

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (5): 965.doi: 10.7503/cjcu20180851
• Physical Chemistry • Previous Articles Next Articles
KANG Huimin, WANG Hongqiang, WANG Huiying, WU Lixin, QIU Yongqing*( )
)
Received:2018-12-20
Online:2019-04-17
Published:2019-04-17
Contact:
QIU Yongqing
E-mail:qiuyq466@nenu.edu.cn
Supported by:CLC Number:
TrendMD:
KANG Huimin,WANG Hongqiang,WANG Huiying,WU Lixin,QIU Yongqing. Theoretical Study on the Second-order Nonlinear Optical Properties of a Porphyrin-o-Carborane-Boron-dipyrromethene Triad Compound†[J]. Chem. J. Chinese Universities, 2019, 40(5): 965.
| Compound | d(C1—C2)/ nm | d(C1—C3)/ nm | d(C2—C4)/ nm | d(Zn—Nmean)/ nm | d(B—N1)/ nm | d(B—N2)/ nm | d(B—F1)/ nm | d(B—F2)/ nm |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.1217 | 0.1424 | 0.1424 | 0.2070 | 0.1557 | 0.1557 | 0.1394 | 0.1394 |
| 1+ | 0.1217 | 0.1420 | 0.1423 | 0.2076 | 0.1561 | 0.1561 | 0.1389 | 0.1389 |
| 1- | 0.1221 | 0.1418 | 0.1418 | 0.2076 | 0.1549 | 0.1549 | 0.1404 | 0.1404 |
| 2 | 0.3160 | 0.1516 | 0.1516 | 0.2070 | 0.1558 | 0.1558 | 0.1394 | 0.1394 |
| 2+ | 0.3155 | 0.1515 | 0.1516 | 0.2075 | 0.1561 | 0.1561 | 0.1389 | 0.1389 |
| 2- | 0.3169 | 0.1514 | 0.1515 | 0.2076 | 0.1549 | 0.1549 | 0.1404 | 0.1404 |
| 3 | 0.1748 | 0.1508 | 0.1509 | 0.2069 | 0.1558 | 0.1558 | 0.1394 | 0.1393 |
| Exp.[ | 0.1708 | 0.1510 | 0.1509 | 0.2062 | 0.1542 | 0.1524 | 0.1439 | 0.1393 |
| 3+ | 0.1737 | 0.1510 | 0.1510 | 0.2076 | 0.1562 | 0.1560 | 0.1391 | 0.1388 |
| 3- | 0.2343 | 0.1466 | 0.1471 | 0.2073 | 0.1553 | 0.1553 | 0.1399 | 0.1399 |
| 4 | 0.1759 | 0.1506 | 0.1507 | 0.2070 | 0.1558 | 0.1558 | 0.1394 | 0.1394 |
| 4+ | 0.1760 | 0.1507 | 0.1506 | 0.2076 | 0.1561 | 0.1560 | 0.1391 | 0.1389 |
| 4- | 0.2325 | 0.1469 | 0.1468 | 0.2072 | 0.1553 | 0.1553 | 0.1398 | 0.1398 |
Table 1 Partial bond lengths of the studied compounds as obtained using DFT(B3LYP functional) employing the 6-31G*/LANL2DZ basis set
