

高等学校化学学报 ›› 2018, Vol. 39 ›› Issue (11): 2534.doi: 10.7503/cjcu20180260
收稿日期:2018-04-04
出版日期:2018-11-10
发布日期:2018-08-23
作者简介:联系人简介: 董晓燕, 女, 博士, 教授, 博士生导师, 主要从事淀粉样蛋白聚集抑制剂方面的研究. E-mail: 基金资助:Received:2018-04-04
Online:2018-11-10
Published:2018-08-23
Contact:
DONG Xiaoyan
E-mail:d_xy@tju.edu.cn
Supported by:摘要:
研究了表没食子儿茶素没食子酸酯(EGCG)在不同pH值(5.0, 6.0和7.4)下对β-淀粉样蛋白(Aβ42)聚集的抑制作用. 结果表明, 虽然在上述pH范围内EGCG均可抑制Aβ42聚集和细胞毒性, 但不同pH值下EGCG与Aβ42的作用方式不同. 当pH=5.0时, Aβ42可在数秒内聚集, EGCG-Aβ42相互作用最弱, 因此聚集前期EGCG不能有效抑制Aβ42纤维化; 培养24 h时, 产生35.1%的β-折叠结构和50%的硫黄素T(ThT)荧光; 但此pH值下EGCG可通过降低Aβ42表面疏水性使聚集体重塑, 因此在聚集后期可阻碍Aβ42纤维化. 当pH=6.0时, Aβ42聚集速度降低, EGCG-Aβ42相互作用增强, EGCG对Aβ42纤维化的抑制作用较pH=5.0时更加显著, 几乎可完全抑制Aβ42向β-折叠构象转换和ThT荧光的产生. 当pH=7.4时, Aβ42聚集速度最慢, EGCG与Aβ42结合作用最强, 因而能够增加Aβ42稳定性和聚集延滞期, 显著抑制Aβ42纤维化.
中图分类号:
TrendMD:
张焕, 董晓燕. EGCG对β-淀粉样蛋白聚集的抑制作用: pH的影响. 高等学校化学学报, 2018, 39(11): 2534.
ZHANG Huan, DONG Xiaoyan. Effects of EGCG on Amyloid β-Protein Fibrillogenesis and Cytotoxicity at Different pH Values†. Chem. J. Chinese Universities, 2018, 39(11): 2534.
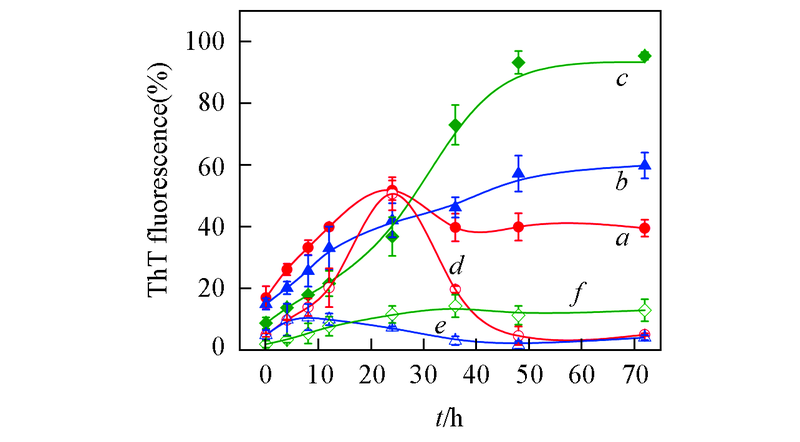
Fig.2 Aggregation kinetics of Aβ42 incubated in the absence and presence of equimolar EGCG under different pH valuesa―c. Aβ42 under pH=5.0(a), 6.0(b), 7.4(c), respectively; d―f. Aβ42 with equimolar EGCG under pH=5.0(d), 6.0(e), 7.4(f). The incubations were done at 37 ℃, 150 r/min. Concentrations of Aβ42 and EGCG were 25 μmol/L.
