

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (1): 47.doi: 10.7503/cjcu20160550
• Organic Chemistry • Previous Articles Next Articles
BAI Xinfa, MA Xuan, XIE Xiaoxia, SHAO Mingsha, GUO Ningning, YAN Ning, YAO Lei*( )
)
Received:2016-07-29
Online:2017-01-10
Published:2016-10-31
Contact:
YAO Lei
E-mail:yaoleiytu@163.com
Supported by:CLC Number:
TrendMD:
BAI Xinfa, MA Xuan, XIE Xiaoxia, SHAO Mingsha, GUO Ningning, YAN Ning, YAO Lei. Synthesis and Anti-tumor Activity of Tubulysins Analogues†[J]. Chem. J. Chinese Universities, 2017, 38(1): 47.
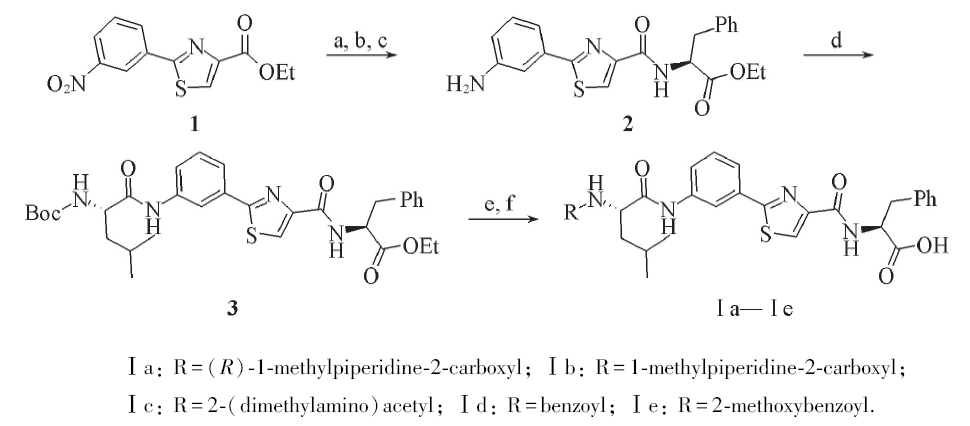
Scheme 1 Synthesis of Tubulysins derivatives Ⅰa—Ⅰea. NaOH/H+, r. t.; b. SOCl2, Et3N, L-Phe-OEt; c. H2/Pd, 35 ℃; d. EDC, HOBt, DIPEA, BOC-L-Leu, r. t.;e. EDC, HOBt, TFA, DIPEA, R-COOH, r. t.; f. LiOH/H+, r. t.
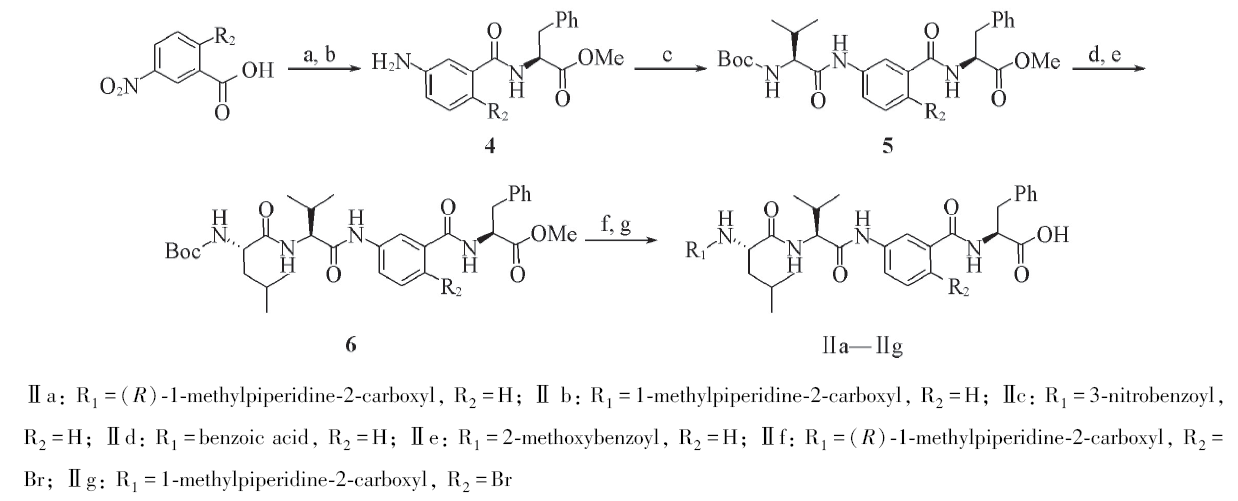
Scheme 2 Synthesis of Tubulysins derivatives Ⅱa—Ⅱga. SOCl2, Et3N, L-Phe-OMe; b. H2/Pd, 35 ℃; c. EDC, HOBt, DIPEA, Boc-L-Val, r. t.; d. TFA, r. t.;e. EDC, HOBt, DIPEA, BOC-L-Leu, r .t.; f. EDC, HOBt, TFA, DIPEA, R-COOH, r. t.; g. LiOH/H+, r. t.
| Compd. | m. p. /℃ | IR(KBr), | 1 H NMR(400 MHz, CDCl3), δ |
|---|---|---|---|
| 2 | 109.1—110.3 | 3339, 3051, 2999, 2925, 1732, 1645, 1527, 1448, 1352, 1225, 1109, 795, 760, 703, 697 | 8.79(t, J=1.9 Hz, 1H, ArH), 8.34(ddd, J=8.2, 2.2, 0.9 Hz, 1H, ArH), 8.29—8.22(m, 1H, ArH), 8.20(s, 1H, ArH), 7.84(d, J=8.2 Hz, 1H, ArH), 7.69(t, J=8.0 Hz, 1H, ArH), 7.40—7.34(m, 2H, ArH), 7.34—7.30(m, 1H, ArH), 7.27—7.23(m, 2H, ArH), 5.07(dt, J=8.2, 6.1 Hz, 1H, NH), 4.23(q, J=7.1 Hz, 2H, CH2CH3), 3.38—3.22(m, 2H, CH2Ar), 1.28( t, J=7.1 Hz, 3H, CH2CH3) |
| 3 | 89.3—90.2 | 3401, 3372, 3369, 3053, 2997, 2925, 2989, 1730, 1681, 1666, 1645, 1527, 1448, 1396, 1380, 1372, 1367, 1352, 1226, 1108, 796, 761, 699, 690 | 9.44(s, 1H, ArH), 8.49(d, J=7.6 Hz, 1H, ArH), 7.92(s, 1H, ArH), 7.84(s, 1H, ArH), 7.74(d, J=7.8 Hz, 1H, ArH), 7.36(d, J=7.3 Hz, 1H, ArH), 7.29(s, 2H, ArH), 7.26—7.21(m, 1H, ArH), 7.18(d, J=7.6 Hz, 1H, ArH), 5.75(s, 1H, NH), 5.17(dd, J=15.0, 7.2 Hz, 1H, NH), 4.53(s, 1H, CH), 4.23(q, J=7.1 Hz, 2H, CH2CH3), 3.41—3.22(m, 2H, CH2Ar), 2.02(d, J=15.8 Hz, 1H, CH), 1.82(ddd, J=25.6, 13.7, 7.0 Hz, 3H, CH2CH3), 1.46—1.36 [m, 9H,(CH3)3], 1.26(t, J=7.1 Hz, 2H, CH2), 1.03 [dd, J=13.3, 6.3 Hz, 6H,(CH3)2] |
| 6 | 92.1—93.5 | 3398, 3389, 3374, 3370, 3052, 2999, 2926, 2990, 1731, 1686, 1675, 1662, 1645, 1397, 1381, 1375, 1366, 1352, 1225, 1109, 794, 765, 698, 693 | 9.42(s, 1H, ArH), 8.00(d, J=7.7 Hz, 1H, ArH), 7.92(s, 1H, ArH), 7.30(dd, J=14.6, 7.6 Hz, 4H, ArH), 7.25(d, J=7.2 Hz, 1H, NH), 7.16(d, J=6.9 Hz, 2H, ArH), 6.91(d, J=7.2 Hz, 1H, NH), 6.85(d, J=7.6 Hz, 1H, NH), 5.74(s, 1H, NH), 5.06(dd, J=13.4, 6.0 Hz, 1H, CH), 4.67—4.37(m, 1H, CH), 4.15(m, 1H, CH), 3.73(s, 3H, OMe), 3.25(qd, J=13.9, 5.9 Hz, 2H, CH2Ar), 2.25(m, 1H, CH), 1.74(m, 1H, CH), 1.62(dd, J=9.4, 4.9 Hz, 2H, CH2), 1.37 [s, 9H,(CH3)3], 1.00 [d, J=6.7 Hz, 6H,(CH3)2], 0.91 [d, J=6.5 Hz, 6H,(CH3)2] |
Table 1 Melting points, IR and 1H NMR data of intermediates 2, 3 and 6
| Compd. | m. p. /℃ | IR(KBr), | 1 H NMR(400 MHz, CDCl3), δ |
|---|---|---|---|
| 2 | 109.1—110.3 | 3339, 3051, 2999, 2925, 1732, 1645, 1527, 1448, 1352, 1225, 1109, 795, 760, 703, 697 | 8.79(t, J=1.9 Hz, 1H, ArH), 8.34(ddd, J=8.2, 2.2, 0.9 Hz, 1H, ArH), 8.29—8.22(m, 1H, ArH), 8.20(s, 1H, ArH), 7.84(d, J=8.2 Hz, 1H, ArH), 7.69(t, J=8.0 Hz, 1H, ArH), 7.40—7.34(m, 2H, ArH), 7.34—7.30(m, 1H, ArH), 7.27—7.23(m, 2H, ArH), 5.07(dt, J=8.2, 6.1 Hz, 1H, NH), 4.23(q, J=7.1 Hz, 2H, CH2CH3), 3.38—3.22(m, 2H, CH2Ar), 1.28( t, J=7.1 Hz, 3H, CH2CH3) |
| 3 | 89.3—90.2 | 3401, 3372, 3369, 3053, 2997, 2925, 2989, 1730, 1681, 1666, 1645, 1527, 1448, 1396, 1380, 1372, 1367, 1352, 1226, 1108, 796, 761, 699, 690 | 9.44(s, 1H, ArH), 8.49(d, J=7.6 Hz, 1H, ArH), 7.92(s, 1H, ArH), 7.84(s, 1H, ArH), 7.74(d, J=7.8 Hz, 1H, ArH), 7.36(d, J=7.3 Hz, 1H, ArH), 7.29(s, 2H, ArH), 7.26—7.21(m, 1H, ArH), 7.18(d, J=7.6 Hz, 1H, ArH), 5.75(s, 1H, NH), 5.17(dd, J=15.0, 7.2 Hz, 1H, NH), 4.53(s, 1H, CH), 4.23(q, J=7.1 Hz, 2H, CH2CH3), 3.41—3.22(m, 2H, CH2Ar), 2.02(d, J=15.8 Hz, 1H, CH), 1.82(ddd, J=25.6, 13.7, 7.0 Hz, 3H, CH2CH3), 1.46—1.36 [m, 9H,(CH3)3], 1.26(t, J=7.1 Hz, 2H, CH2), 1.03 [dd, J=13.3, 6.3 Hz, 6H,(CH3)2] |
