

高等学校化学学报 ›› 2017, Vol. 38 ›› Issue (10): 1841.doi: 10.7503/cjcu20170065
收稿日期:2017-01-26
出版日期:2017-10-10
发布日期:2017-09-22
作者简介:联系人简介: 李越湘, 男, 博士, 教授, 博士生导师, 主要从事无机纳米材料及能源催化方面的研究. E-mail:基金资助:
TIAN Yi, LI Yuexiang*( ), PENG Shaoqin
), PENG Shaoqin
Received:2017-01-26
Online:2017-10-10
Published:2017-09-22
Contact:
LI Yuexiang
E-mail:liyx@ncu.edu.cn
Supported by:摘要:
采用NaBH4还原法将纳米金属Cu负载在Y2O3上. 通过X射线衍射(XRD)、 X射线光电子能谱(XPS)、 透射电子显微镜(TEM)和测定氢氧根在Y2O3和Cu上的吸附等手段对样品进行了表征. 结果表明, Y2O3表面存在一层薄的Y(OH)3, 能优先吸附OH-使其在Y2O3表面“富集”; 金属Cu以纳米粒子形式负载在Y2O3上. 在碱性甲醛溶液中考察了Y2O3负载Cu(Cu/Y2O3)的催化性能. 结果表明, 在低碱度条件下, 相对于未负载纳米Cu的Y2O3, Cu/Y2O3具有较高的产氢活性. 当氢氧化钠浓度为0.050 mol/L时, 在氮气气氛下Cu/Y2O3产氢量约为未负载纳米Cu的7.8倍; 而在空气气氛下约为4.3倍. 在2种气氛下, Cu/Y2O3产氢的最佳氢氧化钠浓度均为0.25 mol/L. 这可归结为Y2O3表面对OH-的“富集”效应, 使其表面产生更多的活性物种CH2(OH)O-(甲二醇负离子). 相对于未负载纳米Cu的Y2O3, Y2O3负载Cu提高了催化剂的稳定性, 其原因是Y2O3负载阻止了Cu粒子在反应过程的生长.
中图分类号:
TrendMD:
田毅, 李越湘, 彭绍琴. Y2O3负载金属Cu在催化甲醛水溶液制氢反应中的Y2O3载体效应. 高等学校化学学报, 2017, 38(10): 1841.
TIAN Yi, LI Yuexiang, PENG Shaoqin. Effect of Y2O3 Supporter on the Catalytic Hydrogen Production from an Aqueous Formaldehyde Solution Catalyzed by Metal Cu Loaded on Y2O3†. Chem. J. Chinese Universities, 2017, 38(10): 1841.
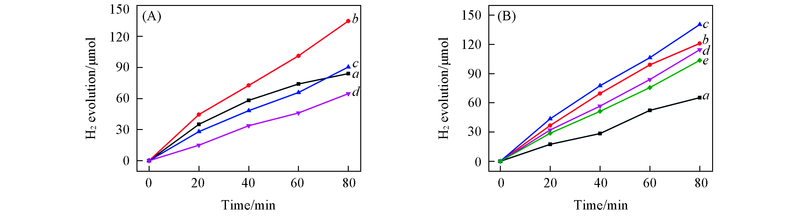
Fig.5 Effects of Cu loading of x-Cu/Y2O3(A) and formaldehyde concentration(B) on hydrogen generation under N2 atmosphere(A) x(%): a. 5; b. 10; c. 15; d. 20. Reaction conditions: 0.50 mol/L of NaOH; 0.60 mol/L of HCHO; a given amount of x-Cu/Y2O3 Cu containing 5 mg Cu. (B) cHCHO/(mol·L-1): a. 0.10; b. 0.30; c. 0.60; d. 0.90; e. 1.20. Reaction conditions: 0.50 mol/L of NaOH; 50 mg of 10-Cu/Y2O3.
