

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (10): 2111.doi: 10.7503/cjcu20190108
• Organix Chemistry • Previous Articles Next Articles
FANG Fang1,XUE Liangmin1,CONG Jing1,TIAN Chao1,WANG Xiaowei1,LIU Junyi1,2,ZHANG Zhili1,*( )
)
Received:2019-02-17
Online:2019-10-08
Published:2019-06-01
Contact:
ZHANG Zhili
E-mail:Lilybmu@bjmu.edu.cn
Supported by:CLC Number:
TrendMD:
FANG Fang,XUE Liangmin,CONG Jing,TIAN Chao,WANG Xiaowei,LIU Junyi,ZHANG Zhili. Synthesis and Anti-tumor Activity Evaluation of a Series of 2- or 4-Substituted Pyrido[3,2-d]pyrimidines as Nonclassical Antifolates †[J]. Chem. J. Chinese Universities, 2019, 40(10): 2111.
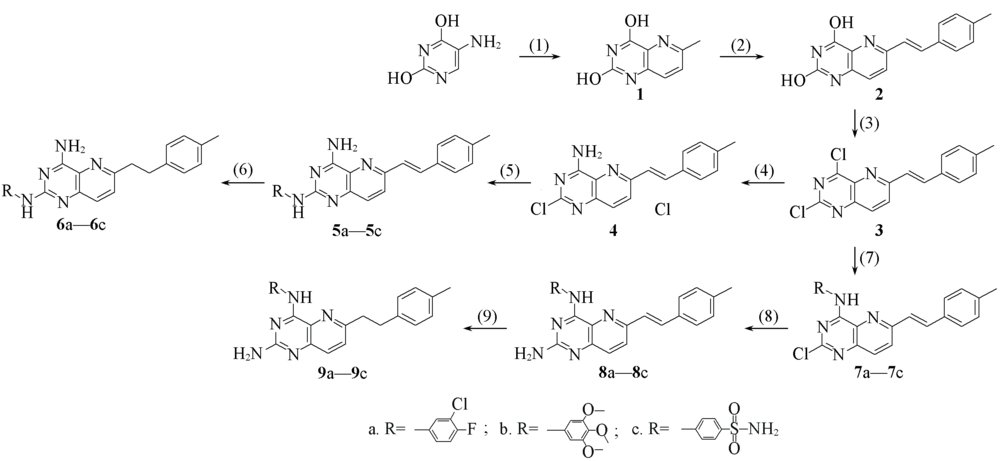
Fig.3 Scheme 1 Synthesis of 2- or 4-substituted 6-(4'-methylphenyl) ethylpyrido [3,2-d]pyrimidines Reagents and conditions: (1) CH3CH=CHCHO, 20%HCl; (2) 4-tolylaldehyde, PTSA, DMAC, 160 ℃, 36 h; (3) POCl3, NEt3; (4) NH3, CH3OH; (5) R1NH2, TFA, TFE, microwave, 140 ℃; (6) Pd/C, H2, CH3CH2OH; (7) R1NH2, CH3OH or CH3CH2OH; (8) NH3/CH3OH, 130 ℃, 130 h; (9) Pd/C, H2, CH3CH2OH.
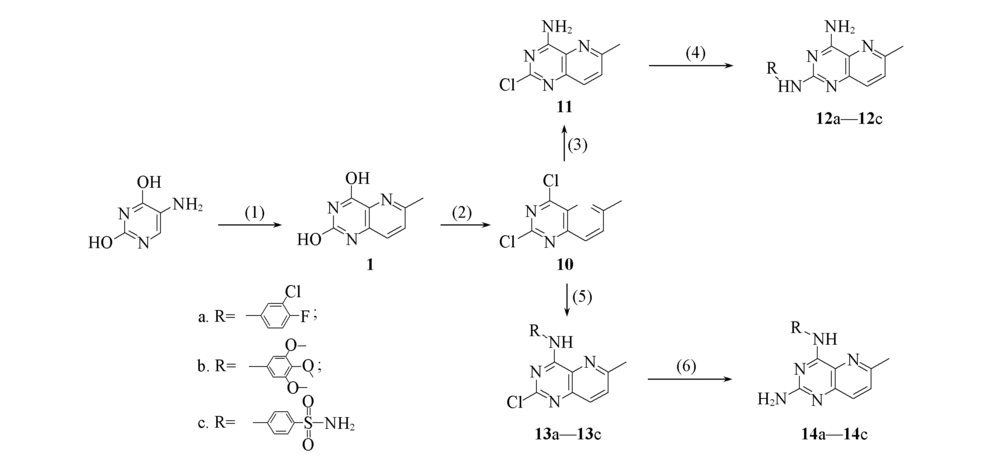
Fig.4 Scheme 2 Synthesis of 2- or 4-substituted 6-methylpyrido [3,2-d] pyrimidines Reagents and conditions: (1) CH3CH=CHCHO, 20%HCl; (2) POCl3, NEt3; (3) NH3, CH3OH; (4) R1NH2, TFA, TFE, microwave, 140 ℃; (5) R1NH2, CH3OH or CH3CH2OH; (6) NH3/CH3OH, 130 ℃, 130 h.