| 6 | 92.1—93.5 | 3398, 3389, 3374, 3370, 3052, 2999, 2926, 2990, 1731, 1686, 1675, 1662, 1645, 1397, 1381, 1375, 1366, 1352, 1225, 1109, 794, 765, 698, 693 | 9.42(s, 1H, ArH), 8.00(d, J=7.7 Hz, 1H, ArH), 7.92(s, 1H, ArH), 7.30(dd, J=14.6, 7.6 Hz, 4H, ArH), 7.25(d, J=7.2 Hz, 1H, NH), 7.16(d, J=6.9 Hz, 2H, ArH), 6.91(d, J=7.2 Hz, 1H, NH), 6.85(d, J=7.6 Hz, 1H, NH), 5.74(s, 1H, NH), 5.06(dd, J=13.4, 6.0 Hz, 1H, CH), 4.67—4.37(m, 1H, CH), 4.15(m, 1H, CH), 3.73(s, 3H, OMe), 3.25(qd, J=13.9, 5.9 Hz, 2H, CH2Ar), 2.25(m, 1H, CH), 1.74(m, 1H, CH), 1.62(dd, J=9.4, 4.9 Hz, 2H, CH2), 1.37 [s, 9H,(CH3)3], 1.00 [d, J=6.7 Hz, 6H,(CH3)2], 0.91 [d, J=6.5 Hz, 6H,(CH3)2] |
| Compd. | m. p. /℃ | Yield (%) | (c 0.20, CH3OH) | HRMS,m/z [M+1]+ | IR(KBr), | |||
|---|---|---|---|---|---|---|---|---|
| Ⅰa | 98.0—99.3 | 43 | -38.2 | 606.2755 (606.2750) | 3455, 3360, 3310, 3088, 3051, 2999, 2966, 2926, 2891, 1761, 1678, 1661, 1655, 1645, 1554, 1495, 1446, 1385, 1376, 795, 760, 703, 697 | |||
| Ⅰb | 78.6—79.5 | 45 | 606.2755 (606.2750) | 3445, 3370, 3310, 3078, 3050, 2996, 2966, 2925, 2890, 1760, 1673, 1661, 1652, 1641, 1557, 1508, 1448, 1388, 1376, 805, 766, 713, 690 | ||||
| Compd. | m. p. /℃ | Yield (%) | (c 0.20, CH3OH) | HRMS,m/z [M+1]+ | IR(KBr), | |||
| Ⅰc | 88.7—89.8 | 44 | -26.3 | 566.2434 (566.2437) | 3355, 3261, 3112, 3079, 3051, 3002, 2966, 2925, 2893, 1758, 1657, 1641, 1635, 1620, 1552, 1491, 1446, 1387, 1376, 795, 763, 705, 697 | |||
| Ⅰd | 135.5—136.4 | 47 | -28.5 | 585.2092 (585.2172) | 3452, 3350, 3291, 3067, 3045, 2988, 2969, 2927, 2890, 1759, 1674, 1658, 1643, 1625, 1550, 1495, 1437, 1385, 1371, 803, 765, 703, 688 | |||
| Ⅰe | 126.3—127.2 | 44 | -30.8 | 615.2281 (615.2277) | 3353, 3294, 3280, 3069, 3051, 3001, 2970, 2922, 2891, 1758, 1672, 1657, 1655, 1645, 1548, 1495, 1438, 1384, 1373, 800, 770, 703, 687 | |||
| Ⅱ a | 121.5—122.6 | 35 | +35.1 | 622.3599 (622.3605) | 3455, 3360, 3310, 3220, 3088, 3051, 2999, 2968, 2927, 2895, 1761, 1678, 1661, 1658, 1655, 1645, 1554, 1495, 1446, 1383, 1378, 795, 760, 703, 697 | |||
| Ⅱ b | 114.2—115.5 | 38 | 622.3599 (622.3605) | 3457, 3359, 3310, 3222, 3086, 3050, 3001, 2963, 2927, 2889, 1761, 1678, 1663, 1659, 1654, 1642, 1554, 1493, 1447, 1385, 1376, 791, 770, 703, 687 | ||||
| Ⅱ c | 105.5—106.2 | 36 | -32.2 | 646.2880 (646.2877) | 3433, 3362, 3298, 3226, 3099, 3046, 3005, 2966, 2930, 2890, 1759, 1666, 1660, 1652, 1646, 1638, 1556, 1525, 1491, 1445, 1389, 1372, 791, 776, 701, 689 | |||
| Ⅱ d | 142.1—143.5 | 39 | -35.3 | 601.3029 (601.3026) | 3455, 3346, 3332, 3202, 3096, 3040, 2998, 2973, 2929, 2888, 1765, 1672, 1669, 1657, 1650, 1641, 1558, 1491, 1445, 1389, 1376, 796, 771, 700, 691 | |||
| Ⅱ e | 95.3—96.5 | 40 | -36.7 | 631.3136 (631.3132) | 3448, 3344, 3308, 3231, 3068, 3048, 3000, 2966, 2928, 2891, 1762, 1675, 1660, 1656, 1653, 1647, 1552, 1491, 1445, 1385, 1371, 792, 776, 701, 689 | |||
| Ⅱ f | 108.2—109.4 | 37 | -38.1 | 700.2631 (700.2710) | 3436, 3346, 3229, 3210, 3069, 3038, 3002, 2969, 2926, 2890, 1765, 1677, 1661, 1656, 1649, 1640, 1551, 1490, 1445, 1386, 1379, 795, 774, 700, 678 | |||
| Ⅱ g | 103.5—104.5 | 36 | 700.2631 (700.2710) | 3440, 3398, 3329, 3237, 3076, 3049, 2997, 2969, 2922, 2888, 1760, 1673, 1665, 1651, 1648, 1640, 1550, 1497, 1442, 1387, 1374, 789, 768, 699, 678 | ||||
Table 2 Melting points, yield, optical rotation, HRMS and IR data for target compounds Ⅰand Ⅱ
| Compd. | m. p. /℃ | Yield (%) | (c 0.20, CH3OH) | HRMS,m/z [M+1]+ | IR(KBr), | |||
|---|---|---|---|---|---|---|---|---|
| Ⅰa | 98.0—99.3 | 43 | -38.2 | 606.2755 (606.2750) | 3455, 3360, 3310, 3088, 3051, 2999, 2966, 2926, 2891, 1761, 1678, 1661, 1655, 1645, 1554, 1495, 1446, 1385, 1376, 795, 760, 703, 697 | |||
| Ⅰb | 78.6—79.5 | 45 | 606.2755 (606.2750) | 3445, 3370, 3310, 3078, 3050, 2996, 2966, 2925, 2890, 1760, 1673, 1661, 1652, 1641, 1557, 1508, 1448, 1388, 1376, 805, 766, 713, 690 | ||||
| Compd. | m. p. /℃ | Yield (%) | (c 0.20, CH3OH) | HRMS,m/z [M+1]+ | IR(KBr), | |||
