

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (12): 2159.doi: 10.7503/cjcu20160568
• Organic Chemistry • Previous Articles Next Articles
ZHANG Jie, ZHOU Changjian, XIE Jianwei*( ), DAI Bin
), DAI Bin
Received:2016-08-11
Online:2016-12-10
Published:2016-11-15
Contact:
XIE Jianwei
E-mail:cesxjw@foxmail.com
Supported by:CLC Number:
TrendMD:
ZHANG Jie, ZHOU Changjian, XIE Jianwei, DAI Bin. Synthesis and Antitumor Activities of Rhein-Valine Adducts†[J]. Chem. J. Chinese Universities, 2016, 37(12): 2159.
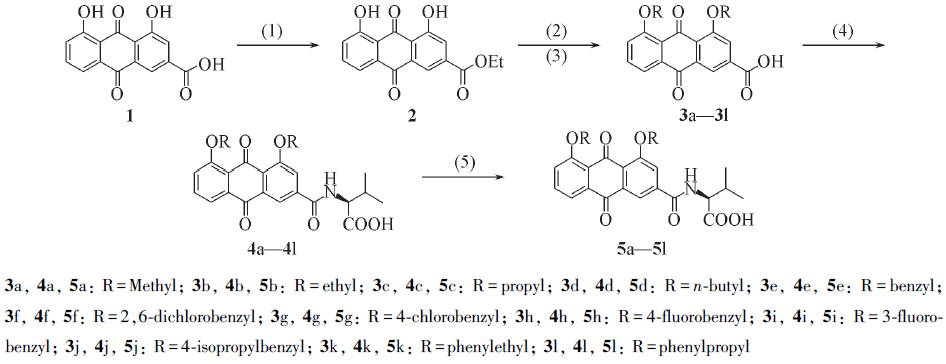
Scheme 1 Synthetic routes of target compounds 5a—5l(1) SOCl2, EtOH, reflux; (2) RX, NaH, DMF; (3) 1 mol/L NaOH(aq), then 1 mol/L HCl(aq);(4) NH2CH(R2)COOEt, EDCI, DMAP, CH2Cl2; (5) 1 mol/L NaOH, then 1 mol/L HCl(aq).
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 3a | Yellow solid | 85 | 301.0—301.7 | 3g | Yellow solid | 90 | 230.1—230.7 |
| 3b | Yellow solid | 72 | 249.9—250.9 | 3h | Yellow solid | 92 | 199.7—200.4 |
| 3c | Yellow solid | 54 | 181.9—182.8 | 3i | Yellow solid | 93 | 222.9—223.7 |
| 3d | Yellow solid | 46 | 231.1—232.1 | 3j | Yellow solid | 95 | 194.3—195.2 |
| 3e | Yellow solid | 86 | 181.1—181.9 | 3k | Yellow solid | 92 | 166.6—167.5 |
| 3f | Yellow solid | 95 | 231.4—232.2 | 3l | Yellow solid | 95 | 179.5—180.3 |
Table 1 Appearances, yields and melting points of compounds 3a—3l
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 3a | Yellow solid | 85 | 301.0—301.7 | 3g | Yellow solid | 90 | 230.1—230.7 |
| 3b | Yellow solid | 72 | 249.9—250.9 | 3h | Yellow solid | 92 | 199.7—200.4 |
| 3c | Yellow solid | 54 | 181.9—182.8 | 3i | Yellow solid | 93 | 222.9—223.7 |
| 3d | Yellow solid | 46 | 231.1—232.1 | 3j | Yellow solid | 95 | 194.3—195.2 |
| 3e | Yellow solid | 86 | 181.1—181.9 | 3k | Yellow solid | 92 | 166.6—167.5 |
| 3f | Yellow solid | 95 | 231.4—232.2 | 3l | Yellow solid | 95 | 179.5—180.3 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ |
|---|---|
| 3a | 13.73(s, 1H), 8.17(d, J=1.6 Hz, 1H), 7.90(d, J=1.6 Hz, 1H), 7.78(t, J=8.0 Hz, 1H), 7.70(dd, J=1.2, 7.6 Hz, 1H), 7.56(dd, J=0.8, 8.4 Hz, 1H), 3.98(s, 3H), 3.93(s, 3H) |
| 3b | 13.70(s, 1H), 8.16(d, J=1.2 Hz, 1H), 7.87(d, J=1.2 Hz, 1H), 7.74(t, J=7.6 Hz, 1H), 7.69(dd, J=1.2, 7.6 Hz, 1H), 7.55(dd, J=1.2, 8.0 Hz, 1H), 4.28(q, J=7.2 Hz, 2H), 4.23(q, J=7.2 Hz, 2H), 1.43(t, J=7.2 Hz, 3H), 1.42(t, J=7.2 Hz, 3H) |
| 3c | 8.17(d, J=1.6 Hz, 1H), 7.88(d, J=1.6 Hz, 1H), 7.74(t, J=8.0 Hz, 1H), 7.69(dd, J=1.6, 7.6 Hz, 1H), 7.54(dd, J=1.2, 8.4 Hz, 1H), 4.16(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 1.78—1.84(m, 4H), 1.05—1.10(m, 6H) |
| 3d | 8.15(d, J=1.2 Hz, 1H), 7.86(s, 1H), 7.72(t, J=7.6 Hz, 1H), 7.67(d, J=6.8 Hz, 1H), 7.52(d, J=8.4 Hz, 1H), 4.18(t, J=6.0 Hz, 2H), 4.12(t, J=6.0 Hz, 2H), 1.72—1.80(m, 4H), 1.52—1.59(m, 4H), 0.96(t, J=7.6 Hz, 6H) |
| 3e | 8.25(d, J=1.2 Hz, 1H), 8.05(d, J=1.2 Hz, 1H), 7.73—7.79(m, 2H), 7.63—7.67(m, 5H), 7.35—7.43(m, 6H), 5.37(s, 2H), 5.33(s, 2H) |
| 3f | 8.27(d, J=1.2 Hz, 1H), 8.06(d, J=1.2 Hz, 1H), 7.79(d, J=1.2 Hz, 1H), 7.78(s, 1H), 7.69(t, J=4.8 Hz, 1H), 7.42—7.50(m, 6H), 5.36(s, 2H), 5.33(s, 2H) |
| 3g | 8.24(d, J=1.6 Hz, 1H), 8.02(d, J=1.6 Hz, 1H), 7.75—7.80(m, 2H), 7.62—7.69(m, 5H), 7.17—7.23(m, 4H), 5.36(s, 2H), 5.30(s, 2H) |
| 3h | 8.23(s, 1H), 8.01(s, 1H), 7.50—7.69(m, 7H), 7.36—7.44(m, 4H), 5.24(s, 4H) |
| 3i | 8.24(d, J=1.6 Hz, 1H), 8.02(d, J=1.2 Hz, 1H), 7.76—7.83(m, 2H), 7.65(dd, J=1.6, 8.0 Hz, 1H), 7.42—7.56(m, 6H), 7.16—7.21(m, 2H), 5.43(s, 2H), 5.37(s, 2H) |
| 3j | 8.22(d, J=1.2 Hz, 1H), 8.03(d, J=1.2 Hz, 1H), 7.73—7.80(m, 2H), 7.64(dd, J=1.2, 7.6 Hz, 1H), 7.57(t, J=8.4 Hz, 4H), 7.29(d, J=7.2 Hz, 4H), 5.34(s, 2H), 5.28(s, 2H), 2.89—2.95(m, 2H), 1.25(s, 6H), 1.23(s, 6H) |
| 3k | 8.17(d, J=1.6 Hz, 1H), 7.89(d, J=1.2 Hz, 1H), 7.68—7.75(m, 2H), 7.50(dd, J=1.6, 8.0 Hz, 4H), 7.29—7.34(m, 5H), 7.20—7.24(m, 2H), 4.41(t, J=6.8 Hz, 2H), 4.35(t, J=6.8 Hz, 2H), 3.16(q, J=6.4 Hz, 4H) |