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | (E)-2-Chloro-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-amine | 8.54(s, 1H), 8.44(s, 1H), 8.06(d,J=16.1 Hz, 1H), 7.98(s, 2H), 7.59(d, J=8.1 Hz, 2H), 7.37(d, J=16.1 Hz, 1H), 7.26(d, J=8.0 Hz, 2H), 2.34(s, 3H) | 163.60, 156.86, 154.69, 145.23, 138.97, 135.55, 135.27, 133.95, 130.36, 130.01, 128.58, 127.70, 126.08, 21.42 | ||||||||
| 5a | (E)-N2-(3-Chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.39(s, 1H), 8.29(dd,J=6.9, 2.5 Hz, 1H), 7.88(m, J=10.0 Hz, 1H), 7.85(d, J=2.8 Hz, 1H), 7.83(s, 1H), 7.7(d, J=8.6 Hz, 2H),7.71(s, 1H), 7.57(d, J=7.9 Hz, 2H), 7.33(d, J=2.8 Hz, 1H), 7.31—7.27(m, 1H), 7.24(d, J=7.8 Hz,2H), 2.34(s, 3H) | 162.25, 156.93, 153.20, 150.90(d, J=21.1 Hz), 150.55, 145.33, 139.04, 138.25, 134.33, 133.68, 132.96, 129.92, 129.02, 127.69, 127.36, 126.80, 119.95, 119.34(d, J=17.1 Hz), 116.79(d, J=21.1 Hz), 21.37 | ||||||||
| 5b | (E)-6-(4-Methylstyryl)-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.04(s, 1H), 7.85(d,J=16.3 Hz, 1H), 7.82(d, J=8.8 Hz, 1H), 7.76(s, 1H), 7.70(d, J=28.3 Hz, 2H), 7.57(d, J=8.0 Hz, 2H), 7.40(s, 2H), 7.31(d, J=16.2 Hz, 1H), 7.24(d, J=7.9 Hz, 2H), 3.79(s, 6H), 3.62(s, 3H), 2.34(s, 3H) | 162.09, 157.14, 153.00, 150.22, 138.28, 137.66, 134.38, 133.61, 132.72, 132.35, 129.95, 128.93, 127.63, 127.36, 126.92, 97.19, 60.53, 56.02, 21.33 | ||||||||
| 5c | (E)-4-((4-Amino-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-2-yl)amino)benzenesulfonamide | 9.61(s, 1H), 8.10(d,J=8.7 Hz, 2H),7.90(d, J=16.3 Hz, 2H), 7.84(d, J=10.1 Hz, 2H), 7.79(s, 1H), 7.71(d, J=8.8 Hz, 2H), 7.58(d, J=7.9 Hz, 2H), 7.33(d, J=16.2 Hz, 1H), 7.25(d, J=7.9 Hz, 2H), 7.17(s, 2H), 2.34(s, 3H) | 162.33, 156.93, 150.81, 144.82, 138.38, 135.99, 134.33, 133.84, 133.13, 129.95, 129.04, 127.76, 127.41, 126.80, 118.30, 70.34, 21.40 | ||||||||
| 7a | (E)-2-Chloro-N-(3-chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-amine | 10.45(s, 1H), 8.27(dd,J=6.8, 2.6 Hz, 1H), 8.17(d, J=16.2 Hz, 1H), 8.11(t, J=5.9 Hz, 2H), 8.01(m, J=9.0, 4.2, 2.7 Hz, 1H), 7.64(d, J=8.0 Hz, 2H), 7.55(t, J= 9.1 Hz, 1H), 7.47(d, J=16.2 Hz, 1H), 7.29(d, J=7.9 Hz, 2H), 2.36(s, 3H) | 158.66, 155.79, 155.61(s, J=227 Hz), 145.33, 139.22, 136.50, 135.75, 135.57, 133.84, 130.49, 130.07, 128.93, 127.84, 126.08, 124.70, 123.57, 123.50, 117.39(d, J=25 Hz), 21.45 | ||||||||
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
| 7b | (E)-2-Chloro-6-(4-methylstyryl)-N-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidin-4-amine | 10.18(s, 1H), 8.11(d, J=16.5 Hz, 2H), 8.07(s, 1H), 7.63(d, J=8.0 Hz, 2H), 7.49(s, 2H), 7.45(d, J=16.3 Hz, 1H), 7.28(d, J=8.0 Hz, 2H), 3.85(s, 6H), 3.71(s, 3H), 2.35(s, 3H) | 158.37, 155.99, 155.43, 153.08, 145.19, 139.20, 136.26, 135.66, 134.97, 134.22, 133.83, 130.55, 130.03, 128.45, 127.80, 126.22, 100.67, 60.64, 56.36, 21.45 | ||||||||
| 7c | (E)-4-((2-Chloro-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 10.55(s, 1H), 8.22—8.13(m, 5H), 7.91(d,J=8.8 Hz, 2H), 7.65(d, J=8.0 Hz, 2H), 7.50(d, J=16.2 Hz, 1H), 7.37(s, 2H), 7.30(d, J=8.0 Hz, 2H), 2.36(s, 3H) | 158.73, 155.75, 145.50, 141.26, 140.07, 139.32, 136.61, 135.83, 133.83, 130.55, 130.08, 128.95, 127.86, 126.86, 126.12, 122.55, 21.45 | ||||||||
| 8a | (E)-N4-(3-Chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.79(s, 1H), 8.46(dd,J=6.8, 2.7 Hz, 1H), 8.21(m, J=9.1, 4.3, 2.7 Hz, 1H), 7.91(d, J=16.3 Hz, 1H), 7.88(d, J=8.8 Hz, 1H), 7.68(d, J=8.7 Hz, 1H), 7.62(d, J=8.1 Hz, 2H), 7.45(t, J=9.1 Hz, 1H), 7.38(d, J=16.3 Hz, 1H), 7.28(d, J=8.0 Hz, 2H), 6.73(s, 2H), 2.37(s, 3H) | 160.82, 157.73, 154.72, 152.31(d, J=241 Hz), 149.47, 146.57, 138.53, 136.92, 134.35, 132.94, 132.82, 129.93, 127.70, 127.35, 126.90, 122.85, 121.80, 119.47(d, J=18.2 Hz), 116.94(d, J=21.7 Hz), 21.35 | ||||||||
| 8b | (E)-6-(4-Methylstyryl)-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.49(s, 1H), 7.88(d,J=8.8 Hz, 1H), 7.83(d, J=16.4 Hz, 1H), 7.64(d, J=8.7 Hz, 1H), 7.60(d, J=8.0 Hz, 2H), 7.56(s, 2H), 7.36(d, J=16.4 Hz, 1H), 7.25(d, J=8.0 Hz, 2H), 6.63(s, 2H), 3.86(s, 6H), 3.67(s, 3H), 2.34(s, 3H) | 160.23, 157.61, 153.14, 149.30, 146.62, 138.28, 135.56, 134.35, 133.76, 133.01, 132.56, 129.93, 127.86, 127.35, 127.17, 99.21, 60.59, 56.48, 21.39 | ||||||||