| Compound | d(C1—C2)/ nm | d(C1—C3)/ nm | d(C2—C4)/ nm | d(Zn—Nmean)/ nm | d(B—N1)/ nm | d(B—N2)/ nm | d(B—F1)/ nm | d(B—F2)/ nm |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.1217 | 0.1424 | 0.1424 | 0.2070 | 0.1557 | 0.1557 | 0.1394 | 0.1394 |
| 1+ | 0.1217 | 0.1420 | 0.1423 | 0.2076 | 0.1561 | 0.1561 | 0.1389 | 0.1389 |
| 1- | 0.1221 | 0.1418 | 0.1418 | 0.2076 | 0.1549 | 0.1549 | 0.1404 | 0.1404 |
| 2 | 0.3160 | 0.1516 | 0.1516 | 0.2070 | 0.1558 | 0.1558 | 0.1394 | 0.1394 |
| 2+ | 0.3155 | 0.1515 | 0.1516 | 0.2075 | 0.1561 | 0.1561 | 0.1389 | 0.1389 |
| 2- | 0.3169 | 0.1514 | 0.1515 | 0.2076 | 0.1549 | 0.1549 | 0.1404 | 0.1404 |
| 3 | 0.1748 | 0.1508 | 0.1509 | 0.2069 | 0.1558 | 0.1558 | 0.1394 | 0.1393 |
| Exp.[ | 0.1708 | 0.1510 | 0.1509 | 0.2062 | 0.1542 | 0.1524 | 0.1439 | 0.1393 |
| 3+ | 0.1737 | 0.1510 | 0.1510 | 0.2076 | 0.1562 | 0.1560 | 0.1391 | 0.1388 |
| 3- | 0.2343 | 0.1466 | 0.1471 | 0.2073 | 0.1553 | 0.1553 | 0.1399 | 0.1399 |
| 4 | 0.1759 | 0.1506 | 0.1507 | 0.2070 | 0.1558 | 0.1558 | 0.1394 | 0.1394 |
| 4+ | 0.1760 | 0.1507 | 0.1506 | 0.2076 | 0.1561 | 0.1560 | 0.1391 | 0.1389 |
| 4- | 0.2325 | 0.1469 | 0.1468 | 0.2072 | 0.1553 | 0.1553 | 0.1398 | 0.1398 |
| Compound | Functional | βx/a.u. | βy/a.u. | βz/a.u. | βtot/a.u. |
|---|---|---|---|---|---|
| 1 | ωB97XD | -1007.03 | 0 | 0 | 1007.03 |
| M06-2X | -1608.93 | 0 | 0 | 1608.93 | |
| 2 | ωB97XD | 138.90 | -11.58 | -46.30 | 150.48 |
| m06-2X | -243.08 | -23.15 | -46.30 | 243.08 | |
| 3 | ωB97XD | -300.95 | 2754.85 | -347.25 | 2801.15 |
| M06-2X | -601.90 | 2963.20 | -381.98 | 3044.23 | |
| 4 | ωB97XD | -219.93 | 3842.90 | 0 | 3854.48 |
| M06-2X | -578.75 | 4305.90 | 0 | 4340.63 |
Table 2 Computed static first hyperpolarizability for compounds 1—4 employing the 6-31G*/LANL2DZ basis set
| Compound | Functional | βx/a.u. | βy/a.u. | βz/a.u. | βtot/a.u. |
|---|---|---|---|---|---|
| 1 | ωB97XD | -1007.03 | 0 | 0 | 1007.03 |