| 3l | 8.19(d, J=1.6 Hz, 1H), 7.87(d, J=1.6 Hz, 1H), 7.71—7.77(m, 2H), 7.53(dd, J=2.0, 7.6 Hz, 1H), 7.12—7.28(m, 10H), 4.18(t, J=6.0 Hz, 2H), 4.12(t, J=6.0 Hz, 2H), 2.91(t, J=7.2 Hz, 4H), 2.04—2.13(m, 4H) |
Table 2 1H NMR data of compounds 3a—3l
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ |
|---|---|
| 3a | 13.73(s, 1H), 8.17(d, J=1.6 Hz, 1H), 7.90(d, J=1.6 Hz, 1H), 7.78(t, J=8.0 Hz, 1H), 7.70(dd, J=1.2, 7.6 Hz, 1H), 7.56(dd, J=0.8, 8.4 Hz, 1H), 3.98(s, 3H), 3.93(s, 3H) |
| 3b | 13.70(s, 1H), 8.16(d, J=1.2 Hz, 1H), 7.87(d, J=1.2 Hz, 1H), 7.74(t, J=7.6 Hz, 1H), 7.69(dd, J=1.2, 7.6 Hz, 1H), 7.55(dd, J=1.2, 8.0 Hz, 1H), 4.28(q, J=7.2 Hz, 2H), 4.23(q, J=7.2 Hz, 2H), 1.43(t, J=7.2 Hz, 3H), 1.42(t, J=7.2 Hz, 3H) |
| 3c | 8.17(d, J=1.6 Hz, 1H), 7.88(d, J=1.6 Hz, 1H), 7.74(t, J=8.0 Hz, 1H), 7.69(dd, J=1.6, 7.6 Hz, 1H), 7.54(dd, J=1.2, 8.4 Hz, 1H), 4.16(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 1.78—1.84(m, 4H), 1.05—1.10(m, 6H) |
| 3d | 8.15(d, J=1.2 Hz, 1H), 7.86(s, 1H), 7.72(t, J=7.6 Hz, 1H), 7.67(d, J=6.8 Hz, 1H), 7.52(d, J=8.4 Hz, 1H), 4.18(t, J=6.0 Hz, 2H), 4.12(t, J=6.0 Hz, 2H), 1.72—1.80(m, 4H), 1.52—1.59(m, 4H), 0.96(t, J=7.6 Hz, 6H) |
| 3e | 8.25(d, J=1.2 Hz, 1H), 8.05(d, J=1.2 Hz, 1H), 7.73—7.79(m, 2H), 7.63—7.67(m, 5H), 7.35—7.43(m, 6H), 5.37(s, 2H), 5.33(s, 2H) |
| 3f | 8.27(d, J=1.2 Hz, 1H), 8.06(d, J=1.2 Hz, 1H), 7.79(d, J=1.2 Hz, 1H), 7.78(s, 1H), 7.69(t, J=4.8 Hz, 1H), 7.42—7.50(m, 6H), 5.36(s, 2H), 5.33(s, 2H) |
| 3g | 8.24(d, J=1.6 Hz, 1H), 8.02(d, J=1.6 Hz, 1H), 7.75—7.80(m, 2H), 7.62—7.69(m, 5H), 7.17—7.23(m, 4H), 5.36(s, 2H), 5.30(s, 2H) |
| 3h | 8.23(s, 1H), 8.01(s, 1H), 7.50—7.69(m, 7H), 7.36—7.44(m, 4H), 5.24(s, 4H) |
| 3i | 8.24(d, J=1.6 Hz, 1H), 8.02(d, J=1.2 Hz, 1H), 7.76—7.83(m, 2H), 7.65(dd, J=1.6, 8.0 Hz, 1H), 7.42—7.56(m, 6H), 7.16—7.21(m, 2H), 5.43(s, 2H), 5.37(s, 2H) |
| 3j | 8.22(d, J=1.2 Hz, 1H), 8.03(d, J=1.2 Hz, 1H), 7.73—7.80(m, 2H), 7.64(dd, J=1.2, 7.6 Hz, 1H), 7.57(t, J=8.4 Hz, 4H), 7.29(d, J=7.2 Hz, 4H), 5.34(s, 2H), 5.28(s, 2H), 2.89—2.95(m, 2H), 1.25(s, 6H), 1.23(s, 6H) |
| 3k | 8.17(d, J=1.6 Hz, 1H), 7.89(d, J=1.2 Hz, 1H), 7.68—7.75(m, 2H), 7.50(dd, J=1.6, 8.0 Hz, 4H), 7.29—7.34(m, 5H), 7.20—7.24(m, 2H), 4.41(t, J=6.8 Hz, 2H), 4.35(t, J=6.8 Hz, 2H), 3.16(q, J=6.4 Hz, 4H) |
| 3l | 8.19(d, J=1.6 Hz, 1H), 7.87(d, J=1.6 Hz, 1H), 7.71—7.77(m, 2H), 7.53(dd, J=2.0, 7.6 Hz, 1H), 7.12—7.28(m, 10H), 4.18(t, J=6.0 Hz, 2H), 4.12(t, J=6.0 Hz, 2H), 2.91(t, J=7.2 Hz, 4H), 2.04—2.13(m, 4H) |
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 4a | Yellow solid | 68 | 223.5—224.3 | 4g | Yellow solid | 70 | 216.8—217.6 |
| 4b | Yellow solid | 96 | 184.5—185.1 | 4h | Yellow solid | 44 | 221.8—222.6 |
| 4c | Yellow solid | 89 | 151.9—152.8 | 4i | Yellow solid | 49 | 193.7—194.6 |
| 4d | Yellow solid | 79 | 161.2—162.4 | 4j | Yellow solid | 39 | 98.7—99.4 |
| 4e | Yellow solid | 97 | 172.3—173.1 | 4k | Yellow solid | 72 | 72.3—73.1 |
| 4f | Yellow solid | 51 | 100.2—101.1 | 4l | Yellow solid | 81 | 125.5—126.4 |
Table 3 Appearances, yields and melting points of compounds 4a—4l
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 4a | Yellow solid | 68 | 223.5—224.3 | 4g | Yellow solid | 70 | 216.8—217.6 |
| 4b | Yellow solid | 96 | 184.5—185.1 | 4h | Yellow solid | 44 | 221.8—222.6 |
| 4c | Yellow solid | 89 | 151.9—152.8 | 4i | Yellow solid | 49 | 193.7—194.6 |
| 4d | Yellow solid | 79 | 161.2—162.4 | 4j | Yellow solid | 39 | 98.7—99.4 |
| 4e | Yellow solid | 97 | 172.3—173.1 | 4k | Yellow solid | 72 | 72.3—73.1 |
| 4f | Yellow solid | 51 | 100.2—101.1 | 4l | Yellow solid | 81 | 125.5—126.4 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ |
|---|---|
| 4a | 8.10(d, J=1.6 Hz, 1H), 7.88(d, J=1.6 Hz, 1H), 7.86(dd, J=0.8, 7.6 Hz, 1H), 7.66(t, J=8.0 Hz, 1H), 7.32(dd, J=0.8, 8.4 Hz, 1H), 6.84(d, J=8.4 Hz, 1H), 4.77(q, J=5.2 Hz, 1H), 4.23—4.31(m, 2H), 4.06(s, 3H), 4.01(s, 3H), 2.28—2.37(m, 1H), 1.33(t, J=7.2 Hz, 3H), 1.06(d, J=4.4 Hz, 3H), 1.04(d, J=4.0 Hz, 3H) |
