

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (12): 2262.doi: 10.7503/cjcu20170331
• Physical Chemistry • Previous Articles Next Articles
KANG Yanshuang1,*( ), SUN Zongli2,*(
), SUN Zongli2,*( ), TAN Shanshan2
), TAN Shanshan2
Received:2017-05-26
Online:2017-12-10
Published:2017-11-21
Contact:
KANG Yanshuang,SUN Zongli
E-mail:kang_yanshuang@163.com;sunzl@ncepu.edu.cn
Supported by:CLC Number:
TrendMD:
KANG Yanshuang, SUN Zongli, TAN Shanshan. Depletion Potential and Adsorption Stability of a Colloidal Particle in Confined Solvent Mixture†[J]. Chem. J. Chinese Universities, 2017, 38(12): 2262.
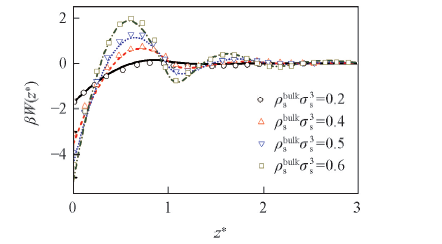
Fig.1 Comparison of calculated results with those from the Monte-Carlo simulation The lines correspond the results from this work, while the symbols to those from Monte-Carlo simulation.
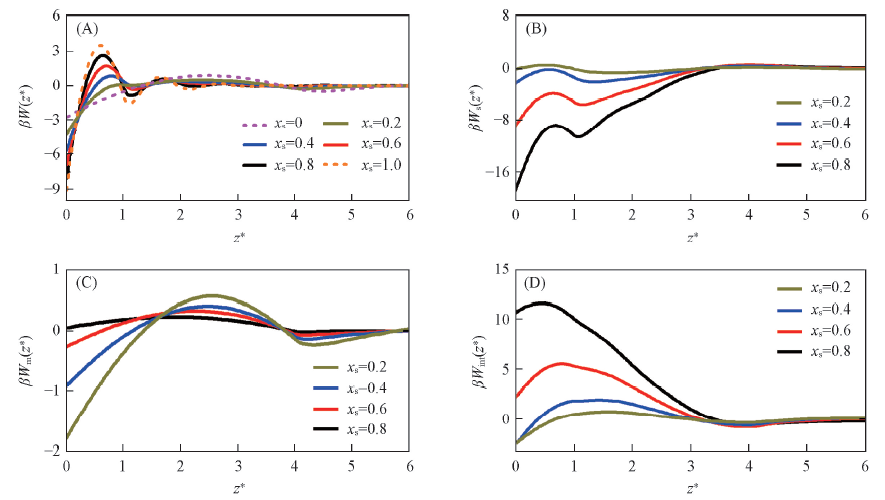
Fig.2 Depletion potential and its contributions between colloidal particle and the wall under different conditions of relative volume fraction xs(A) The results for the total depletion potential W(z); (B)—(D) the contributions Ws(z), Wm(z) and Wint(z), respectively.
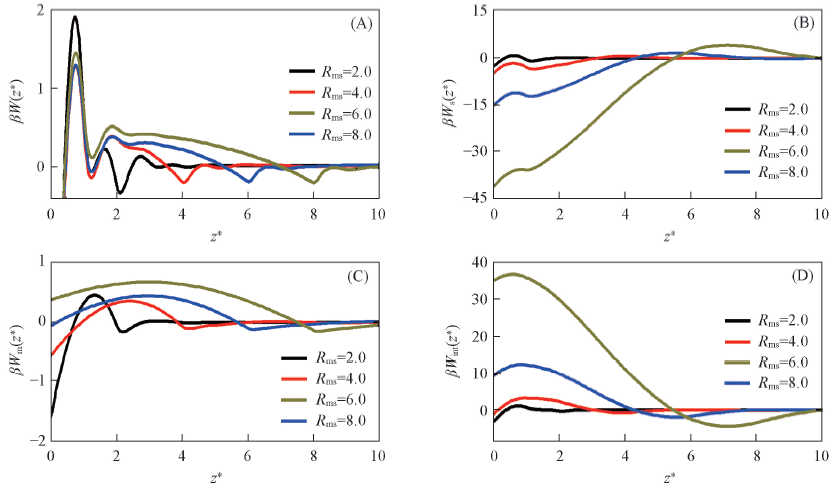
Fig.3 Depletion potential and its contributions between colloidal particle and the wall under different conditions of size ratio Rms(A) The results for the total depletion potential W(z); (B)—(D) the contributions Ws(z), Wm(z) and Wint(z), respectively.