| 8c | (E)-4-((2-Amino-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 9.88(s, 1H), 8.39(d,J=8.4 Hz, 2H), 7.91(d, J=11.3 Hz, 1H), 7.86(d, J=18.3 Hz, 2H), 7.81(s, 1H), 7.68(d, J=9.0 Hz, 1H), 7.61(d, J=8.0 Hz, 2H), 7.38(d, J=16.3 Hz, 1H), 7.31(s, 2H), 7.26(d, J=7.6 Hz), 6.78(s, 2H), 2.35(s, 3H) | 160.14, 157.69, 149.57, 146.95, 142.66, 138.36, 138.26, 134.34, 133.21, 132.91, 129.95, 127.73, 127.68, 127.40, 126.95, 126.79, 120.69, 21.40 | ||||||||
| 13a | 2-Chloro-N-(3-chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidin-4-amine | 10.46(s, 1H), 8.29—8.23(m, 1H), 8.08(d,J=8.6 Hz, 1H), 7.99(m, J=9.0, 4.3, 2.7 Hz, 1H), 7.85(d, J=8.6 Hz, 1H), 7.52(t, J=9.1 Hz, 1H), 2.78(s, 3H) | 159.30, 158.56, 155.61(d,J=243 Hz), 153.15, 144.92, 135.63(d, J=3.0 Hz), 135.38, 130.55, 129.79, 124.39, 123.20, 119.43(d, J=18.5 Hz), 117.14(d, J=21.7 Hz), 24.93 | ||||||||
| 13b | 2-Chloro-6-methyl-N-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidin-4-amine | 10.15(s, 1H, NH-4), 8.06(d,J=8.5 Hz, 1H), 7.83(d, J=8.6 Hz, 1H), 7.52(s, 2H), 3.84(s, 6H), 3.70(s, 3H), 2.77(s, 3H) | 159.07, 158.23, 155.80, 152.98, 144.68, 135.37, 134.71, 134.33, 130.36, 129.88, 100.25, 60.55, 56.49, 24.83 | ||||||||
| 13c | 4-((2-Chloro-6-methylpyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 10.51(s, 1H), 8.13(d,J=8.8 Hz, 2H), 8.10—8.04(m, 1H), 7.87(d, J=8.8 Hz, 2H), 7.83(m, J=5.4, 3.1 Hz, 1H), 7.33(s, 2H), 2.77(s, 3H) | 159.49, 158.69, 155.67, 145.06, 141.29, 139.92, 135.45, 130.68, 129.85, 126.80, 122.35, 24.89 | ||||||||
| 6a | N2-(3-Chloro-4-fluorophenyl)-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.32(s, 1H), 8.28(d,J=6.7 Hz, 1H), 7.84(d, J=9.1 Hz, 1H), 7.70(d, J=8.5 Hz, 1H), 7.58(s, 2H), 7.52(s, J=8.7 Hz, 1H), 7.29(t, J=9.1 Hz, 1H), 7.14(d, J=7.6 Hz, 2H), 7.07(d, J=7.6 Hz, 2H), 3.11(d, J=6.6 Hz, 2H), 3.07(d, J=5.7 Hz, 2H), 2.25(s, 3H) | 162.14, 156.81, 156.30, 153.20(d, J=238 Hz), 150.82, 144.85, 139.23, 138.90, 135.10, 133.63, 129.27, 128.77, 128.73, 128.42, 119.78, 119.00(d, J=20.9, 12.1 Hz), 116.77(d, J=21.4 Hz), 34.57, 21.29, 14.23 | ||||||||
| 6b | 6-(4-Methylphenethyl)-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 8.96(s, 1H), 7.68(d,J=8.5 Hz, 1H), 7.48(s, 1H), 7.45(d, J=11.3 Hz, 2H), 7.42(s, 2H), 7.12(d, J=7.9 Hz, 2H), 7.05(d, J=7.9 Hz, 2H), 3.79(s, 6H), 3.62(s, 3H), 3.09(t, 2H), 3.05(t, 2H), 2.23(s, 3H) | 161.96, 157.13, 155.84, 152.97, 145.03, 138.85, 137.94, 135.07, 133.66, 132.14, 129.24, 128.69, 128.61, 128.35, 97.02, 60.54, 56.10, 34.65, 21.01, 18.99 | ||||||||
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
| 6c | 4-((4-Amino-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidin-2-yl)amino)benzenesulfonamide | 9.57(s, 1H), 8.12(d,J=8.8 Hz, 2H), 7.78(d, J=8.6 Hz, 1H), 7.71(d, J=8.8 Hz, 2H), 7.64(d, J=10.2 Hz, 2H), 7.58(d, J=8.6 Hz, 1H), 7.18(d, J=9.7 Hz, 4H), 7.10(d, J=7.9 Hz, 2H), 3.15(t, 2H), 3.10(t, 2H), 2.28(s, 3H) | 162.19, 156.80, 156.57, 144.96, 144.88, 138.89, 135.78, 135.12, 133.79, 129.29, 128.83, 128.75, 128.41, 126.78, 118.08, 34.52, 31.75, 21.09 | ||||||||
| 9a | N4-(3-Chloro-4-fluorophenyl)-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.62(s, 1H), 8.41(dd,J=6.8, 2.5 Hz, 1H), 8.18—8.09(m, 1H), 7.61(d, J=8.6 Hz, 1H), 7.53(d, J=8.6 Hz, 1H), 7.43(t, J=9.1 Hz, 1H), 7.18(d, J=7.8 Hz, 2H), 7.10(d, J=7.8 Hz, 2H), 6.62(s, 2H), 3.23—3.14(t, 2H), 3.13—3.05(t, 2H), 2.27(s, 3H) | 159.96, 157.63, 155.15, 154.63(d, J=241 Hz), 152.22, 146.03, 138.90, 136.93, 135.11, 132.96, 129.27, 128.73, 127.11, 122.64, 121.57, 119.45(d, J=18.2 Hz), 116.91(d, J=21.6 Hz), 36.64, 30.39, 22.03 | ||||||||
| 9b | 6-(4-Methylphenethyl)-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.34(s, 1H), 7.59(d,J=8.5 Hz, 1H), 7.52(d, 1H), 7.50(s, 2H), 7.17(d, J=6.9 Hz, 2H), 7.09(d, J=7.4 Hz, 2H), 6.53(s, 2H), 3.86(s, 6H), 3.67(s, 3H), 3.17(t, 2H), 3.09(t, 2H), 2.26(s, 3H) | 160.07, 157.54, 154.94, 153.15, 145.91, 138.84, 135.56, 135.15, 133.67, 133.00, 129.28, 128.76, 127.30, 98.98, 60.59, 56.47, 34.86, 30.48, 21.10 | ||||||||