| 4b | 8.08(d, J=1.6 Hz, 1H), 7.86(d, J=1.2 Hz, 1H), 7.84(dd, J=0.8, 7.6 Hz, 1H), 7.63(t, J=8.0 Hz, 1H), 7.31(d, J=8.4 Hz, 1H), 6.86(d, J=8.4 Hz, 1H), 4.78(q, J=4.8 Hz, 1H), 4.23—4.34(m, 6H), 2.28—2.36(m, 1H), 1.56(t, J=6.8 Hz, 6H), 1.33(t, J=6.8 Hz, 3H), 1.06(d, J=5.2 Hz, 3H), 1.04(d, J=4.8 Hz, 3H) |
| 4c | 8.07(d, J=1.6 Hz, 1H), 7.85(d, J=1.6 Hz, 1H), 7.83(dd, J=0.8, 7.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.30(dd, J=1.2, 8.4 Hz, 1H), 6.85(d, J=8.4 Hz, 1H), 4.78(q, J=4.8 Hz, 1H), 4.23—4.31(m, 2H), 4.17(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 2.28—2.36(m, 1H), 1.90—2.00(m, 4H), 1.33(t, J=7.2 Hz, 3H), 1.10—1.15(m, 6H), 1.05(d, J=4.8 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4d | 8.07(d, J=1.6 Hz, 1H), 7.85(d, J=1.6 Hz, 1H), 7.82(dd, J=1.2, 7.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.30(dd, J=0.8, 8.4 Hz, 1H), 6.85(d, J=8.4 Hz, 1H), 4.77(q, J=4.8 Hz, 1H), 4.23—4.30(m, 2H), 4.20(t, J=6.4 Hz, 2H), 4.15(t, J=6.4 Hz, 2H), 2.93—2.36(m, 1H), 1.87—1.94(m, 4H), 1.58—1.66(m, 4H), 1.33(t, J=7.2 Hz, 3H), 0.99—1.06(m, 12H) |
| 4e | 8.13(d, J=1.6 Hz, 1H), 7.94(d, J=1.6 Hz, 1H), 7.88(dd, J=0.8, 7.6 Hz, 1H), 7.61—7.67(m, 5H), 7.33—7.42(m, 7H), 6.84(d, J=8.4 Hz, 1H), 5.38(s, 2H), 5.33(s, 2H), 4.77(q, J=5.2 Hz, 1H), 4.26—4.29(m, 2H), 2.28—2.36(m, 1H), 1.33(t, J=7.2 Hz, 3H), 1.05(d, J=4.4 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4f | 8.18(s, 1H), 7.99(s, 1H), 7.92(d, J=8.0 Hz, 1H), 7.63(t, J=8.0 Hz, 1H), 7.44(d, J=8.4 Hz, 1H), 7.27—7.32(m, 4H), 7.18—7.24(m, 2H), 6.81(d, J=8.4 Hz, 1H), 5.49(s, 2H), 5.42(s, 2H), 4.80(q, J=5.2 Hz, 1H), 3.80(s, 3H), 2.28—2.36(m, 1H), 1.05(d, J=4.4 Hz, 3H), 1.03(d, J=4.4 Hz, 3H) |
| 4g | 8.13(d, J=1.2 Hz, 1H), 7.90(dd, J=1.6, 6.4 Hz, 2H), 7.65(t, J=8.0 Hz, 1H), 7.55—7.59(m, 4H), 7.33—7.38(m, 5H), 6.86(d, J=8.4 Hz, 1H), 5.31(s, 2H), 5.27(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.81(s, 3H), 2.28—2.36(m, 1H), 1.05(d, J=3.2 Hz, 3H), 1.03(d, J=3.2 Hz, 3H) |
| 4h | 8.13(d, J=1.6 Hz, 1H), 7.92(d, J=1.6 Hz, 1H), 7.90(dd, J=0.8, 7.6 Hz, 1H), 7.58—7.67(m, 5H), 7.36(dd, J=1.2, 8.4 Hz, 1H), 7.05—7.10(m, 4H), 6.88(d, J=8.4 Hz, 1H), 5.31(s, 2H), 5.27(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.81(s, 3H), 2.30—2.35(m, 1H), 1.05(d, J=3.2 Hz, 3H), 1.03(d, J=3.2 Hz, 3H) |
| 4i | 8.14(d, J=1.6 Hz, 1H), 7.89—7.91(m, 2H), 7.65(t, J=8.0 Hz, 1H), 7.34—7.45(m, 7H), 7.00—7.06(m, 2H), 6.91(d, J=8.8 Hz, 1H), 5.34(s, 2H), 5.29(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.82(s, 3H), 2.30—2.35(m, 1H), 1.05(d, J=2.4 Hz, 3H), 1.03(d, J=2.4 Hz, 3H) |
| 4j | 8.12(d, J=1.6 Hz, 1H), 7.94(d, J=1.6 Hz, 1H), 7.87(dd, J=0.8, 7.6 Hz, 1H), 7.55—7.64(m, 5H), 7.37(dd, J=1.2, 8.4 Hz, 1H), 7.28(s, 2H), 7.26(s, 2H), 6.79(d, J=8.8 Hz, 1H), 5.35(s, 2H), 5.30(s, 2H), 4.79(q, J=4.8 Hz, 1H), 3.80(s, 3H), 2.89—2.97(m, 2H), 2.27—2.35(m, 1H), 1.28(d, J=2.0 Hz, 6H), 1.26(d, J=2.4 Hz, 6H), 1.04(d, J=4.4 Hz, 3H), 1.02(d, J=4.4 Hz, 3H) |
| 4k | 8.07(s, 1H), 7.72—7.89(m, 2H), 7.57(t, J=8.0 Hz, 1H), 7.41—7.45(m, 4H), 7.32(q, J=7.2 Hz, 4H), 7.20—7.26(m, 3H), 7.02(d, J=8.8 Hz, 1H), 4.75—4.80(m, 1H), 4.39(t, J=7.2 Hz, 2H), 4.32(t, J=7.2 Hz, 2H), 4.23—4.29(m, 2H), 3.28(t, J=6.8 Hz, 4H), 2.28—2.36(m, 1H), 1.32(t, J=7.2 Hz, 3H), 1.05(d, J=4.8 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4l | 8.09(d, J=1.6 Hz, 1H), 7.85(dd, J=0.8, 7.6 Hz, 1H), 7.82(d, J=1.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.28(d, J=0.8 Hz, 1H), 7.20—7.26(m, 8H), 7.14—7.18(m, 2H), 6.81(d, J=8.4 Hz, 1H), 4.79(q, J=5.2 Hz, 1H), 4.19(t, J=6.0 Hz, 2H), 4.13(t, J=6.0 Hz, 2H), 3.80(s, 3H), 2.95—3.00(m, 4H), 2.29—2.33(m, 1H), 2.20—2.27(m, 4H), 1.04(d, J=3.6 Hz, 3H), 1.02(d, J=3.6 Hz, 3H) |
Table 4 1H NMR data of compounds 4a—4l
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ |
|---|---|