| t/h | pH=5.0 | pH=6.0 | pH=7.4 | |||
|---|---|---|---|---|---|---|
| — | EGCG | — | EGCG | — | EGCG | |
| 0 | 29.5±5.2 | 27.0±4.4 | 24.7±1.8 | 22.8±1.5 | 23.0±2.2 | 18.4±2.6 |
| 6 | 41.5±3.7 | 38.4±2.8 | 33.5±3.4 | 23.4±2.3 | 27.9±1.7 | 25.3±2.7 |
| 12 | 44.9±7.3 | 35.6±3.2 | 42.0±3.9 | 23.7±5.6 | 38.5±1.6 | 30.9±2.6 |
| 24 | 39.7±1.8 | 35.1±2.2 | 45.9±5.7 | 23.8±2.4 | 49.2±4.2 | 33.9±2.2 |
| 48 | 41.8±4.9 | 30.5±5.4 | 40.9±2.0 | 19.1±0.6 | 70.9±5.8 | 39.3±2.8 |
Table 1 β-Sheet content(%) of Aβ42 in absence and presence of EGCG under different pH values
| t/h | pH=5.0 | pH=6.0 | pH=7.4 | |||
|---|---|---|---|---|---|---|
| — | EGCG | — | EGCG | — | EGCG | |
| 0 | 29.5±5.2 | 27.0±4.4 | 24.7±1.8 | 22.8±1.5 | 23.0±2.2 | 18.4±2.6 |
| 6 | 41.5±3.7 | 38.4±2.8 | 33.5±3.4 | 23.4±2.3 | 27.9±1.7 | 25.3±2.7 |
| 12 | 44.9±7.3 | 35.6±3.2 | 42.0±3.9 | 23.7±5.6 | 38.5±1.6 | 30.9±2.6 |
| 24 | 39.7±1.8 | 35.1±2.2 | 45.9±5.7 | 23.8±2.4 | 49.2±4.2 | 33.9±2.2 |
| 48 | 41.8±4.9 | 30.5±5.4 | 40.9±2.0 | 19.1±0.6 | 70.9±5.8 | 39.3±2.8 |
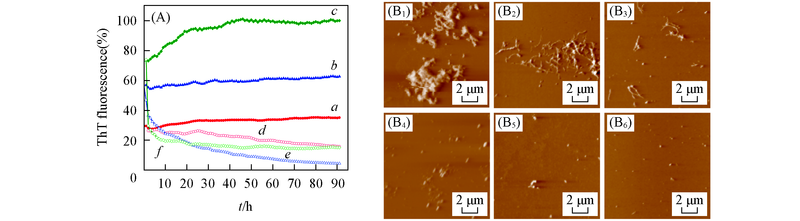
Fig.3 EGCG-induced remodeling of Aβ42 mature fibrils under different pH values(A) Loss of ThT fluorescence of Aβ42 fibrils measured in the absence and presence of EGCG at different conditions. a―c. Aβ42 aggregates under pH=5.0, 6.0, 7.4; d―f. Aβ42 aggregates with equimolar EGCG under pH=5.0, 6.0, 7.4. (B1—B6): AFM images of Aβ42 aggregates in the absence and presence of EGCG under different pH. (B1―B3) Aβ42 aggregates under pH=5.0, 6.0, 7.4, (B4―B6) Aβ42 aggregates with equimolar EGCG under pH=5.0, 6.0, 7.4. The samples were incubated at 37 ℃ for 4 d. Concentrations of Aβ42 aggregates and EGCG were 25 μmol/L.
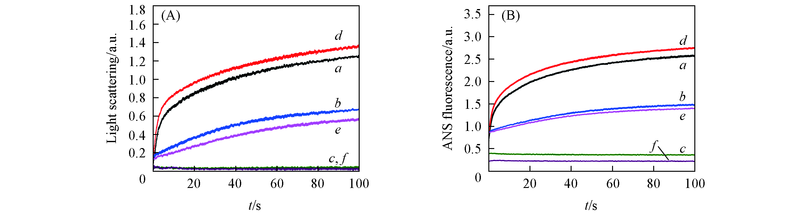
Fig.4 Effect of EGCG on Aβ42 rapid aggregation kinetics under different pH values(A) Rayleigh light scatting; (B) ANS fluorescence. a.―c. Aβ42 under pH=5.0, 6.0, 7.4; d.―f. Aβ42 with equimolar EGCG under pH=5.0, 6.0, 7.4. The detections were performed at 37 ℃. Final concentrations of Aβ42 and EGCG were 10 μmol/L.
