

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (3): 20210727.doi: 10.7503/cjcu20210727
陈瀚翔1,2,3, 边绍菊1,2, 胡斌1,2( ), 李武1,2
), 李武1,2
收稿日期:2021-10-19
出版日期:2022-03-10
发布日期:2022-01-07
通讯作者:
胡斌
E-mail:hubin@isl.ac.cn
基金资助:
CHEN Hanxiang1,2,3, BIAN Shaoju1,2, HU Bin1,2( ), LI Wu1,2
), LI Wu1,2
Received:2021-10-19
Online:2022-03-10
Published:2022-01-07
Contact:
HU Bin
E-mail:hubin@isl.ac.cn
Supported by:摘要:
盐湖卤水中含有大量的锂、 钾资源, 发展卤水的渗透压预测模型和方法, 对于资源的综合利用具有重要的指导意义. 本文采用分子动力学(MD)方法结合水溶液渗透压模拟(OPAS)技术, 研究了LiCl-NaCl-KCl-H2O体系的渗透压模拟方法, 计算了298.15 K下体系多种组成和浓度的渗透压, 并将计算结果与实验值和Pitzer模型计算值进行了对比. 结果显示, 模拟计算值与实验值和Pitzer模型计算值渗透压的趋势基本一致, 包括同种溶液不同浓度和同浓度不同溶液之间的渗透压关系. 该方法可以应用于多元混合物体系对渗透压进行定性和半定量的计算.
中图分类号:
TrendMD:
陈瀚翔, 边绍菊, 胡斌, 李武. LiCl-NaCl-KCl-H2O溶液体系渗透压的分子动力学模拟. 高等学校化学学报, 2022, 43(3): 20210727.
CHEN Hanxiang, BIAN Shaoju, HU Bin, LI Wu. Molecular Simulation of the Osmotic Pressures for LiCl-NaCl-KCl-H2O Solution System. Chem. J. Chinese Universities, 2022, 43(3): 20210727.
| System | Number of models | Molality/(mol?kg-1) | Number of particles in models | r |
|---|---|---|---|---|
| LiCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| NaCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| KCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| LiCl?NaCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| LiCl?KCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| NaCl?KCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| LiCl?NaCl?KCl?H2O | 24 | 0.8—4.7 | 8000 water a, 160—640 ion pairs | 2∶1∶1, 1∶2∶1, 1∶1∶2 c |
Table 1 Number of models, molalities and number of particles in models of all solution systems for simulation
| System | Number of models | Molality/(mol?kg-1) | Number of particles in models | r |
|---|---|---|---|---|
| LiCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| NaCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| KCl?H2O | 8 | 0.5—4.7 | 8000 water a, 80—640 ion pairs | |
| LiCl?NaCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| LiCl?KCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| NaCl?KCl?H2O | 28 | 1.1—4.7 | 8000 water a, 160—640 ion pairs | 1∶4, 2∶3, 3∶2, 4∶1 b |
| LiCl?NaCl?KCl?H2O | 24 | 0.8—4.7 | 8000 water a, 160—640 ion pairs | 2∶1∶1, 1∶2∶1, 1∶1∶2 c |
| Solution | Molality/(mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[ |
|---|---|---|---|---|---|
| LiCl | 0.56 | -0.174 | 2.80 | 3.12 | 2.75 |
| LiCl | 1.13 | -0.248 | 6.31 | 5.30 | 5.79 |
| LiCl | 1.70 | -0.364 | 9.27 | 8.71 | 9.23 |
| LiCl | 2.28 | -0.570 | 10.11 | 14.77 | 13.11 |
| LiCl | 2.87 | -0.752 | 16.12 | 20.09 | 17.47 |
| LiCl | 3.48 | -0.720 | 12.14 | 19.16 | 22.34 |
| LiCl | 4.08 | -0.992 | 21.11 | 27.05 | 27.82 |
| LiCl | 4.70 | -1.240 | 26.17 | 34.20 | 33.89 |
| NaCl | 0.56 | -0.133 | 1.84 | 2.96 | 2.62 |
| NaCl | 1.12 | -0.262 | 7.00 | 5.07 | 5.30 |
| NaCl | 1.69 | -0.406 | 10.07 | 7.62 | 8.13 |
| NaCl | 2.27 | -0.611 | 13.97 | 11.58 | 11.14 |
