

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (3): 555.doi: 10.7503/cjcu20130543
• Organic Chemistry • Previous Articles Next Articles
YANG Haikui1, XU Wanfu2, DUAN Anna1, YOU Wenwei1,*( ), ZHAO Peiliang1,*(
), ZHAO Peiliang1,*( )
)
Received:2013-06-13
Online:2014-03-10
Published:2013-09-02
Contact:
YOU Wenwei,ZHAO Peiliang
E-mail:youww@smu.edu.cn;plzhao@smu.edu.cn
Supported by:CLC Number:
TrendMD:
YANG Haikui, XU Wanfu, DUAN Anna, YOU Wenwei, ZHAO Peiliang. Syntheses and Biological Activities of Novel Imine and Imide Derivatives Bearing 1,2,4-Triazole Moiety†[J]. Chem. J. Chinese Universities, 2014, 35(3): 555.
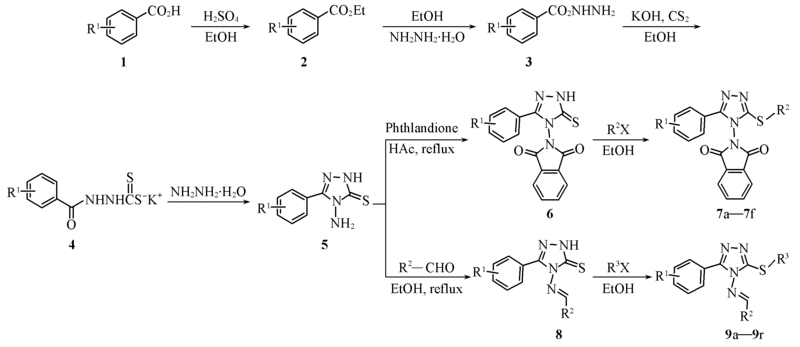
Scheme 1 Synthetic routes of the title compounds7a: R1=H, R2=4-FC6H4CH2; 7b: R1=H, R2=CH≡≡CCH2; 7c: R1=H, R2=4-ClC6H4COCH2; 7d: R1=H, R2=C6H5CH2; 7e: R1=H, R2=Me; 7f: R1=H, R2=4-Me C6H4COCH2; 9a: R1=H, R2=C6H5, R3=Me; 9b: R1=H, R2=C6H5, R3=C6H5CH2; 9c: R1=H, R2=C6H5, R3=CH2C=CH2; 9d: R1=H, R2=C6H5, R3=4-FC6H4CH2; 9e: R1=H, R2=C6H5, R3=CH≡≡CCH2; 9f: R1=3,4,5-(MeO)3, R2=3,4,5-(MeO)3C6H2, R3=Me; 9g: R1=3,4,5-(MeO)3, R2=3,4,5-(MeO)3C6H2, R3=CH2C=CH2; 9h: R1=3,4,5-(MeO)3, R2=3,4,5-(MeO)3C6H2, R3=CH≡≡CCH2; 9i: R1=3,4,5-(MeO)3, R2=3,4,5-(MeO)3C6H2, R3=4-FC6H4CH2; 9j: R1=3,4,5-(MeO)3, R2=3,4,5-(MeO)3C6H2, R3=2-ClC6H4CH2; 9k: R1=3,4,5-(MeO)3, R2=3,4,5-(MeO)3C6H2, R3=4-ClC6H4COCH2; 9l: R1=3,4,5-(MeO)3, R2=3,4,5-(MeO)3C6H2, R3=C6H5CH2; 9m: R1=3,4,5-(MeO)3, R2=4-FC6H4, R3=Me; 9n: R1=3,4,5-(MeO)3, R2=4-FC6H4, R3=C6H5CH2; 9o: R1=3,4,5-(MeO)3, R2=4-FC6H4, R3=2-ClC6H4CH2; 9p: R1=3,4,5-(MeO)3, R2=4-FC6H4, R3=4-FC6H4CH2; 9q: R1=3,4,5-(MeO)3, R2=4-FC6H4, R3=CH≡≡CCH2; 9r: R1=3,4,5-(MeO)3, R2=4-FC6H4, R3=CH2C=CH2.