| M06-2X | -1608.93 | 0 | 0 | 1608.93 | |
| 2 | ωB97XD | 138.90 | -11.58 | -46.30 | 150.48 |
| m06-2X | -243.08 | -23.15 | -46.30 | 243.08 | |
| 3 | ωB97XD | -300.95 | 2754.85 | -347.25 | 2801.15 |
| M06-2X | -601.90 | 2963.20 | -381.98 | 3044.23 | |
| 4 | ωB97XD | -219.93 | 3842.90 | 0 | 3854.48 |
| M06-2X | -578.75 | 4305.90 | 0 | 4340.63 |
| Compound | λ/nm | Egm/eV | fos | Main transitiona |
|---|---|---|---|---|
| 1 | 553.6(506)b | 2.24 | 0.1087 | H-1→L+2(32%), H→L+1(67%) |
| 420.0(424)b | 2.95 | 2.0288 | H-1→L+2(45%), H→L+1(23%), H→L+3(25%) | |
| 2 | 548.7 | 2.26 | 0.0346 | H-1→L+1(37%), H→L+2(63%) |
| 404.9 | 3.06 | 1.9836 | H-1→L+2(63%), H→L+1(34%) | |
| 3 | 551.4(506)b,(548.6)c | 2.25 | 0.0610 | H-1→L+2(34%), H→L+1(65%) |
| 408.8(424)b,(385)c | 3.03 | 1.6190 | H-1→L+2(56%), H→L+1(30%), H→L+3(11%) | |
| 4 | 552.1 | 2.25 | 0.0774 | H-1→L+2(34%), H→L+1(66%) |
| 414.7 | 2.99 | 1.3802 | H-1→L+2(39%), H→L+1(21%), H→L+3(33%) |
Table 3 Detailed TD-DFT calculations for compounds 1—4 employing the 6-31G*/LANL2DZ basis set
| Compound | λ/nm | Egm/eV | fos | Main transitiona |
|---|---|---|---|---|
| 1 | 553.6(506)b | 2.24 | 0.1087 | H-1→L+2(32%), H→L+1(67%) |
| 420.0(424)b | 2.95 | 2.0288 | H-1→L+2(45%), H→L+1(23%), H→L+3(25%) | |
| 2 | 548.7 | 2.26 | 0.0346 | H-1→L+1(37%), H→L+2(63%) |
| 404.9 | 3.06 | 1.9836 | H-1→L+2(63%), H→L+1(34%) | |
| 3 | 551.4(506)b,(548.6)c | 2.25 | 0.0610 | H-1→L+2(34%), H→L+1(65%) |
| 408.8(424)b,(385)c | 3.03 | 1.6190 | H-1→L+2(56%), H→L+1(30%), H→L+3(11%) | |
| 4 | 552.1 | 2.25 | 0.0774 | H-1→L+2(34%), H→L+1(66%) |
| 414.7 | 2.99 | 1.3802 | H-1→L+2(39%), H→L+1(21%), H→L+3(33%) |
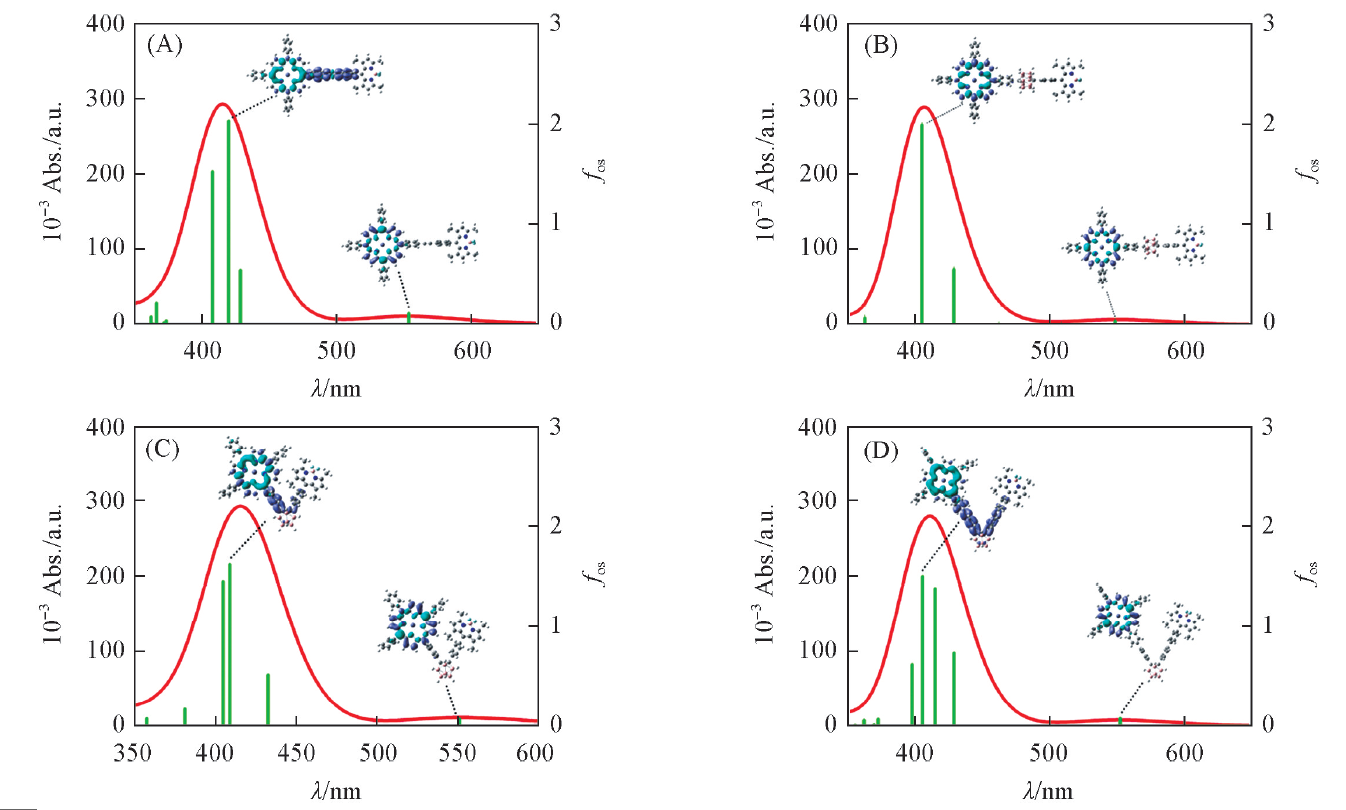
Fig.2 UV-Vis spectra of the compounds 1(A), 2(B), 3(C) and 4(D) along with electron density difference maps(EDDM) of corresponding transitionsPurple and blue colors indicate accumulation and depletion of electron density, respectively.