| NaCl | 2.86 | -0.807 | 15.43 | 15.75 | 14.33 |
| NaCl | 3.45 | -0.819 | 18.76 | 16.04 | 17.75 |
| NaCl | 4.05 | -1.087 | 24.00 | 22.37 | 21.42 |
| NaCl | 4.67 | -1.175 | 25.76 | 24.62 | 25.32 |
| KCl | 0.56 | -0.113 | 2.79 | 2.53 | 2.54 |
| KCl | 1.13 | -0.331 | 7.15 | 5.18 | 5.05 |
| KCl | 1.71 | -0.466 | 10.11 | 7.46 | 7.58 |
| KCl | 2.30 | -0.600 | 13.42 | 10.18 | 10.16 |
| KCl | 2.90 | -0.699 | 14.47 | 12.50 | 12.81 |
| KCl | 3.51 | -0.912 | 19.66 | 18.38 | 15.55 |
| KCl | 4.15 | -0.902 | 20.39 | 18.07 | 18.36 |
| KCl | 4.78 | -0.934 | 23.97 | 19.05 | 21.29 |
Table 2 Main numeric results of binary solution system simulations
| Solution | Molality/(mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[ |
|---|---|---|---|---|---|
| LiCl | 0.56 | -0.174 | 2.80 | 3.12 | 2.75 |
| LiCl | 1.13 | -0.248 | 6.31 | 5.30 | 5.79 |
| LiCl | 1.70 | -0.364 | 9.27 | 8.71 | 9.23 |
| LiCl | 2.28 | -0.570 | 10.11 | 14.77 | 13.11 |
| LiCl | 2.87 | -0.752 | 16.12 | 20.09 | 17.47 |
| LiCl | 3.48 | -0.720 | 12.14 | 19.16 | 22.34 |
| LiCl | 4.08 | -0.992 | 21.11 | 27.05 | 27.82 |
| LiCl | 4.70 | -1.240 | 26.17 | 34.20 | 33.89 |
| NaCl | 0.56 | -0.133 | 1.84 | 2.96 | 2.62 |
| NaCl | 1.12 | -0.262 | 7.00 | 5.07 | 5.30 |
| NaCl | 1.69 | -0.406 | 10.07 | 7.62 | 8.13 |
| NaCl | 2.27 | -0.611 | 13.97 | 11.58 | 11.14 |
| NaCl | 2.86 | -0.807 | 15.43 | 15.75 | 14.33 |
| NaCl | 3.45 | -0.819 | 18.76 | 16.04 | 17.75 |
| NaCl | 4.05 | -1.087 | 24.00 | 22.37 | 21.42 |
| NaCl | 4.67 | -1.175 | 25.76 | 24.62 | 25.32 |
| KCl | 0.56 | -0.113 | 2.79 | 2.53 | 2.54 |
| KCl | 1.13 | -0.331 | 7.15 | 5.18 | 5.05 |
| KCl | 1.71 | -0.466 | 10.11 | 7.46 | 7.58 |
| KCl | 2.30 | -0.600 | 13.42 | 10.18 | 10.16 |
| KCl | 2.90 | -0.699 | 14.47 | 12.50 | 12.81 |
| KCl | 3.51 | -0.912 | 19.66 | 18.38 | 15.55 |
| KCl | 4.15 | -0.902 | 20.39 | 18.07 | 18.36 |
| KCl | 4.78 | -0.934 | 23.97 | 19.05 | 21.29 |
| Solution | c2 | c1 | c0 |
|---|---|---|---|
| LiCl | -7.884 | -124.7 | -2.066 |
| NaCl | 85.21 | -60.30 | 0.9623 |
| KCl | 231.1 | -26.40 | 1.648 |
Table 3 Final fitted parameters of MEF method
| Solution | c2 | c1 | c0 |
|---|---|---|---|
| LiCl | -7.884 | -124.7 | -2.066 |
| NaCl | 85.21 | -60.30 | 0.9623 |
| KCl | 231.1 | -26.40 | 1.648 |
| Solution | RMSE MD?FS(%) | RMSE MD?MEF(%) |
|---|---|---|
| LiCl?KCl?H2O | 7.9 | 2.6 |
| LiCl?NaCl?H2O | 8.6 | 2.7 |
| NaCl?KCl?H2O | 12.4 | 2.6 |
| LiCl?NaCl?KCl?H2O | 6.8 | 2.0 |
Table 4 Error analysis of computed osmotic pressures of ternary and quaternary systems
| Solution | RMSE MD?FS(%) | RMSE MD?MEF(%) |
|---|---|---|
| LiCl?KCl?H2O | 7.9 | 2.6 |
| LiCl?NaCl?H2O | 8.6 | 2.7 |
| NaCl?KCl?H2O | 12.4 | 2.6 |
| LiCl?NaCl?KCl?H2O | 6.8 | 2.0 |
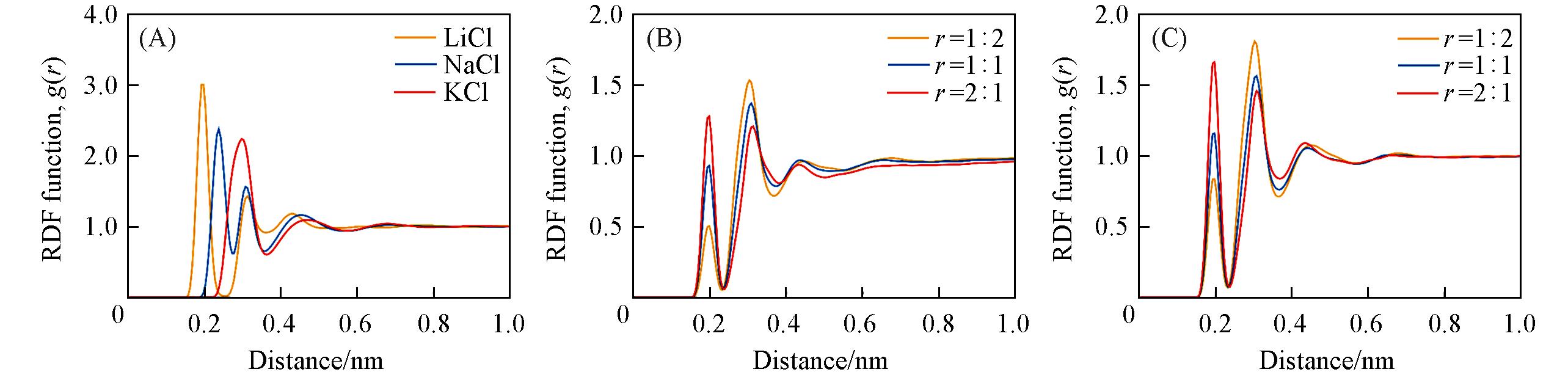
Fig.8 Radial distribution functions of water?ion pairs of binary systems and ternary system LiCl?KCl?H2O(A) 4.4 mol/kg MCl?H2O water?ion RDF; (B) 6.0 mol/kg LiCl?KCl?H2O water?ion RDF; (C) 3.0 mol/kg LiCl?KCl?H2O water?ion RDF.
