

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (1): 56.doi: 10.7503/cjcu20160699
• Physical Chemistry • Previous Articles Next Articles
LI Lei, LI Shushi*( ), WANG Changsheng*(
), WANG Changsheng*( )
)
Received:2016-09-29
Online:2017-01-10
Published:2016-12-19
Contact:
LI Shushi,WANG Changsheng
E-mail:lishushi101@163.com;chwangcs@lnnu.edu.cn
Supported by:CLC Number:
TrendMD:
LI Lei, LI Shushi, WANG Changsheng. Theoretical Studies on Noncovalent Interactions Between Charged Histidine Side Chain and DNA Base†[J]. Chem. J. Chinese Universities, 2017, 38(1): 56.
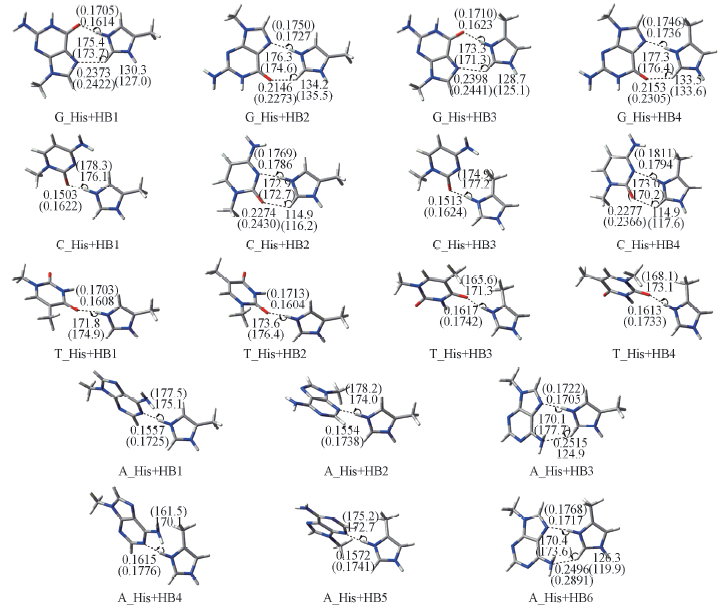
Fig.1 Structures of H-bonded complexes composed of histidine side chain and four basesThe hydrogen bond distances RNH…X(nm) and bond angles ∠NHX(°) in gas phase and in water solvent are given in the corresponding positions and in parentheses where X=N or O. The optimal structures of hydrogen-bonded complexes in gas phase are obtained at the MP2/6-31+G(d,p) level and the optimal structures in water solvent were obtained at the MP2/6-31+G(d,p) level with PCM model.
| Complex | Gas phase | Water solvent | |||||
|---|---|---|---|---|---|---|---|
| Eb(g)CCSD(T)/ (kJ·mol-1) | Eb(g)MP2/ (kJ·mol-1) | Eb(g)M06/ (kJ·mol-1) | Eb1(w)MP2/ (kJ·mol-1) | Eb1(w)M06/ (kJ·mol-1) | Eb2(w)MP2/ (kJ·mol-1) | ||
| G_His+HB1 | -153.80 | -153.57 | -54.94 | -49.24 | -52.67 | ||
| G_His+HB2 | -154.76 | -153.88 | -57.73 | -51.41 | -55.99 | ||
| G_His+HB3 | -154.77 | -154.43 | -56.04 | -50.13 | -53.65 | ||
| G_His+HB4 | -154.71 | -153.99 | -59.10 | -52.87 | -57.02 | ||
| C_His+HB1 | -145.98 | -146.81 | -56.52 | -51.69 | -56.04 | ||
| C_His+HB2 | -139.75 | -141.80 | -55.14 | -51.12 | -54.60 | ||
| C_His+HB3 | -146.90 | -147.54 | -59.28 | -53.63 | -58.07 | ||
| C_His+HB4 | -139.32 | -141.11 | -56.41 | -51.91 | -54.48 | ||
| T_His+HB1 | -95.65 | -93.99 | -35.72 | -29.62 | -37.54 | ||