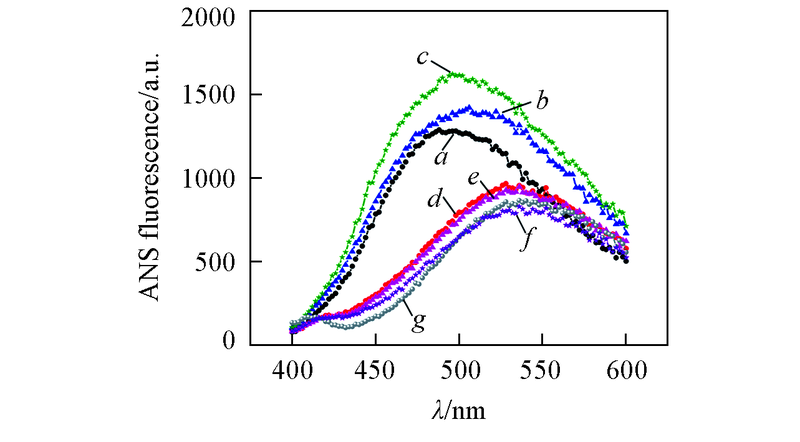
Fig.5 ANS fluorescence spectra of Aβ42 species in the absence and presence of equimolar EGCG under different pHa—c. Aβ42 under pH=5.0, 6.0, 7.4; d—f. Aβ42 with equimolar EGCG under pH=5.0, 6.0, 7.4; g. ANS control. The samples were incubated at 37 ℃, 150 r/min for 48 h. Concentrations of Aβ42 and EGCG were 25 μmol/L.
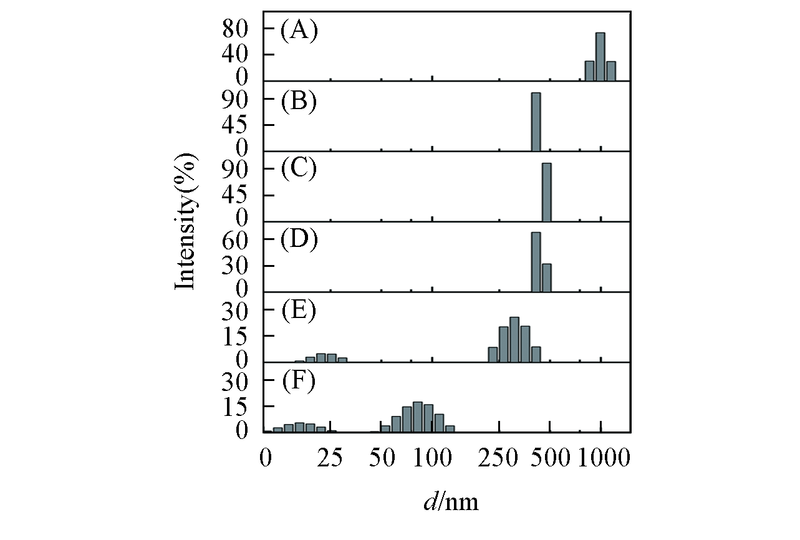
Fig.6 DLS analysis of the Aβ42 species in the absence and presence of equimolar EGCG under different pH values(A), (C), (E): Aβ42 under pH=5.0, 6.0, 7.4; (B), (D), (F): Aβ42 with equimolar EGCG under pH=5.0, 6.0, 7.4. The samples were incubated at 37 ℃, 150 r/min for 48 h. Aβ42 concentration was 25 μmol/L.