| Ⅰc | 88.7—89.8 | 44 | -26.3 | 566.2434 (566.2437) | 3355, 3261, 3112, 3079, 3051, 3002, 2966, 2925, 2893, 1758, 1657, 1641, 1635, 1620, 1552, 1491, 1446, 1387, 1376, 795, 763, 705, 697 | |||
| Ⅰd | 135.5—136.4 | 47 | -28.5 | 585.2092 (585.2172) | 3452, 3350, 3291, 3067, 3045, 2988, 2969, 2927, 2890, 1759, 1674, 1658, 1643, 1625, 1550, 1495, 1437, 1385, 1371, 803, 765, 703, 688 | |||
| Ⅰe | 126.3—127.2 | 44 | -30.8 | 615.2281 (615.2277) | 3353, 3294, 3280, 3069, 3051, 3001, 2970, 2922, 2891, 1758, 1672, 1657, 1655, 1645, 1548, 1495, 1438, 1384, 1373, 800, 770, 703, 687 | |||
| Ⅱ a | 121.5—122.6 | 35 | +35.1 | 622.3599 (622.3605) | 3455, 3360, 3310, 3220, 3088, 3051, 2999, 2968, 2927, 2895, 1761, 1678, 1661, 1658, 1655, 1645, 1554, 1495, 1446, 1383, 1378, 795, 760, 703, 697 | |||
| Ⅱ b | 114.2—115.5 | 38 | 622.3599 (622.3605) | 3457, 3359, 3310, 3222, 3086, 3050, 3001, 2963, 2927, 2889, 1761, 1678, 1663, 1659, 1654, 1642, 1554, 1493, 1447, 1385, 1376, 791, 770, 703, 687 | ||||
| Ⅱ c | 105.5—106.2 | 36 | -32.2 | 646.2880 (646.2877) | 3433, 3362, 3298, 3226, 3099, 3046, 3005, 2966, 2930, 2890, 1759, 1666, 1660, 1652, 1646, 1638, 1556, 1525, 1491, 1445, 1389, 1372, 791, 776, 701, 689 | |||
| Ⅱ d | 142.1—143.5 | 39 | -35.3 | 601.3029 (601.3026) | 3455, 3346, 3332, 3202, 3096, 3040, 2998, 2973, 2929, 2888, 1765, 1672, 1669, 1657, 1650, 1641, 1558, 1491, 1445, 1389, 1376, 796, 771, 700, 691 | |||
| Ⅱ e | 95.3—96.5 | 40 | -36.7 | 631.3136 (631.3132) | 3448, 3344, 3308, 3231, 3068, 3048, 3000, 2966, 2928, 2891, 1762, 1675, 1660, 1656, 1653, 1647, 1552, 1491, 1445, 1385, 1371, 792, 776, 701, 689 | |||
| Ⅱ f | 108.2—109.4 | 37 | -38.1 | 700.2631 (700.2710) | 3436, 3346, 3229, 3210, 3069, 3038, 3002, 2969, 2926, 2890, 1765, 1677, 1661, 1656, 1649, 1640, 1551, 1490, 1445, 1386, 1379, 795, 774, 700, 678 | |||
| Ⅱ g | 103.5—104.5 | 36 | 700.2631 (700.2710) | 3440, 3398, 3329, 3237, 3076, 3049, 2997, 2969, 2922, 2888, 1760, 1673, 1665, 1651, 1648, 1640, 1550, 1497, 1442, 1387, 1374, 789, 768, 699, 678 | ||||
| Compd. | 1H NMR(CD3OD, 400 MHz), δ | 13C NMR(CD3OD, 100 MHz), δ |
|---|---|---|
| Ⅰa | 8.19(s, 1H, ArH), 8.07(s, 1H, ArH), 7.61(dd, J=16.0, 8.0 Hz, 2H, ArH), 7.35(t, J=7.9 Hz, 1H, ArH), 7.16(dd, J=17.6, 7.2 Hz, 4H, ArH), 7.08(t, J=6.8 Hz, 1H, ArH), 4.66—4.59(m, 1H, CH), 4.45(dd, J=9.8, 4.6 Hz, 1H, CH), 3.30—3.25(m, 1H, CH2Ar), 2.97(m, 1H, CH2Ar), 2.69(s, 3H, NCH3), 1.83(m, 3H, CHCH2), 1.66 [m, 6H,(CH2)3], 1.51(d, J=12.5 Hz, 2H, CH2), 1.33—1.14(m, 1H, CH), 0.91 [dd, J=10.8, 6.1 Hz, 6H,(CH3)2] | 20.2, 22.0, 24.7, 29.7, 37.9, 40.5, 42.8, 52.6, 54.3, 55.2, 55.9, 68.4, 115.3, 117.5, 118.1, 121.8, 123.2, 126.1, 127.8, 129.3, 133.2, 137.7, 139.1, 150.1, 161.0, 161.5, 161.7, 167.9, 171.8 |
| Ⅰb | 8.29(s, 1H, ArH), 8.16(d, J=2.5 Hz, 1H, ArH), 7.71(d, J=6.6 Hz, 1H, ArH), 7.64(t, J=7.3 Hz, 1H, ArH), 7.44(t, J=7.9 Hz, 1H, ArH), 7.31—7.22(m, 4H, ArH), 7.19(d, J=6.5 Hz, 1H, ArH), 4.63(m, 1H, CH), 4.45(dd, J=9.8, 4.6 Hz, 1H, CH), 3.30—3.25(m, 1H, CH2Ar), 2.97(m, 1H, CH2Ar), 2.69(s, 3H, NCH3), 1.83(m, 3H, CHCH2), 1.66 [m, 6H,(CH2)3], 1.51(d, J=12.5 Hz, 2H, CH2), 1.33—1.14(m, 1H, CH), 0.91 [dd, J=10.8, 6.1 Hz, 6H,(CH3)2] | 20.3, 22.0, 24.7, 29.6, 37.9, 40.5, 42.8, 52.6, 54.3, 55.2, 55.9, 68.4, 115.3, 117.5, 118.2, 121.8, 123.2, 126.1, 127.8, 129.3, 133.2, 137.7, 139.1, 150.2, 161.0, 161.4, 161.7, 167.9, 171.9 |
| Compd. | 1H NMR(CD3OD, 400 MHz), δ | 13C NMR(CD3OD, 100 MHz), δ |
| Ⅰc | 8.23(d, J=1.7 Hz, 1H, ArH), 8.09(s, 1H, ArH), 7.65(d, J=7.7 Hz, 1H, ArH), 7.59(dd, J=8.2, 1.1 Hz, 1H, ArH), 7.36(t, J=8.0 Hz, 1H, ArH), 7.18(d, J=4.4 Hz, 4H, ArH), 7.11(m, 1H, ArH), 4.58—4.45(m, 1H, CH), 3.93(q, J=15.6 Hz, 1H, CH), 3.34—3.23(m, 1H, CH2Ar), 3.12(m, 1H, CH2Ar), 2.84 [s, 6H, N(CH3)2], 1.62(m, 3H, CHCH2), 1.24—1.16(m, 2H, CH2), 0.92 [t, J=6.2 Hz, 6H,(CH3)2] | 20.4, 22.0, 24.6, 36.9, 40.6, 43.0, 52.9, 57.8, 117.5, 121.9, 122.1, 123.7, 126.4, 128.1, 129.1, 132.9, 136.6, 138.9, 149.7, 161.2, 164.5, 168.1, 171.8 |