| T_His+HB2 | -88.24 | -86.02 | -34.97 | -28.96 | -36.58 | ||
| T_His+HB3 | -95.98 | -94.06 | -36.90 | -30.76 | -40.62 | ||
| T_His+HB4 | -88.20 | -85.94 | -36.27 | -30.25 | -38.90 | ||
| A_His+HB1 | -99.62 | -96.64 | -41.72 | -34.97 | -43.39 | ||
| A_His+HB2 | -95.40 | -94.97 | -43.97 | -39.46 | -44.99 | ||
| A_His+HB3 | -95.48 | -90.78 | -39.45 | -30.48 | -43.56 | ||
| A_His+HB4 | -101.42 | -97.40 | -44.84 | -36.86 | -45.66 | ||
| A_His+HB5 | -95.40 | -94.63 | -46.68 | -41.67 | -47.18 | ||
| A_His+HB6 | -94.93 | -89.81 | -40.60 | -31.18 | -42.46 | ||
| G_His+ST | -68.8 | -72.05 | -69.12 | -25.52 | -18.02 | ||
| C_His+ST | -61.4 | -63.09 | -61.81 | -21.77 | -16.96 | ||
| T_His+ST | -34.8 | -38.36 | -36.04 | -26.26 | -20.71 | ||
| A_His+ST | -54.4 | -62.58 | -57.47 | -39.44 | -28.93 | ||
| G_His+TS1 | -108.9 | -108.49 | -109.36 | -22.40 | -18.82 | ||
| G_His+TS2 | -85.6 | -84.66 | -84.86 | -22.92 | -17.92 | ||
| C_His+TS1 | -97.8 | -96.03 | -99.45 | -23.13 | -21.53 | ||
| C_His+TS2 | -88.1 | -87.63 | -87.34 | -26.27 | -22.21 | ||
| T_His+TS1 | -54.8 | -53.61 | -55.08 | -13.62 | -11.76 | ||
| T_His+TS2 | -51.0 | -51.41 | -50.71 | -21.23 | -17.64 | ||
| A_His+TS1 | -43.0 | -44.59 | -42.37 | -16.19 | -12.61 | ||
| A_His+TS2 | -63.6 | -65.98 | -60.16 | -26.08 | -18.99 | ||
Table 1 Binding energies(Eb) for the H-bonded, stacking and T-shaped complexes*
| Complex | Gas phase | Water solvent | |||||
|---|---|---|---|---|---|---|---|
| Eb(g)CCSD(T)/ (kJ·mol-1) | Eb(g)MP2/ (kJ·mol-1) | Eb(g)M06/ (kJ·mol-1) | Eb1(w)MP2/ (kJ·mol-1) | Eb1(w)M06/ (kJ·mol-1) | Eb2(w)MP2/ (kJ·mol-1) | ||
| G_His+HB1 | -153.80 | -153.57 | -54.94 | -49.24 | -52.67 | ||
| G_His+HB2 | -154.76 | -153.88 | -57.73 | -51.41 | -55.99 | ||
| G_His+HB3 | -154.77 | -154.43 | -56.04 | -50.13 | -53.65 | ||
| G_His+HB4 | -154.71 | -153.99 | -59.10 | -52.87 | -57.02 | ||