| 9c | 4-((2-Amino-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 9.71(s, 1H), 8.31(d,J=8.5 Hz,2H), 7.80(d, J=8.6 Hz, 2H), 7.62(d, J=8.6 Hz, 1H), 7.54(d, J=8.7 Hz, 1H), 7.31(s, 2H), 7.17(d, J=7.6 Hz, 2H), 7.09(d, J=7.5 Hz, 2H), 6.69(s, 2H), 3.17(t, 2H), 3.09(t, 2H), 2.25(s, 3H) | 159.79, 157.48, 155.27, 145.56, 142.28, 138.72, 137.99, 135.10, 132.98, 129.29, 128.95, 128.77, 127.08, 126.79, 120.32, 34.55, 29.48, 21.12 | ||||||||
| 12a | N2-(3-Chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidine-2,4-diamine | 9.34(s, 1H), 8.32(dd,J=6.9, 2.5 Hz, 1H), 7.91—7.83(m, 1H), 7.75(d, J=8.6 Hz, 1H), 7.57(d, J=8.6 Hz, 1H), 7.54(s, 2H), 7.32(t, J=9.1 Hz, 1H), 2.62(s, 3H) | 162.06, 156.74, 153.43, 153.31(d, J=238 Hz), 144.66, 139.27, 133.77, 129.20, 128.40, 119.74(d, J=29 Hz), 118.99(d, J=23.6 Hz), 116.88, 116.67, 24.44 | ||||||||
| 12b | 6-Methyl-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 8.96(s, 1H), 7.74(d,J=8.5 Hz, 1H), 7.56(d, J=8.6 Hz, 1H), 7.45(s, 2H), 7.44(s, 2H), 3.83(s, 6H), 3.66(s, 3H), 2.62(s, 3H) | 161.87, 157.06, 152.97, 144.88, 137.95, 133.81, 132.14, 129.06, 128.31, 97.03, 60.58, 56.13, 24.43 | ||||||||
| 12c | 4-((4-Amino-6-methylpyrido[3,2-d]pyri-midin-2-yl)amino)benzenesulfonamide | 10.95(s, 1H), 9.27(d,J=97.5 Hz, 2H), 7.95(d, J=8.6 Hz, 1H), 7.87(m, J=20.7, 8.9 Hz, 4H), 7.77(d, J=8.6 Hz, 1H), 7.38(s, 2H), 2.65(s, 3H) | 162.93, 156.57, 153.04, 150.33, 140.90, 139.87, 131.09, 127.17, 126.99, 126.40, 121.56, 24.07 | ||||||||
| 14a | N4-(3-Chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidine-2,4-diamine | 9.68(s, 1H), 8.43(dd,J=6.8, 2.3 Hz, 1H), 8.18—8.04(m, 1H), 7.62(d, J=8.6 Hz, 1H), 7.53(d, J=8.6 Hz, 1H), 7.41(t, J=9.1 Hz, 1H), 6.58(s, 2H), 2.64(s, 3H) | 159.95, 157.58, 154.55, 152.14(d, J=236 Hz), 145.97, 137.09, 133.11, 129.26, 127.15, 122.54, 121.47, 119.40(d, J=18.2 Hz), 116.87(d, J=21.5 Hz), 24.29 | ||||||||
| 14b | 6-Methyl-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.37(s, 1H), 7.60(d,J=8.6 Hz, 1H), 7.53(s, 2H), 7.51(d, J=8.6 Hz, 1H), 6.51(s, 2H), 3.85(s, 6H), 3.66(s, 3H), 2.63(s, 3H) | 159.99, 157.44, 153.12, 151.85, 145.66, 135.67, 133.53, 133.08, 129.09, 127.29, 98.78, 60.55, 56.40, 24.72 | ||||||||
| 14c | 4-((2-Amino-6-methylpyrido[3,2-d]pyri-midin-4-yl)amino)benzenesulfonamide | 9.78(s, 1H, NH-4), 8.34(d, J=8.8 Hz, 2H), 7.81(d, J=8.8 Hz, 2H), 7.65(d, J=8.6 Hz, 1H), 7.56(d, J=8.6 Hz, 1H), 7.32(s, 2H), 6.65(s, 2H), 2.65(s, 3H) | 159.90, 157.44, 152.29, 146.07, 142.63, 137.96, 133.10, 129.39, 127.08, 126.76, 120.46, 24.32 | ||||||||
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | (E)-2-Chloro-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-amine | 8.54(s, 1H), 8.44(s, 1H), 8.06(d,J=16.1 Hz, 1H), 7.98(s, 2H), 7.59(d, J=8.1 Hz, 2H), 7.37(d, J=16.1 Hz, 1H), 7.26(d, J=8.0 Hz, 2H), 2.34(s, 3H) | 163.60, 156.86, 154.69, 145.23, 138.97, 135.55, 135.27, 133.95, 130.36, 130.01, 128.58, 127.70, 126.08, 21.42 | ||||||||
| 5a | (E)-N2-(3-Chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.39(s, 1H), 8.29(dd,J=6.9, 2.5 Hz, 1H), 7.88(m, J=10.0 Hz, 1H), 7.85(d, J=2.8 Hz, 1H), 7.83(s, 1H), 7.7(d, J=8.6 Hz, 2H),7.71(s, 1H), 7.57(d, J=7.9 Hz, 2H), 7.33(d, J=2.8 Hz, 1H), 7.31—7.27(m, 1H), 7.24(d, J=7.8 Hz,2H), 2.34(s, 3H) | 162.25, 156.93, 153.20, 150.90(d, J=21.1 Hz), 150.55, 145.33, 139.04, 138.25, 134.33, 133.68, 132.96, 129.92, 129.02, 127.69, 127.36, 126.80, 119.95, 119.34(d, J=17.1 Hz), 116.79(d, J=21.1 Hz), 21.37 | ||||||||
| 5b | (E)-6-(4-Methylstyryl)-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.04(s, 1H), 7.85(d,J=16.3 Hz, 1H), 7.82(d, J=8.8 Hz, 1H), 7.76(s, 1H), 7.70(d, J=28.3 Hz, 2H), 7.57(d, J=8.0 Hz, 2H), 7.40(s, 2H), 7.31(d, J=16.2 Hz, 1H), 7.24(d, J=7.9 Hz, 2H), 3.79(s, 6H), 3.62(s, 3H), 2.34(s, 3H) | 162.09, 157.14, 153.00, 150.22, 138.28, 137.66, 134.38, 133.61, 132.72, 132.35, 129.95, 128.93, 127.63, 127.36, 126.92, 97.19, 60.53, 56.02, 21.33 | ||||||||