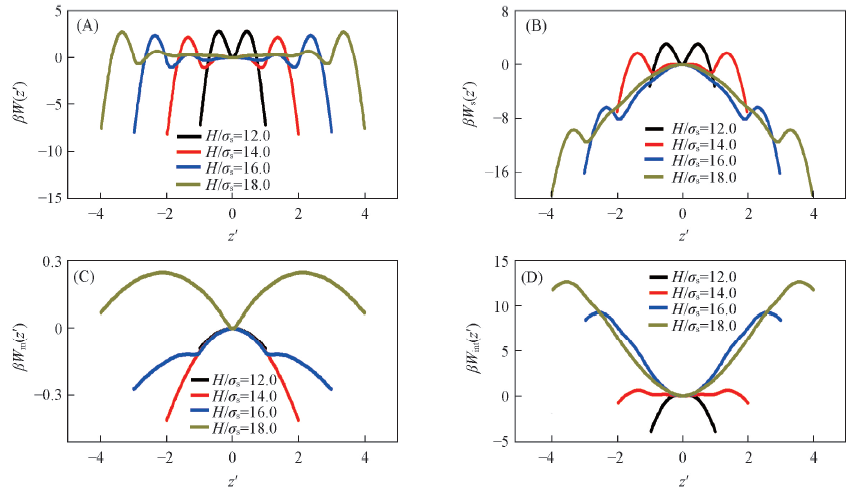
Fig.4 Depletion potential and its contributions between colloidal particle and the slit pore under different conditions of pore width H(A) The results for the total depletion potential W(z); (B)—(D) the contributions Ws(z), Wm(z) and Wint(z), respectively.
| [1] | Lekkerkerker H.N. W., Tuinier R., Colloids and the Depletion Interaction, Springer, Heidelberg, 2011, 16—50 |
| [2] | Asakura S., Oosawa F., J. Chem. Phys., 1954, 22(7), 1255—1256 |
| [3] | Zhao K., Mason T. G., Phys. Rev. Lett., 2007, 99(26), 268301 |
| [4] | Ashton D. J., Jacka R. L., Wilding N. B., Soft Matter, 2013, 9(40), 9661—9666 |
| [5] | Tuinier R., Rieger J., de Kruif C. G., Adv. Colloid Interface Sci., 2003, 103(1), 1—31 |
| [6] | Jamie E. A. G., Dullens R. P. A., Aarts D. G. A. L., J. Phys. : Condens. Matter,2011, 23(19), 194115 |
| [7] | Jorge A. F., Nunes S. C. C., Cova T. F. G. G., Pais A. A. C. C., Curr. Opin. Coll. Interf. Sci., 2016, 26, 66—74 |
| [8] | Tanaka S., Ataka M., Onuma K., Kubota T., Biophys. J., 2003, 84(5), 3299—3306 |
| [9] | Tanaka S., Ataka M., J. Chem. Phys., 2002, 117(7), 3504—3510 |
| [10] | Dmitry B., Angela F., van Marijn H., Cinzia G., Andrea F., Ugo L., Henny Z., Marco Z., Roberto C., Liberato M., Nano Lett., 2010, 10(2), 743—749 |
| [11] | Zhang J. W., Song L. R., Madsen G. K. H., Fischer K. F. F., Zhang W. Q., Shi X., Iversen B. B., Nat Commun., 2016, 7, 10892 |
| [12] | Roth R., van Roij R., Andrienko D., Mecke K. R., Dietrich S., Phys. Rev. Lett., 2002, 89(8), 088301 |
| [13] | Moncho-Jordá A., Odriozola G., Curr. Opin. Coll. Interf. Sci., 2015, 20(1), 24—31 |
| [14] | Trokhymchuk A., Henderson D., Curr. Opin. Coll. Interf. Sci., 2015, 20(1), 32—38 |
| [15] | Ji S. X., Walz J. Y., Curr. Opin. Coll. Interf. Sci., 2015, 20(1), 39—45 |
| [16] | Briscoe W. H., Curr. Opin. Coll. Interf. Sci., 2015, 20(1), 46—53 |
| [17] | Tuinier R., Fan T. H., Taniguchi T., Curr. Opin. Coll. Interf. Sci., 2015, 20(1), 66—70 |
| [18] | Yang S. A., Yan D. D., Tan H. G., Shi A. C., Phys. Rev. E, 2006, 74(4), 041808 |
| [19] | Yethiraj A., Hall C. K., Dickman R., J. Colloid Interface Sci., 1992, 151(1), 102—117 |
| [20] | Chatterjee A. P., Schweizer K. S., J. Chem. Phys., 1998, 109(23), 10464—10476 |
| [21] | López-Sánchez E., Estrada-Álvarez C. D., Pérez-Ángel G., Méndez-Alcaraz J. M., Mozuelos P. G., J. Chem. Phys., 2013, 139(10), 104908 |
| [22] | Zhou S. Q., Phys. Rev. E, 2006, 74(1), 011402 |
| [23] | Roth R., Evans R., Dietrich S., Phys. Rev. E, 2000, 62(4), 5360—5376 |
| [24] | Patel N., Egorov S. A., J. Chem. Phys., 2004, 121(10), 4987—4997 |
| [25] | Chen X., Cai J., Liu H., Hu Y., Mol. Simul., 2006, 32(10/11), 877—885 |
| [26] | Ashton D. J., Wilding N. B., Roth R., Evans R., Phys. Rev. E, 2011, 84(6), 061136 |
| [27] | Sun Z. L., Kang Y. S., Kang Y. M., J. Phys. Chem. B, 2014, 118(40), 11826—11834 |
| [28] | Xiao C. M., Guo J. Y., Li C. S., Europhys. Lett., 2006, 73(3), 443—449 |