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
|---|---|---|---|---|---|---|
| LiCl?KCl | 1∶4 | 1.13 | -0.270 | 7.73 | 4.63 | 5.17 |
| LiCl?KCl | 1∶4 | 1.71 | -0.378 | 10.78 | 6.57 | 7.82 |
| LiCl?KCl | 1∶4 | 2.29 | -0.521 | 12.15 | 9.48 | 10.59 |
| LiCl?KCl | 1∶4 | 2.89 | -0.806 | 20.80 | 16.59 | 13.46 |
| LiCl?KCl | 1∶4 | 3.51 | -0.919 | 25.48 | 19.88 | 16.42 |
| LiCl?KCl | 1∶4 | 4.13 | -1.040 | 30.94 | 23.68 | 19.55 |
| LiCl?KCl | 1∶4 | 4.76 | -1.113 | 30.67 | 26.13 | 22.82 |
| LiCl?KCl | 2∶3 | 1.13 | -0.296 | 9.94 | 5.50 | 5.30 |
| LiCl?KCl | 2∶3 | 1.71 | -0.506 | 16.14 | 10.10 | 8.11 |
| LiCl?KCl | 2∶3 | 2.29 | -0.545 | 17.59 | 11.01 | 11.09 |
| LiCl?KCl | 2∶3 | 2.89 | -0.792 | 22.55 | 17.44 | 14.23 |
| LiCl?KCl | 2∶3 | 3.50 | -0.809 | 22.10 | 17.94 | 17.55 |
| LiCl?KCl | 2∶3 | 4.12 | -0.844 | 24.73 | 18.95 | 21.10 |
| LiCl?KCl | 2∶3 | 4.75 | -1.157 | 35.18 | 28.70 | 24.82 |
| LiCl?KCl | 3∶2 | 1.13 | -0.327 | 7.51 | 6.63 | 5.44 |
| LiCl?KCl | 3∶2 | 1.70 | -0.371 | 11.43 | 7.67 | 8.44 |
| LiCl?KCl | 3∶2 | 2.29 | -0.572 | 13.64 | 12.73 | 11.67 |
| LiCl?KCl | 3∶2 | 2.88 | -0.655 | 20.31 | 14.95 | 15.17 |
| LiCl?KCl | 3∶2 | 3.49 | -0.742 | 20.81 | 17.31 | 18.92 |
| LiCl?KCl | 3∶2 | 4.10 | -0.875 | 22.01 | 21.11 | 22.99 |
| LiCl?KCl | 3∶2 | 4.73 | -1.185 | 32.23 | 30.63 | 27.35 |
| LiCl?KCl | 4∶1 | 1.13 | -0.276 | 6.95 | 5.78 | 5.61 |
| LiCl?KCl | 4∶1 | 1.70 | -0.326 | 8.24 | 7.11 | 8.81 |
| LiCl?KCl | 4∶1 | 2.29 | -0.492 | 11.90 | 11.58 | 12.34 |
| LiCl?KCl | 4∶1 | 2.88 | -0.719 | 19.43 | 17.92 | 16.24 |
| LiCl?KCl | 4∶1 | 3.48 | -0.868 | 28.16 | 22.20 | 20.52 |
| LiCl?KCl | 4∶1 | 4.09 | -1.034 | 26.05 | 27.08 | 25.23 |
| LiCl?KCl | 4∶1 | 4.71 | -1.226 | 30.80 | 32.89 | 30.38 |
| LiCl?NaCl | 1∶4 | 1.12 | -0.274 | 7.43 | 5.43 | 5.42 |
| LiCl?NaCl | 1∶4 | 1.69 | -0.513 | 14.15 | 10.34 | 8.38 |
| LiCl?NaCl | 1∶4 | 2.27 | -0.583 | 16.73 | 11.84 | 11.57 |
| LiCl?NaCl | 1∶4 | 2.86 | -0.689 | 19.53 | 14.22 | 15.01 |
| LiCl?NaCl | 1∶4 | 3.45 | -0.950 | 28.48 | 20.40 | 18.75 |
| LiCl?NaCl | 1∶4 | 4.06 | -1.027 | 31.16 | 22.34 | 22.77 |
| LiCl?NaCl | 1∶4 | 4.66 | -1.338 | 39.75 | 30.57 | 27.13 |
| LiCl?NaCl | 2∶3 | 1.12 | -0.261 | 11.24 | 5.32 | 5.52 |
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
| LiCl?NaCl | 2∶3 | 1.69 | -0.502 | 14.38 | 10.76 | 8.61 |
| LiCl?NaCl | 2∶3 | 2.27 | -0.604 | 18.93 | 13.18 | 12.00 |
| LiCl?NaCl | 2∶3 | 2.86 | -0.686 | 20.43 | 15.15 | 15.66 |
| LiCl?NaCl | 2∶3 | 3.46 | -0.812 | 28.41 | 18.25 | 19.67 |
| LiCl?NaCl | 2∶3 | 4.06 | -1.022 | 29.51 | 23.64 | 24.07 |
| LiCl?NaCl | 2∶3 | 4.67 | -1.200 | 30.19 | 28.38 | 28.85 |
| LiCl?NaCl | 3∶2 | 1.12 | -0.296 | 11.00 | 6.29 | 5.62 |
| LiCl?NaCl | 3∶2 | 1.70 | -0.494 | 15.00 | 11.24 | 8.82 |
| LiCl?NaCl | 3∶2 | 2.28 | -0.476 | 12.19 | 10.78 | 12.37 |
| LiCl?NaCl | 3∶2 | 2.86 | -0.678 | 21.28 | 15.95 | 16.30 |
| LiCl?NaCl | 3∶2 | 3.46 | -0.869 | 26.12 | 20.97 | 20.61 |
| LiCl?NaCl | 3∶2 | 4.07 | -1.119 | 31.95 | 27.70 | 25.36 |
| LiCl?NaCl | 3∶2 | 4.68 | -1.215 | 35.19 | 30.36 | 30.56 |
| LiCl?NaCl | 4∶1 | 1.13 | -0.224 | 7.82 | 4.55 | 5.71 |
| LiCl?NaCl | 4∶1 | 1.70 | -0.409 | 10.65 | 9.57 | 9.03 |
| LiCl?NaCl | 4∶1 | 2.28 | -0.440 | 12.07 | 10.41 | 12.75 |
| LiCl?NaCl | 4∶1 | 2.87 | -0.935 | 25.25 | 24.07 | 16.90 |
| LiCl?NaCl | 4∶1 | 3.47 | -0.860 | 25.68 | 21.98 | 21.49 |