| 5c | (E)-4-((4-Amino-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-2-yl)amino)benzenesulfonamide | 9.61(s, 1H), 8.10(d,J=8.7 Hz, 2H),7.90(d, J=16.3 Hz, 2H), 7.84(d, J=10.1 Hz, 2H), 7.79(s, 1H), 7.71(d, J=8.8 Hz, 2H), 7.58(d, J=7.9 Hz, 2H), 7.33(d, J=16.2 Hz, 1H), 7.25(d, J=7.9 Hz, 2H), 7.17(s, 2H), 2.34(s, 3H) | 162.33, 156.93, 150.81, 144.82, 138.38, 135.99, 134.33, 133.84, 133.13, 129.95, 129.04, 127.76, 127.41, 126.80, 118.30, 70.34, 21.40 | ||||||||
| 7a | (E)-2-Chloro-N-(3-chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-amine | 10.45(s, 1H), 8.27(dd,J=6.8, 2.6 Hz, 1H), 8.17(d, J=16.2 Hz, 1H), 8.11(t, J=5.9 Hz, 2H), 8.01(m, J=9.0, 4.2, 2.7 Hz, 1H), 7.64(d, J=8.0 Hz, 2H), 7.55(t, J= 9.1 Hz, 1H), 7.47(d, J=16.2 Hz, 1H), 7.29(d, J=7.9 Hz, 2H), 2.36(s, 3H) | 158.66, 155.79, 155.61(s, J=227 Hz), 145.33, 139.22, 136.50, 135.75, 135.57, 133.84, 130.49, 130.07, 128.93, 127.84, 126.08, 124.70, 123.57, 123.50, 117.39(d, J=25 Hz), 21.45 | ||||||||
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
| 7b | (E)-2-Chloro-6-(4-methylstyryl)-N-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidin-4-amine | 10.18(s, 1H), 8.11(d, J=16.5 Hz, 2H), 8.07(s, 1H), 7.63(d, J=8.0 Hz, 2H), 7.49(s, 2H), 7.45(d, J=16.3 Hz, 1H), 7.28(d, J=8.0 Hz, 2H), 3.85(s, 6H), 3.71(s, 3H), 2.35(s, 3H) | 158.37, 155.99, 155.43, 153.08, 145.19, 139.20, 136.26, 135.66, 134.97, 134.22, 133.83, 130.55, 130.03, 128.45, 127.80, 126.22, 100.67, 60.64, 56.36, 21.45 | ||||||||
| 7c | (E)-4-((2-Chloro-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 10.55(s, 1H), 8.22—8.13(m, 5H), 7.91(d,J=8.8 Hz, 2H), 7.65(d, J=8.0 Hz, 2H), 7.50(d, J=16.2 Hz, 1H), 7.37(s, 2H), 7.30(d, J=8.0 Hz, 2H), 2.36(s, 3H) | 158.73, 155.75, 145.50, 141.26, 140.07, 139.32, 136.61, 135.83, 133.83, 130.55, 130.08, 128.95, 127.86, 126.86, 126.12, 122.55, 21.45 | ||||||||
| 8a | (E)-N4-(3-Chloro-4-fluorophenyl)-6-(4-methylstyryl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.79(s, 1H), 8.46(dd,J=6.8, 2.7 Hz, 1H), 8.21(m, J=9.1, 4.3, 2.7 Hz, 1H), 7.91(d, J=16.3 Hz, 1H), 7.88(d, J=8.8 Hz, 1H), 7.68(d, J=8.7 Hz, 1H), 7.62(d, J=8.1 Hz, 2H), 7.45(t, J=9.1 Hz, 1H), 7.38(d, J=16.3 Hz, 1H), 7.28(d, J=8.0 Hz, 2H), 6.73(s, 2H), 2.37(s, 3H) | 160.82, 157.73, 154.72, 152.31(d, J=241 Hz), 149.47, 146.57, 138.53, 136.92, 134.35, 132.94, 132.82, 129.93, 127.70, 127.35, 126.90, 122.85, 121.80, 119.47(d, J=18.2 Hz), 116.94(d, J=21.7 Hz), 21.35 | ||||||||
| 8b | (E)-6-(4-Methylstyryl)-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.49(s, 1H), 7.88(d,J=8.8 Hz, 1H), 7.83(d, J=16.4 Hz, 1H), 7.64(d, J=8.7 Hz, 1H), 7.60(d, J=8.0 Hz, 2H), 7.56(s, 2H), 7.36(d, J=16.4 Hz, 1H), 7.25(d, J=8.0 Hz, 2H), 6.63(s, 2H), 3.86(s, 6H), 3.67(s, 3H), 2.34(s, 3H) | 160.23, 157.61, 153.14, 149.30, 146.62, 138.28, 135.56, 134.35, 133.76, 133.01, 132.56, 129.93, 127.86, 127.35, 127.17, 99.21, 60.59, 56.48, 21.39 | ||||||||
| 8c | (E)-4-((2-Amino-6-(4-methylstyryl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 9.88(s, 1H), 8.39(d,J=8.4 Hz, 2H), 7.91(d, J=11.3 Hz, 1H), 7.86(d, J=18.3 Hz, 2H), 7.81(s, 1H), 7.68(d, J=9.0 Hz, 1H), 7.61(d, J=8.0 Hz, 2H), 7.38(d, J=16.3 Hz, 1H), 7.31(s, 2H), 7.26(d, J=7.6 Hz), 6.78(s, 2H), 2.35(s, 3H) | 160.14, 157.69, 149.57, 146.95, 142.66, 138.36, 138.26, 134.34, 133.21, 132.91, 129.95, 127.73, 127.68, 127.40, 126.95, 126.79, 120.69, 21.40 | ||||||||
| 13a | 2-Chloro-N-(3-chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidin-4-amine | 10.46(s, 1H), 8.29—8.23(m, 1H), 8.08(d,J=8.6 Hz, 1H), 7.99(m, J=9.0, 4.3, 2.7 Hz, 1H), 7.85(d, J=8.6 Hz, 1H), 7.52(t, J=9.1 Hz, 1H), 2.78(s, 3H) | 159.30, 158.56, 155.61(d,J=243 Hz), 153.15, 144.92, 135.63(d, J=3.0 Hz), 135.38, 130.55, 129.79, 124.39, 123.20, 119.43(d, J=18.5 Hz), 117.14(d, J=21.7 Hz), 24.93 | ||||||||
| 13b | 2-Chloro-6-methyl-N-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidin-4-amine | 10.15(s, 1H, NH-4), 8.06(d,J=8.5 Hz, 1H), 7.83(d, J=8.6 Hz, 1H), 7.52(s, 2H), 3.84(s, 6H), 3.70(s, 3H), 2.77(s, 3H) | 159.07, 158.23, 155.80, 152.98, 144.68, 135.37, 134.71, 134.33, 130.36, 129.88, 100.25, 60.55, 56.49, 24.83 | ||||||||