| [29] | Ashton D. J., Sánchez-Gil V., Wilding N. B., J. Chem. Phys., 2013, 139(14), 144102 |
| [30] | Miao H., Li Y., Ma H. R., J. Chem. Phys., 2014, 140(15), 154904 |
| [31] | Kamp M., Hermes M., van Kats C. M., Kraft D. J., Kegel W. K., Dijkstra M., van Blaaderen A., Langmuir,2016, 32(5), 1233—1240 |
| [32] | Widom B., J. Phys. Chem., 1982, 86(6), 869—872 |
| [33] | Egorov S. A., Phys. Rev. E, 2004, 70(3), 031402 |
| [34] | Oettel M., Hansen-Goos H., Bryk P., Roth R., Europhys. Lett., 2009, 85(3), 36003 |
| [35] | Gu F., Wang H. J., Chem. J. Chinese Universities, 2011, 32(7), 1567—1572 |
| (顾芳, 王海军. 高等学校化学学报, 2011, 32(7), 1567—1572) | |
| [36] | Gu F., Wang H. J., Fu D., Acta Chim. Sin., 2011, 69(19), 2215—2220 |
| (顾芳, 王海军, 付东. 化学学报, 2011, 69(19), 2215—2220) | |
| [37] | Boţan V., Pesth F., Schilling T., Oettel M., Phys. Rev. E, 2009, 79(6), 061402 |
| [38] | Roth R Kinoshita M., J. Chem. Phys., 2006, 125(8), 084910 |
| [39] | Karino Y., Akiyama R., Kinoshita M., J. Phys. Soc. Jap., 2009, 78(4), 044801 |
| [40] | Karino Y., Akiyama R., Kinoshita M., J. Phys. Soc. Jap., 2011, 80(11), 114802 |
| [41] | Rowlinson J.S., Widom B., The Molecular Theory of Capillarity, Oxford, Clarendon, 1982, 69—85 |
| [42] | Hansen J.P., McDonald I. R., Theory of Simple Liquids 4th Ed., Elsevier, New York, 2013, 70—72 |
| [43] | Evans R., Adv. Phys., 1979, 28(2), 143—200 |
| [44] | Rosenfeld Y., Phys. Rev. Lett., 1989, 63(9), 980—983 |
| [45] | Yu Y. X., Wu J. Z., J. Chem. Phys., 2002, 117(22), 10156—10164 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [3] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [4] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [5] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [6] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [7] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [8] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [9] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [10] | ZHENG Ruoxin, ZHANG Igor Ying, XU Xin. Development and Benchmark of Lower Scaling Doubly Hybrid Density Functional XYG3 [J]. Chem. J. Chinese Universities, 2021, 42(7): 2210. |
| [11] | YING Fuming, JI Chenru, SU Peifeng, WU Wei. λ-DFCAS: A Hybrid Density Functional Complete Active Space Self Consistent Field Method [J]. Chem. J. Chinese Universities, 2021, 42(7): 2218. |
| [12] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [13] | HU Wei, LIU Xiaofeng, LI Zhenyu, YANG Jinlong. Surface and Size Effects of Nitrogen-vacancy Centers in Diamond Nanowires [J]. Chem. J. Chinese Universities, 2021, 42(7): 2178. |
| [14] | YANG Yiying, ZHU Rongxiu, ZHANG Dongju, LIU Chengbu. Theoretical Study on Gold-catalyzed Cyclization of Alkynyl Benzodioxin to 8-Hydroxy-isocoumarin [J]. Chem. J. Chinese Universities, 2021, 42(7): 2299. |
| [15] | LIU Changhui, LIANG Guojun, LI Yanlu, CHENG Xiufeng, ZHAO Xian. Density Functional Theory Study of NH3 Adsorption on Boron Nanotubes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2263. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||