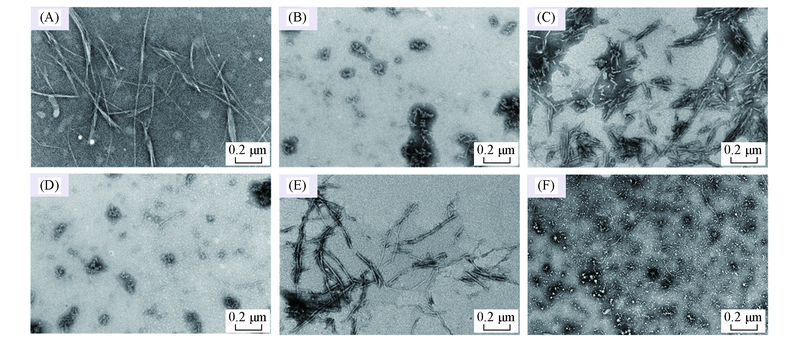
Fig.7 TEM images of Aβ42 species in the absence and presence of EGCG under different pH values(A), (C), (E) Aβ42 under pH=5.0, 6.0, 7.4; (B), (D), (F) Aβ42 with equimolar EGCG under pH=5.0, 6.0, 7.4.The samples were incubated at 37 ℃, 150 r/min for 48 h. Aβ42 concentration was 25 μmol/L.
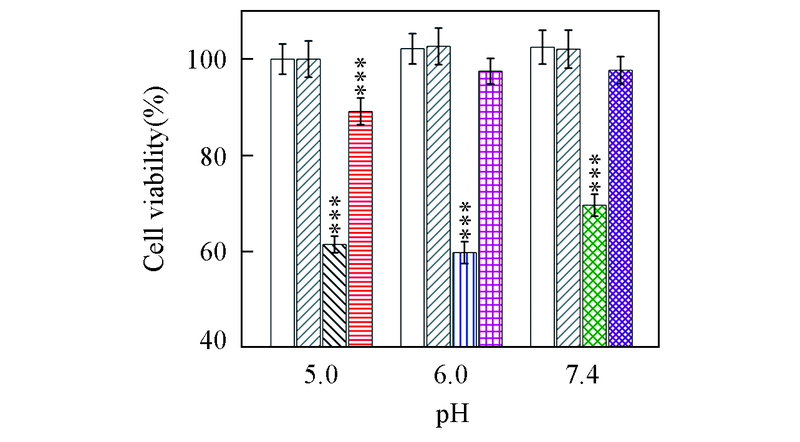
Fig.8 Viability of SH-SY5Y cells incubated with Aβ42 species in the absence and presence of equimolar EGCG under different pH valuesBuffer-treated group in the absence() and presence() of EGCG; Aβ42 under pH=5.0(), 6.0(), 7.4(); Aβ42 with equimolar EGCG under pH 5.0(), 6.0(), 7.4(). The incubations were performed at 37 ℃, 150 r/min. The aged-concentration of Aβ42 was 25 μmol/L, and the final concentration of Aβ42 was 5 μmol/L in cells. *** P<0.001 as compared to buffer-treated group.
| [1] | Chiti F., Dobson C. M., Annu. Rev. Biochem., 2017, 86(1), 27—68 |
| [2] | Walsh D. M., Lomakin A., Benedek G. B., Condron M. M., Teplow D. B., J. Biol. Chem., 1999, 274(36), 25945—25952 |
| [3] | Jakob-Roetne R., Jacobsen H., Angew. Chem. Int. Ed. Engl., 2009, 48(17), 3030—3059 |
| [4] | Zheng L., Cedazo-Minguez A., Hallbeck M., Jerhammar F., Marcusson J., Terman A., Transl. Neurodegener., 2012, 1(1), 1—7 |
| [5] | Tam J. H., Seah C., Pasternak S. H., Mol. Brain, 2014, 7(1), 54 |
| [6] | Peralvarez-Marin A., Barth A., Graslund A., J. Mol. Biol., 2008, 379(3), 589—596 |
| [7] | Brännström K., Öhman A., Nilsson L., Pihl M., Sandblad L., Olofsson A., J. Am. Chem. Soc., 2014, 136(31), 10956—10964 |
| [8] | Gorman P. M., Yip C. M., Fraser P. E., Chakrabartty A. P., J. Mol. Biol., 2003, 325(4), 743—757 |
| [9] | Khandogin J., Brooks C. L., Proc. Natl. Acad. Sci. USA, 2007, 104(43), 16880—16885 |
| [10] | Su Y., Chang P. T., Brain Res., 2001, 893(1/2), 287—291 |
| [11] | Du W. J., Guo J. J., Gao M. T., Hu S. Q., Dong X. Y., Han Y. F., Liu F. F., Jiang S., Sun Y., Sci. Rep., 2015, 5, 7992 |
| [12] | Song S. M., Ma X. W., Zhou Y. H., Xu M. T., Shuang S. M., Dong C., Chem. Res. Chinese Universities, 2016, 32(2), 172—177 |
| [13] | Xiong N., Dong X. Y., Zheng J., Liu F. F., Sun Y., ACS Appl. Mater. Interfaces, 2015, 7(10), 5650—5662 |