| 4a | 8.10(d, J=1.6 Hz, 1H), 7.88(d, J=1.6 Hz, 1H), 7.86(dd, J=0.8, 7.6 Hz, 1H), 7.66(t, J=8.0 Hz, 1H), 7.32(dd, J=0.8, 8.4 Hz, 1H), 6.84(d, J=8.4 Hz, 1H), 4.77(q, J=5.2 Hz, 1H), 4.23—4.31(m, 2H), 4.06(s, 3H), 4.01(s, 3H), 2.28—2.37(m, 1H), 1.33(t, J=7.2 Hz, 3H), 1.06(d, J=4.4 Hz, 3H), 1.04(d, J=4.0 Hz, 3H) |
| 4b | 8.08(d, J=1.6 Hz, 1H), 7.86(d, J=1.2 Hz, 1H), 7.84(dd, J=0.8, 7.6 Hz, 1H), 7.63(t, J=8.0 Hz, 1H), 7.31(d, J=8.4 Hz, 1H), 6.86(d, J=8.4 Hz, 1H), 4.78(q, J=4.8 Hz, 1H), 4.23—4.34(m, 6H), 2.28—2.36(m, 1H), 1.56(t, J=6.8 Hz, 6H), 1.33(t, J=6.8 Hz, 3H), 1.06(d, J=5.2 Hz, 3H), 1.04(d, J=4.8 Hz, 3H) |
| 4c | 8.07(d, J=1.6 Hz, 1H), 7.85(d, J=1.6 Hz, 1H), 7.83(dd, J=0.8, 7.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.30(dd, J=1.2, 8.4 Hz, 1H), 6.85(d, J=8.4 Hz, 1H), 4.78(q, J=4.8 Hz, 1H), 4.23—4.31(m, 2H), 4.17(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 2.28—2.36(m, 1H), 1.90—2.00(m, 4H), 1.33(t, J=7.2 Hz, 3H), 1.10—1.15(m, 6H), 1.05(d, J=4.8 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4d | 8.07(d, J=1.6 Hz, 1H), 7.85(d, J=1.6 Hz, 1H), 7.82(dd, J=1.2, 7.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.30(dd, J=0.8, 8.4 Hz, 1H), 6.85(d, J=8.4 Hz, 1H), 4.77(q, J=4.8 Hz, 1H), 4.23—4.30(m, 2H), 4.20(t, J=6.4 Hz, 2H), 4.15(t, J=6.4 Hz, 2H), 2.93—2.36(m, 1H), 1.87—1.94(m, 4H), 1.58—1.66(m, 4H), 1.33(t, J=7.2 Hz, 3H), 0.99—1.06(m, 12H) |
| 4e | 8.13(d, J=1.6 Hz, 1H), 7.94(d, J=1.6 Hz, 1H), 7.88(dd, J=0.8, 7.6 Hz, 1H), 7.61—7.67(m, 5H), 7.33—7.42(m, 7H), 6.84(d, J=8.4 Hz, 1H), 5.38(s, 2H), 5.33(s, 2H), 4.77(q, J=5.2 Hz, 1H), 4.26—4.29(m, 2H), 2.28—2.36(m, 1H), 1.33(t, J=7.2 Hz, 3H), 1.05(d, J=4.4 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4f | 8.18(s, 1H), 7.99(s, 1H), 7.92(d, J=8.0 Hz, 1H), 7.63(t, J=8.0 Hz, 1H), 7.44(d, J=8.4 Hz, 1H), 7.27—7.32(m, 4H), 7.18—7.24(m, 2H), 6.81(d, J=8.4 Hz, 1H), 5.49(s, 2H), 5.42(s, 2H), 4.80(q, J=5.2 Hz, 1H), 3.80(s, 3H), 2.28—2.36(m, 1H), 1.05(d, J=4.4 Hz, 3H), 1.03(d, J=4.4 Hz, 3H) |
| 4g | 8.13(d, J=1.2 Hz, 1H), 7.90(dd, J=1.6, 6.4 Hz, 2H), 7.65(t, J=8.0 Hz, 1H), 7.55—7.59(m, 4H), 7.33—7.38(m, 5H), 6.86(d, J=8.4 Hz, 1H), 5.31(s, 2H), 5.27(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.81(s, 3H), 2.28—2.36(m, 1H), 1.05(d, J=3.2 Hz, 3H), 1.03(d, J=3.2 Hz, 3H) |
| 4h | 8.13(d, J=1.6 Hz, 1H), 7.92(d, J=1.6 Hz, 1H), 7.90(dd, J=0.8, 7.6 Hz, 1H), 7.58—7.67(m, 5H), 7.36(dd, J=1.2, 8.4 Hz, 1H), 7.05—7.10(m, 4H), 6.88(d, J=8.4 Hz, 1H), 5.31(s, 2H), 5.27(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.81(s, 3H), 2.30—2.35(m, 1H), 1.05(d, J=3.2 Hz, 3H), 1.03(d, J=3.2 Hz, 3H) |
| 4i | 8.14(d, J=1.6 Hz, 1H), 7.89—7.91(m, 2H), 7.65(t, J=8.0 Hz, 1H), 7.34—7.45(m, 7H), 7.00—7.06(m, 2H), 6.91(d, J=8.8 Hz, 1H), 5.34(s, 2H), 5.29(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.82(s, 3H), 2.30—2.35(m, 1H), 1.05(d, J=2.4 Hz, 3H), 1.03(d, J=2.4 Hz, 3H) |
| 4j | 8.12(d, J=1.6 Hz, 1H), 7.94(d, J=1.6 Hz, 1H), 7.87(dd, J=0.8, 7.6 Hz, 1H), 7.55—7.64(m, 5H), 7.37(dd, J=1.2, 8.4 Hz, 1H), 7.28(s, 2H), 7.26(s, 2H), 6.79(d, J=8.8 Hz, 1H), 5.35(s, 2H), 5.30(s, 2H), 4.79(q, J=4.8 Hz, 1H), 3.80(s, 3H), 2.89—2.97(m, 2H), 2.27—2.35(m, 1H), 1.28(d, J=2.0 Hz, 6H), 1.26(d, J=2.4 Hz, 6H), 1.04(d, J=4.4 Hz, 3H), 1.02(d, J=4.4 Hz, 3H) |
| 4k | 8.07(s, 1H), 7.72—7.89(m, 2H), 7.57(t, J=8.0 Hz, 1H), 7.41—7.45(m, 4H), 7.32(q, J=7.2 Hz, 4H), 7.20—7.26(m, 3H), 7.02(d, J=8.8 Hz, 1H), 4.75—4.80(m, 1H), 4.39(t, J=7.2 Hz, 2H), 4.32(t, J=7.2 Hz, 2H), 4.23—4.29(m, 2H), 3.28(t, J=6.8 Hz, 4H), 2.28—2.36(m, 1H), 1.32(t, J=7.2 Hz, 3H), 1.05(d, J=4.8 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4l | 8.09(d, J=1.6 Hz, 1H), 7.85(dd, J=0.8, 7.6 Hz, 1H), 7.82(d, J=1.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.28(d, J=0.8 Hz, 1H), 7.20—7.26(m, 8H), 7.14—7.18(m, 2H), 6.81(d, J=8.4 Hz, 1H), 4.79(q, J=5.2 Hz, 1H), 4.19(t, J=6.0 Hz, 2H), 4.13(t, J=6.0 Hz, 2H), 3.80(s, 3H), 2.95—3.00(m, 4H), 2.29—2.33(m, 1H), 2.20—2.27(m, 4H), 1.04(d, J=3.6 Hz, 3H), 1.02(d, J=3.6 Hz, 3H) |