| C_His+HB1 | -145.98 | -146.81 | -56.52 | -51.69 | -56.04 | ||
| C_His+HB2 | -139.75 | -141.80 | -55.14 | -51.12 | -54.60 | ||
| C_His+HB3 | -146.90 | -147.54 | -59.28 | -53.63 | -58.07 | ||
| C_His+HB4 | -139.32 | -141.11 | -56.41 | -51.91 | -54.48 | ||
| T_His+HB1 | -95.65 | -93.99 | -35.72 | -29.62 | -37.54 | ||
| T_His+HB2 | -88.24 | -86.02 | -34.97 | -28.96 | -36.58 | ||
| T_His+HB3 | -95.98 | -94.06 | -36.90 | -30.76 | -40.62 | ||
| T_His+HB4 | -88.20 | -85.94 | -36.27 | -30.25 | -38.90 | ||
| A_His+HB1 | -99.62 | -96.64 | -41.72 | -34.97 | -43.39 | ||
| A_His+HB2 | -95.40 | -94.97 | -43.97 | -39.46 | -44.99 | ||
| A_His+HB3 | -95.48 | -90.78 | -39.45 | -30.48 | -43.56 | ||
| A_His+HB4 | -101.42 | -97.40 | -44.84 | -36.86 | -45.66 | ||
| A_His+HB5 | -95.40 | -94.63 | -46.68 | -41.67 | -47.18 | ||
| A_His+HB6 | -94.93 | -89.81 | -40.60 | -31.18 | -42.46 | ||
| G_His+ST | -68.8 | -72.05 | -69.12 | -25.52 | -18.02 | ||
| C_His+ST | -61.4 | -63.09 | -61.81 | -21.77 | -16.96 | ||
| T_His+ST | -34.8 | -38.36 | -36.04 | -26.26 | -20.71 | ||
| A_His+ST | -54.4 | -62.58 | -57.47 | -39.44 | -28.93 | ||
| G_His+TS1 | -108.9 | -108.49 | -109.36 | -22.40 | -18.82 | ||
| G_His+TS2 | -85.6 | -84.66 | -84.86 | -22.92 | -17.92 | ||
| C_His+TS1 | -97.8 | -96.03 | -99.45 | -23.13 | -21.53 | ||
| C_His+TS2 | -88.1 | -87.63 | -87.34 | -26.27 | -22.21 | ||
| T_His+TS1 | -54.8 | -53.61 | -55.08 | -13.62 | -11.76 | ||
| T_His+TS2 | -51.0 | -51.41 | -50.71 | -21.23 | -17.64 | ||
| A_His+TS1 | -43.0 | -44.59 | -42.37 | -16.19 | -12.61 | ||
| A_His+TS2 | -63.6 | -65.98 | -60.16 | -26.08 | -18.99 | ||
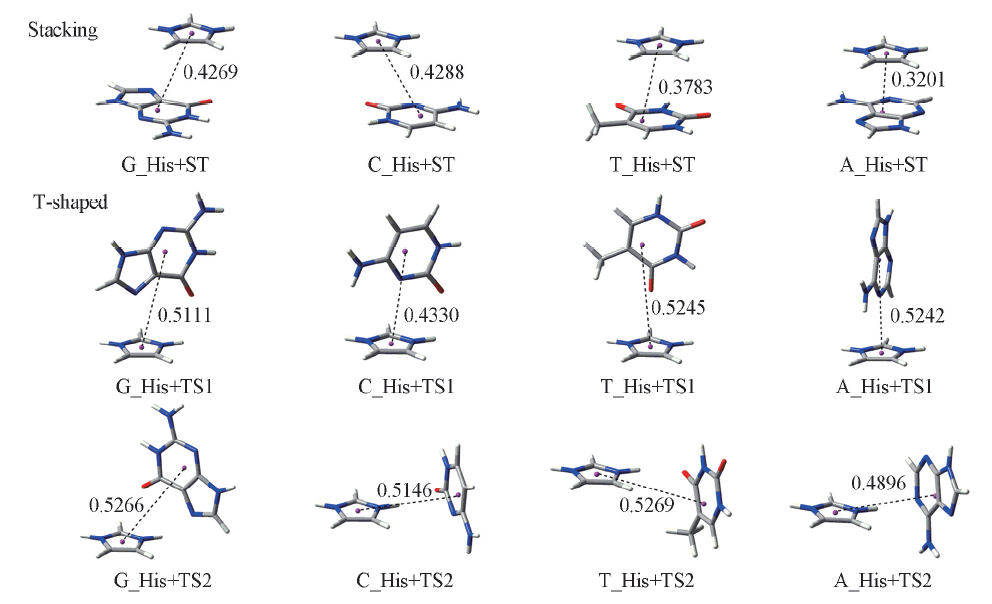
Fig.2 Structures of stacking and T-shaped complexes composed of histidine side chain and four bases The structures of π-π+ complexes are obtained from ref. [29]. The equilibrium intermolecular distances are in nm.
