36(11), 2171— 2178
doi: 10.7503/cjcu20150546 URL |
|
( 郭凯, 张恒, 孙继超, 苑世领, 刘成卜 . 高等学校化学学报, 2015,36(11), 2171— 2178)
doi: 10.7503/cjcu20150546 URL |
|
| [21] | Accelrys Software Inc., Materials Studio, Release 4.4, Accelrys Software Inc., San Diego, 2008 |
| [22] |
Madadrang C. J., Kim H. Y., Gao G., Wang N., Zhu J., Feng H., Gorring M ., ACS Appl. Mater. Inter., 2012,4(3), 1186— 1193
doi: 10.1021/am201645g URL pmid: 22304446 |
| [23] |
Essmann U., Perera L., Berkowiz ML., Darden T., Lee H., Pedersen L. G ., J. Chem. Phys., 1995,103(19), 8577— 8593
doi: 10.1063/1.470117 URL |
| [24] |
Berendsen H. J. C., Postma J. P. M., van Gunsteren W. F., DiNola A., Haak J. R ., J. Chem. Phys., 1984,81(8), 3684— 3690
doi: 10.1063/1.448118 URL |
| [25] |
Hess B., Bekker H., Berendsen H. J. C., Fraaije J. G. E. M ., J. Comput. Chem., 1997,18(12), 1463— 1472
doi: 10.1002/(ISSN)1096-987X URL |
| [26] |
Qing S., Yi H., White A. D., Shaoyi J ., J. Phys. Chem. B, 2010,114(49), 16625— 16631
doi: 10.1021/jp107272n URL pmid: 21086974 |
| [27] |
Yi H., Jason H., Shengfu C., Bernards M. T., Yung C., Shaoyi J ., Langmuir the ACS J. Surfaces & Colloids, 2008,24(18), 10358— 10364
doi: 10.1016/j.jcis.2010.10.034 URL pmid: 21056425 |
| [28] |
Yi H., Yung C., Hower J. C., Jie Z., Shengfu C., Shaoyi J ., Phys.Chem.Chem.Phys., 2008,10(36), 5539— 5544
doi: 10.1039/b807129b URL pmid: 18956088 |
| [29] |
Hower J. C., Yi H., Bernards M. T., Shaoyi J ., J. Chem. Phys., 2006,125(21), 214704
doi: 10.1063/1.2397681 URL pmid: 17166037 |
| [1] | 王瑞娜, 孙瑞粉, 钟添华, 池毓务. 大尺寸石墨烯量子点组装体的制备及电化学发光性能[J]. 高等学校化学学报, 2022, 43(8): 20220161. |
| [2] | 高志伟, 李军委, 史赛, 付强, 贾钧儒, 安海龙. 基于分子动力学模拟的TRPM8通道门控特性分析[J]. 高等学校化学学报, 2022, 43(6): 20220080. |
| [3] | 郝宏蕾, 孟繁雨, 李若钰, 李迎秋, 贾明君, 张文祥, 袁晓玲. 生物质基氮掺杂多孔炭材料的制备及对水中亚甲基蓝的吸附性能[J]. 高等学校化学学报, 2022, 43(6): 20220055. |
| [4] | 曾晛阳, 赵熹, 黄旭日. 细胞松弛素B对葡萄糖/质子共转运蛋白GlcPSe的抑制机理[J]. 高等学校化学学报, 2022, 43(4): 20210822. |
| [5] | 刘嘉欣, 闵杰, 许华杰, 任海生, 谈宁馨. 基于反应力场分子模拟的乙烯燃烧自由基与氮气相互作用研究[J]. 高等学校化学学报, 2022, 43(4): 20210834. |
| [6] | 陈瀚翔, 边绍菊, 胡斌, 李武. LiCl-NaCl-KCl-H2O溶液体系渗透压的分子动力学模拟[J]. 高等学校化学学报, 2022, 43(3): 20210727. |
| [7] | 杨隽阁, 高成乾, 李博鑫, 尹德忠. 改性氧化石墨烯稳定Pickering乳液法制备高导热相变整体材料[J]. 高等学校化学学报, 2022, 43(2): 20210593. |
| [8] | 张志博, 尚涵, 徐文轩, 韩广东, 崔金声, 杨皓然, 李瑞鑫, 张生辉, 徐欢. 氧化石墨烯插层聚β-羟基丁酸酯复合材料的结晶形态与宏观性能[J]. 高等学校化学学报, 2022, 43(2): 20210566. |
| [9] | 胡波, 朱昊辰. 双层氧化石墨烯纳米体系中受限水的介电常数[J]. 高等学校化学学报, 2022, 43(2): 20210614. |
| [10] | 俞彬, 谌小燕, 赵越, 陈卫昌, 肖新颜, 刘海洋. 氧化石墨烯基钴卟啉复合材料的电催化析氢反应[J]. 高等学校化学学报, 2022, 43(2): 20210549. |
| [11] | 王雪丽, 宋相伟, 解颜宁, 杜妮阳, 王振新. 部分还原氧化石墨烯的制备、 表征及对人宫颈癌HeLa细胞的体外杀伤作用[J]. 高等学校化学学报, 2022, 43(2): 20210595. |
| [12] | 张伶育, 张继龙, 曲泽星. RDX分子内振动能量重分配的动力学研究[J]. 高等学校化学学报, 2022, 43(10): 20220393. |
| [13] | 柳雪广, 杨晓珊, 马菁菁, 刘伟生. 铕基金属有机框架材料从混合染料中选择性分离亚甲基蓝[J]. 高等学校化学学报, 2022, 43(1): 20210715. |
| [14] | 朱德帅, 赵剑英, 杨正慧, 郭海泉, 高连勋. 基于多层结构设计的高储能密度氧化石墨烯/聚酰亚胺复合材料[J]. 高等学校化学学报, 2021, 42(8): 2694. |
| [15] | 雷晓彤, 金怡卿, 孟烜宇. 基于分子模拟方法预测PIP2在双孔钾通道TREK-1上结合位点的研究[J]. 高等学校化学学报, 2021, 42(8): 2550. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||