| Ⅰd | 8.17(s, 1H, ArH), 8.09(d, J=3.3 Hz, 1H, ArH), 7.85(dd, J=7.7, 1.8 Hz, 1H, ArH), 7.69(dd, J=11.0, 4.1 Hz, 2H, ArH), 7.55—7.19(m, 1H, ArH), 7.40(t, J=7.5 Hz, 1H, ArH), 7.23—7.10(m, 5H, ArH), 7.03(t, J=7.1 Hz, 2H, ArH), 6.98(t, J=7.5 Hz, 1H, ArH), 4.87—4.79(m, 2H, CH, CH), 3.32(d, J=5.1 Hz, 1H, CH2Ar), 3.21(dd, J=13.2, 7.1 Hz, 1H, CH2Ar), 1.90—1.68(m, 2H, CH2), 1.23(dd, J=13.8, 6.0 Hz, 1H, CH), 1.01 [d, J=5.2 Hz, 6H,(CH3)2] | 20.1, 22.0, 25.2, 35.8, 40.8, 55.1, 111.0, 116.9, 121.1, 121.8, 122.3, 123.5, 128.0, 129.1, 129.5, 130.2, 132.9, 133.7, 135.3, 138.2, 149.8, 158.9, 166.5, 168.4, 172.0 |
| Ⅰe | 8.36(s, 1H, ArH), 8.17(d, J=3.3 Hz, 1H, ArH), 7.96(dd, J=7.8, 1.7 Hz, 1H, ArH), 7.70(dd, J=11.7, 4.6 Hz, 2H, ArH), 7.58—7.48(m, 1H, ArH), 7.44(t, J=7.9 Hz, 1H, ArH), 7.32—7.25(m, 4H, ArH), 7.18(t, J=7.1 Hz, 2H, ArH), 7.08(t, J=7.5 Hz, 1H, ArH), 4.89—4.81(m, 2H, CH, CH), 4.01(s, 3H, OCH3), 3.38(d, J=5.1 Hz, 1H, CH2Ar), 3.25(dd, J=13.8, 7.6 Hz, 1H, CH2Ar), 1.91—1.75(m, 2H, CH2), 1.24(dd, J=13.3, 6.1 Hz, 1H, CH), 1.06 [d, J=5.8 Hz, 6H,(CH3)2] | 20.9, 22.1, 24.9, 36.9, 41.4, 53.1, 55.3, 111.6, 117.6, 120.6, 121.0, 121.9, 122.1, 123.7, 126.5, 128.1, 129.0, 129.4, 130.8, 133.1, 133.2, 136.6, 139.1, 149.6, 157.8, 166.4, 168.0, 172.1 |
| Ⅱa | 7.98(d, J=8.8 Hz, 1H, ArH), 7.72(dd, J=8.0, 7.1 Hz, 1H, ArH), 7.45(dd, J=7.7 Hz, 1.3, 1H, ArH), 7.36(td, J=7.9, 1.6 Hz, 1H, ArH), 7.31—7.19(m, 4H, ArH), 7.15(td, J=7.1, 1.5 Hz, 1H, ArH), 4.78—4.63(m, 1H, CH), 4.53—4.40(m, 1H, CH), 4.36(d, J=7.6 Hz, 1H, CH), 3.35—3.29(m, 2H, CH2Ar), 2.52(d, J=2.9 Hz, 1H, CH), 2.45(d, J=2.7 Hz, 3H, NCH3), 2.15(dd, J=13.9, 6.9 Hz, 1H, CH), 1.94(d, J=8.1 Hz, 1H, CH2), 1.81(d, J=13.0 Hz, 1H, CH2), 1.68 [tdd, J=15.2, 10.9, 4.5 Hz, 6H,(CH2)3], 0.95 [ddd, J=11.5, 8.5, 4.5 Hz, 12H,(CH3)4] | 17.2, 18.4, 20.3, 20.9, 22.0, 24.6, 29.2, 30.5, 39.9, 42.5, 46.3, 52.0, 54.9, 59.2, 67.8, 118.7, 122.4, 122.6, 125.6, 125.9, 127.7, 128.6, 129.1, 135.3, 138.0, 170.0, 170.6, 171.9, 172.1, 173.2 |
| Ⅱ b | 7.95(d, J=12.5 Hz, 1H, ArH), 7.68(dd, J=19.6, 8.2 Hz, 1H, ArH), 7.45(d, J=7.9 Hz, 1H, ArH), 7.37(t, J=7.9 Hz, 1H, ArH), 7.24(q, J=7.2 Hz, 3H, ArH), 7.15(t, J=6.6 Hz, 1H, ArH), 4.60—4.47(m, 1H, CH), 4.48—4.37(m, 1H, CH), 4.38—4.26(m, 1H, CH), 3.50(d, J=9.6 Hz, 1H, CH2Ar), 3.13(s, 1H, CH2Ar), 2.94—2.72(m, 1H, CH), 2.15(ddd, J=40.8, 24.7, 14.9 Hz, 2H, CH2), 1.86(dd, J=26.9, 15.5 Hz, 2H, CH2), 1.69 [dt, J=13.8, 9.6 Hz, 4H,(CH2)2], 1.10—0.84 [m, 12H,(CH3)4] | 17.2, 18.4, 20.2, 21.1, 22.0, 24.6, 29.3, 30.9, 40.1, 42.5, 45.9, 52.1, 55.0, 59.2, 67.8, 118.8, 122.4, 122.7, 125.6, 125.9, 127.7, 128.6, 129.3, 135.4, 138.0, 170.1, 170.3, 171.9, 174.4, 174.9 |
| Ⅱc | 8.63(t, J=1.9 Hz, 1H, ArH), 8.33—8.25(m, 1H, ArH), 8.15(d, J=7.8 Hz, 1H, ArH), 7.63(d, J=8.0 Hz, 1H, ArH), 7.61—7.54(m, 1H, ArH), 7.34(d, J=7.8 Hz, 1H, ArH), 7.27(t, J=7.9 Hz, 1H, ArH), 7.22—7.13(m, 4H, ArH), 7.12—7.05(m, 1H, ArH), 4.73(dd, J=9.5, 4.9 Hz, 1H, CH), 4.65(dd, J=9.4, 4.3 Hz, 1H, CH), 4.26(t, J=6.6 Hz, 1H, CH), 3.30(s, 1H, CH2Ar), 3.13—2.93(m, 1H, CH2Ar), 2.07(dd, J=13.2, 6.7 Hz, 1H, CH), 1.64 [ddd, J=26.5, 10.3, 5.4 Hz, 3H, CHCH2], 0.99—0.81 [m, 12H,(CH3)4] | 20.5, 22.0, 24.8, 30.9, 36.8, 40.1, 52.7, 54.3, 59.5, 118.9, 122.1, 122.7, 123.0, 125.8, 126.3, 128.1, 128.6, 128.8, 129.6, 133.2, 134.8, 135.5, 137.2, 138.1, 148.2, 166.5, 168.5, 170.9, 173.4, 173.6 |
| Compd. | 1H NMR(CD3OD, 400 MHz), δ | 13C NMR(CD3OD, 100 MHz), δ |
| Ⅱd | 7.92—7.83(m, 1H, ArH), 7.76(d, J=7.4 Hz, 2H, ArH), 7.57(d, J=5.3 Hz, 1H, ArH), 7.43(s, 1H, ArH), 7.34(dd, J=14.3, 7.0 Hz, 3H, ArH), 7.23(t, J=7.8 Hz, 1H, ArH), 7.14(dd, J=14.2, 7.4 Hz, 4H, ArH), 7.03(t, J=7.0 Hz, 1H, ArH), 4.63(d, J=9.8 Hz, 2H, CH), 4.27(d, J=7.4 Hz, 1H, CH), 3.00(dd, J=13.7, 8.3 Hz, 1H, CH2Ar), 2.06(dd, J=13.6, 6.8 Hz, 1H, CH), 1.76—1.52(m, 3H, CHCH2), 0.95—0.81 [m, 12H,(CH3)4] | 17.3, 17.9, 18.1, 18.4, 30.5, 37.8, 55.6, 58.9, 59.4, 117.1, 125.8, 127.0, 127.6, 128.1, 128.8, 129.3, 131.3, 134.1, 134.7, 135.7, 137.6, 143.6, 144.3, 161.0, 169.0, 170.5, 171.3, 172.8 |