| [14] | Takahashi T., Mihara H. T., Acc. Chem. Res., 2008, 41(10), 1309—1318 |
| [15] | Xie B. L., Li X., Dong X. Y., Sun Y., Langmuir, 2014, 30(32), 9789—9796 |
| [16] | Kerr M. L., Gasperini R., Gibbs M. E., Hou X., Shepherd C. E., Strickland D. K., Foa L., Lawen A., Small D. H., J. Neurochem., 2010, 112(5), 1199—1209 |
| [17] | Linse S., Cabaleiro-Lago C., Xue W. F., Lynch I., Lindman S., Thulin E., Radford S. E., Dawson K. A., Proc. Natl. Acad. Sci. USA, 2007, 104(21), 8691—8696 |
| [18] | Xie L., Luo Y., Lin D., Xi W., Yang X., Wei G., Nanoscale, 2014, 6(16), 9752—9762 |
| [19] | Mak J. C., Clin. Exp. Pharmacol. Physiol., 2012, 39(3), 265—273 |
| [20] | Nagle D. G., Ferreira D., Zhou Y. D., Phytochemistry, 2006, 67(17), 1849—1855 |
| [21] | Weinreb O., Amit T., Mandel S., Youdim M. B., Genes Nutr., 2009, 4(4), 283—296 |
| [22] | Ehrnhoefer D. E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E. E., Nat. Struct. Mol. Biol., 2008, 15(6), 558—566 |
| [23] | Liu Y., Liu Y., Wang S. H., Dong S. Z., Chang P., Jiang Z. F., RSC Adv., 2015, 5(77), 62402—62413 |
| [24] | Bieschke J., Russ J., Friedrich R. P., Ehrnhoefer D. E., Wobst H., Neugebauer K., Wanker E. E., Proc. Natl. Acad. Sci. USA, 2010, 107(17), 7710—7715 |
| [25] | Palhano F. L., Lee J., Grimster N. P., Kelly J. W., J. Am. Chem. Soc., 2013, 135(20), 7503—7510 |
| [26] | Wang S. H., Liu F. F., Dong X. Y., Sun Y., J. Phys. Chem. B, 2010, 114(35), 11576—11583 |
| [27] | Wang Q., Shah N., Zhao J., Wang C., Zhao C., Liu L., Li L., Zhou F., Zheng J., Phys. Chem. Chem. Phys., 2011, 13(33), 15200—15210 |
| [28] | Tomaselli S., Esposito V., Vangone P., van Nuland N. A., Bonvin A. M., Guerrini R., Tancredi T., Temussi P. A., Picone D., Chem. Bio. Chem., 2006, 7(2), 257—267 |
| [29] | LeVine H., Amyloid, 1995, 2(1), 1—6 |
| [30] | Ji S. R., Wu Y., Sui S. F., Gen. Physiol. Biophys., 2002, 21(4), 415—427 |
| [31] | Roychaudhuri R., Lomakin A., Bernstein S., Zheng X., Condron M. M., Benedek G. B., Bowers M., Teplow D. B., J. Mol. Biol., 2014, 426(13), 2422—2441 |
| [32] | Micsonai A., Wien F., Kernya L., Lee Y. H., Goto Y., Réfrégiers M., Kardos J., Proc. Natl. Acad. Sci. USA, 2015, 112(24), E3095—E3103 |