| Compd. | Inhibitory ratio(%) | ||||
|---|---|---|---|---|---|
| Fusarium oxysporum f.sp.cubense | Pestalotiopsis palmarum | Corynespora cmssiicola (Berk & Curt.)Wei | Colletotrichum gloeosporioides PenZ | Rhizoctonia solani | |
| 7a | ++ | - | - | - | + |
| 7b | + | - | - | - | - |
| 7c | - | - | - | - | + |
| 7d | - | - | - | + | + |
| 7e | + | - | - | - | - |
| 7f | + | - | - | - | + |
| 9a | ++ | + | + | + | ++++ |
| 9b | - | - | - | - | ++ |
| 9c | + | - | + | + | ++ |
| 9d | ++ | ++ | - | + | ++++ |
| 9e | + | - | + | + | ++++ |
| 9f | - | - | - | - | + |
| 9g | - | - | + | - | ++ |
| 9h | - | - | - | + | ++ |
| 9i | - | - | + | - | + |
| 9j | - | + | - | - | + |
| 9k | - | - | - | - | + |
| 9l | - | - | - | ++ | ++ |
| 9m | - | - | - | - | + |
| 9n | - | + | - | - | ++ |
| 9o | ++ | - | - | - | + |
| 9p | - | - | + | - | ++ |
| 9q | ++ | - | - | - | + |
| 9r | - | - | + | - | ++ |
| Flusilazole | ++++ | ++++ | ++++ | +++ | ++++ |
Table 1 Fungicidal activities of compounds 7a—7f and 9a—9r*
| Compd. | Inhibitory ratio(%) | ||||
|---|---|---|---|---|---|
| Fusarium oxysporum f.sp.cubense | Pestalotiopsis palmarum | Corynespora cmssiicola (Berk & Curt.)Wei | Colletotrichum gloeosporioides PenZ | Rhizoctonia solani | |
| 7a | ++ | - | - | - | + |
| 7b | + | - | - | - | - |
| 7c | - | - | - | - | + |
| 7d | - | - | - | + | + |
| 7e | + | - | - | - | - |
| 7f | + | - | - | - | + |
| 9a | ++ | + | + | + | ++++ |
| 9b | - | - | - | - | ++ |
| 9c | + | - | + | + | ++ |
| 9d | ++ | ++ | - | + | ++++ |
| 9e | + | - | + | + | ++++ |
| 9f | - | - | - | - | + |
| 9g | - | - | + | - | ++ |
| 9h | - | - | - | + | ++ |
| 9i | - | - | + | - | + |
| 9j | - | + | - | - | + |
| 9k | - | - | - | - | + |
| 9l | - | - | - | ++ | ++ |
| 9m | - | - | - | - | + |
| 9n | - | + | - | - | ++ |
| 9o | ++ | - | - | - | + |
| 9p | - | - | + | - | ++ |
| 9q | ++ | - | - | - | + |
| 9r | - | - | + | - | ++ |
| Flusilazole | ++++ | ++++ | ++++ | +++ | ++++ |
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|---|---|
| A549 | MD-MBA-231 | PC-3M | A549 | MD-MBA-231 | PC-3M | ||
| 7a | >200 | >200 | >200 | 9h | >200 | >200 | >200 |
| 7b | >200 | >200 | >200 | 9i | >200 | >200 | 91.6 |
| 7c | 38.3 | >200 | >200 | 9j | >200 | >200 | 98.0 |
| 7d | >200 | >200 | >200 | 9k | 36.7 | 147.5 | 60.7 |
| 7e | >200 | >200 | >200 | 9l | >200 | >200 | >200 |
| 7f | 44.6 | >200 | >200 | 9m | >200 | >200 | >200 |
| 9a | >200 | >200 | 34.1 | 9n | >200 | 89.6 | >200 |
| 9b | >200 | >200 | 97.9 | 9o | >200 | >200 | 59.8 |
| 9c | >200 | >200 | >200 | 9p | >200 | >200 | >200 |
| 9d | 104.6 | 59.8 | >200 | 9q | >200 | >200 | 61.3 |
| 9e | >200 | >200 | 41.1 | 9r | >200 | >200 | >200 |
| 9f | >200 | >200 | 99.2 | 5-Fu | 35.4 | 14.2 | 22.6 |
| 9g | >200 | >200 | 73.2 | ||||
Table 2 IC50 values of target compounds 7a—7f and 9a—9r against A549, MD-MBA-231 and PC-3M*
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|---|---|
| A549 | MD-MBA-231 | PC-3M | A549 | MD-MBA-231 | PC-3M | ||
| 7a | >200 | >200 | >200 | 9h | >200 | >200 | >200 |
| 7b | >200 | >200 | >200 | 9i | >200 | >200 | 91.6 |
| 7c | 38.3 | >200 | >200 | 9j | >200 | >200 | 98.0 |
| 7d | >200 | >200 | >200 | 9k | 36.7 | 147.5 | 60.7 |
| 7e | >200 | >200 | >200 | 9l | >200 | >200 | >200 |