| LiCl?NaCl | 4∶1 | 4.07 | -1.018 | 24.98 | 26.40 | 26.60 |
| LiCl?NaCl | 4∶1 | 4.69 | -1.084 | 31.94 | 28.22 | 32.23 |
| NaCl?KCl | 1∶4 | 1.12 | -0.282 | 9.00 | 4.66 | 5.10 |
| NaCl?KCl | 1∶4 | 1.69 | -0.505 | 17.10 | 8.46 | 7.70 |
| NaCl?KCl | 1∶4 | 2.28 | -0.478 | 14.66 | 7.94 | 10.36 |
| NaCl?KCl | 1∶4 | 2.86 | -0.662 | 21.18 | 11.81 | 13.12 |
| NaCl?KCl | 1∶4 | 3.46 | -0.777 | 24.48 | 14.64 | 15.96 |
| NaCl?KCl | 1∶4 | 4.06 | -1.145 | 34.74 | 25.73 | 18.95 |
| NaCl?KCl | 1∶4 | 4.68 | -1.249 | 37.30 | 29.42 | 22.01 |
| NaCl?KCl | 2∶3 | 1.13 | -0.263 | 10.25 | 4.57 | 5.13 |
| NaCl?KCl | 2∶3 | 1.70 | -0.542 | 16.49 | 9.44 | 7.76 |
| NaCl?KCl | 2∶3 | 2.28 | -0.568 | 16.18 | 9.99 | 10.48 |
| NaCl?KCl | 2∶3 | 2.87 | -0.750 | 23.77 | 14.08 | 13.30 |
| NaCl?KCl | 2∶3 | 3.47 | -0.829 | 26.98 | 16.06 | 16.23 |
| NaCl?KCl | 2∶3 | 4.08 | -0.923 | 29.60 | 18.61 | 19.30 |
| NaCl?KCl | 2∶3 | 4.71 | -1.160 | 34.48 | 25.73 | 22.48 |
| NaCl?KCl | 3∶2 | 1.13 | -0.284 | 7.54 | 5.07 | 5.18 |
| NaCl?KCl | 3∶2 | 1.70 | -0.481 | 14.36 | 8.50 | 7.85 |
| NaCl?KCl | 3∶2 | 2.29 | -0.568 | 16.58 | 10.23 | 10.63 |
| NaCl?KCl | 3∶2 | 2.88 | -0.799 | 23.79 | 15.39 | 13.54 |
| NaCl?KCl | 3∶2 | 3.49 | -0.866 | 25.25 | 17.06 | 16.58 |
| NaCl?KCl | 3∶2 | 4.08 | -0.981 | 29.55 | 20.09 | 19.76 |
| NaCl?KCl | 3∶2 | 4.73 | -1.154 | 34.85 | 25.06 | 23.15 |
| NaCl?KCl | 4∶1 | 1.13 | -0.308 | 7.74 | 5.65 | 5.22 |
| NaCl?KCl | 4∶1 | 1.70 | -0.392 | 13.18 | 7.11 | 7.95 |
| NaCl?KCl | 4∶1 | 2.29 | -0.572 | 15.97 | 10.55 | 10.82 |
| NaCl?KCl | 4∶1 | 2.89 | -0.684 | 17.87 | 12.90 | 13.82 |
| NaCl?KCl | 4∶1 | 3.49 | -0.806 | 21.23 | 15.66 | 17.03 |
| NaCl?KCl | 4∶1 | 4.12 | -0.897 | 28.55 | 17.84 | 20.39 |
| NaCl?KCl | 4∶1 | 4.76 | -1.001 | 30.74 | 20.45 | 23.97 |
| LiCl?NaCl?KCl | 1∶1∶2 | 0.90 | -0.282 | 9.00 | 5.17 | 4.39 |
| LiCl?NaCl?KCl | 1∶1∶2 | 1.36 | -0.332 | 8.97 | 6.12 | 6.75 |
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
| LiCl?NaCl?KCl | 1∶1∶2 | 1.82 | -0.455 | 11.78 | 8.61 | 9.21 |
| LiCl?NaCl?KCl | 1∶1∶2 | 2.29 | -0.561 | 15.12 | 10.94 | 11.78 |
| LiCl?NaCl?KCl | 1∶1∶2 | 2.76 | -0.709 | 20.41 | 14.50 | 14.42 |
| LiCl?NaCl?KCl | 1∶1∶2 | 3.24 | -0.813 | 25.55 | 17.18 | 17.14 |
| LiCl?NaCl?KCl | 1∶1∶2 | 3.73 | -0.909 | 26.39 | 19.84 | 19.96 |
| LiCl?NaCl?KCl | 1∶1∶2 | 4.22 | -0.894 | 30.80 | 19.42 | 22.87 |
| LiCl?NaCl?KCl | 1∶1∶2 | 4.73 | -1.202 | 32.89 | 28.77 | 25.85 |
| LiCl?NaCl?KCl | 1∶2∶1 | 0.90 | -0.260 | 9.95 | 4.97 | 4.43 |
| LiCl?NaCl?KCl | 1∶2∶1 | 1.35 | -0.307 | 8.22 | 5.89 | 6.83 |
| LiCl?NaCl?KCl | 1∶2∶1 | 1.81 | -0.389 | 12.59 | 7.53 | 9.36 |
| LiCl?NaCl?KCl | 1∶2∶1 | 2.28 | -0.643 | 17.73 | 13.13 | 12.00 |
| LiCl?NaCl?KCl | 1∶2∶1 | 2.75 | -0.693 | 23.24 | 14.32 | 14.75 |
| LiCl?NaCl?KCl | 1∶2∶1 | 3.23 | -0.859 | 26.71 | 18.47 | 17.58 |
| LiCl?NaCl?KCl | 1∶2∶1 | 3.71 | -0.957 | 26.29 | 21.07 | 20.55 |
| LiCl?NaCl?KCl | 1∶2∶1 | 4.21 | -0.952 | 28.65 | 20.92 | 23.59 |
| LiCl?NaCl?KCl | 1∶2∶1 | 4.70 | -1.191 | 31.36 | 27.68 | 26.77 |
| LiCl?NaCl?KCl | 2∶1∶1 | 0.90 | -0.246 | 7.44 | 4.82 | 4.51 |
| LiCl?NaCl?KCl | 2∶1∶1 | 1.35 | -0.330 | 10.92 | 6.71 | 7.01 |
| LiCl?NaCl?KCl | 2∶1∶1 | 1.82 | -0.360 | 11.20 | 7.40 | 9.66 |
| LiCl?NaCl?KCl | 2∶1∶1 | 2.28 | -0.654 | 18.29 | 14.59 | 12.47 |
| LiCl?NaCl?KCl | 2∶1∶1 | 2.76 | -0.708 | 22.02 | 15.98 | 15.42 |