| [33] | Noy D., Solomonov I., Sinkevich O., Arad T., Kjaer K., Sagi I., J. Am. Chem. Soc., 2008, 130(4), 1376—1383 |
| [34] | Meng J., Zhang H., Dong X. Y., Liu F. F., Sun Y., J. Inorg. Biochem., 2018, 181, 56—64 |
| [35] | Fotakis G., Timbrell J. A., Toxicol. Lett., 2006, 160(2), 171—177 |
| [36] | Lin M. S., Chen L. Y., Tsai H. T., Wang S. S., Chang Y., Higuchi A., Chen W. Y., Langmuir, 2008, 24(11), 5802—5808 |
| [37] | Khan M. V., Rabbani G., Ahmad E., Khan R. H., Int. J. Biol. Macromol., 2014, 70(8), 606—614 |
| [38] | Hills R. D. Jr, Brooks Ⅲ C. L., J. Mol. Biol., 2007, 368(3), 894—901 |
| [39] | Jarrett J. T., Berger E. P., Lansbury P. T. Jr., Biochemistry, 1993, 32(18), 4693—4697 |
| [40] | Picotti P., De Franceschi G., Frare E., Spolaore B., Zambonin M., Chiti F., de Laureto P. P., Fontana A., J. Mol. Biol., 2007, 367(5), 1237—1245 |
| [41] | Guo M., Gorman P. M., Rico M., Chakrabartty A., Laurents D. V., FEBS Lett., 2005, 579(17), 3574—3578 |
| [42] | Ma K., Clancy E. L., Zhang Y. B., Ray D. G., Wollenberg K., Zagorski M. G., J. Am. Chem. Soc., 1999, 121(38), 8698—8706 |
| [43] | Bujacz A., Acta Cryst., 2012, 68(10), 1278—1289 |
| [44] | Arya P., Srivastava A., Vasaikar S. V., Mukherjee G., Mishra P., Kundu B., ACS Chem. Neurosci., 2014, 5(10), 982—992 |
| [45] | Walsh D. M., Selkoe D. J., J. Neurochem., 2007, 101(5), 1172—1184 |
| [46] | Wogulis M., Wright S., Cunningham D., Chilcote T., Powell K., Rydel R. E., J. Neurosci., 2005, 25(5), 1071—1080 |
| [47] | Tabner B. J., El-Agnaf O. M., Turnbull S., German M. J., Paleologou K. E., Hayashi Y., Cooper L. J., Fullwood N. J., Allsop D., J. Biol. Chem., 2005, 280(43), 35789—35792 |
| [48] | Christen Y., Am. J. Clin. Nutr., 2000, 71(2), 621S—629S |
| [49] | Miranda S., Opazo C., Larrondo L. F., Muñoz F. J., Ruiz F., Leighton F., Inestrosa N. C., Prog. Neurobiol., 2000, 62(6), 633—648 |
| [50] | Arispe N., Pollard H. B., Rojas E., Proc. Natl. Acad. Sci. USA, 1993, 90(22), 10573—10577 |
| [51] | Levites Y., Amit T., Mandel S., Youdim M. B., FASEB J., 2003, 17(8), 952—954 |