| 7f | 44.6 | >200 | >200 | 9m | >200 | >200 | >200 |
| 9a | >200 | >200 | 34.1 | 9n | >200 | 89.6 | >200 |
| 9b | >200 | >200 | 97.9 | 9o | >200 | >200 | 59.8 |
| 9c | >200 | >200 | >200 | 9p | >200 | >200 | >200 |
| 9d | 104.6 | 59.8 | >200 | 9q | >200 | >200 | 61.3 |
| 9e | >200 | >200 | 41.1 | 9r | >200 | >200 | >200 |
| 9f | >200 | >200 | 99.2 | 5-Fu | 35.4 | 14.2 | 22.6 |
| 9g | >200 | >200 | 73.2 | ||||
| [1] | Voronkov A., Holsworth D. D., Waaler J., Wilson S. R., Ekblad B., Perdreau-Dahl H., Dinh H., Drewes G., Hopf C., Morth J. P., Krauss S., J. Med. Chem., 2013, 56(7), 3012—3023 |
| [2] | Carlsson J., Tosh D. K., Phan K., Gao Z. G., Jacobson K. A., ACS Med. Chem. Lett., 2012, 3(9), 715—720 |
| [3] | Uzgören-Baran A., Tel B. C., Sarıgöl D., Oztürk E.I., Kazkayası I., Okay G., Ertan M., Tozkoparan B., Eur. J. Med. Chem., 2012, 57, 398—406 |
| [4] | Zhao P. L., Wang Y. Z., Zhang B., Yang G. F., Chin. J. Org. Chem., 2008, 28(5), 875—880 |
| (赵培亮, 王亚洲, 章博, 杨光富. 有机化学, 2008, 28(5), 875—880) | |
| [5] | Shang J., Wang W. M., Li Y. H., Song H. B., Li Z. M., Wang J. G., J. Agric. Food Chem., 2012, 60(34), 8286—8293 |
| [6] | Liu Z., Pan L., Li Y. H., Wang S. H., Li Z. M., Chem. J. Chinese Universities,2013, 29(3), 466—472 |
| (刘卓, 潘里, 李永红, 王素华, 李正名. 高等学校化学学报, 2013, 29(3), 466—472) | |
| [7] | Kalhor M., Mobinikhaledi A., Dadras A., Tohidpour M., J. Heterocycl. Chem., 2011, 48(6), 1366—1370 |
| [8] | Vijesh A. M., Isloor A. M., Shetty P., Sundershan S., Fun H. K., Eur. J. Med. Chem., 2013, 62, 410—415 |
| [9] | Alam M. M., Shaharyar M., Hamid H., Nazreen S., Haider S., Alam M. S., Med. Chem., 2011, 7(6), 663—673 |
| [10] | Jubie S., Ramesh P. N., Dhanabal P., Kalirajan R., Muruganantham N., Antony A. S., Eur. J. Med. Chem., 2012, 54, 931—935 |
| [11] | Chang J. J., Wang Y., Zhang H. Z., Zhou C. H., Geng R. X., Ji Q. G., Chem. J. Chinese Universities,2011, 32(9), 1970—1985 |
| (常娟娟, 王艳, 张慧珍, 周成合, 耿蓉霞, 吉庆刚. 高等学校化学学报, 2011, 32(9), 1970—1985) | |
| [12] | Köberle D., Thürlimann B., Expert Rev. Anticancer Ther., 2001, 1(2), 169—176 |
| [13] | Yamada K., Yajima O., Yoshizawa Y., Oh K., Bioorg. Med. Chem., 2013, 21(9), 2451—2461 |
| [14] | Capitosti S. M., Hansen T. P., Brown M. L., Bioorg. Med. Chem., 2004, 12(2), 327—336 |
| [15] | Singh P., Kaur S., Kumar V., Bedi P. M., Mahajan M. P., Sehar I., Pal H.C., Saxena A. K., Bioorg. Med. Chem. Lett., 2011, 21(10), 3017—3020 |
| [16] | Chu K. C., Tarone R. E., Freeman H. P., Cancer,2003, 97, 1507—1516 |
| [17] | Bansode T. N., Shelke J. V., Dongre V. G., Eur. J. Med. Chem., 2009, 44, 5094—5098 |
| [18] | Wu J., Yu W., Fu L., He W., Wang Y., Chai B., Song C., Chang J., Eur. J. Med. Chem., 2013, 63, 739—745 |
| [19] | Zhao P. L., Ma W. F., Duan A. N., Zou M., Yan Y. C., You W. W., Wu S. G., Eur. J. Med. Chem., 2012, 54, 813—822 |
| [20] | Zhao P. L., Duan A. N., Zou M., Yang H. K., You W. W., Wu S. G., Bioorg. Med. Chem. Lett., 2012, 22(13), 4471—4474 |
| [21] | Li Q. Z., Song B. A., Cai X. J., Zheng Y. G., Guo Q. Q., Chin. J. Org. Chem., 2010, 30(4), 569—575 |
| (李黔柱, 宋宝安, 蔡学建, 郑玉国, 郭晴晴. 有机化学, 2010, 30(4), 569—575) | |
| [22] | Sahoo P. K., Sharma R., Pattanayak P., Med. Chem. Res., 2010, 19, 127—135 |