| [1] | Müller-Dethlefs K., Hobza P., Chem. Rev., 2000, 100(1), 143—167 |
| [2] | Li Y., Wang C. S., J. Comput. Chem., 2011, 32(13), 2765—2773 |
| [3] | Wang C. S., Yu N., Liu P., Acta Phys. Chim. Sin., 2013, 29(6), 1173—1182 |
| (王长生, 于楠, 刘朋. 物理化学学报, 2013, 29(6), 1173—1182) | |
| [4] | Liu P., Li S., Wang C. S., Chem. J. Chinese Universities,2014, 35(1), 154—160 |
| (刘朋, 刘帅, 王长生. 高等学校化学学报, 2014, 35(1), 154—160) | |
| [5] | Zhao G. J., Han K. L., Acc. Chem. Res., 2012, 45(3), 404—413 |
| [6] | Ganguly A., Manahan C. C., Top D., Yee E. F., Lin C., Young M. W., Thiel W., Crane B. R., Proceedings of the National Academy of Sciences,2016, 113(36), 10073—10078 |
| [7] | Chou S. T., Hom K., Zhang D., Leng Q., Tricoli L. J., Hustedt J. M., Lee A., Shapiro M. J., Seog J., Kahn J. D., Mixson A. J., Biomaterials,2014, 35(2), 846—855 |
| [8] | Liu D. J., Wang C. S., Acta Phys. Chim. Sin., 2012, 28(12), 2809—2816 |
| (刘东佳, 王长生. 物理化学学报, 2012, 28(12), 2809—2816) | |
| [9] | Sun L., Cukier R. I., Bu Y., J. Phys. Chem. B,2007, 111(7), 1802—1808 |
| [10] | Liu C., Yu G., Huang C. Y., Wang C. S., Acta Chim. Sinica,2015, 73(4), 357—365 |
| (刘畅, 于歌, 黄翠英, 王长生. 化学学报, 2015, 73(4), 357—365) | |
| [11] | Rutledge L. R., Wetmore S. D., Can. J. Chem., 2010, 88(8), 815—830 |
| [12] | Li X. H., Ma H. M., Dong S. Y., Duan X. J., Nie L. H., Sun M., Chem. J. Chinese Universities,2003, 24(11), 154—160 |
| (李晓花, 马会民, 董素英, 段学军, 聂丽华, 孙铭. 高等学校化学学报, 2003, 24(11), 154—160) | |
| [13] | Churchill C. D. M., Wetmore S. D., J. Phys. Chem. B,2009, 113(49), 16046—16058 |
| [14] | Meot-Ner M., Chem. Rev., 2012, 112(1), 22—103 |
| [15] | Biedermann F., Schneider H. J., Chem. Rev., 2016, 116(9), 5216—5300 |
| [16] | Okada C., Yamashita E., Lee S. J., Shibata S., Katahira J., Nakagawa A., Yoneda Y., Tsukihara T., Science,2009, 236(5957), 1275—1279 |
| [17] | Wang P., Zhang J. Z. H., J. Phys. Chem. B,2010, 114(40), 12958—129640 |
| [18] | Wilson K. A., Kellie J. L., Wetmore S. D., Nucl. Acids Res., 2014, 42(10), 6726—6740 |
| [19] | Wilson K. A., Wells R. A., Abendong M. N., Anderson C. B., Kung R. W., Wetmore S. D., J. Biomol. Struct. Dyn., 2016, 34(1), 184—197 |
| [20] | Gu J., Wang J., Leszczynski J., J. Phys. Chem. B,2006, 110(27), 13590—13596 |
| [21] | Nguyen T. T., Mai B. K., Li M. S., J. Chem. Inf. Model., 2011, 51(9), 2266—2276 |
| [22] | Ramírez-Salinas G. L., García-Machorro J., Quiliano M., Zimic M., Briz V., Rojas-Hernández S., Correa-Basurto J., J. Mol. Model,2015, 21(11), 292—305 |
| [23] | Wang J., Gu J., Leszczynski J., Chem. Phys. Lett., 2007, 442(1—3), 124—127 |
| [24] | Baker C. M., Grant G. H., Biopolymers,2007, 85(5/6), 456—470 |
| [25] | Lambert A. R., Sussman D., Shen B., Maunus R., Nix J., Samuelson J., Xu S. Y., Stoddard B. L., Structure,2008, 16(4), 558—569 |
| [26] | Kaus-Drobek M., Czapinska H., Sokołowska M., Tamulaitis G., Szczepanowski R. H., Urbanke C., Siksnys V., Bochtler M., Nucl. Acids Res., 2007, 35(6), 2035—2046 |
| [27] | Golovenko D., Manakova E., Tamulaitiene G., Grazulis S., Siksnys V., Nucl. Acids Res., 2009, 37(19), 6613—6624 |
| [28] | Huber E. M., Scharf D. H., Hortschansky P., Groll M., Brakhage A. A., Structure,2012, 20(10), 1757—1768 |
| [29] | Cauet E., Rooman M., Wintjens R., Lievin J., Biot C., J. Chem. Theory Comput., 2005, 1(3), 472—483 |