| LiCl?NaCl?KCl | 2∶1∶1 | 3.23 | -0.977 | 29.43 | 23.35 | 18.53 |
| LiCl?NaCl?KCl | 2∶1∶1 | 3.72 | -0.851 | 24.05 | 19.82 | 21.78 |
| LiCl?NaCl?KCl | 2∶1∶1 | 4.21 | -0.883 | 23.87 | 20.70 | 25.18 |
| LiCl?NaCl?KCl | 2∶1∶1 | 4.72 | -1.090 | 28.52 | 26.60 | 28.74 |
Table 5 Main numeric results of ternary solution simulations
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
|---|---|---|---|---|---|---|
| LiCl?KCl | 1∶4 | 1.13 | -0.270 | 7.73 | 4.63 | 5.17 |
| LiCl?KCl | 1∶4 | 1.71 | -0.378 | 10.78 | 6.57 | 7.82 |
| LiCl?KCl | 1∶4 | 2.29 | -0.521 | 12.15 | 9.48 | 10.59 |
| LiCl?KCl | 1∶4 | 2.89 | -0.806 | 20.80 | 16.59 | 13.46 |
| LiCl?KCl | 1∶4 | 3.51 | -0.919 | 25.48 | 19.88 | 16.42 |
| LiCl?KCl | 1∶4 | 4.13 | -1.040 | 30.94 | 23.68 | 19.55 |
| LiCl?KCl | 1∶4 | 4.76 | -1.113 | 30.67 | 26.13 | 22.82 |
| LiCl?KCl | 2∶3 | 1.13 | -0.296 | 9.94 | 5.50 | 5.30 |
| LiCl?KCl | 2∶3 | 1.71 | -0.506 | 16.14 | 10.10 | 8.11 |
| LiCl?KCl | 2∶3 | 2.29 | -0.545 | 17.59 | 11.01 | 11.09 |
| LiCl?KCl | 2∶3 | 2.89 | -0.792 | 22.55 | 17.44 | 14.23 |
| LiCl?KCl | 2∶3 | 3.50 | -0.809 | 22.10 | 17.94 | 17.55 |
| LiCl?KCl | 2∶3 | 4.12 | -0.844 | 24.73 | 18.95 | 21.10 |
| LiCl?KCl | 2∶3 | 4.75 | -1.157 | 35.18 | 28.70 | 24.82 |
| LiCl?KCl | 3∶2 | 1.13 | -0.327 | 7.51 | 6.63 | 5.44 |
| LiCl?KCl | 3∶2 | 1.70 | -0.371 | 11.43 | 7.67 | 8.44 |
| LiCl?KCl | 3∶2 | 2.29 | -0.572 | 13.64 | 12.73 | 11.67 |
| LiCl?KCl | 3∶2 | 2.88 | -0.655 | 20.31 | 14.95 | 15.17 |
| LiCl?KCl | 3∶2 | 3.49 | -0.742 | 20.81 | 17.31 | 18.92 |
| LiCl?KCl | 3∶2 | 4.10 | -0.875 | 22.01 | 21.11 | 22.99 |
| LiCl?KCl | 3∶2 | 4.73 | -1.185 | 32.23 | 30.63 | 27.35 |
| LiCl?KCl | 4∶1 | 1.13 | -0.276 | 6.95 | 5.78 | 5.61 |
| LiCl?KCl | 4∶1 | 1.70 | -0.326 | 8.24 | 7.11 | 8.81 |
| LiCl?KCl | 4∶1 | 2.29 | -0.492 | 11.90 | 11.58 | 12.34 |
| LiCl?KCl | 4∶1 | 2.88 | -0.719 | 19.43 | 17.92 | 16.24 |
| LiCl?KCl | 4∶1 | 3.48 | -0.868 | 28.16 | 22.20 | 20.52 |
| LiCl?KCl | 4∶1 | 4.09 | -1.034 | 26.05 | 27.08 | 25.23 |
| LiCl?KCl | 4∶1 | 4.71 | -1.226 | 30.80 | 32.89 | 30.38 |
| LiCl?NaCl | 1∶4 | 1.12 | -0.274 | 7.43 | 5.43 | 5.42 |
| LiCl?NaCl | 1∶4 | 1.69 | -0.513 | 14.15 | 10.34 | 8.38 |
| LiCl?NaCl | 1∶4 | 2.27 | -0.583 | 16.73 | 11.84 | 11.57 |
| LiCl?NaCl | 1∶4 | 2.86 | -0.689 | 19.53 | 14.22 | 15.01 |
| LiCl?NaCl | 1∶4 | 3.45 | -0.950 | 28.48 | 20.40 | 18.75 |
| LiCl?NaCl | 1∶4 | 4.06 | -1.027 | 31.16 | 22.34 | 22.77 |
| LiCl?NaCl | 1∶4 | 4.66 | -1.338 | 39.75 | 30.57 | 27.13 |
| LiCl?NaCl | 2∶3 | 1.12 | -0.261 | 11.24 | 5.32 | 5.52 |
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
| LiCl?NaCl | 2∶3 | 1.69 | -0.502 | 14.38 | 10.76 | 8.61 |
| LiCl?NaCl | 2∶3 | 2.27 | -0.604 | 18.93 | 13.18 | 12.00 |
| LiCl?NaCl | 2∶3 | 2.86 | -0.686 | 20.43 | 15.15 | 15.66 |
| LiCl?NaCl | 2∶3 | 3.46 | -0.812 | 28.41 | 18.25 | 19.67 |
| LiCl?NaCl | 2∶3 | 4.06 | -1.022 | 29.51 | 23.64 | 24.07 |
| LiCl?NaCl | 2∶3 | 4.67 | -1.200 | 30.19 | 28.38 | 28.85 |
| LiCl?NaCl | 3∶2 | 1.12 | -0.296 | 11.00 | 6.29 | 5.62 |
| LiCl?NaCl | 3∶2 | 1.70 | -0.494 | 15.00 | 11.24 | 8.82 |
| LiCl?NaCl | 3∶2 | 2.28 | -0.476 | 12.19 | 10.78 | 12.37 |
| LiCl?NaCl | 3∶2 | 2.86 | -0.678 | 21.28 | 15.95 | 16.30 |
| LiCl?NaCl | 3∶2 | 3.46 | -0.869 | 26.12 | 20.97 | 20.61 |
| LiCl?NaCl | 3∶2 | 4.07 | -1.119 | 31.95 | 27.70 | 25.36 |
| LiCl?NaCl | 3∶2 | 4.68 | -1.215 | 35.19 | 30.36 | 30.56 |