| [1] | DONG Xinrui, XIA Zhe, WANG Zhenxue, BIAN Qiang, LI Huabin. Design, Synthesis and Biological Activity of Pyrazole-4-carboxamides Compounds Containing 1,2,4,5-Tetrasubstituted Phenyl [J]. Chem. J. Chinese Universities, 2020, 41(12): 2759. |
| [2] | XIAO Yanhua, ZHANG Guangjie, ZONG Liang, LIU Guohong, REN Lijun, DONG Junxing. Chemical Constituents and Antitumor Activity of Tupistra chinensis † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1897. |
| [3] | LÜ Mingjun,LI Wen,YANG Xinying,FANG Hao. Synthesis and Antitumor Activity of N9 Position Aromatic Substituted Purine-8-one Derivatives† [J]. Chem. J. Chinese Universities, 2019, 40(2): 254. |
| [4] | FANG Fang,XUE Liangmin,CONG Jing,TIAN Chao,WANG Xiaowei,LIU Junyi,ZHANG Zhili. Synthesis and Anti-tumor Activity Evaluation of a Series of 2- or 4-Substituted Pyrido[3,2-d]pyrimidines as Nonclassical Antifolates † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2111. |
| [5] | ZHANG Peiquan,YANG Qianqian,LONG Huidan,CHEN Xin. Synthesis and Antitumor Activity of Auranofin Derivatives † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2097. |
| [6] | LIU Xiaoyu, XU Yi, TANG Liangfu. Synthesis and Biological Activity of 3-Alkylphosphonate Substituted Isoindolinone Derivatives† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2433. |
| [7] | TAN Ying, XIAO Mengwu, YE Jiao, HU Aixi, ZENG Ziqing, OU Xiaoming. Synthesis, Crystal Structure and Fungicidal Activity of (Z)-3,3-Dimethyl-1-(1H-1,2,4-triazol-1-yl)butan-2-one O-[(5-Aryl-1,3,4-oxadiazol-2-yl)methyl] oxime [J]. Chem. J. Chinese Universities, 2017, 38(8): 1375. |
| [8] | BAI Xinfa, MA Xuan, XIE Xiaoxia, SHAO Mingsha, GUO Ningning, YAN Ning, YAO Lei. Synthesis and Anti-tumor Activity of Tubulysins Analogues† [J]. Chem. J. Chinese Universities, 2017, 38(1): 47. |
| [9] | GUO Liang, CAO Rihui, FAN Wenxi, GAN Ziyun, MA Qin. Design, Synthesis and in vitro Antitumor Activities of Novel Bivalent β-Carbolines† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1093. |
| [10] | JIA Changqing, YANG Dongyan, CHE Chuanliang, MA Yongqiang, RUI Changhui, YAN Xiaojing, QIN Zhaohai. Synthesis, Structural Characterization, Insecticidal and Fungicidal Activity of (1H-1,2,4-Triazol-5-yl)carbamates† [J]. Chem. J. Chinese Universities, 2016, 37(5): 892. |
| [11] | ZHANG Jie, ZHOU Changjian, XIE Jianwei, DAI Bin. Synthesis and Antitumor Activities of Rhein-Valine Adducts† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2159. |
| [12] | ZHOU Hao, DUAN Zhigang, ZHAO Shuang, BAO Meiying, LI Zhiwei, PEI Yazhong. Design and Synthesis of Phenylpyrimidine and Their Anticancer Activity† [J]. Chem. J. Chinese Universities, 2015, 36(9): 1694. |
| [13] | CHEN Wei, WEI Wei, LI Yuxin, WAN Yingying, LIU Qiaoxia, LI Yonghong, YU Shujing, LI Zhengming. Synthesis and Biological Activity of 2-Methyl-6-nitrobenzenesulfonylurea Derivatives† [J]. Chem. J. Chinese Universities, 2015, 36(5): 907. |
| [14] | LI Xiuyun, WAN Chuan, DU Shijie, LI Hong, YUAN Huizhu, JIANG Jiazhen, XIAO Yumei, QIN Zhaohai. Synthesis and Fungicidal Activities of Biaryl Methanone O-Benzyl Oximes† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2415. |
| [15] | WANG Gang, HAN Leiqiang, FANG Hao. Syntheses and Antitumor Activities of Phenylpiperazine Derivatives† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2435. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||