| [30] | Wintjens R., Rooman M., Wodak S. J., J. Mol. Biol., 1996, 255(1), 235—253 |
| [31] | Biot C., Buisine E., Kwasigroch J. M., Wintjens R., Rooman M., J. Biol. Chem., 2002, 277(43), 40816—40822 |
| [32] | Rooman M., Lievin J., Buisine E., Wintjens R., J. Mol. Biol., 2002, 319(1), 67—76 |
| [33] | Riley K. E., Hobza P., Comput. Mol. Sci., 2011, 1(1), 3—7 |
| [34] | Tomasia J., Mennucci B., Cancèsb E., J. Mol. Struct. Theochem., 1999, 464(1—3), 211—226 |
| [35] | Steindal A. H., Ruud K., Frediani L., Aidas K., Kongsted J., J. Phys. Chem. B,2011, 115(12), 3027—3037 |
| [36] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaor F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision D. 01, Gaussian Inc., Wallingford, CT, 2013 |
| [37] | Riley K. E., Hobza P., J. Phys. Chem. A,2007, 111(33), 8257—8263 |
| [1] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [2] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [3] | WANG Sijia, HOU Lu, LI Chenglong, LI Wencui, LU Anhui. Recent Advances in Synthesis and Applications of Hollow Nano-carbons [J]. Chem. J. Chinese Universities, 0, (): 20220637. |
| [4] | WU Qingying, ZHU Zhenyu, WU Jianming, XU Xin. A Dataset Representativeness Metric and A Slicing Sampling Strategy for the Kennard-Stone Algorithm [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220397. |
| [5] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [6] | ZHANG Lingyu, ZHANG Jilong, QU Zexing. Dynamics Study of Intramolecular Vibrational Energy Redistribution in RDX Molecule [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220393. |
| [7] | SHEN Qi, CHEN Haiyao, GAO Denghui, ZHAO Xi, NA Risong, LIU Jia, HUANG Xuri. A Study on the Interaction Mechanism of the Natural Product Falcarindiol with Human GABAA Receptor [J]. Chem. J. Chinese Universities, 0, (): 0. |
| [8] | CHEN Shaochen, CHENG Min, WANG Shihui, WU Jinkui, LUO Lei, XUE Xiaoyu, JI Xu, ZHANG Changchun, ZHOU Li. Transfer Learning Modeling for Predicting the Methane and Hydrogen Delivery Capacity of Metal-Organic Frameworks [J]. Chem. J. Chinese Universities, 0, (): 20220459. |
| [9] | PENG Xinzhe, GE Jiaoyang, WANG Fangli, YU Guojing, ZHOU Dong, RAN Xueqin, YANG Lei, XIE Linghai. A Theoretical Study on Tension and Reorganization Energy of Benzothiophene Grid [J]. Chem. J. Chinese Universities, 0, (): 20220313. |
| [10] | GUO Cheng, ZHANG Wei, TANG Yun. Ordered Mesoporous Materials: History, Progress and Perspective [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220167. |
| [11] | TANG Qiaowei, CAI Xiaoqing, LI Jiang, ZHU Ying, WANG Lihua, TIAN Yang, FAN Chunhai, HU Jun. Synchrotron-based X-ray Microscopy for Brain Imaging [J]. Chem. J. Chinese Universities, 0, (): 20220379. |
| [12] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [13] | DAI Wei, HOU Hua, WANG Baoshan. Theoretical Investigations on the Electronic Structures and Reactivity of Heptafluoro-iso-butyronitrile Anion [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220044. |
| [14] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [15] | REN Nana, XUE Jie, WANG Zhifan, YAO Xiaoxia, WANG Fan. Effects of Thermodynamic Data on Combustion Characters of 1,3-Butadiene [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220151. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||