| Compound | NBO charge/e | Compound | NBO charge/e | ||||
|---|---|---|---|---|---|---|---|
| Porphyrin | BODIPY | Ethynel/carborane | Porphyrin | BODIPY | Ethynel/carborane | ||
| 1 | 0.001 | -0.021 | 0.020 | 3 | 0.121 | 0.103 | -0.224 |
| 1+ | 0.719 | 0.218 | 0.039 | 3+ | 0.951 | 0.241 | -0.193 |
| 1- | -0.521 | -0.475 | -0.004 | 3- | -0.136 | -0.233 | -0.789 |
| 2 | 0.097 | 0.087 | -0.183 | 4 | 0.120 | 0.114 | -0.234 |
| 2+ | 0.827 | 0.339 | -0.166 | 4+ | 0.765 | 0.216 | 0.020 |
| 2- | -0.424 | -0.367 | -0.209 | 4- | -0.162 | -0.166 | -0.672 |
Table 4 NBO charge of all the complexes employing the 6-31G*/LANL2DZ basis set
| Compound | NBO charge/e | Compound | NBO charge/e | ||||
|---|---|---|---|---|---|---|---|
| Porphyrin | BODIPY | Ethynel/carborane | Porphyrin | BODIPY | Ethynel/carborane | ||
| 1 | 0.001 | -0.021 | 0.020 | 3 | 0.121 | 0.103 | -0.224 |
| 1+ | 0.719 | 0.218 | 0.039 | 3+ | 0.951 | 0.241 | -0.193 |
| 1- | -0.521 | -0.475 | -0.004 | 3- | -0.136 | -0.233 | -0.789 |
| 2 | 0.097 | 0.087 | -0.183 | 4 | 0.120 | 0.114 | -0.234 |
| 2+ | 0.827 | 0.339 | -0.166 | 4+ | 0.765 | 0.216 | 0.020 |
| 2- | -0.424 | -0.367 | -0.209 | 4- | -0.162 | -0.166 | -0.672 |
| Compound | βx/a.u. | βy/a.u. | βz/a.u. | βtot/a.u. |
|---|---|---|---|---|
| 1+ | 63755.10 | -752.38 | 254.65 | 63766.68 |
| 1- | -1624111.40 | 48094.13 | 10255.45 | 1624852.20 |
| 2+ | 7280.68 | -57.88 | 46.30 | 7280.68 |
| 2- | -46566.23 | 393.55 | -1250.10 | 46577.80 |
| 3+ | -706.08 | 1666.82 | -312.52 | 1840.43 |
| 3- | 59564.95 | -85770.75 | 69.45 | 104429.65 |
| 4+ | 10984.68 | -10903.65 | -231.50 | 15475.78 |
| 4- | 22524.95 | -73489.68 | -567.18 | 76858.00 |
Table 5 Computed static first hyperpolarizability for oxidized and reduced of compounds 1—4 employing the 6-31G*/LANL2DZ basis set
| Compound | βx/a.u. | βy/a.u. | βz/a.u. | βtot/a.u. |
|---|---|---|---|---|
| 1+ | 63755.10 | -752.38 | 254.65 | 63766.68 |
| 1- | -1624111.40 | 48094.13 | 10255.45 | 1624852.20 |
| 2+ | 7280.68 | -57.88 | 46.30 | 7280.68 |
| 2- | -46566.23 | 393.55 | -1250.10 | 46577.80 |
| 3+ | -706.08 | 1666.82 | -312.52 | 1840.43 |
| 3- | 59564.95 | -85770.75 | 69.45 | 104429.65 |
| 4+ | 10984.68 | -10903.65 | -231.50 | 15475.78 |
| 4- | 22524.95 | -73489.68 | -567.18 | 76858.00 |
| Compound | ω=0.0239 a.u. | ω=0.0340 a.u. | ||
|---|---|---|---|---|
| β(-ω; ω,0) | β(-2ω; ω,ω) | β(-ω; ω,0) | β(-2ω; ω,ω) | |
| 1 | 1030.18 | 1284.83 | 1157.50 | 2002.48 |
| 2 | 138.90 | 104.18 | 127.33 | 150.48 |
| 3 | 2963.20 | 3379.90 | 3159.98 | 4363.78 |
| 4 | 4085.98 | 4606.85 | 4340.63 | 5787.50 |
Table 6 Estimated values of β(-ω; ω,0) and β(-2ω; ω,ω) at specific frequencies calculated at 6-31G*/LANL2DZ level
| Compound | ω=0.0239 a.u. | ω=0.0340 a.u. | ||