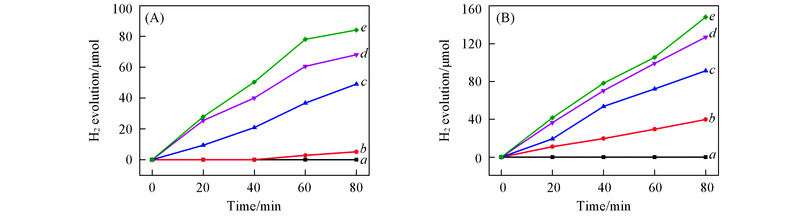
Fig.6 Effect of NaOH concentration on hydrogen generation over nano Cu(A) and 10-Cu/Y2O3(B) under N2 atmospherecNaOH/(mol·L-1): a. 0; b. 0.05; c. 0.10; d. 0.25; e. 0.50. Reaction conditions: 0.60 mol/L of HCHO;5 mg of nano Cu or 50 mg of 10-Cu/Y2O3.
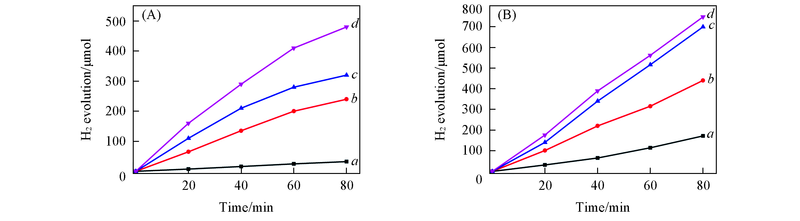
Fig.7 Effect of NaOH concentration on hydrogen generation over nano Cu(A) and 10-Cu/Y2O3(B) under air atmospherecNaOH/(mol·L-1): a. 0.05; b. 0.10; c. 0.25; d. 0.50. Reaction conditions are the same as those in Fig.6.
| Catalyst | Reaction condition | Minimum concentration/ (mol·L-1) | Optimal or used concentration/ (mol·L-1) | Optimal H2 evolution rate/ (mL·g-1·min-1) | Ref. |
|---|---|---|---|---|---|
| 10-Cu/Y2O3 | 0.60 mol/L HCHO; 50 mg catalyst | 0.050 | 0.25 | 39.8 | This work |
| Nano Cu | 0.48 mol/L HCHO; 10 mg catalyst | 0.25 | 1.0 | 29.5 | [ |
| Nano Cu | 0.50 mol/L HCHO; 20 mg catalyst | 1.0 | 9.6 | [ | |
| Hollow Pd nanotube | 0.48 mol/L HCHO; 10 mg catalyst | 0.25 | 1.0 | 168.0 | [ |
| Rh nanotube | 0.50 mol/L HCHO; 5 mg catalyst | 1.0 | 275.0 | [ | |
| Nano Au | 0.50 mol/L HCHO; 10 mg catalyst | 1.0 | 29.5 | [ | |
| Au nanotube | 0.50 mol/L HCHO; 10 mg catalyst | 1.0 | 154.0 | [ | |
| Nano Ag | 0.50 mol/L HCHO; 10 mg catalyst | 0.25 | 0.50 | 164.0 | [ |
| Ag/γ-Al2O3 | 0.87 mol/L HCHO; 50 mg catalyst | >0.10 | 2.0 | 415.0 | [ |
| AgPd4@C-72 | 0.26 mol/L HCHO; 15 mg catalyst | 0.50 | 1.0 | 237.0 | [ |
| Pd/TiO2 | 0.60 mol/L HCHO; 15 mg catalyst | 0.25 | 1.0 | 250.0 | [ |
Table 1 Comparison of minimum and optimal(used) NaOH concentrations and optimal H2 evolution rate under air atmosphere from formaldehyde solution over various catalysts at room temperature
| Catalyst | Reaction condition | Minimum concentration/ (mol·L-1) | Optimal or used concentration/ (mol·L-1) | Optimal H2 evolution rate/ (mL·g-1·min-1) | Ref. |
|---|---|---|---|---|---|
| 10-Cu/Y2O3 | 0.60 mol/L HCHO; 50 mg catalyst | 0.050 | 0.25 | 39.8 | This work |