| LiCl?NaCl | 4∶1 | 1.13 | -0.224 | 7.82 | 4.55 | 5.71 |
| LiCl?NaCl | 4∶1 | 1.70 | -0.409 | 10.65 | 9.57 | 9.03 |
| LiCl?NaCl | 4∶1 | 2.28 | -0.440 | 12.07 | 10.41 | 12.75 |
| LiCl?NaCl | 4∶1 | 2.87 | -0.935 | 25.25 | 24.07 | 16.90 |
| LiCl?NaCl | 4∶1 | 3.47 | -0.860 | 25.68 | 21.98 | 21.49 |
| LiCl?NaCl | 4∶1 | 4.07 | -1.018 | 24.98 | 26.40 | 26.60 |
| LiCl?NaCl | 4∶1 | 4.69 | -1.084 | 31.94 | 28.22 | 32.23 |
| NaCl?KCl | 1∶4 | 1.12 | -0.282 | 9.00 | 4.66 | 5.10 |
| NaCl?KCl | 1∶4 | 1.69 | -0.505 | 17.10 | 8.46 | 7.70 |
| NaCl?KCl | 1∶4 | 2.28 | -0.478 | 14.66 | 7.94 | 10.36 |
| NaCl?KCl | 1∶4 | 2.86 | -0.662 | 21.18 | 11.81 | 13.12 |
| NaCl?KCl | 1∶4 | 3.46 | -0.777 | 24.48 | 14.64 | 15.96 |
| NaCl?KCl | 1∶4 | 4.06 | -1.145 | 34.74 | 25.73 | 18.95 |
| NaCl?KCl | 1∶4 | 4.68 | -1.249 | 37.30 | 29.42 | 22.01 |
| NaCl?KCl | 2∶3 | 1.13 | -0.263 | 10.25 | 4.57 | 5.13 |
| NaCl?KCl | 2∶3 | 1.70 | -0.542 | 16.49 | 9.44 | 7.76 |
| NaCl?KCl | 2∶3 | 2.28 | -0.568 | 16.18 | 9.99 | 10.48 |
| NaCl?KCl | 2∶3 | 2.87 | -0.750 | 23.77 | 14.08 | 13.30 |
| NaCl?KCl | 2∶3 | 3.47 | -0.829 | 26.98 | 16.06 | 16.23 |
| NaCl?KCl | 2∶3 | 4.08 | -0.923 | 29.60 | 18.61 | 19.30 |
| NaCl?KCl | 2∶3 | 4.71 | -1.160 | 34.48 | 25.73 | 22.48 |
| NaCl?KCl | 3∶2 | 1.13 | -0.284 | 7.54 | 5.07 | 5.18 |
| NaCl?KCl | 3∶2 | 1.70 | -0.481 | 14.36 | 8.50 | 7.85 |
| NaCl?KCl | 3∶2 | 2.29 | -0.568 | 16.58 | 10.23 | 10.63 |
| NaCl?KCl | 3∶2 | 2.88 | -0.799 | 23.79 | 15.39 | 13.54 |
| NaCl?KCl | 3∶2 | 3.49 | -0.866 | 25.25 | 17.06 | 16.58 |
| NaCl?KCl | 3∶2 | 4.08 | -0.981 | 29.55 | 20.09 | 19.76 |
| NaCl?KCl | 3∶2 | 4.73 | -1.154 | 34.85 | 25.06 | 23.15 |
| NaCl?KCl | 4∶1 | 1.13 | -0.308 | 7.74 | 5.65 | 5.22 |
| NaCl?KCl | 4∶1 | 1.70 | -0.392 | 13.18 | 7.11 | 7.95 |
| NaCl?KCl | 4∶1 | 2.29 | -0.572 | 15.97 | 10.55 | 10.82 |
| NaCl?KCl | 4∶1 | 2.89 | -0.684 | 17.87 | 12.90 | 13.82 |
| NaCl?KCl | 4∶1 | 3.49 | -0.806 | 21.23 | 15.66 | 17.03 |
| NaCl?KCl | 4∶1 | 4.12 | -0.897 | 28.55 | 17.84 | 20.39 |
| NaCl?KCl | 4∶1 | 4.76 | -1.001 | 30.74 | 20.45 | 23.97 |
| LiCl?NaCl?KCl | 1∶1∶2 | 0.90 | -0.282 | 9.00 | 5.17 | 4.39 |
| LiCl?NaCl?KCl | 1∶1∶2 | 1.36 | -0.332 | 8.97 | 6.12 | 6.75 |
| Solution | r | Molality/ (mol?kg-1) | Membrane energy/(kJ?mol-1) | Pressure from FS method/MPa | Pressure from MEF method/MPa | Referenced pressure[44―46]/MPa |
| LiCl?NaCl?KCl | 1∶1∶2 | 1.82 | -0.455 | 11.78 | 8.61 | 9.21 |
| LiCl?NaCl?KCl | 1∶1∶2 | 2.29 | -0.561 | 15.12 | 10.94 | 11.78 |
| LiCl?NaCl?KCl | 1∶1∶2 | 2.76 | -0.709 | 20.41 | 14.50 | 14.42 |
| LiCl?NaCl?KCl | 1∶1∶2 | 3.24 | -0.813 | 25.55 | 17.18 | 17.14 |
| LiCl?NaCl?KCl | 1∶1∶2 | 3.73 | -0.909 | 26.39 | 19.84 | 19.96 |
| LiCl?NaCl?KCl | 1∶1∶2 | 4.22 | -0.894 | 30.80 | 19.42 | 22.87 |
| LiCl?NaCl?KCl | 1∶1∶2 | 4.73 | -1.202 | 32.89 | 28.77 | 25.85 |
| LiCl?NaCl?KCl | 1∶2∶1 | 0.90 | -0.260 | 9.95 | 4.97 | 4.43 |
| LiCl?NaCl?KCl | 1∶2∶1 | 1.35 | -0.307 | 8.22 | 5.89 | 6.83 |
| LiCl?NaCl?KCl | 1∶2∶1 | 1.81 | -0.389 | 12.59 | 7.53 | 9.36 |
| LiCl?NaCl?KCl | 1∶2∶1 | 2.28 | -0.643 | 17.73 | 13.13 | 12.00 |
| LiCl?NaCl?KCl | 1∶2∶1 | 2.75 | -0.693 | 23.24 | 14.32 | 14.75 |
| LiCl?NaCl?KCl | 1∶2∶1 | 3.23 | -0.859 | 26.71 | 18.47 | 17.58 |
| LiCl?NaCl?KCl | 1∶2∶1 | 3.71 | -0.957 | 26.29 | 21.07 | 20.55 |
| LiCl?NaCl?KCl | 1∶2∶1 | 4.21 | -0.952 | 28.65 | 20.92 | 23.59 |