|---|---|---|---|---|
| β(-ω; ω,0) | β(-2ω; ω,ω) | β(-ω; ω,0) | β(-2ω; ω,ω) | |
| 1 | 1030.18 | 1284.83 | 1157.50 | 2002.48 |
| 2 | 138.90 | 104.18 | 127.33 | 150.48 |
| 3 | 2963.20 | 3379.90 | 3159.98 | 4363.78 |
| 4 | 4085.98 | 4606.85 | 4340.63 | 5787.50 |
| [1] | Cheng W. D., Xiang K. H., Pandey R., Pernisz U. C., J. Phys. Chem. B,2000, 104(29), 6737—6742 |
| [2] | Zhang C. C., Xu H. L., Hu Y. Y., Sun S. L., Su Z. M., J. Phys. Chem. A,2011, 115(10), 2035—2040 |
| [3] | Kim H. M., Cho B. R., J. Mater. Chem.,2009, 19(40), 7402—7409 |
| [4] | Tykwinski R. R., Gubler U., Martin R. E., Diederich F., Bosshard C., Günter P., J. Phys. Chem. B,1998, 102(23), 4451—4465 |
| [5] | Yu Y. Z, Cao J. P., Xu Y., Chem. Res. Chinese Universities,2019, 35(1), 1—4 |
| [6] | Zhang F. Y., Xu H. L., Su Z. M., Org. Electron.,2018, 57, 68—73 |
| [7] | Amudha K., Latha Mageshwari P. S., Mohan Kumar R., Umarani P. R., Mater. Lett.,2018, 223, 33—36 |
| [8] | Wu J., Wang H. Q., Liu X. Y., Shi Z. Y., Qiu Y. Q., Chem. J. Chinese Universities,2018, 39(7), 1490—1496 |
| (吴娟, 王洪强, 刘晓云, 史志圆, 仇永清. 高等学校化学学报,2018, 39(7), 1490—1496) | |
| [9] | Li X., Wang H. Y., Wang H. Q., Ye J. T., Qiu Y. Q., Chem. J. Chinese Universities,2018, 39(10), 2221—2229 |
| (李想, 王慧莹, 王洪强, 叶近婷, 仇永清. 高等学校化学学报,2018, 39(10), 2221—2229) | |
| [10] | Oudar J. L., Chemla D. S., J. Chem. Phys.,1977, 66(6), 2664—2668 |
| [11] | Wu K. C., Chen C. T., Appl. Phys. A,1992, 54(3), 209—220 |
| [12] | Gao F. W., Xu H. L., Muhammad S., Su Z. M., Phys. Chem. Chem. Phys.,2018, 20, 18699—18706 |
| [13] | Fukui H., Shigeta Y., Nakano M., Kubo T., Kamada K., Ohta K., Champagne B., Botek E., J. Phys. Chem. A,2011, 115(6), 1117—1124 |
| [14] | Wang L., Wang W. Y., Fang X. Y., Zhu C. L., Qiu Y. Q., Org. Electron.,2016, 33, 290—299 |
| [15] | Ye J. T., Wang L., Wang H. Q., Pan X. M., Xie H. M., Qiu Y. Q., New J. Chem.,2018, 42, 6091—6100 |
| [16] | Bharati M. S. S., Bhattacharya S., Suman Krishna J. V., Giribabu L., Venugopal Rao S., Opt. Laser Technol.,2018, 108, 418—425 |
| [17] | Hu Z. B., Sun Z. R., Sun H. T., Int. J. Quantum Chem.,2018, 118(10), 25536 |
| [18] | Zarate X., Schott E., Gomez T., Arratia-Pérez R., J. Phys. Chem. A,2013, 117(2), 430—438 |
| [19] | Guo X. M., Guo B., Chem. Res. Chinese Universities,2017, 33(4), 530—533 |
| [20] | Jin G. F., Cho Y. J., Wee K. R., Hong S. A., Suh I. H., Son H. J., Lee J. D., Han W. S., Cho D. W., Kang S. O., Dalton Trans., 2015, 44(6), 2780—2787 |
| [21] | Sun T. T., Guan X. G., Zheng M., Jing X. B., Xie Z. G., ACS Med. Chem. Lett., 2015, 6(4), 430—433 |
| [22] | Filarowski A., Lopatkova M., Lipkowski P., Van der Auweraer M., Leen V., Dehaen W., J. Phys. Chem. B,2015, 119(6), 2576—2584 |