| Nano Cu | 0.48 mol/L HCHO; 10 mg catalyst | 0.25 | 1.0 | 29.5 | [ |
| Nano Cu | 0.50 mol/L HCHO; 20 mg catalyst | 1.0 | 9.6 | [ | |
| Hollow Pd nanotube | 0.48 mol/L HCHO; 10 mg catalyst | 0.25 | 1.0 | 168.0 | [ |
| Rh nanotube | 0.50 mol/L HCHO; 5 mg catalyst | 1.0 | 275.0 | [ | |
| Nano Au | 0.50 mol/L HCHO; 10 mg catalyst | 1.0 | 29.5 | [ | |
| Au nanotube | 0.50 mol/L HCHO; 10 mg catalyst | 1.0 | 154.0 | [ | |
| Nano Ag | 0.50 mol/L HCHO; 10 mg catalyst | 0.25 | 0.50 | 164.0 | [ |
| Ag/γ-Al2O3 | 0.87 mol/L HCHO; 50 mg catalyst | >0.10 | 2.0 | 415.0 | [ |
| AgPd4@C-72 | 0.26 mol/L HCHO; 15 mg catalyst | 0.50 | 1.0 | 237.0 | [ |
| Pd/TiO2 | 0.60 mol/L HCHO; 15 mg catalyst | 0.25 | 1.0 | 250.0 | [ |
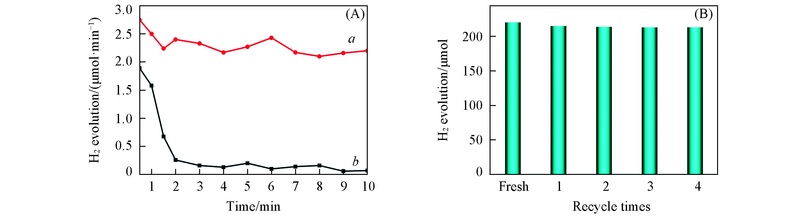
Fig.8 Time curves of 10-Cu/Y2O3(a) and nano Cu(b) for H2 production from formaldehyde solution(A) and reusability of 10-Cu/Y2O3 for H2 production from formaldehyde solutionReaction conditions are the same as those in Fig.5(A) except the reaction time. (B) Reaction conditions: 0.50 mol/L NaOH; 0.60 mol/L of HCHO; 100 mg 10-Cu/Y2O3, N2 atmosphere.
| [1] | Li Y. X., Hou Y. L., Fu Q. Y., Peng S. Q., Hu Y. H., Appl. Catal. B: Environ., 2017, 206, 726—733 |
| [2] | Li Y. X., Wang J. X., Peng S. Q., Lu G. X., Li S. B.,Int. J. Hydrogen Energy,2010, 35(13), 7116—7126 |
| [3] | Li Y. X., Wang H., Peng S. Q., J. Phys. Chem. C,2014, 118(34), 19842—19848 |
| [4] | Zhang W. Y., Li Y. X., Peng S. Q., ACS Appl. Mater. Interfaces,2016, 8(24), 15187—15195 |
| [5] | Zhang X., Zhong X., Yang Z., Song J., Lu H., Chem. Res. Chinese Universities,2016, 32(6), 1016—1018 |
| [6] | Ma Y., Li X. Z., Li Y. T., Liu D. M., Zhang Q. A., Si T. Z., Chem. J. Chinese Universities,2016, 37(10), 1776—1783 |
| (马勇, 李祥志, 李永涛, 柳东明, 张庆安, 斯庭智. 高等学校化学学报, 2016, 37(10), 1776—1783) | |
| [7] | Heim L. E., Schlörer N. E., Choi J. H., Prechtl M. H., Nat. Commun., 2014, 5, 4621 |
| [8] | Tsang M. H., Jwu T. C., Gene H. L., Yu W., J. Am. Chem. Soc., 1993, 115(3), 1170—1171 |