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z[M-H]- |
|---|---|---|---|---|
| 5a | Yellow solid | 88 | 159.2—159.8 | 410.1246(410.1245) |
| 5b | Yellow solid | 86 | 102.6—103.2 | 438.1561(438.1558) |
| 5c | Yellow solid | 83 | 183.3—184.2 | 466.1873(466.1871) |
| 5d | Yellow solid | 92 | 169.8—170.4 | 494.2178(494.2184) |
| 5e | Yellow solid | 74 | 132.5—133.4 | 562.1865(562.1871) |
| 5f | Yellow solid | 85 | 153.9—154.8 | 698.0313(698.0312) |
| 5g | Yellow solid | 81 | 229.9—230.7 | 630.1085(630.1092) |
| 5h | Yellow solid | 83 | 181.3—182.2 | 598.1677(598.1683) |
| 5i | Yellow solid | 80 | 227.1—227.9 | 598.1675(598.1677) |
| 5j | Yellow solid | 67 | 109.4—110.3 | 646.2802(646.2810) |
| 5k | Yellow solid | 93 | 79.1—80.0 | 590.2174(590.2184) |
| 5l | Yellow solid | 93 | 127.1—128.4 | 618.2491(618.2497) |
Table 5 Appearances, yields, melting points and HRMS data of target compounds 5a—5l
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z[M-H]- |
|---|---|---|---|---|
| 5a | Yellow solid | 88 | 159.2—159.8 | 410.1246(410.1245) |
| 5b | Yellow solid | 86 | 102.6—103.2 | 438.1561(438.1558) |
| 5c | Yellow solid | 83 | 183.3—184.2 | 466.1873(466.1871) |
| 5d | Yellow solid | 92 | 169.8—170.4 | 494.2178(494.2184) |
| 5e | Yellow solid | 74 | 132.5—133.4 | 562.1865(562.1871) |
| 5f | Yellow solid | 85 | 153.9—154.8 | 698.0313(698.0312) |
| 5g | Yellow solid | 81 | 229.9—230.7 | 630.1085(630.1092) |
| 5h | Yellow solid | 83 | 181.3—182.2 | 598.1677(598.1683) |
| 5i | Yellow solid | 80 | 227.1—227.9 | 598.1675(598.1677) |
| 5j | Yellow solid | 67 | 109.4—110.3 | 646.2802(646.2810) |
| 5k | Yellow solid | 93 | 79.1—80.0 | 590.2174(590.2184) |
| 5l | Yellow solid | 93 | 127.1—128.4 | 618.2491(618.2497) |
| Compd. | 1H NMR(400 MHz), δa | 13C NMR(100 MHz), δb |
|---|---|---|
| 5a | 8.92(d, J=8.0 Hz, 1H), 8.19(s, 1H), 7.88(s, 1H), 7.77(t, J=7.6 Hz, 1H), 7.70(d, J=7.2 Hz, 1H), 7.55(d, J=8.0 Hz, 1H), 4.34(t, J=7.6 Hz, 1H), 4.00(s, 3H), 3.93(s, 3H), 2.20—2.28(m, 1H), 1.00(t, J=6.0 Hz, 6H) | 182.9, 180.9, 173.1, 165.2, 158.7, 158.6, 138.9, 134.4, 134.1, 125.1, 123.4, 119.0, 118.2, 117.3, 117.2, 58.9, 56.5, 56.3, 29.7, 19.4, 18.9 |
| 5b | 12.73(s, 1H), 8.97(d, J=8.4 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 7.88(d, J=0.8 Hz, 1H), 7.75(t, J=7.6 Hz, 1H), 7.72(dd, J=1.2, 7.6 Hz, 1H), 7.55(dd, J=1.2, 8.0 Hz, 1H), 4.34(t, J=7.6 Hz, 1H), 4.30(q, J=6.8 Hz, 2H), 4.23(q, J=6.8 Hz, 2H), 2.19—2.27(m, 1H), 1.40—1.46(m, 6H), 1.00(t, J=6.8 Hz, 6H) | 183.0, 180.7, 172.9, 165.3, 158.1, 158.0, 138.7, 134.4, 134.2, 125.2, 123.5, 120.1, 118.4, 118.2, 117.4, 65.0, 64.7, 58.7, 29.5, 19.4, 19.0, 14.6 |
| 5c | 8.91(d, J=8.4 Hz, 1H), 8.18(d, J=1.2 Hz, 1H ), 7.87(d, J=1.2 Hz, 1H), 7.75(t, J=7.6 Hz, 1H), 7.71(dd, J=1.2, 7.6 Hz, 1H), 7.54(dd, J=1.2, 8.0 Hz, 1H), 4.33(t, J=7.6 Hz, 1H), 4.19(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 2.19—2.27(m, 1H), 1.76—1.87(m, 4H), 1.08(q, J=7.6 Hz, 6H), 1.00(d, J=5.6 Hz, 3H), 0.98(d, J=5.2 Hz, 3H) | 183.1, 180.6, 173.0, 165.2, 158.3, 158.2, 138.8, 134.3, 134.2, 134.1, 125.4, 123.7, 120.1, 118.4, 118.1, 117.2, 70.6, 70.4, 58.8, 29.6, 22.1, 19.4, 18.9, 10.4, 10.3 |
| 5d | 8.87(d, J=8.4 Hz, 1H), 8.18(d, J=1.6 Hz, 1H ), 7.87(d, J=1.6 Hz, 1H), 7.74(t, J=8.0 Hz, 1H), 7.71(dd, J=1.6, 7.6 Hz, 1H), 7.54(dd, J=1.6, 8.0 Hz, 1H), 4.35(t, J=7.6 Hz, 1H), 4.22(t, J=6.0 Hz, 2H), 4.15(t, J=6.0 Hz, 2H), 2.19—2.27(m, 1H), 1.74—1.83(m, 4H), 1.54—1.61(m, 4H), 0.96—1.01(m, 12H) | 183.0, 180.7,172.9, 165.3, 158.2, 158.1, 138.7, 134.3, 134.2, 134.1, 125.5, 123.8, 120.1, 118.4, 118.1, 117.3, 68.9, 68.7, 58.7, 30.8, 30.7, 29.6, 19.4, 18.9, 18.7, 13.7 |