| [23] | Li R. R., Wang H. Q., Wang L., Wu J., Qiu Y. Q., Chem. J. Chinese Universities,2017, 38(10), 1796—1803 |
| (李荣荣, 王洪强, 王丽, 吴娟, 仇永清. 高等学校化学学报,2017, 38(10), 1796—1803) | |
| [24] | Wang H. Q., Wang W. Y., Fang X. Y., Wang L., Zhu C. L., Chen Z. Z., Chen H., Qiu Y. Q., J. Mol. Graph. Modell., 2016, 67, 111—118 |
| [25] | Berksun E., Nar I., Atsay A., Özçeşmeci I., Gelir A., Hamuryudan E., Inorg. Chem. Front.,2018, 5(1), 200—207 |
| [26] | Ma N. N., Liu C. G., Qiu Y. Q., Sun S. L., Su Z. M., J. Comput. Chem.,2012, 33, 211—219 |
| [27] | Misra R., J. Phys. Chem. C, 2017, 121, 5731—5739 |
| [28] | Wang H. Q., Wang L., Xia Y. Y., Ye J. T., Zhao H. Y., Qiu Y. Q., J. Phys. Chem. C,2017, 121(30), 16470—16480 |
| [29] | Becke A. D., J. Chem. Phys., 1993, 98(7), 5648—5652 |
| [30] | Miertu$\widetilde{s}$ S., Tomasi J., Chem. Phys., 1982, 65(2), 239—245 |
| [31] | Bonaccorsi R., Cimiraglia R., Tomasi J., Chem. Phys. Lett.,1983, 99(1), 77—82 |
| [32] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J. Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Dapprich S., Daniela A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision D.01, Gaussian Inc., Wallingford CT, 2013 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [3] | ZHENG Xuelian, YANG Cuicui, TIAN Weiquan. The Second Order Nonlinear Optical Properties of Azulene-defect Graphene Nanosheets with Full Armchair Edge [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210806. |
| [4] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [5] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [6] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [7] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [8] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [9] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [10] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [11] | ZHUO Zengqing, PAN Feng. Progress of Key Electronic States in Lithium Ion Battery Materials Probed Through Soft X-ray Spectroscopy [J]. Chem. J. Chinese Universities, 2021, 42(8): 2332. |
| [12] | LIU Yang, LI Qingbo, SUN Jie, ZHAO Xian. Direct Synthesis of Graphene on AlN Substrates via Ga Remote Catalyzation [J]. Chem. J. Chinese Universities, 2021, 42(7): 2271. |
| [13] | ZHENG Ruoxin, ZHANG Igor Ying, XU Xin. Development and Benchmark of Lower Scaling Doubly Hybrid Density Functional XYG3 [J]. Chem. J. Chinese Universities, 2021, 42(7): 2210. |
| [14] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [15] | HU Wei, LIU Xiaofeng, LI Zhenyu, YANG Jinlong. Surface and Size Effects of Nitrogen-vacancy Centers in Diamond Nanowires [J]. Chem. J. Chinese Universities, 2021, 42(7): 2178. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||