

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (9): 1911.doi: 10.7503/cjcu20190292
• Physical Chemistry • Previous Articles Next Articles
ZHANG Lin,ZHANG Wei,YUE Xin,LI Pengjie,YANG Zuoyin,PU Min,LEI Ming( )
)
Received:2019-05-22
Online:2019-09-10
Published:2019-09-09
Contact:
LEI Ming
E-mail:leim@mail.buc
Supported by:CLC Number:
TrendMD:
ZHANG Lin, ZHANG Wei, YUE Xin, LI Pengjie, YANG Zuoyin, PU Min, LEI Ming. Theoretical Study on Mechanism of CO2 Hydrogenation to Formic Acid Catalyzed by Manganese Complex †[J]. Chem. J. Chinese Universities, 2019, 40(9): 1911.
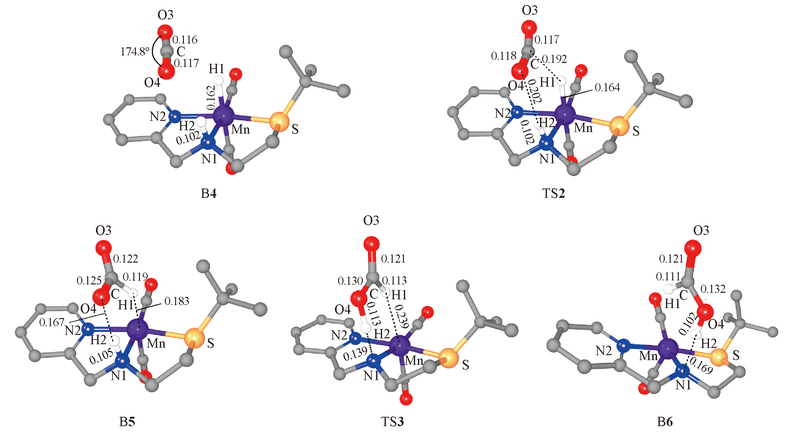
Fig.5 Geometric parameters of key intermediates and transition states in the hydrogenation of carbon dioxide Bond lengths are in nm, angles are in degree.
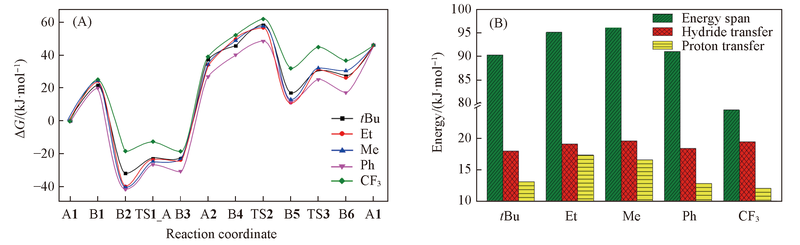
Fig.6 Effect of R group modulation on Gbbis free energy in the whole catalytic cycle(A) and effect of R group modulation on the energy span, the energy barrier of hydride and proton transfer(B)
| [1] | Kennedy J. F., Shimizu J., ,. Carbohyd. Polym. 1995, 26( 1), 81— 82 |
| [2] | Klein H. F., ,. Ber. Bunsen-Ges. Phys. Chem. 1989, 93( 7), 827 |
| [3] | Aresta M., Quaranta E., Tommasi I., Giannoccaro P., Ciccarese A., ,. Gazz. Chim. Ital. 1995, 125( 11), 509— 538 |
| [4] | Baiker A., ,. Appl. Organomet. Chem. 2000, 14( 12), 751— 762 |
| [5] | Schuchmann K., Müller V ., Science 2013, 342( 6164), 1382— 1385 |
| [6] | Wang W., Wang S. P., Ma X. B., Gong J. L., ,. Chem. Soc. Rev. 2011, 40( 7), 3703— 3727 |
| [7] | Mellmann D., Sponholz P., Junge H., Beller M., ,. Chem. Soc. Rev. 2016, 45( 14), 3954— 3988 |
| [8] | Eppinger J., Huang K. W., ,. ACS Energy Lett. 2016, 2( 1), 188— 195 |
| [9] | Moret S., Dyson P. J., Laurenczy G., ,. Nat. Commun. 2014, 5, 4017 |
| [10] | Kayaki Y., Shimokawatoko Y., Ikariya T., ,. Inorg. Chem. 2007, 46( 14), 5791— 5797 |
| [11] | Darensbourg D. J., Yoder J. C., Holtcamp M. W., Klausmeyer K. K., Reibenspies J. H., ,. Inorg. Chem. 1996, 35( 16), 4764— 4769 |
| [12] | Walther D., Ruben M., Rau S., ,. Coord. Chem. Rev. 1999, 182( 1), 67— 100 |
| [13] | Hutschka F., Dedieu A., Eichberger M., Fornika R., Leitner W., ,. J. Am. Chem. Soc. 1997, 119( 19), 4432— 4443 |
| [14] | Munshi P., Main A. D., Linehan J. C., Tai C. C., Jessop P. G., ,. J. Am. Chem. Soc. 2002, 124( 27), 7963— 7971 |
| [15] | Ohnishi Y. Y., Nakao Y., Sato H., Sakaki S ., Organometallics 2006, 25( 14), 3352— 3363 |
| [16] | Arup M., Alexander N., Gregory L., Shimon L. J. W., Yehoshoa B. D., Angel E. J. N., Milstein D., ,. J. Am. Chem. Soc. 2016, 138( 13), 4298— 4301 |
| [17] | Elangovan S., Topf C., Fischer S., Jiao H., Spannenberg A., Baumann W., Ludwig R., Junge K., Beller M., ,. J. Am. Chem. Soc. 2016, 138( 28), 8809— 8814 |
| [18] | Das K., Mondal A., Srimani D., ,. Chem. Commun. 2018, 54( 75), 10582— 10585 |
| [19] | Das K., Mondal A., Pal D., Srivastava H. K., Srimani D ., Organometallics 2019, 38( 8), 1815— 1825 |