| Ⅱe | 8.06(d, J=6.2 Hz, 1H, ArH), 8.03—7.95(m, 1H, ArH), 7.85—7.77(m, 1H, ArH), 7.66—7.59(m, 1H, ArH), 7.56(d, J=7.8 Hz, 1H, ArH), 7.49(t, J=7.8 Hz, 1H, ArH), 7.44—7.34(m, 4H, ArH), 7.34—7.24(m, 2H, ArH), 7.17(t, J=7.5 Hz, 1H, ArH), 4.93(dd, J=9.3, 4.6 Hz, 1H, CH), 4.86(t, J=7.1 Hz, 1H, CH), 4.46(d, J=7.5 Hz, 1H, CH), 4.10(s, 3H, OCH3), 3.47(d, J=4.8 Hz, 1H, CH2Ar), 3.22(dd, J=13.8, 9.4 Hz, 1H, CH2Ar), 2.34—2.24(m, 1H, CH), 1.89—1.77(m, 3H, CHCH2), 1.13 [ddd, J=18.5,8.7, 4.4 Hz, 12H,(CH3)4] | 19.3, 20.7, 22.0, 24.7, 30.8, 36.8, 40.8, 52.3, 55.1, 59.3, 111.5, 118.9, 120.5, 121.1, 122.7, 123.0, 126.3, 128.0, 128.6, 128.9, 130.6, 132.9, 134.9, 137.4, 138.2, 139.5, 157.6, 166.7, 170.7, 171.5, 173.5 |
| Ⅱf | 7.73(s, 1H, ArH), 7.40(d, J=8.6 Hz, 1H, ArH), 7.32(d, J=7.4 Hz, 2H, ArH), 7.21(t, J=7.6 Hz, 2H, ArH), 7.14(s, 1H, ArH), 7.00(t, J=7.3 Hz, 1H, ArH), 4.64(dd, J=9.5, 4.0 Hz, 1H, CH), 4.33(d, J=8.1 Hz, 1H, CH), 4.28—4.19(m, 1H, CH), 3.35(d, J=9.3 Hz, 2H, CH2Ar), 2.91(dd, J=13.3, 9.6 Hz, 1H, CH), 2.14(d, J=7.3 Hz, 1H, CH), 1.82—1.69 [m, 4H,(CH2)2], 1.70—1.48 [m, 4H,(CH2)2], 1.40(d, J=11.1 Hz, 3H, CHCH2), 1.02—0.64 [m, 12H,(CH3)4] | 18.9, 21.1, 22.5, 24.4, 25.1, 25.6, 31.2, 36.6, 41.5, 41.8, 56.3, 56.8, 59.7, 62.1, 70.9, 114.5, 119.3, 125.6, 125.9, 127.6, 128.9, 131.9, 136.8, 137.0, 140.3, 167.4, 171.1, 172.4, 173.1, 174.9 |
| Ⅱg | 7.73(s, 1H, ArH), 7.40(d, J=8.6 Hz, 1H, ArH), 7.32(d, J=7.4 Hz, 2H, ArH), 7.21(t, J=7.6 Hz, 2H, ArH), 7.14(s, 1H, ArH), 7.00(t, J=7.3 Hz, 1H, ArH), 4.64(dd, J=9.5, 4.0 Hz, 1H, CH), 4.33(d, J=8.1 Hz, 1H, CH), 4.28—4.19(m, 1H, CH), 3.35(d, J=9.3 Hz, 2H, CH2Ar), 2.91(dd, J=13.3, 9.6 Hz, 1H, CH), 2.14(d, J=7.3 Hz, 1H, CH), 1.82—1.69 [m, 4H,(CH2)2], 1.70—1.48 [m, 4H,(CH2)2], 1.40(d, J=11.1 Hz, 3H, CHCH2), 1.02—0.64 [m, 12H,(CH3)4] | 18.8, 21.1, 22.6, 24.4, 25.1, 25.6, 31.2, 36.6, 41.5, 41.9, 56.3, 56.8, 59.7, 62.0, 70.9, 114.5, 119.5, 125.6, 125.9, 127.4, 128.9, 131.9, 136.8, 137.0, 140.3, 167.9, 171.1, 172.1, 173.6, 174.9 |
Table 3 1H NMR and 13C NMR data of target compounds Ⅰ and Ⅱ
| Compd. | 1H NMR(CD3OD, 400 MHz), δ | 13C NMR(CD3OD, 100 MHz), δ |
|---|---|---|
| Ⅰa | 8.19(s, 1H, ArH), 8.07(s, 1H, ArH), 7.61(dd, J=16.0, 8.0 Hz, 2H, ArH), 7.35(t, J=7.9 Hz, 1H, ArH), 7.16(dd, J=17.6, 7.2 Hz, 4H, ArH), 7.08(t, J=6.8 Hz, 1H, ArH), 4.66—4.59(m, 1H, CH), 4.45(dd, J=9.8, 4.6 Hz, 1H, CH), 3.30—3.25(m, 1H, CH2Ar), 2.97(m, 1H, CH2Ar), 2.69(s, 3H, NCH3), 1.83(m, 3H, CHCH2), 1.66 [m, 6H,(CH2)3], 1.51(d, J=12.5 Hz, 2H, CH2), 1.33—1.14(m, 1H, CH), 0.91 [dd, J=10.8, 6.1 Hz, 6H,(CH3)2] | 20.2, 22.0, 24.7, 29.7, 37.9, 40.5, 42.8, 52.6, 54.3, 55.2, 55.9, 68.4, 115.3, 117.5, 118.1, 121.8, 123.2, 126.1, 127.8, 129.3, 133.2, 137.7, 139.1, 150.1, 161.0, 161.5, 161.7, 167.9, 171.8 |
| Ⅰb | 8.29(s, 1H, ArH), 8.16(d, J=2.5 Hz, 1H, ArH), 7.71(d, J=6.6 Hz, 1H, ArH), 7.64(t, J=7.3 Hz, 1H, ArH), 7.44(t, J=7.9 Hz, 1H, ArH), 7.31—7.22(m, 4H, ArH), 7.19(d, J=6.5 Hz, 1H, ArH), 4.63(m, 1H, CH), 4.45(dd, J=9.8, 4.6 Hz, 1H, CH), 3.30—3.25(m, 1H, CH2Ar), 2.97(m, 1H, CH2Ar), 2.69(s, 3H, NCH3), 1.83(m, 3H, CHCH2), 1.66 [m, 6H,(CH2)3], 1.51(d, J=12.5 Hz, 2H, CH2), 1.33—1.14(m, 1H, CH), 0.91 [dd, J=10.8, 6.1 Hz, 6H,(CH3)2] | 20.3, 22.0, 24.7, 29.6, 37.9, 40.5, 42.8, 52.6, 54.3, 55.2, 55.9, 68.4, 115.3, 117.5, 118.2, 121.8, 123.2, 126.1, 127.8, 129.3, 133.2, 137.7, 139.1, 150.2, 161.0, 161.4, 161.7, 167.9, 171.9 |
| Compd. | 1H NMR(CD3OD, 400 MHz), δ | 13C NMR(CD3OD, 100 MHz), δ |
| Ⅰc | 8.23(d, J=1.7 Hz, 1H, ArH), 8.09(s, 1H, ArH), 7.65(d, J=7.7 Hz, 1H, ArH), 7.59(dd, J=8.2, 1.1 Hz, 1H, ArH), 7.36(t, J=8.0 Hz, 1H, ArH), 7.18(d, J=4.4 Hz, 4H, ArH), 7.11(m, 1H, ArH), 4.58—4.45(m, 1H, CH), 3.93(q, J=15.6 Hz, 1H, CH), 3.34—3.23(m, 1H, CH2Ar), 3.12(m, 1H, CH2Ar), 2.84 [s, 6H, N(CH3)2], 1.62(m, 3H, CHCH2), 1.24—1.16(m, 2H, CH2), 0.92 [t, J=6.2 Hz, 6H,(CH3)2] | 20.4, 22.0, 24.6, 36.9, 40.6, 43.0, 52.9, 57.8, 117.5, 121.9, 122.1, 123.7, 126.4, 128.1, 129.1, 132.9, 136.6, 138.9, 149.7, 161.2, 164.5, 168.1, 171.8 |
| Ⅰd | 8.17(s, 1H, ArH), 8.09(d, J=3.3 Hz, 1H, ArH), 7.85(dd, J=7.7, 1.8 Hz, 1H, ArH), 7.69(dd, J=11.0, 4.1 Hz, 2H, ArH), 7.55—7.19(m, 1H, ArH), 7.40(t, J=7.5 Hz, 1H, ArH), 7.23—7.10(m, 5H, ArH), 7.03(t, J=7.1 Hz, 2H, ArH), 6.98(t, J=7.5 Hz, 1H, ArH), 4.87—4.79(m, 2H, CH, CH), 3.32(d, J=5.1 Hz, 1H, CH2Ar), 3.21(dd, J=13.2, 7.1 Hz, 1H, CH2Ar), 1.90—1.68(m, 2H, CH2), 1.23(dd, J=13.8, 6.0 Hz, 1H, CH), 1.01 [d, J=5.2 Hz, 6H,(CH3)2] | 20.1, 22.0, 25.2, 35.8, 40.8, 55.1, 111.0, 116.9, 121.1, 121.8, 122.3, 123.5, 128.0, 129.1, 129.5, 130.2, 132.9, 133.7, 135.3, 138.2, 149.8, 158.9, 166.5, 168.4, 172.0 |