| LiCl?NaCl?KCl | 1∶2∶1 | 4.70 | -1.191 | 31.36 | 27.68 | 26.77 |
| LiCl?NaCl?KCl | 2∶1∶1 | 0.90 | -0.246 | 7.44 | 4.82 | 4.51 |
| LiCl?NaCl?KCl | 2∶1∶1 | 1.35 | -0.330 | 10.92 | 6.71 | 7.01 |
| LiCl?NaCl?KCl | 2∶1∶1 | 1.82 | -0.360 | 11.20 | 7.40 | 9.66 |
| LiCl?NaCl?KCl | 2∶1∶1 | 2.28 | -0.654 | 18.29 | 14.59 | 12.47 |
| LiCl?NaCl?KCl | 2∶1∶1 | 2.76 | -0.708 | 22.02 | 15.98 | 15.42 |
| LiCl?NaCl?KCl | 2∶1∶1 | 3.23 | -0.977 | 29.43 | 23.35 | 18.53 |
| LiCl?NaCl?KCl | 2∶1∶1 | 3.72 | -0.851 | 24.05 | 19.82 | 21.78 |
| LiCl?NaCl?KCl | 2∶1∶1 | 4.21 | -0.883 | 23.87 | 20.70 | 25.18 |
| LiCl?NaCl?KCl | 2∶1∶1 | 4.72 | -1.090 | 28.52 | 26.60 | 28.74 |
| 1 | Zheng M. P., Acta Geo. Sin., 2010, 84(11), 1613—1622(郑绵平. 地质学报, 2010, 84(11), 1613—1622) |
| 2 | Nie Z., Bu L. Z., Zheng M. P., Acta Geosci. Sin., 2010, 31(1), 95—101(乜贞, 卜令忠, 郑绵平. 地球学报, 2010, 31(1), 95—101) |
| 3 | Yan H., J. Salt Lake Res., 2018, 26(4), 85—90(颜辉. 盐湖研究, 2018, 26(4), 85—90) |
| 4 | Catlow C. R. A., Diller K. M., Norgett M. J., J. Phys. C: Solid State Physics, 1977, 10(9), 1395—1412 |
| 5 | Dang L. X., Chang T. M., J. Chem. Phys., 1997, 106(19), 8149—8159 |
| 6 | Maginn E. J., J. Phys.: Condensed Matter., 2009, 21(37), 373101 |
| 7 | Yang J. M., Yao Y., Zhang A. Y., Song P. S., Chem. J. Chinese Universities, 2006, 27(4), 735—738(杨吉民, 姚燕, 张爱云, 宋彭生. 高等学校化学学报, 2006, 27(4), 735—738) |
| 8 | Zhang A. Y., Yao Y., Song P. S., Chem. J. Chinese Universities, 2004, 25(10), 1934—1936(张爱云, 姚燕, 宋彭生. 高等学校化学学报, 2004, 25(10), 1934—1936) |
| 9 | Song P. S., Yao Y., Sun B., Li W., Sci. Sin. Chim., 2010, 40(9), 1286—1296(宋彭生, 姚燕, 孙柏, 李武. 中国科学: 化学, 2010, 40(9), 1286—1296) |
| 10 | Song P. S., Yao Y., J. Salt Lake Res., 2003, 11(3), 1—8(宋彭生, 姚燕. 盐湖研究, 2003, 11(3), 1—8) |
| 11 | Song P. S., Yao Y., J. Salt Lake Res., 2003, 11(4), 1—12,19(宋彭生, 姚燕. 盐湖研究, 2003, 11(4), 1—12,19) |
| 12 | Taylor R. S., Dang L. X., Garrett B. C., J. Phys. Chem., 1996, 100(28), 11720—11725 |
| 13 | Takagi R., Sakurai M., Z. Naturforsh. A, 1998, 53(1/2), 13—16 |
| 14 | Smith P. E., Van Gunsteren W. F., Chem. Phys. Lett., 1993, 215(4), 315—318 |
| 15 | Satarifard V., Kashefolgheta S., Vila Verde A., GrafmüLler A., J. Chem. Theor. Comput., 2017, 13(5), 2112—2122 |
| 16 | Saric D., Kohns M., Vrabec J., J. Chem. Phys., 2020, 152(1), 164502 |
| 17 | Sanz E., Vega C., J. Chem. Phys., 2007, 126(1), 014507 |
| 18 | Rajabpour A., Akizi F. Y., Heyhat M. M., Gordiz K., Int. Nano Lett., 2013, 3(58), 1—6 |
| 19 | Raim V., Srebnik S., J. Membrane Sci., 2018, 563(14), 183—190 |
| 20 | Song T., Liu S. J., Xiao L. P., Chen Q. Y., Chem. J. Chinese Universities, 2012, 33(1), 114—118(宋婷, 刘士军, 肖刘萍, 陈启元. 高等学校化学学报, 2012, 33(1), 114—118) |
| 21 | Liu Q. Z., Yang D. F., Hu Y. D., Chem. J. Chinese Universities, 2009, 30(3), 568—572(刘清芝, 杨登峰, 胡仰栋. 高等学校化学学报, 2009, 30(3), 568—572) |
| 22 | Kohns M., Reiser S., Horsch M., Hasse H., J. Chem. Phys., 2016, 144(8), 084112 |
| 23 | Kohns M., Horsch M., Hasse H., J. Chem. Phys., 2017, 147(14), 144108 |
| 24 | Moucka F., Lisal M., Skvor J., Jirsak J., Nezbeda I., Smith W. R., J. Phys. Chem. B, 2011, 115(24), 7849—7861 |
| 25 | Luo Y., Roux B., J. Phys. Chem. Lett., 2009, 1(1), 183—189 |
| 26 | Luo Y., Jiang W., Yu H., Mackerell A. D., Roux B., Faraday Discuss., 2013, 160(9), 135—149 |