| [20] | Chai J. D., Head-Gordon M., ,. Phys. Chem. Chem. Phys. 2008, 10( 44), 6615— 6620 |
| [21] | Frisch M J. . Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision B, Gaussian Inc., Wallingford, CT, 2010 |
| [22] | Wang M., Zhang X., Chen Z., Tang Y. H., Lei M., ,. Sci. China Chem. 2014, 57( 9), 1264— 1275 |
| [23] | Lei M., Wang Z. D., Du X. J., Zhang X., Tang Y. H., ,. J. Phys. Chem. A 2014, 118( 39), 8960— 8970 |
| [24] | Ma X. L., Tang Y. H., Lei M., ,. Dalton Trans. 2014, 43( 30), 11658— 11666 |
| [25] | Lei M., Ma X. L., Pan Y. H., ,. Eur. J. Inorg. Chem. 2015, 2015( 5), 794— 803 |
| [26] | Ma X. L., Lei M., Liu S. B ., Organometallics 2015, 34( 7), 1255— 1263 |
| [27] | Li H., Ma X. L., Lei M., ,. Dalton Trans. 2016, 45( 20), 8506— 8512 |
| [28] | Li L. F., Pan Y. H., Lei M., ,. Catal. Sci. Technol. 2016, 6( 12), 4450— 4457 |
| [29] |
Zhang Y. W., Ma X. L., Zhang X., Lei M., ,Acta Chim. Sinica, 2016,74( 4), 340— 350
doi: 10.6023/A15120781 |
|
(张益伟, 马雪璐, 张欣, 雷鸣.化学学报,2016,74(4), 340— 350)
doi: 10.6023/A15120781 |
|
| [30] | Yue X., Li L. F., Li P. J., Luo C. G., Pu M., Yang Z. Y., Lei M., ,. Chinese J. Chem. 2019, 37( 8), 883— 886 |
| [31] | Xiao M., Yue X., Xu R. R., Tang W. J., Xue D., Li C. Q., Lei M., Xiao J. L., Wang C., ,. Angew. Chem. Int. Ed. 2019, 58( 31), 10528— 10536 |
| [32] | Musashi Y., Sakaki S., ,. J. Am. Chem. Soc. 2002, 124( 25), 7588— 7603 |
| [33] | Filonenko G. A., Hensen E. J. M., Pidko E. A., ,. Catal. Sci. Technol. 2014, 4( 10), 3474— 3485 |
| [34] | Yin C. Q., Xu Z. T., Yang S. Y., Ng S. M., Wong K. Y., Lin Z. Y., Lau C. P ., Organometallics 2001, 20( 6), 1216— 1222 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [3] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [4] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [5] | SUN Cuihong, LYU Liqiang, LIU Ying, WANG Yan, YANG Jing, ZHANG Shaowen. Mechanism and Kinetics on the Reaction of Isopropyl Nitrate with Cl, OH and NO3 Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210591. |
| [6] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [7] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [8] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [9] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [10] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [11] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [12] | MENG Fanwei, GAO Qi, YE Qing, LI Chenxi. Potassium Poisoning Mechanism of Cu-SAPO-18 Catalyst for Selective Catalytic Reduction of NOx by Ammonia [J]. Chem. J. Chinese Universities, 2021, 42(9): 2832. |
| [13] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [14] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [15] | LI Xinyi, LIU Yongjun. Theoretical Insights into the Cleavage of β-Hydroxy Ketone Catalyzed by Artificial Retro-aldolase RA95.5-8F [J]. Chem. J. Chinese Universities, 2021, 42(7): 2306. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||