| Ⅰe | 8.36(s, 1H, ArH), 8.17(d, J=3.3 Hz, 1H, ArH), 7.96(dd, J=7.8, 1.7 Hz, 1H, ArH), 7.70(dd, J=11.7, 4.6 Hz, 2H, ArH), 7.58—7.48(m, 1H, ArH), 7.44(t, J=7.9 Hz, 1H, ArH), 7.32—7.25(m, 4H, ArH), 7.18(t, J=7.1 Hz, 2H, ArH), 7.08(t, J=7.5 Hz, 1H, ArH), 4.89—4.81(m, 2H, CH, CH), 4.01(s, 3H, OCH3), 3.38(d, J=5.1 Hz, 1H, CH2Ar), 3.25(dd, J=13.8, 7.6 Hz, 1H, CH2Ar), 1.91—1.75(m, 2H, CH2), 1.24(dd, J=13.3, 6.1 Hz, 1H, CH), 1.06 [d, J=5.8 Hz, 6H,(CH3)2] | 20.9, 22.1, 24.9, 36.9, 41.4, 53.1, 55.3, 111.6, 117.6, 120.6, 121.0, 121.9, 122.1, 123.7, 126.5, 128.1, 129.0, 129.4, 130.8, 133.1, 133.2, 136.6, 139.1, 149.6, 157.8, 166.4, 168.0, 172.1 |
| Ⅱa | 7.98(d, J=8.8 Hz, 1H, ArH), 7.72(dd, J=8.0, 7.1 Hz, 1H, ArH), 7.45(dd, J=7.7 Hz, 1.3, 1H, ArH), 7.36(td, J=7.9, 1.6 Hz, 1H, ArH), 7.31—7.19(m, 4H, ArH), 7.15(td, J=7.1, 1.5 Hz, 1H, ArH), 4.78—4.63(m, 1H, CH), 4.53—4.40(m, 1H, CH), 4.36(d, J=7.6 Hz, 1H, CH), 3.35—3.29(m, 2H, CH2Ar), 2.52(d, J=2.9 Hz, 1H, CH), 2.45(d, J=2.7 Hz, 3H, NCH3), 2.15(dd, J=13.9, 6.9 Hz, 1H, CH), 1.94(d, J=8.1 Hz, 1H, CH2), 1.81(d, J=13.0 Hz, 1H, CH2), 1.68 [tdd, J=15.2, 10.9, 4.5 Hz, 6H,(CH2)3], 0.95 [ddd, J=11.5, 8.5, 4.5 Hz, 12H,(CH3)4] | 17.2, 18.4, 20.3, 20.9, 22.0, 24.6, 29.2, 30.5, 39.9, 42.5, 46.3, 52.0, 54.9, 59.2, 67.8, 118.7, 122.4, 122.6, 125.6, 125.9, 127.7, 128.6, 129.1, 135.3, 138.0, 170.0, 170.6, 171.9, 172.1, 173.2 |
| Ⅱ b | 7.95(d, J=12.5 Hz, 1H, ArH), 7.68(dd, J=19.6, 8.2 Hz, 1H, ArH), 7.45(d, J=7.9 Hz, 1H, ArH), 7.37(t, J=7.9 Hz, 1H, ArH), 7.24(q, J=7.2 Hz, 3H, ArH), 7.15(t, J=6.6 Hz, 1H, ArH), 4.60—4.47(m, 1H, CH), 4.48—4.37(m, 1H, CH), 4.38—4.26(m, 1H, CH), 3.50(d, J=9.6 Hz, 1H, CH2Ar), 3.13(s, 1H, CH2Ar), 2.94—2.72(m, 1H, CH), 2.15(ddd, J=40.8, 24.7, 14.9 Hz, 2H, CH2), 1.86(dd, J=26.9, 15.5 Hz, 2H, CH2), 1.69 [dt, J=13.8, 9.6 Hz, 4H,(CH2)2], 1.10—0.84 [m, 12H,(CH3)4] | 17.2, 18.4, 20.2, 21.1, 22.0, 24.6, 29.3, 30.9, 40.1, 42.5, 45.9, 52.1, 55.0, 59.2, 67.8, 118.8, 122.4, 122.7, 125.6, 125.9, 127.7, 128.6, 129.3, 135.4, 138.0, 170.1, 170.3, 171.9, 174.4, 174.9 |
| Ⅱc | 8.63(t, J=1.9 Hz, 1H, ArH), 8.33—8.25(m, 1H, ArH), 8.15(d, J=7.8 Hz, 1H, ArH), 7.63(d, J=8.0 Hz, 1H, ArH), 7.61—7.54(m, 1H, ArH), 7.34(d, J=7.8 Hz, 1H, ArH), 7.27(t, J=7.9 Hz, 1H, ArH), 7.22—7.13(m, 4H, ArH), 7.12—7.05(m, 1H, ArH), 4.73(dd, J=9.5, 4.9 Hz, 1H, CH), 4.65(dd, J=9.4, 4.3 Hz, 1H, CH), 4.26(t, J=6.6 Hz, 1H, CH), 3.30(s, 1H, CH2Ar), 3.13—2.93(m, 1H, CH2Ar), 2.07(dd, J=13.2, 6.7 Hz, 1H, CH), 1.64 [ddd, J=26.5, 10.3, 5.4 Hz, 3H, CHCH2], 0.99—0.81 [m, 12H,(CH3)4] | 20.5, 22.0, 24.8, 30.9, 36.8, 40.1, 52.7, 54.3, 59.5, 118.9, 122.1, 122.7, 123.0, 125.8, 126.3, 128.1, 128.6, 128.8, 129.6, 133.2, 134.8, 135.5, 137.2, 138.1, 148.2, 166.5, 168.5, 170.9, 173.4, 173.6 |
| Compd. | 1H NMR(CD3OD, 400 MHz), δ | 13C NMR(CD3OD, 100 MHz), δ |
| Ⅱd | 7.92—7.83(m, 1H, ArH), 7.76(d, J=7.4 Hz, 2H, ArH), 7.57(d, J=5.3 Hz, 1H, ArH), 7.43(s, 1H, ArH), 7.34(dd, J=14.3, 7.0 Hz, 3H, ArH), 7.23(t, J=7.8 Hz, 1H, ArH), 7.14(dd, J=14.2, 7.4 Hz, 4H, ArH), 7.03(t, J=7.0 Hz, 1H, ArH), 4.63(d, J=9.8 Hz, 2H, CH), 4.27(d, J=7.4 Hz, 1H, CH), 3.00(dd, J=13.7, 8.3 Hz, 1H, CH2Ar), 2.06(dd, J=13.6, 6.8 Hz, 1H, CH), 1.76—1.52(m, 3H, CHCH2), 0.95—0.81 [m, 12H,(CH3)4] | 17.3, 17.9, 18.1, 18.4, 30.5, 37.8, 55.6, 58.9, 59.4, 117.1, 125.8, 127.0, 127.6, 128.1, 128.8, 129.3, 131.3, 134.1, 134.7, 135.7, 137.6, 143.6, 144.3, 161.0, 169.0, 170.5, 171.3, 172.8 |
| Ⅱe | 8.06(d, J=6.2 Hz, 1H, ArH), 8.03—7.95(m, 1H, ArH), 7.85—7.77(m, 1H, ArH), 7.66—7.59(m, 1H, ArH), 7.56(d, J=7.8 Hz, 1H, ArH), 7.49(t, J=7.8 Hz, 1H, ArH), 7.44—7.34(m, 4H, ArH), 7.34—7.24(m, 2H, ArH), 7.17(t, J=7.5 Hz, 1H, ArH), 4.93(dd, J=9.3, 4.6 Hz, 1H, CH), 4.86(t, J=7.1 Hz, 1H, CH), 4.46(d, J=7.5 Hz, 1H, CH), 4.10(s, 3H, OCH3), 3.47(d, J=4.8 Hz, 1H, CH2Ar), 3.22(dd, J=13.8, 9.4 Hz, 1H, CH2Ar), 2.34—2.24(m, 1H, CH), 1.89—1.77(m, 3H, CHCH2), 1.13 [ddd, J=18.5,8.7, 4.4 Hz, 12H,(CH3)4] | 19.3, 20.7, 22.0, 24.7, 30.8, 36.8, 40.8, 52.3, 55.1, 59.3, 111.5, 118.9, 120.5, 121.1, 122.7, 123.0, 126.3, 128.0, 128.6, 128.9, 130.6, 132.9, 134.9, 137.4, 138.2, 139.5, 157.6, 166.7, 170.7, 171.5, 173.5 |