| 27 | Joung I. S., Thomas E., Cheatham I., J. Phys. Chem. B, 2009, 113(1), 13279—13290 |
| 28 | Joung I. S., Thomas E. Cheatham I., J. Phys. Chem. B, 2008, 112(30), 9020—9041 |
| 29 | Yagasaki T., Matsumoto M., Tanaka H., J. Chem. Thero. Comput., 2020, 16(4), 2460—2473 |
| 30 | Boda D., Henderson D., Mol. Phys., 2008, 106(20), 2367—2370 |
| 31 | Delhommelle J., Millie P., Mol. Phys., 2001, 99(8), 619—625 |
| 32 | Kulkarni M., Yang C., Pak Y., B. Korean Chem. Soc., 2018, 39(8), 931—935 |
| 33 | Aragones J. L., Sanz E., Vega C., J. Chem. Phys., 2012, 136(24), 244508 |
| 34 | Mester Z., Panagiotopoulos A. Z., J. Chem. Phys., 2015, 142(4), 044507 |
| 35 | Moučka F., Nezbeda I., Smith W. R., J. Chem. Theor. Comput., 2015, 11(4), 1756—1764 |
| 36 | Price D. J., Brooks Iii C. L., J. Chem. Phys., 2004, 121(20), 10096—10103 |
| 37 | Mark P., Nilsson L., J. Phys. Chem. A, 2001, 105(43), 9954—9960 |
| 38 | Kiss P. T., Baranyai A., J. Chem. Phys., 2011, 134(5), 054106 |
| 39 | Jorgensen W. L., Jenson C., J. Comput. Chem., 1998, 19(10), 1179—1186 |
| 40 | Harrach M. F., Drossel B., J. Chem. Phys., 2014, 140(17), 174501 |
| 41 | Guendouzi M. E., Dinane A., Mounir A., Calphad, 2001, 33(9), 1059—1072 |
| 42 | Martínez L., Andrade R., Birgin E. G., Martínez J. M., J. Comput. Chem., 2009, 30(13), 2157—2164 |
| 43 | Thompson A. P., Aktulga H. M., Berger R., Bolintineanu D. S., Brown W. M., Crozier P. S., In 'T Veld P. J., Kohlmeyer A., Moore S. G., Nguyen T. D., Shan R., Stevens M. J., Tranchida J., Trott C., Plimpton S. J., Comput. Phys. Comm., 2022, 271, 108171 |
| 44 | Dinane A., J. Chem. Thermodyn., 2007, 39(1), 96—103 |
| 45 | Guendouzi M. E., Benbiyi A., Dinane A., Azougen R., Calphad, 2004, 28(1), 97—103 |
| 46 | Guendouzi M. E., Benbiyi A., Dinane A., Azougen R., Calphad, 2003, 27(23), 213—219 |
| 47 | Dinane A., Guendouzi M. E., Mounir A., J. Chem. Thermodyn., 2002, 34(4), 423—441 |
| [1] | 高志伟, 李军委, 史赛, 付强, 贾钧儒, 安海龙. 基于分子动力学模拟的TRPM8通道门控特性分析[J]. 高等学校化学学报, 2022, 43(6): 20220080. |
| [2] | 曾晛阳, 赵熹, 黄旭日. 细胞松弛素B对葡萄糖/质子共转运蛋白GlcPSe的抑制机理[J]. 高等学校化学学报, 2022, 43(4): 20210822. |
| [3] | 刘嘉欣, 闵杰, 许华杰, 任海生, 谈宁馨. 基于反应力场分子模拟的乙烯燃烧自由基与氮气相互作用研究[J]. 高等学校化学学报, 2022, 43(4): 20210834. |
| [4] | 胡波, 朱昊辰. 双层氧化石墨烯纳米体系中受限水的介电常数[J]. 高等学校化学学报, 2022, 43(2): 20210614. |
| [5] | 张伶育, 张继龙, 曲泽星. RDX分子内振动能量重分配的动力学研究[J]. 高等学校化学学报, 2022, 43(10): 20220393. |
| [6] | 李聪聪, 刘明皓, 韩佳睿, 朱镜璇, 韩葳葳, 李婉南. 基于分子动力学模拟的VmoLac非特异性底物催化活性的理论研究[J]. 高等学校化学学报, 2021, 42(8): 2518. |
| [7] | 雷晓彤, 金怡卿, 孟烜宇. 基于分子模拟方法预测PIP2在双孔钾通道TREK-1上结合位点的研究[J]. 高等学校化学学报, 2021, 42(8): 2550. |
| [8] | 刘沙沙, 张恒, 苑世领, 刘成卜. 脉冲电场O/W乳状液破乳的分子动力学模拟[J]. 高等学校化学学报, 2021, 42(7): 2170. |
| [9] | 曾永辉, 言天英. 质子水合结构的振动态密度分析[J]. 高等学校化学学报, 2021, 42(6): 1855. |
| [10] | 齐人睿, 李明昊, 常浩, 付学奇, 高波, 韩葳葳, 韩璐, 李婉南. 基于拉伸分子动力学模拟的黄嘌呤氧化酶抑制剂解离途径的理论研究[J]. 高等学校化学学报, 2021, 42(3): 758. |
| [11] | 刘爱清, 徐文生, 徐晓雷, 陈继忠, 安立佳. 高分子/棒状纳米粒子复合物的分子动力学模拟[J]. 高等学校化学学报, 2021, 42(3): 875. |
| [12] | 肖宇情,李申慧,汤晶,徐君,邓风. 金属有机框架材料的结构、 动力学行为和主客体相互作用的固体核磁共振研究[J]. 高等学校化学学报, 2020, 41(2): 204. |
| [13] | 朱玉荃, 赵晓婕, 钟嫄, 陈子茹, 鄢红, 段雪. 类水滑石材料主客体插层结构的构筑及特性的理论研究[J]. 高等学校化学学报, 2020, 41(11): 2287. |
| [14] | 瞿思颖, 徐沁. 凝血因子Xa的S4口袋部分关键残基对利伐沙班结合的不同作用机制[J]. 高等学校化学学报, 2019, 40(9): 1918. |
| [15] | 马玉聪, 樊保民, 王满曼, 杨彪, 郝华, 孙辉, 张慧娟. 曲唑酮的两步法制备及对碳钢的缓蚀机理[J]. 高等学校化学学报, 2019, 40(8): 1706. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||