| [9] | Hu H. Y., Jiao Z. B., Ye J. H., Lu G. X., Bi Y. P., Nano Energy,2014, 8, 103—109 |
| [10] | Bi Y. P., Hu H., Li Q., Lu G. X., Int. J. Hydrogen Energy,2010, 35(13), 7177—7182 |
| [11] | Wang T., Liu L., Zhu Z., Papakonstantinou P., Hu J., Liu H., Li M., Energy Environ. Sci., 2013, 6, 625—633 |
| [12] | Li Y. W., Chen T., Wang T., Zhang Y. P., Lu G. X., Bi Y. P., Int. J. Hydrogen Energy,2014, 39, 9114—9120 |
| [13] | Pan X., Wang L., Ling F., Li Y., Han D., Pang Q., Jia L., Int. J. Hydrogen Energy,2015, 40(4), 1752—1759 |
| [14] | Bi Y. P., Lu G. X., Int. J. Hydrogen Energy,2008, 33(9), 2225—2232 |
| [15] | Preti D., Squarcialupi S., Fachinetti G. Aerobic., Angew. Chem., Int. Ed., 2009, 48(26), 4763—4766 |
| [16] | Patil N. S., Uphade B. S., McCulloh D. G., Bhargava S. K., Choudhary V. R., Catal. Commun., 2004, 5(11), 681—685 |
| [17] | Biesinger M. C., Lau L. W. M., Gerson A. R., Appl. Surf. Sci., 2010, 257(3), 887—898 |
| [18] | Nakamura T., Tomizuka H., Takahashi M., J. Surf. Sci. Soc. Jpn., 1995, 16(8), 515—520 |
| [19] | Craciun V., Howard J., Lambers E. S., Singh R. K., Craciun D., Perriere J., Appl. Phys. A,1999, 69(1), 535—538 |
| [20] | Nefedov V. I., Firsov M. N., Shaplygin I. S., J. Electron. Spectrosc. Relat. Phenom., 1982, 26(1), 65—78 |
| [21] | Barreca D., Battiston G. A., Berto D., Gerbasi R., Tondello E., Surf. Sci. Spectra,2001, 8(3), 234—239 |
| [22] | Kuroda Y., Hamano H., Mori T., Yoshikawa Y., Nagao M., Langmuir,2000, 16(17), 6937—6947 |
| [23] | Li Y. X., Guo M. M., Peng S. Q., Lu G. X., Li S. B., Int. J. Hydrogen Energy,2009, 34(14), 5629—5636 |
| [24] | Işik M., Colloids Surf. B, 2008, 62(1), 97—104 |
| [25] | Bi Y. P., Lu G. X., Chem. Commun., 2008, 47, 6402—6404 |
| [26] | Bi Y. P., Lu G. X., Mater. Lett., 2008, 62(17), 2696—2699 |
| [27] | Bi Y. P., Lu G. X., Nanotechnology,2008, 19(27), 275306 |
| [28] | Gao S., Feng T., Wu Q., Feng C., Shang N., Wang C., RSC Adv., 2016, 6(107), 105638—105643 |
| [29] | Li S., Hu H., Bi Y. P., J. Mater. Chem. A,2016, 4(3), 796—800 |