| [52] | Choi Y. T., Jung C. H., Lee S. R., Bae J. H., Baek W. K., Suh M. H., Park J., Park C. W., Suh S. I., Life Sci., 2001, 70(5), 603—614 |
| [53] | del Amo J. M. L., Fink U., Dasari M., Grelle G., Wanker E. E., Bieschke J., Reif B., J. Mol. Biol., 2012, 421(4/5), 517—524 |
| [54] | Zhou L., Elias R. J., Food Chem., 2013, 138(2/3), 1503—1509 |
| [55] | Li N., Taylor L. S., Ferruzzi M. G., Mauer L. J., J. Agric. Food Chem., 2012, 60(51), 12531—12539 |
| [56] | Liu F. F., Dong X. Y., He L., Middelberg A. P., Sun Y., J. Phys. Chem. B, 2011, 115(41), 11879—11887 |
| [57] | Bae M. J., Ishii T., Minoda K., Kawada Y., Ichikawa T., Mori T., Kamihira M., Nakayama T., Mol. Nutr. Food Res., 2009, 53(6), 709—715 |
| [58] | An T. T., Feng S., Zeng C. M., Redox Biol., 2017, 11(C), 315—321 |
| [1] | 赵永梅, 穆叶舒, 洪琛, 罗稳, 田智勇. 双萘酰亚胺衍生物用于检测水溶液中的苦味酸[J]. 高等学校化学学报, 2022, 43(3): 20210765. |
| [2] | 伍泽鑫, 朱渊杰, 王泓中, 王均安, 贺英. 甲基修饰的咔唑/二苯砜基AIE-TADF蓝光材料及其OLED器件[J]. 高等学校化学学报, 2022, 43(11): 20220371. |
| [3] | 刘威, 姚伟, 周明明, 游淇, 聂永, 蒋绪川. 9,10⁃二(N⁃苯基吲哚⁃3⁃乙烯基)蒽的合成及聚集诱导荧光和压致荧光变色性质[J]. 高等学校化学学报, 2021, 42(8): 2668. |
| [4] | 李根, 王克亮, 逯春晶. 颗粒聚集体对两性SiO2颗粒无水泡沫表面性质的影响[J]. 高等学校化学学报, 2020, 41(9): 2038. |
| [5] | 黄加玲,刘凤娇,王婷婷,刘翠娥,郑凤英,王振红,李顺兴. 氮硫共掺杂碳量子点对胃液pH值的精确检测[J]. 高等学校化学学报, 2020, 41(7): 1513. |
| [6] | 李红红,耿科颖,田芙月,顾芳,王海军. 偶极Janus粒子的相态结构调控及应用[J]. 高等学校化学学报, 2020, 41(5): 1042. |
| [7] | 李婧影, 陈琛, 李娟, 杨黄浩. 人工调控细胞表面受体聚集状态及功能[J]. 高等学校化学学报, 2020, 41(5): 892. |
| [8] | 沈扬, 朱方, 沈湾湾, 范倩倩, 李乙文, 程义云. 植物多酚基元辅助递送siRNA的构效关系研究[J]. 高等学校化学学报, 2020, 41(4): 633. |
| [9] | 白兰, 翟磊, 王畅鸥, 何民辉, 莫松, 范琳. 含酰胺结构超低膨胀聚酰亚胺薄膜的热膨胀行为[J]. 高等学校化学学报, 2020, 41(4): 795. |
| [10] | 杜宪超, 郝红霞, 秦安军, 唐本忠. 聚集诱导发光分子/适配体/外切酶Ⅰ体系对可卡因的检测[J]. 高等学校化学学报, 2020, 41(3): 411. |
| [11] | 武文博,刘斌. 可双光子激发的聚集诱导发光光敏剂及其生物医学应用[J]. 高等学校化学学报, 2020, 41(2): 191. |
| [12] | 姜童, 任佳骏, 帅志刚. 频域空间密度矩阵重正化群的研究进展[J]. 高等学校化学学报, 2020, 41(12): 2610. |
| [13] | 刘秋娜, 许文文, 刘茂祝, 王惠钢, 郑旭明. 高分辨偏正拉曼光谱对丙酸酐C=O振动模的拉曼光谱非一致效应研究[J]. 高等学校化学学报, 2019, 40(5): 932. |
| [14] | 张雨, 荆江博, 邵玥明, 殷鑫, 徐斌, 温晓玉. 基于聚集诱导发光的纳米粒子用于肝癌细胞靶向成像[J]. 高等学校化学学报, 2019, 40(11): 2382. |
| [15] | 魏良晨, 胡伟康, 周世雄, 束俊, 周会东, 胡旭成, 姜毅, 童碧海, 张千峰. 含亚磷酸酯和联吡啶羧酸酯配体的铱配合物及其聚集诱导发光增强和电致发光性能[J]. 高等学校化学学报, 2018, 39(7): 1371. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