| 13c | 4-((2-Chloro-6-methylpyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 10.51(s, 1H), 8.13(d,J=8.8 Hz, 2H), 8.10—8.04(m, 1H), 7.87(d, J=8.8 Hz, 2H), 7.83(m, J=5.4, 3.1 Hz, 1H), 7.33(s, 2H), 2.77(s, 3H) | 159.49, 158.69, 155.67, 145.06, 141.29, 139.92, 135.45, 130.68, 129.85, 126.80, 122.35, 24.89 | ||||||||
| 6a | N2-(3-Chloro-4-fluorophenyl)-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.32(s, 1H), 8.28(d,J=6.7 Hz, 1H), 7.84(d, J=9.1 Hz, 1H), 7.70(d, J=8.5 Hz, 1H), 7.58(s, 2H), 7.52(s, J=8.7 Hz, 1H), 7.29(t, J=9.1 Hz, 1H), 7.14(d, J=7.6 Hz, 2H), 7.07(d, J=7.6 Hz, 2H), 3.11(d, J=6.6 Hz, 2H), 3.07(d, J=5.7 Hz, 2H), 2.25(s, 3H) | 162.14, 156.81, 156.30, 153.20(d, J=238 Hz), 150.82, 144.85, 139.23, 138.90, 135.10, 133.63, 129.27, 128.77, 128.73, 128.42, 119.78, 119.00(d, J=20.9, 12.1 Hz), 116.77(d, J=21.4 Hz), 34.57, 21.29, 14.23 | ||||||||
| 6b | 6-(4-Methylphenethyl)-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 8.96(s, 1H), 7.68(d,J=8.5 Hz, 1H), 7.48(s, 1H), 7.45(d, J=11.3 Hz, 2H), 7.42(s, 2H), 7.12(d, J=7.9 Hz, 2H), 7.05(d, J=7.9 Hz, 2H), 3.79(s, 6H), 3.62(s, 3H), 3.09(t, 2H), 3.05(t, 2H), 2.23(s, 3H) | 161.96, 157.13, 155.84, 152.97, 145.03, 138.85, 137.94, 135.07, 133.66, 132.14, 129.24, 128.69, 128.61, 128.35, 97.02, 60.54, 56.10, 34.65, 21.01, 18.99 | ||||||||
| Compd. | Name | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ | ||||||||
| 6c | 4-((4-Amino-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidin-2-yl)amino)benzenesulfonamide | 9.57(s, 1H), 8.12(d,J=8.8 Hz, 2H), 7.78(d, J=8.6 Hz, 1H), 7.71(d, J=8.8 Hz, 2H), 7.64(d, J=10.2 Hz, 2H), 7.58(d, J=8.6 Hz, 1H), 7.18(d, J=9.7 Hz, 4H), 7.10(d, J=7.9 Hz, 2H), 3.15(t, 2H), 3.10(t, 2H), 2.28(s, 3H) | 162.19, 156.80, 156.57, 144.96, 144.88, 138.89, 135.78, 135.12, 133.79, 129.29, 128.83, 128.75, 128.41, 126.78, 118.08, 34.52, 31.75, 21.09 | ||||||||
| 9a | N4-(3-Chloro-4-fluorophenyl)-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.62(s, 1H), 8.41(dd,J=6.8, 2.5 Hz, 1H), 8.18—8.09(m, 1H), 7.61(d, J=8.6 Hz, 1H), 7.53(d, J=8.6 Hz, 1H), 7.43(t, J=9.1 Hz, 1H), 7.18(d, J=7.8 Hz, 2H), 7.10(d, J=7.8 Hz, 2H), 6.62(s, 2H), 3.23—3.14(t, 2H), 3.13—3.05(t, 2H), 2.27(s, 3H) | 159.96, 157.63, 155.15, 154.63(d, J=241 Hz), 152.22, 146.03, 138.90, 136.93, 135.11, 132.96, 129.27, 128.73, 127.11, 122.64, 121.57, 119.45(d, J=18.2 Hz), 116.91(d, J=21.6 Hz), 36.64, 30.39, 22.03 | ||||||||
| 9b | 6-(4-Methylphenethyl)-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.34(s, 1H), 7.59(d,J=8.5 Hz, 1H), 7.52(d, 1H), 7.50(s, 2H), 7.17(d, J=6.9 Hz, 2H), 7.09(d, J=7.4 Hz, 2H), 6.53(s, 2H), 3.86(s, 6H), 3.67(s, 3H), 3.17(t, 2H), 3.09(t, 2H), 2.26(s, 3H) | 160.07, 157.54, 154.94, 153.15, 145.91, 138.84, 135.56, 135.15, 133.67, 133.00, 129.28, 128.76, 127.30, 98.98, 60.59, 56.47, 34.86, 30.48, 21.10 | ||||||||
| 9c | 4-((2-Amino-6-(4-methylphenethyl)pyrido[3,2-d]pyrimidin-4-yl)amino)benzenesulfonamide | 9.71(s, 1H), 8.31(d,J=8.5 Hz,2H), 7.80(d, J=8.6 Hz, 2H), 7.62(d, J=8.6 Hz, 1H), 7.54(d, J=8.7 Hz, 1H), 7.31(s, 2H), 7.17(d, J=7.6 Hz, 2H), 7.09(d, J=7.5 Hz, 2H), 6.69(s, 2H), 3.17(t, 2H), 3.09(t, 2H), 2.25(s, 3H) | 159.79, 157.48, 155.27, 145.56, 142.28, 138.72, 137.99, 135.10, 132.98, 129.29, 128.95, 128.77, 127.08, 126.79, 120.32, 34.55, 29.48, 21.12 | ||||||||
| 12a | N2-(3-Chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidine-2,4-diamine | 9.34(s, 1H), 8.32(dd,J=6.9, 2.5 Hz, 1H), 7.91—7.83(m, 1H), 7.75(d, J=8.6 Hz, 1H), 7.57(d, J=8.6 Hz, 1H), 7.54(s, 2H), 7.32(t, J=9.1 Hz, 1H), 2.62(s, 3H) | 162.06, 156.74, 153.43, 153.31(d, J=238 Hz), 144.66, 139.27, 133.77, 129.20, 128.40, 119.74(d, J=29 Hz), 118.99(d, J=23.6 Hz), 116.88, 116.67, 24.44 | ||||||||
| 12b | 6-Methyl-N2-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 8.96(s, 1H), 7.74(d,J=8.5 Hz, 1H), 7.56(d, J=8.6 Hz, 1H), 7.45(s, 2H), 7.44(s, 2H), 3.83(s, 6H), 3.66(s, 3H), 2.62(s, 3H) | 161.87, 157.06, 152.97, 144.88, 137.95, 133.81, 132.14, 129.06, 128.31, 97.03, 60.58, 56.13, 24.43 | ||||||||
| 12c | 4-((4-Amino-6-methylpyrido[3,2-d]pyri-midin-2-yl)amino)benzenesulfonamide | 10.95(s, 1H), 9.27(d,J=97.5 Hz, 2H), 7.95(d, J=8.6 Hz, 1H), 7.87(m, J=20.7, 8.9 Hz, 4H), 7.77(d, J=8.6 Hz, 1H), 7.38(s, 2H), 2.65(s, 3H) | 162.93, 156.57, 153.04, 150.33, 140.90, 139.87, 131.09, 127.17, 126.99, 126.40, 121.56, 24.07 | ||||||||