| [30] | Walker J.F., Formaldehyde, 3th. Ed., Reinhold Publishing Corporation, London, 1964, 59—73 |
| [31] | Li Y. X., Lü G. X., Li S. B., Yu F., J. Mol. Catal.(China), 2002, 16(4), 241—246 |
| (李越湘, 吕功煊, 李树本, 俞飞. 分子催化, 2002, 16(4), 241—246) | |
| [32] | Azizi S. N., Ghasemi S., Amiripour F., Sens. Actuators B,2016, 227, 1—10 |
| [33] | Zhao C., Li M., Jiao K., J. Anal. Chem., 2006, 61(12), 1204—1208 |
| [34] | Starodubov S. S., Nechaev I. V., Vvedenskii A. V., Russ. J. Phys. Chem. A,2016, 90(1), 122—129 |
| [1] | 陈长利, 米万良, 李煜璟. 单原子催化材料在电化学氢循环应用中的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220065. |
| [2] | 陈望松, 罗兰, 刘玉广, 周华, 孔祥贵, 栗振华, 段昊泓. 光电解水制氢耦合生物质醇/醛氧化的研究进展[J]. 高等学校化学学报, 2022, 43(2): 20210683. |
| [3] | 李淑蓉, 王琳, 陈玉贞, 江海龙. 金属-有机框架材料在液相催化化学制氢中的研究进展[J]. 高等学校化学学报, 2022, 43(1): 20210575. |
| [4] | 吴启亮, 梅晋豪, 李铮, 范海东, 张彦威. 多种纳米结构Fe掺杂TiO2光热耦合水分解制氢研究[J]. 高等学校化学学报, 2021, 42(6): 1837. |
| [5] | 桂晨, 王颢霖, 邵柏璇, 杨育景, 徐光青. 熔盐辅助法制备g-C3N4纳米结构及其光催化制氢性能[J]. 高等学校化学学报, 2021, 42(3): 827. |
| [6] | 肖兆忠, 马智, 朴玲钰. 不同半导体体系中磷化镍光催化甲酸分解的助催化作用[J]. 高等学校化学学报, 2021, 42(12): 3692. |
| [7] | 王乙舒, 李雪, 闫丽, 徐红赟, 祝玉鑫, 宋艳华, 崔言娟. 二维Z型BCN/Sn3O4复合材料的光催化还原性能[J]. 高等学校化学学报, 2021, 42(12): 3722. |
| [8] | 孙亚光, 张含烟, 明涛, 徐宝彤, 高雨, 丁茯, 徐振和. ZnIn2S4/g-C3N4复合材料的制备及可见光催化制氢性能[J]. 高等学校化学学报, 2021, 42(10): 3160. |
| [9] | 祝玉鑫, 欧阳杰, 宋艳华, 唐盛, 崔言娟. 硼碘共掺杂氮化碳的制备及光解水制氢性能[J]. 高等学校化学学报, 2020, 41(7): 1645. |
| [10] | 罗威, 梁佑才, 胡志诚, 唐浩然, 刘孝诚, 邢晔彤, 黄飞. 新型亲水性共轭聚合物的制备及光催化制氢性能[J]. 高等学校化学学报, 2020, 41(3): 456. |
| [11] | 宁湫洋, 冯微, 吴国光. 瓶状InN-In2O3纳米复合材料的制备及增强的甲醛气敏性能[J]. 高等学校化学学报, 2020, 41(12): 2804. |
| [12] | 段亚军, 程岩岩, 隋光辉, 朱燕超, 王晓峰, 郭玉鹏, 王子忱. 木质素对木质素-脲醛共聚树脂的影响及反应机理[J]. 高等学校化学学报, 2019, 40(5): 1058. |
| [13] | 黄锐, 姚志龙, 孙培永, 张胜红. CuO-WO3-ZrO2的结构和性质对苯甲醛加氢反应催化性能的影响[J]. 高等学校化学学报, 2019, 40(5): 1005. |
| [14] | 王志鹏, 牛珠珠, 班丽君, 郝全爱, 张鸿喜, 李海涛, 赵永祥. 不同晶相TiO2负载Cu2O催化甲醛乙炔化反应[J]. 高等学校化学学报, 2019, 40(2): 334. |
| [15] | 鲁礼林, 舒红飞, 阮祝华, 倪嘉琪, 张海军. 石墨烯负载Pt-Pd催化剂的制备、催化制氢性能及机理研究[J]. 高等学校化学学报, 2018, 39(5): 949. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||