| 5e | 8.94(d, J=8.4 Hz, 1H), 8.23(s, 1H), 8.00(s, 1H), 7.74—7.80(m, 2H), 7.63—7.68(m, 4H), 7.36—7.43(m, 7H), 5.42(s, 2H), 5.34(s, 2H), 4.34(t, J=7.2 Hz, 1H), 2.20—2.27(m, 1H), 0.99(t, J=7.6 Hz, 6H) | 182.9, 181.0, 173.0, 165.1, 157.7, 157.6, 138.9, 136.8, 136.7, 134.4, 134.3, 134.2, 128.5, 128.3, 128.2, 127.7, 127.6, 127.0, 126.9, 126.8, 125.6, 123.9, 120.5, 118.9, 118.6, 117.7, 70.4, 70.1, 58.8, 29.7, 19.4, 18.9 |
| 5f | 8.69(d, J=6.8 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 8.03(d, J=1.6 Hz, 1H), 7.79(d, J=1.2 Hz, 1H), 7.78(s, 1H), 7.69(t, J=4.8 Hz, 1H), 7.42—7.50(m, 6H), 5.41(s, 2H), 5.33(s, 2H), 4.29(q, J=6.4 Hz, 1H), 2.19—2.27(m, 1H), 0.99(d, J=2.4 Hz, 3H), 0.97(d, J=2.4 Hz, 3H) | 182.6, 180.4, 164.8, 157.8, 157.7, 139.2, 136.2, 134.3, 134.2, 134.1, 131.4, 131.3, 131.2, 128.7, 126.2, 124.7, 121.6, 119.6, 119.4, 118.3, 67.1, 67.0, 59.2, 40.2, 30.0, 19.5, 18.8 |
| 5g | 8.75(d, J=7.6 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 7.97(d, J=1.2 Hz, 1H), 7.73—7.80(m, 2H), 7.60—7.67(m, 5H), 7.44(s, 2H), 7.42(s, 2H), 5.37(s, 2H), 5.30(s, 2H), 4.32(q, J=6.4 Hz, 1H), 2.20—2.28(m, 1H), 1.00(d, J=5.2 Hz, 3H), 0.98(t, J=5.2 Hz, 3H) | 182.8, 181.0, 165.0, 157.5, 157.4, 139.0, 135.9, 135.8, 134.5, 134.2, 134.1, 132.2, 132.1, 128.9, 128.8, 128.3, 128.2, 125.6, 123.9, 120.4, 118.8, 118.7, 117.8, 69.7, 69.4, 58.9, 29.7, 19.4, 18.9 |
| 5h | 8.46(s, 1H), 8.10(s, 1H), 7.93(s, 1H), 7.55—7.71(m, 7H), 7.18(q, J=8.0 Hz, 4H), 5.31(s, 2H), 5.24(s, 2H), 4.25(t, J=6.8 Hz, 1H), 2.19—2.28(m, 1H), 0.94(s, 3H), 0.93(s, 3H) | 182.7, 180.8, 164.5, 162.8, 160.4, 157.6, 139.5, 134.3, 134.0, 133.0, 129.0, 125.2, 123.7, 120.4, 118.7, 117.4, 115.1, 114.9, 69.8, 69.5, 59.7, 30.5, 19.7, 18.7 |
| 5i | 12.75(s, 1H), 8.99(d, J=8.4 Hz, 1H), 8.28(d, J=1.6 Hz, 1H), 8.01(d, J=1.6 Hz, 1H), 7.78—7.84(m, 2H), 7.67(dd, J=2.0, 8.0 Hz, 1H), 7.42—7.56(m, 6H), 7.16—7.21(m, 2H), 5.45(s, 2H), 5.38(s, 2H), 4.36(q, J=7.2 Hz, 1H), 2.20—2.28(m, 1H), 1.01(d, J=6.8 Hz, 3H), 0.99(d, J=6.8 Hz, 3H) | 182.8, 181.0, 172.9, 165.2, 163.5, 161.1, 157.6, 157.5, 139.8, 139.7, 139.6, 139.6, 138.9, 134.6, 134.2, 134.3, 130.3, 130.2, 125.3, 123.6, 122.5, 120.4, 118.8, 117.9, 114.4, 114.2, 113.6, 113.3, 69.6, 69.4, 58.7, 29.6, 19.4, 18.9 |
| 5j | 8.90(d, J=7.2 Hz, 1H), 8.23(s, 1H), 8.01(s, 1H), 7.74—7.80(m, 2H), 7.65(d, J=8.0 Hz, 1H), 7.58(t, J=8.8 Hz, 4H), 7.29(d, J=7.6 Hz, 4H), 5.36(s, 2H), 5.29(s, 2H), 4.34(t, J=7.6 Hz, 1H), 2.89—2.96(m, 2H), 2.20—2.29(m, 1H), 1.24(s, 6H), 1.23(s, 6H), 0.99(t, J=7.6 Hz, 6H) | 182.9, 180.9, 165.1, 157.8, 157.7, 147.8, 147.7, 138.9, 134.4, 134.2, 134.1, 134.0, 127.1, 127.0, 126.2, 126.1, 125.5, 123.8, 120.5, 118.8, 118.6, 117.6, 70.4, 70.1, 58.9, 33.3, 29.7, 23.9, 23.8, 19.4, 18.9 |
| 5k | 8.26(d, J=1.2 Hz, 1H), 7.91(s, 1H), 7.79(d, J=7.6 Hz, 1H), 7.58(t, J=8.0 Hz, 1H), 7.41—7.45(m, 4H), 7.27—7.38(m, 6H), 7.20—7.24(m, 2H), 4.90(q, J=5.2 Hz, 1H), 4.41(t, J=7.2 Hz, 2H), 4.34(t, J=6.8 Hz, 2H), 3.26—3.30(m, 4H), 2.35—2.41(m, 1H), 1.09(d, J=3.6 Hz, 3H), 1.07(d, J=3.2 Hz, 3H) | 184.6, 181.6, 174.9, 165.7, 159.2, 158.8, 138.3, 138.1, 138.0, 134.7, 134.6, 134.1, 129.5, 128.7, 128.6, 126.8, 126.7, 126.6, 124.5, 120.1, 119.5, 119.3, 116.5, 100.1, 71.0, 70.9, 57.9, 36.0, 35.9, 31.8, 19.2, 18.3 |
| 5l | 8.30(d, J=7.6 Hz, 1H), 8.10(s, 1H), 7.83(s, 1H), 7.67—7.73(m, 2H), 7.49(dd, J=1.2, 7.2 Hz, 1H), 7.12—7.26(m, 10H), 4.21—4.27(m, 1H), 4.18(t, J=6.0 Hz, 2H), 4.11(t, J=6.0 Hz, 2H), 2.90(t, J=7.6 Hz, 4H), 2.17—2.24(m, 1H), 2.04—2.10(m, 4H), 0.93(s, 3H), 0.92(s, 3H) | 182.9, 180.7, 164.4, 158.2, 141.6, 141.5, 139.6, 134.2, 134.1, 134.0, 128.4, 128.3, 128.2, 125.7, 125.1, 123.6, 119.9, 118.2, 118.0, 116.9, 68.0, 67.8, 59.7, 31.3, 30.6, 30.5, 19.7, 18.7 |
Table 6 1H NMR and 13C NMR data of target compounds 5a—5l
| Compd. | 1H NMR(400 MHz), δa | 13C NMR(100 MHz), δb |
|---|---|---|
| 5a | 8.92(d, J=8.0 Hz, 1H), 8.19(s, 1H), 7.88(s, 1H), 7.77(t, J=7.6 Hz, 1H), 7.70(d, J=7.2 Hz, 1H), 7.55(d, J=8.0 Hz, 1H), 4.34(t, J=7.6 Hz, 1H), 4.00(s, 3H), 3.93(s, 3H), 2.20—2.28(m, 1H), 1.00(t, J=6.0 Hz, 6H) | 182.9, 180.9, 173.1, 165.2, 158.7, 158.6, 138.9, 134.4, 134.1, 125.1, 123.4, 119.0, 118.2, 117.3, 117.2, 58.9, 56.5, 56.3, 29.7, 19.4, 18.9 |