| 14a | N4-(3-Chloro-4-fluorophenyl)-6-methylpyrido[3,2-d]pyrimidine-2,4-diamine | 9.68(s, 1H), 8.43(dd,J=6.8, 2.3 Hz, 1H), 8.18—8.04(m, 1H), 7.62(d, J=8.6 Hz, 1H), 7.53(d, J=8.6 Hz, 1H), 7.41(t, J=9.1 Hz, 1H), 6.58(s, 2H), 2.64(s, 3H) | 159.95, 157.58, 154.55, 152.14(d, J=236 Hz), 145.97, 137.09, 133.11, 129.26, 127.15, 122.54, 121.47, 119.40(d, J=18.2 Hz), 116.87(d, J=21.5 Hz), 24.29 | ||||||||
| 14b | 6-Methyl-N4-(3,4,5-trimethoxyphenyl)pyrido[3,2-d]pyrimidine-2,4-diamine | 9.37(s, 1H), 7.60(d,J=8.6 Hz, 1H), 7.53(s, 2H), 7.51(d, J=8.6 Hz, 1H), 6.51(s, 2H), 3.85(s, 6H), 3.66(s, 3H), 2.63(s, 3H) | 159.99, 157.44, 153.12, 151.85, 145.66, 135.67, 133.53, 133.08, 129.09, 127.29, 98.78, 60.55, 56.40, 24.72 | ||||||||
| 14c | 4-((2-Amino-6-methylpyrido[3,2-d]pyri-midin-4-yl)amino)benzenesulfonamide | 9.78(s, 1H, NH-4), 8.34(d, J=8.8 Hz, 2H), 7.81(d, J=8.8 Hz, 2H), 7.65(d, J=8.6 Hz, 1H), 7.56(d, J=8.6 Hz, 1H), 7.32(s, 2H), 6.65(s, 2H), 2.65(s, 3H) | 159.90, 157.44, 152.29, 146.07, 142.63, 137.96, 133.10, 129.39, 127.08, 126.76, 120.46, 24.32 | ||||||||
| Compd. | m. p./℃ | MS, m/z[M+H]+ | Compd. | m. p./℃ | MS, m/z[M+H]+ |
|---|---|---|---|---|---|
| 6a | 139—140 | 408.13 | 12a | 172—173 | 304.07 |
| 6b | 129—130 | 446.21 | 12b | 171—172 | 342.15 |
| 6c | 233—234 | 435.15 | 12c | >300 | 331.09 |
| 9a | 183—184 | 408.13 | 14a | 221 | 323.20 |
| 9b | 165—166 | 446.21 | 14b | 164 | 361.10 |
| 9c | 266—267 | 435.15 | 14c | 290—291 | 350.02 |
| Compd. | m. p./℃ | MS, m/z[M+H]+ | Compd. | m. p./℃ | MS, m/z[M+H]+ |
|---|---|---|---|---|---|
| 6a | 139—140 | 408.13 | 12a | 172—173 | 304.07 |
| 6b | 129—130 | 446.21 | 12b | 171—172 | 342.15 |
| 6c | 233—234 | 435.15 | 12c | >300 | 331.09 |
| 9a | 183—184 | 408.13 | 14a | 221 | 323.20 |
| 9b | 165—166 | 446.21 | 14b | 164 | 361.10 |
| 9c | 266—267 | 435.15 | 14c | 290—291 | 350.02 |
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|---|---|
| HL-60 | A549 | HCT116 | HL-60 | A549 | HCT116 | ||
| 6a | 12.46±1.97 | 6.72±3.42 | 23.11±2.96 | 12b | 30.75±2.17 | 34.91±18.30 | 35.36±0.08 |
| 6b | 4.09±0.48 | 17.99±7.20 | 14.52±4.74 | 12c | 39.29±5.45 | 62.51±10.23 | >100 |
| 6c | 6.57±0.17 | 22.21±8.99 | >100 | 14a | 15.43±582 | 30.91±0.28 | 16.51±11.23 |
| 9a | 18.39±10.54 | ND | ND | 14b | 6.20±0.60 | 18.86±4.48 | 16.67±7.10 |
| 9b | 6.74±1.44 | 16.57±1.64 | 13.70±1.02 | 14c | 65.35±12.89 | 41.82±7.01 | 39.99±6.67 |
| 9c | 5.28±1.70 | 20.02±2.40 | 22.28±0.01 | wm-5b | 0.07±0.05 | 3.25±1.20 | 4.68±3.93 |
| 12a | 20.35±2.35 | 24.40±4.88 | 32.58±3.43 | MTX | 0.023±0.001 | 0.041±0.02 | 0.281±4.56 |
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|---|---|
| HL-60 | A549 | HCT116 | HL-60 | A549 | HCT116 | ||
| 6a | 12.46±1.97 | 6.72±3.42 | 23.11±2.96 | 12b | 30.75±2.17 | 34.91±18.30 | 35.36±0.08 |
| 6b | 4.09±0.48 | 17.99±7.20 | 14.52±4.74 | 12c | 39.29±5.45 | 62.51±10.23 | >100 |
| 6c | 6.57±0.17 | 22.21±8.99 | >100 | 14a | 15.43±582 | 30.91±0.28 | 16.51±11.23 |
| 9a | 18.39±10.54 | ND | ND | 14b | 6.20±0.60 | 18.86±4.48 | 16.67±7.10 |
| 9b | 6.74±1.44 | 16.57±1.64 | 13.70±1.02 | 14c | 65.35±12.89 | 41.82±7.01 | 39.99±6.67 |
| 9c | 5.28±1.70 | 20.02±2.40 | 22.28±0.01 | wm-5b | 0.07±0.05 | 3.25±1.20 | 4.68±3.93 |
| 12a | 20.35±2.35 | 24.40±4.88 | 32.58±3.43 | MTX | 0.023±0.001 | 0.041±0.02 | 0.281±4.56 |
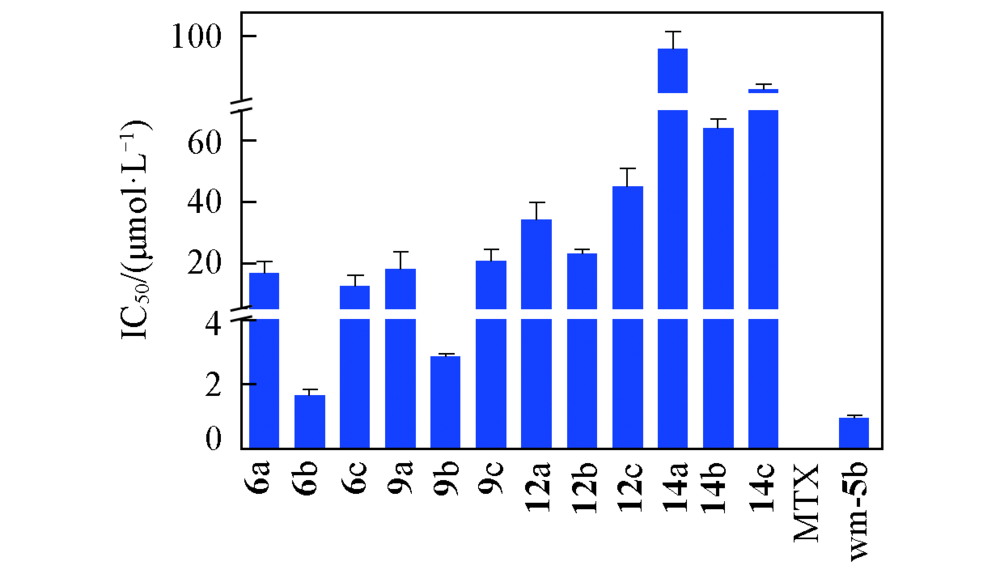
Fig.1 IC50 values of target compounds against DHFR Results are presented as mean values±standard errors from three replicates; MTX showed rhDHFR inhibitory potency with IC50 value of (0.0094±0.0002) μmol/L; calculated by three replicates.
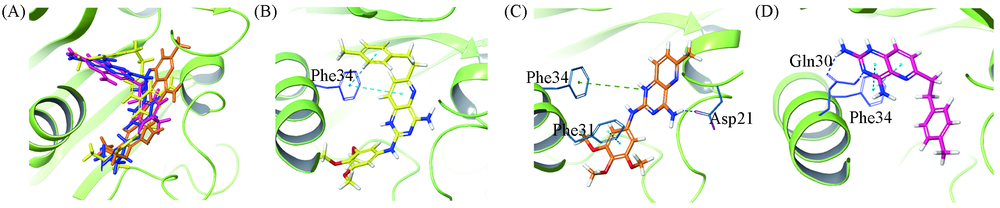
Fig.2 Results of docking of different compouds (A) Docking of the overlay of the original ligand and compounds wm-5b, 6b and 12b in rhDHFR; (B) docking of compound 6b; (C) docking of compound 12b; (D) docking of compound wm-5b. MTX: blue; compound 6b: yellow; compound 12b: orange and compound wm-5b: red(PDB code 4QJC).