| Ⅱf | 7.73(s, 1H, ArH), 7.40(d, J=8.6 Hz, 1H, ArH), 7.32(d, J=7.4 Hz, 2H, ArH), 7.21(t, J=7.6 Hz, 2H, ArH), 7.14(s, 1H, ArH), 7.00(t, J=7.3 Hz, 1H, ArH), 4.64(dd, J=9.5, 4.0 Hz, 1H, CH), 4.33(d, J=8.1 Hz, 1H, CH), 4.28—4.19(m, 1H, CH), 3.35(d, J=9.3 Hz, 2H, CH2Ar), 2.91(dd, J=13.3, 9.6 Hz, 1H, CH), 2.14(d, J=7.3 Hz, 1H, CH), 1.82—1.69 [m, 4H,(CH2)2], 1.70—1.48 [m, 4H,(CH2)2], 1.40(d, J=11.1 Hz, 3H, CHCH2), 1.02—0.64 [m, 12H,(CH3)4] | 18.9, 21.1, 22.5, 24.4, 25.1, 25.6, 31.2, 36.6, 41.5, 41.8, 56.3, 56.8, 59.7, 62.1, 70.9, 114.5, 119.3, 125.6, 125.9, 127.6, 128.9, 131.9, 136.8, 137.0, 140.3, 167.4, 171.1, 172.4, 173.1, 174.9 |
| Ⅱg | 7.73(s, 1H, ArH), 7.40(d, J=8.6 Hz, 1H, ArH), 7.32(d, J=7.4 Hz, 2H, ArH), 7.21(t, J=7.6 Hz, 2H, ArH), 7.14(s, 1H, ArH), 7.00(t, J=7.3 Hz, 1H, ArH), 4.64(dd, J=9.5, 4.0 Hz, 1H, CH), 4.33(d, J=8.1 Hz, 1H, CH), 4.28—4.19(m, 1H, CH), 3.35(d, J=9.3 Hz, 2H, CH2Ar), 2.91(dd, J=13.3, 9.6 Hz, 1H, CH), 2.14(d, J=7.3 Hz, 1H, CH), 1.82—1.69 [m, 4H,(CH2)2], 1.70—1.48 [m, 4H,(CH2)2], 1.40(d, J=11.1 Hz, 3H, CHCH2), 1.02—0.64 [m, 12H,(CH3)4] | 18.8, 21.1, 22.6, 24.4, 25.1, 25.6, 31.2, 36.6, 41.5, 41.9, 56.3, 56.8, 59.7, 62.0, 70.9, 114.5, 119.5, 125.6, 125.9, 127.4, 128.9, 131.9, 136.8, 137.0, 140.3, 167.9, 171.1, 172.1, 173.6, 174.9 |
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||
|---|---|---|---|---|---|
| MDA-MB-231 | MCF7 | MDA-MB-231 | MCF7 | ||
| Ⅰa | >20 | >20 | Ⅱa | 17.1 | >20 |
| Ⅰb | >20 | >20 | Ⅱb | 7.6 | 11.8 |
| Ⅰc | >20 | >20 | Ⅱc | 16.3 | >20 |
| Ⅰd | >20 | >20 | Ⅱd | >20 | 15.5 |
| Ⅰe | >20 | >20 | Ⅱe | 16.3 | >20 |
| Taxol | <0.01 | 0.006 | Ⅱf | >20 | >20 |
| Ⅱg | >20 | >20 | |||
Table 4 Antitumor activity of target compounds in vitro
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||
|---|---|---|---|---|---|
| MDA-MB-231 | MCF7 | MDA-MB-231 | MCF7 | ||
| Ⅰa | >20 | >20 | Ⅱa | 17.1 | >20 |
| Ⅰb | >20 | >20 | Ⅱb | 7.6 | 11.8 |
| Ⅰc | >20 | >20 | Ⅱc | 16.3 | >20 |
| Ⅰd | >20 | >20 | Ⅱd | >20 | 15.5 |
| Ⅰe | >20 | >20 | Ⅱe | 16.3 | >20 |
| Taxol | <0.01 | 0.006 | Ⅱf | >20 | >20 |
| Ⅱg | >20 | >20 | |||
| [1] | Sasse F., Sieinmetz H., Heil J., Höfle G., Reichenbach H., J. Antibiot., 2000, 59(9), 879—885 |
| [2] | Kaur G., Hollingshead M., Holbeck S., Schauer-Vukasinovic V., Camalier R. F., Dömling A., Agarwal S., Biochem. J., 2006, 396(2), 235—242 |
| [3] | Khalil M.W., Sasse F., Luensdorf H., Elnakady Y. A., Reichenbach H.,ChemBioChem, 2006, 7(4), 678—683 |
| [4] | Steinmetz H., Glaser N., Herdtweck E., Sasse F., Reichenbach H., Höfle G., Angew. Chem. Int. Ed., 2004, 43(37), 4888—4892 |
| [5] | Peltier H. M., McMahon J. P., Patterson A. W., Ellman J. A., J. Am. Chem. Soc., 2006, 128(50), 16018—16019 |
| [6] | Sani M., Fossati G., Huguenot F., Zanda M., Angew. Chem., 2007, 119(19), 3596—3599 |
| [7] | Pando O., Dörner S., Preusentanz R., Denkert A., Porzel A., Richter W., Wessjohann L., Org. Lett., 2009, 11(24), 5567—5569 |
| [8] | Ullrich A., Chai Y., Pistorius D., Elnakady Y. A., Herrmann J. E., Weissman K. J., Kazmaier U., Müller R., Angew. Chem. Int. Ed., 2009, 48(24), 4422—4425 |
| [9] | Braig S., Wiedmann R. M., Liebl J., Singer M., Kubisch R., Schreiner L., Abhari B. A., Wagner E., Kazmaier U., Fulda S., Vollmar A. M., Cell Death Dis., 2014, 5(1), e1001 |
| [10] | Kubisch R., von Gamm M., Braig S., Ullrich A., Burkhart J. L., Colling L., Hermann J., Scherer O., Müller R., Werz O., Kazmaier U., Vollmar A. M., J. Nat. Prod., 2014, 77(3), 536—542 |
| [11] | Burkhart J. L., Müller R., Kazmaier U., Eur. J. Org. Chem., 2011, 2011(16), 3050—3059 |
| [12] | Hwang J. Y., Attia R. R., Zhu F.,Yang L., Lemoff A., Jeffries C., Connelly M. C., Guy R. K., J. Med. Chem., 2012, 55(5), 2301—2310 |
| [13] | Shearer B. G., Chao E. Y., Uehling D. E., Deaton D. N., Cowan C., Sherman B. W., Milliken T., Faison W., Brown K., Adkisond K. K., Lee F., J. Med. Chem., 2002, 45(3), 567—583 |