| 5b | 12.73(s, 1H), 8.97(d, J=8.4 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 7.88(d, J=0.8 Hz, 1H), 7.75(t, J=7.6 Hz, 1H), 7.72(dd, J=1.2, 7.6 Hz, 1H), 7.55(dd, J=1.2, 8.0 Hz, 1H), 4.34(t, J=7.6 Hz, 1H), 4.30(q, J=6.8 Hz, 2H), 4.23(q, J=6.8 Hz, 2H), 2.19—2.27(m, 1H), 1.40—1.46(m, 6H), 1.00(t, J=6.8 Hz, 6H) | 183.0, 180.7, 172.9, 165.3, 158.1, 158.0, 138.7, 134.4, 134.2, 125.2, 123.5, 120.1, 118.4, 118.2, 117.4, 65.0, 64.7, 58.7, 29.5, 19.4, 19.0, 14.6 |
| 5c | 8.91(d, J=8.4 Hz, 1H), 8.18(d, J=1.2 Hz, 1H ), 7.87(d, J=1.2 Hz, 1H), 7.75(t, J=7.6 Hz, 1H), 7.71(dd, J=1.2, 7.6 Hz, 1H), 7.54(dd, J=1.2, 8.0 Hz, 1H), 4.33(t, J=7.6 Hz, 1H), 4.19(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 2.19—2.27(m, 1H), 1.76—1.87(m, 4H), 1.08(q, J=7.6 Hz, 6H), 1.00(d, J=5.6 Hz, 3H), 0.98(d, J=5.2 Hz, 3H) | 183.1, 180.6, 173.0, 165.2, 158.3, 158.2, 138.8, 134.3, 134.2, 134.1, 125.4, 123.7, 120.1, 118.4, 118.1, 117.2, 70.6, 70.4, 58.8, 29.6, 22.1, 19.4, 18.9, 10.4, 10.3 |
| 5d | 8.87(d, J=8.4 Hz, 1H), 8.18(d, J=1.6 Hz, 1H ), 7.87(d, J=1.6 Hz, 1H), 7.74(t, J=8.0 Hz, 1H), 7.71(dd, J=1.6, 7.6 Hz, 1H), 7.54(dd, J=1.6, 8.0 Hz, 1H), 4.35(t, J=7.6 Hz, 1H), 4.22(t, J=6.0 Hz, 2H), 4.15(t, J=6.0 Hz, 2H), 2.19—2.27(m, 1H), 1.74—1.83(m, 4H), 1.54—1.61(m, 4H), 0.96—1.01(m, 12H) | 183.0, 180.7,172.9, 165.3, 158.2, 158.1, 138.7, 134.3, 134.2, 134.1, 125.5, 123.8, 120.1, 118.4, 118.1, 117.3, 68.9, 68.7, 58.7, 30.8, 30.7, 29.6, 19.4, 18.9, 18.7, 13.7 |
| 5e | 8.94(d, J=8.4 Hz, 1H), 8.23(s, 1H), 8.00(s, 1H), 7.74—7.80(m, 2H), 7.63—7.68(m, 4H), 7.36—7.43(m, 7H), 5.42(s, 2H), 5.34(s, 2H), 4.34(t, J=7.2 Hz, 1H), 2.20—2.27(m, 1H), 0.99(t, J=7.6 Hz, 6H) | 182.9, 181.0, 173.0, 165.1, 157.7, 157.6, 138.9, 136.8, 136.7, 134.4, 134.3, 134.2, 128.5, 128.3, 128.2, 127.7, 127.6, 127.0, 126.9, 126.8, 125.6, 123.9, 120.5, 118.9, 118.6, 117.7, 70.4, 70.1, 58.8, 29.7, 19.4, 18.9 |
| 5f | 8.69(d, J=6.8 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 8.03(d, J=1.6 Hz, 1H), 7.79(d, J=1.2 Hz, 1H), 7.78(s, 1H), 7.69(t, J=4.8 Hz, 1H), 7.42—7.50(m, 6H), 5.41(s, 2H), 5.33(s, 2H), 4.29(q, J=6.4 Hz, 1H), 2.19—2.27(m, 1H), 0.99(d, J=2.4 Hz, 3H), 0.97(d, J=2.4 Hz, 3H) | 182.6, 180.4, 164.8, 157.8, 157.7, 139.2, 136.2, 134.3, 134.2, 134.1, 131.4, 131.3, 131.2, 128.7, 126.2, 124.7, 121.6, 119.6, 119.4, 118.3, 67.1, 67.0, 59.2, 40.2, 30.0, 19.5, 18.8 |
| 5g | 8.75(d, J=7.6 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 7.97(d, J=1.2 Hz, 1H), 7.73—7.80(m, 2H), 7.60—7.67(m, 5H), 7.44(s, 2H), 7.42(s, 2H), 5.37(s, 2H), 5.30(s, 2H), 4.32(q, J=6.4 Hz, 1H), 2.20—2.28(m, 1H), 1.00(d, J=5.2 Hz, 3H), 0.98(t, J=5.2 Hz, 3H) | 182.8, 181.0, 165.0, 157.5, 157.4, 139.0, 135.9, 135.8, 134.5, 134.2, 134.1, 132.2, 132.1, 128.9, 128.8, 128.3, 128.2, 125.6, 123.9, 120.4, 118.8, 118.7, 117.8, 69.7, 69.4, 58.9, 29.7, 19.4, 18.9 |
| 5h | 8.46(s, 1H), 8.10(s, 1H), 7.93(s, 1H), 7.55—7.71(m, 7H), 7.18(q, J=8.0 Hz, 4H), 5.31(s, 2H), 5.24(s, 2H), 4.25(t, J=6.8 Hz, 1H), 2.19—2.28(m, 1H), 0.94(s, 3H), 0.93(s, 3H) | 182.7, 180.8, 164.5, 162.8, 160.4, 157.6, 139.5, 134.3, 134.0, 133.0, 129.0, 125.2, 123.7, 120.4, 118.7, 117.4, 115.1, 114.9, 69.8, 69.5, 59.7, 30.5, 19.7, 18.7 |
| 5i | 12.75(s, 1H), 8.99(d, J=8.4 Hz, 1H), 8.28(d, J=1.6 Hz, 1H), 8.01(d, J=1.6 Hz, 1H), 7.78—7.84(m, 2H), 7.67(dd, J=2.0, 8.0 Hz, 1H), 7.42—7.56(m, 6H), 7.16—7.21(m, 2H), 5.45(s, 2H), 5.38(s, 2H), 4.36(q, J=7.2 Hz, 1H), 2.20—2.28(m, 1H), 1.01(d, J=6.8 Hz, 3H), 0.99(d, J=6.8 Hz, 3H) | 182.8, 181.0, 172.9, 165.2, 163.5, 161.1, 157.6, 157.5, 139.8, 139.7, 139.6, 139.6, 138.9, 134.6, 134.2, 134.3, 130.3, 130.2, 125.3, 123.6, 122.5, 120.4, 118.8, 117.9, 114.4, 114.2, 113.6, 113.3, 69.6, 69.4, 58.7, 29.6, 19.4, 18.9 |