| [1] | Filiz E. O. B., Hande S., Hulya A. , Pteridines, 2015,13(3), 85— 92 |
| [2] | Claudia M. H., Elisa T., Marilu F., Serena V. , Expert Opinion on Drug Metabolism & Toxicology, 2017,26(3), 245— 257 |
| [3] | Xia Q., Li R. L. , World Pharmacy, 1997,18(3), 135— 140 |
| ( 夏青, 李仁利 . 世界临床药物, 1997,18(3), 135— 140 | |
| [4] | Brigle K. E., Spinella M. J., Sierra E. E., Goldman I. D. , J. Biol. Chem., 1995,270(39), 22974— 22979 |
| [5] | Robert M., Godefridus J. P., David G. P., Yehuda G. A., Stavit D. Ietje K., Paul N., Marlene A. B., Andre R., Jan H. S., Herbert M. P., Gerrit J. , Biochemical Pharmacology, 2002,63(2), 105— 115 |
| [6] | Raz S., Stark M., Assaraf Y. G. , Drug Resistance Updates, 2016,28, 43— 64 |
| [7] | Lilah R., Ilan I., Yotam K., David G. P., Gerrit J., Yehuda G. A. , Biochem. J., 2002,367(3), 741— 750 |
| [8] | Assaraf Y. G., Schimke R. T. , Proc. Nat. Acad. Sci.USA, 1987,84(20), 7154— 7158 |
| [9] | Taylor I. W., Slowiaczek P., Friedlander M. L., Tattersall M. H. , Cancer Research, 1985,45(3), 978— 982 |
| [10] | Webber S., Bartlett C. A., Boritzki T. J., Hillard J. A., Howland E. F. Johnston A. L., Kosa M., Margosiak S. A., Morse C. A., Shetty B. V. , Cancer Chemotherapy and Pharmacology, 1996,37(6), 509— 517 |
| [11] | Carbain B., Coxon C. R. , Lebraud H., Elliott K. J., Matheson C. J., Meschini E., Roberts A. R., Turner D. M., Wong C., Cano C., Griffin R. J., Hardcastle I. R., Golding B. T. , Chem. Eur. J., 2014,20, 2311— 2317 |
| [12] | Andrew J. B., Gibson K. H., Walter G., Andrew A. G., Jeffrey J. B., Mark P. H., James R. W., Susan E. A., Brenda J. C., Lynn S., Lianne H., Laura R. , Bio. & Med. Chem. Lett., 2001,14(11), 1911— 1914 |
| [13] | Wissner A., Mansour T. S. , Arch. Pharm. Chem. Life Sci., 2008,341, 465— 477 |
| [14] | Kong X. L. , J. Int. Oncol., 1992,19(3) , 144— 146 |
| ( 孔祥林 . 国际肿瘤学杂志, 1992,19(3), 144— 146) | |
| [15] | Ernst S., Stephane B., Riazul A., Mathew P. , J. Med. Chem., 2013,56, 3768— 3782 |
| [16] | Liu X. R., Shi S. H., Lam F., Pepper C., Fischer P. M., Wang S. D. , Int. J. Cancer, 2012,130, 1216— 1226 |
| [17] | Lam F., Abbas A. Y., Shao H., Teo T., Adams J. Li P., Bradshaw T. D., Fischer P. M., Walsby E., Pepper C., Chen Y., Ding J., Wang S. D. , Oncotarget, 2014,17(5), 7691— 7704 |
| [18] | Tao Z., Cheng H. B., Zhou J. P., Zhang H. B. , Progress in Pharmaceutical Sciences, 2013,37(6), 241— 248 |
| ( 陶卓, 程海博, 周金培, 张惠斌 . 药学进展, 2013,37(6), 241— 248) | |
| [19] | Wang M., Yang J. J., Yuan M. M., Xue L. M., Li H., Tian C., Wang X. W., Liu J. Y., Zhang L. L. , Eur. J. Med., 2017,128, 88— 97 |
| [20] | El-Subbagh H. I., Hassan G. S., El-Messery S. M., Al-Rashood S. T., Al-Omary F. A. M., Abulfadl Y. S., Shabayek M. , Eur. J. Med. Chem., 2014,74, 234— 245 |
| [21] | He X., Gong H., Li L., Guo Y. D. , Guangdong Chemical Industry, 2015,45(2), 70— 71 |
| ( 何兴, 龚浩, 李玲, 郭义东 . 广东化工, 2015,45(2), 70— 71) | |
| [22] | Carbain B., Coxoh C. R., Lebraud H., Elliott K. J., Matheson C. J., Meschini E., Roberts A. R., Turner D. M., Wong C., Cano C., Griffin R. J., Hardcastle I. R., Golding B. T. , Chem. Eur. J., 2014,20(8), 2311— 2317 |
| [1] | XIAO Yanhua, ZHANG Guangjie, ZONG Liang, LIU Guohong, REN Lijun, DONG Junxing. Chemical Constituents and Antitumor Activity of Tupistra chinensis † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1897. |
| [2] | LÜ Mingjun,LI Wen,YANG Xinying,FANG Hao. Synthesis and Antitumor Activity of N9 Position Aromatic Substituted Purine-8-one Derivatives† [J]. Chem. J. Chinese Universities, 2019, 40(2): 254. |
| [3] | ZHANG Peiquan,YANG Qianqian,LONG Huidan,CHEN Xin. Synthesis and Antitumor Activity of Auranofin Derivatives † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2097. |
| [4] | BAI Xinfa, MA Xuan, XIE Xiaoxia, SHAO Mingsha, GUO Ningning, YAN Ning, YAO Lei. Synthesis and Anti-tumor Activity of Tubulysins Analogues† [J]. Chem. J. Chinese Universities, 2017, 38(1): 47. |
| [5] | GUO Liang, CAO Rihui, FAN Wenxi, GAN Ziyun, MA Qin. Design, Synthesis and in vitro Antitumor Activities of Novel Bivalent β-Carbolines† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1093. |
| [6] | ZHANG Jie, ZHOU Changjian, XIE Jianwei, DAI Bin. Synthesis and Antitumor Activities of Rhein-Valine Adducts† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2159. |
| [7] | ZHOU Hao, DUAN Zhigang, ZHAO Shuang, BAO Meiying, LI Zhiwei, PEI Yazhong. Design and Synthesis of Phenylpyrimidine and Their Anticancer Activity† [J]. Chem. J. Chinese Universities, 2015, 36(9): 1694. |
| [8] | WANG Gang, HAN Leiqiang, FANG Hao. Syntheses and Antitumor Activities of Phenylpiperazine Derivatives† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2435. |
| [9] | GUO Hua, YANG Chengling, WANG Wei, LAI Quanyong, YUAN Zhi. Preparation of Liver-targeted Nano-prodrug Based on Sodium Alginate Derivative and the Study on Antitumor Activity† [J]. Chem. J. Chinese Universities, 2014, 35(8): 1835. |
| [10] | SONG Xiudao, HE Jun, MA Jin, LIU Yunmei, ZHENG Xing, LEI Xiaoyong, GUO Yu. Syntheses and Anticancer Activities of Glycine Derivatives of Chrysin† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1465. |
| [11] | WANG Junhua, WANG Quande, DUN Yanyan, FANG Hao. Syntheses and Antitumor Activities of Purine-sulfonamides Derivatives† [J]. Chem. J. Chinese Universities, 2014, 35(6): 1189. |
| [12] | GUO Liang, CAO Rihui, FAN Wenxi, MA Qin. Synthesis and Biological Evaluation of 1,2,7,9-Tetrasubstituted Harmine Derivatives as Potential Antitumor Agents† [J]. Chem. J. Chinese Universities, 2014, 35(3): 518. |
| [13] | YANG Haikui, XU Wanfu, DUAN Anna, YOU Wenwei, ZHAO Peiliang. Syntheses and Biological Activities of Novel Imine and Imide Derivatives Bearing 1,2,4-Triazole Moiety† [J]. Chem. J. Chinese Universities, 2014, 35(3): 555. |
| [14] | YANG Hongliang, XU Guoxing, BAO Meiying, ZHANG Dapeng, LI Zhiwei, PEI Yazhong. Design and Synthesis of Pyridinylisoxazoles and Their Anticancer Activities† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2584. |
| [15] | DING Guobin, LI Binchun, GUO Yi, XU Li. Spectral Properties and Antitumor Activity of 10-Hydroxycamptothecin and Its Application in Cell Labeling† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2324. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||