| [14] | Wang Z. J., Sun X. H., Liu Y. F., Chen B., Shen S. Q., Jin R. Y., Ma H. X., Chem. J. Chinese Universities,2015, 36(7), 1315—1320 |
| (王子剑, 孙晓红, 刘源发, 陈邦, 沈生强, 靳如意, 马海霞. 高等学校化学学报, 2015, 36(7), 1315—1320) | |
| [15] | Stodulski M., Mamińska A., Mlynarski J., Tetrahedron: Asymmetry,2011, 22(4), 464—467 |
| [16] | Parka Y., Lee J. K., Ryu J. S., Synlett., 2015, 26(8), 1063—1068 |
| [17] | Li Y., Di F., Wang D., Chen W., Wan Y. Y., Li Z. M., Chem. Res. Chinese Universities,2015, 31(6), 952—957 |
| [18] | Chen Q., Lin J., Zhao D., Zhu X., Chem. Res. Chinese Universities,2016, 32(5), 792—796 |
| [19] | Murray B. C., Peterson M. T., Fecik R. A., Nat. Prod. Rep., 2015, 32(5), 654—662 |
| [20] | Xu X. M., Gregory K. F., Yao L., Mini-Rev. Med. Chem., 2013, 13(11), 1572—1578 |
| [1] | GAO Qilong, LIANG Erjun, XING Xianran, CHEN Jun. Negative Thermal Expansion in Prussian Blue Analogues † [J]. Chem. J. Chinese Universities, 2020, 41(3): 388. |
| [2] | XIAO Yanhua, ZHANG Guangjie, ZONG Liang, LIU Guohong, REN Lijun, DONG Junxing. Chemical Constituents and Antitumor Activity of Tupistra chinensis † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1897. |
| [3] | LÜ Mingjun,LI Wen,YANG Xinying,FANG Hao. Synthesis and Antitumor Activity of N9 Position Aromatic Substituted Purine-8-one Derivatives† [J]. Chem. J. Chinese Universities, 2019, 40(2): 254. |
| [4] | FANG Fang,XUE Liangmin,CONG Jing,TIAN Chao,WANG Xiaowei,LIU Junyi,ZHANG Zhili. Synthesis and Anti-tumor Activity Evaluation of a Series of 2- or 4-Substituted Pyrido[3,2-d]pyrimidines as Nonclassical Antifolates † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2111. |
| [5] | ZHANG Peiquan,YANG Qianqian,LONG Huidan,CHEN Xin. Synthesis and Antitumor Activity of Auranofin Derivatives † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2097. |
| [6] | LI Yang, LI Zhiwen, ZHU Junfei, LIU Shihui, HE Junlin. Construction of Pyrenyl Pairs in dsDNA: Fluorescent Properties of Multiple Pyrenyl-attached dsDNAs Through 7-Substituted 8-Aza-7-deaza-2'-deoxyadenosine Analogues† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2206. |
| [7] | GUO Liang, CAO Rihui, FAN Wenxi, GAN Ziyun, MA Qin. Design, Synthesis and in vitro Antitumor Activities of Novel Bivalent β-Carbolines† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1093. |
| [8] | ZHANG Jie, ZHOU Changjian, XIE Jianwei, DAI Bin. Synthesis and Antitumor Activities of Rhein-Valine Adducts† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2159. |
| [9] | QIN Yaoguo, ZHANG Jingpeng, SONG Dunlun, DUAN Hongxia, LING Yun, JIANG Biaobiao, WANG Di, YANG Xinling. Design, Synthesis and Biological Activity of Novel Aphid Alarm Pheromone Analogues Containing Isonicotinic Acid† [J]. Chem. J. Chinese Universities, 2016, 37(11): 1977. |
| [10] | ZHOU Hao, DUAN Zhigang, ZHAO Shuang, BAO Meiying, LI Zhiwei, PEI Yazhong. Design and Synthesis of Phenylpyrimidine and Their Anticancer Activity† [J]. Chem. J. Chinese Universities, 2015, 36(9): 1694. |
| [11] | WANG Gang, HAN Leiqiang, FANG Hao. Syntheses and Antitumor Activities of Phenylpiperazine Derivatives† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2435. |
| [12] | GUO Hua, YANG Chengling, WANG Wei, LAI Quanyong, YUAN Zhi. Preparation of Liver-targeted Nano-prodrug Based on Sodium Alginate Derivative and the Study on Antitumor Activity† [J]. Chem. J. Chinese Universities, 2014, 35(8): 1835. |
| [13] | SONG Xiudao, HE Jun, MA Jin, LIU Yunmei, ZHENG Xing, LEI Xiaoyong, GUO Yu. Syntheses and Anticancer Activities of Glycine Derivatives of Chrysin† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1465. |
| [14] | WANG Junhua, WANG Quande, DUN Yanyan, FANG Hao. Syntheses and Antitumor Activities of Purine-sulfonamides Derivatives† [J]. Chem. J. Chinese Universities, 2014, 35(6): 1189. |
| [15] | YANG Haikui, XU Wanfu, DUAN Anna, YOU Wenwei, ZHAO Peiliang. Syntheses and Biological Activities of Novel Imine and Imide Derivatives Bearing 1,2,4-Triazole Moiety† [J]. Chem. J. Chinese Universities, 2014, 35(3): 555. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||