| 5j | 8.90(d, J=7.2 Hz, 1H), 8.23(s, 1H), 8.01(s, 1H), 7.74—7.80(m, 2H), 7.65(d, J=8.0 Hz, 1H), 7.58(t, J=8.8 Hz, 4H), 7.29(d, J=7.6 Hz, 4H), 5.36(s, 2H), 5.29(s, 2H), 4.34(t, J=7.6 Hz, 1H), 2.89—2.96(m, 2H), 2.20—2.29(m, 1H), 1.24(s, 6H), 1.23(s, 6H), 0.99(t, J=7.6 Hz, 6H) | 182.9, 180.9, 165.1, 157.8, 157.7, 147.8, 147.7, 138.9, 134.4, 134.2, 134.1, 134.0, 127.1, 127.0, 126.2, 126.1, 125.5, 123.8, 120.5, 118.8, 118.6, 117.6, 70.4, 70.1, 58.9, 33.3, 29.7, 23.9, 23.8, 19.4, 18.9 |
| 5k | 8.26(d, J=1.2 Hz, 1H), 7.91(s, 1H), 7.79(d, J=7.6 Hz, 1H), 7.58(t, J=8.0 Hz, 1H), 7.41—7.45(m, 4H), 7.27—7.38(m, 6H), 7.20—7.24(m, 2H), 4.90(q, J=5.2 Hz, 1H), 4.41(t, J=7.2 Hz, 2H), 4.34(t, J=6.8 Hz, 2H), 3.26—3.30(m, 4H), 2.35—2.41(m, 1H), 1.09(d, J=3.6 Hz, 3H), 1.07(d, J=3.2 Hz, 3H) | 184.6, 181.6, 174.9, 165.7, 159.2, 158.8, 138.3, 138.1, 138.0, 134.7, 134.6, 134.1, 129.5, 128.7, 128.6, 126.8, 126.7, 126.6, 124.5, 120.1, 119.5, 119.3, 116.5, 100.1, 71.0, 70.9, 57.9, 36.0, 35.9, 31.8, 19.2, 18.3 |
| 5l | 8.30(d, J=7.6 Hz, 1H), 8.10(s, 1H), 7.83(s, 1H), 7.67—7.73(m, 2H), 7.49(dd, J=1.2, 7.2 Hz, 1H), 7.12—7.26(m, 10H), 4.21—4.27(m, 1H), 4.18(t, J=6.0 Hz, 2H), 4.11(t, J=6.0 Hz, 2H), 2.90(t, J=7.6 Hz, 4H), 2.17—2.24(m, 1H), 2.04—2.10(m, 4H), 0.93(s, 3H), 0.92(s, 3H) | 182.9, 180.7, 164.4, 158.2, 141.6, 141.5, 139.6, 134.2, 134.1, 134.0, 128.4, 128.3, 128.2, 125.7, 125.1, 123.6, 119.9, 118.2, 118.0, 116.9, 68.0, 67.8, 59.7, 31.3, 30.6, 30.5, 19.7, 18.7 |
| Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|
| Hela | MCF7 | HepG2 | KB | HEK293T | |
| Rhein-Na | >100 | >100 | >100 | >100 | >100 |
| 5a | >100 | >100 | >100 | >100 | >100 |
| 5b | >100 | >100 | >100 | >100 | >100 |
| 5c | >100 | >100 | >100 | >100 | >100 |
| 5d | 67.8 | >100 | 54.4 | 28.6 | 43.1 |
| 5e | 44.9 | 36.5 | 36.1 | 15.6 | 21.5 |
| 5f | >100 | >100 | >100 | >100 | >100 |
| 5g | >100 | >100 | >100 | >100 | >100 |
| 5h | >100 | >100 | >100 | >100 | >100 |
| 5i | 35.4 | 38.6 | 29.6 | 17.8 | 12.9 |
| 5j | 37.5 | 13.9 | 5.8 | 9.4 | 1.9 |
| 5k | >100 | >100 | 56.5 | >100 | >100 |
| 5l | 9.4 | 4.5 | 3.6 | 4.9 | 1.6 |
| DDP | 5.5 | 15.8 | 14.3 | 6.1 | 3.3 |
| Doxorubicin | 0.99 | 2.8 | 4.7 | 3.7 | 0.7 |
Table 7 Antitumor activity of target compoundsin vitro*
| Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|
| Hela | MCF7 | HepG2 | KB | HEK293T | |
| Rhein-Na | >100 | >100 | >100 | >100 | >100 |
| 5a | >100 | >100 | >100 | >100 | >100 |
| 5b | >100 | >100 | >100 | >100 | >100 |
| 5c | >100 | >100 | >100 | >100 | >100 |
| 5d | 67.8 | >100 | 54.4 | 28.6 | 43.1 |
| 5e | 44.9 | 36.5 | 36.1 | 15.6 | 21.5 |
| 5f | >100 | >100 | >100 | >100 | >100 |
| 5g | >100 | >100 | >100 | >100 | >100 |
| 5h | >100 | >100 | >100 | >100 | >100 |
| 5i | 35.4 | 38.6 | 29.6 | 17.8 | 12.9 |
| 5j | 37.5 | 13.9 | 5.8 | 9.4 | 1.9 |
| 5k | >100 | >100 | 56.5 | >100 | >100 |
| 5l | 9.4 | 4.5 | 3.6 | 4.9 | 1.6 |
| DDP | 5.5 | 15.8 | 14.3 | 6.1 | 3.3 |
| Doxorubicin | 0.99 | 2.8 | 4.7 | 3.7 | 0.7 |
| [1] | Xu X., Li B. P., Zhang H. F., Shanghai J. Tradit. Chin. Med., 2003, 37(4), 56—59 |
| (徐翔, 郦柏平, 张慧芬. 上海中医药杂志,2003, 37(4), 56—59) | |
| [2] | Fu X. S., Chen F., Liu X. H., Xu H., Zhou Y. Z., Chin. J. New Drugs, 2011, 20(16), 1534—1538 |
| (傅兴圣, 陈菲, 刘渊红, 许虎, 周逸芝. 中国新药杂志,2011, 20(16), 1534—1538) | |
| [3] | Sheng X. Y., Wang M., Lu M., Xi B. L., Sheng H. G., Zang Y. Q., Am. J. Physiol. Endocrinol. Metab., 2011, 300(5), E886—E893 |
| [4] | Tang J. C., Yang H., Song X. Y., Song X. H., Yan S. L., Shao J. Q., Zhang T. L., Zhang J. N., Phytother. Res., 2009, 23(2), 159—164 |
| [5] | Badria F. A., Ibrahim A. S., Drug Discov. Ther., 2013, 7(2), 84—89 |
| [6] | Fernand V. E., Losso J. N., Truax R. E., Villar E. E., Bwambok D. K., Fakayode S. O., Lowry M., Warner I. M., Chem. Biol. Interact., 2011, 192(3), 220—232 |
| [7] | Ip S. W., Weng Y. S., Lin S. Y., Yang M. D., Tang N. Y., Su C. C., Chung J. G., Anticancer Res., 2007, 27(1A), 379—389 |
| [8] | Wang Q., Zhang N. N., Li H. Y., Jiang M., Gao J., Bai G., Acta Pharm. Sin., 2012, 47(12), 1618—1622 |
| (王倩, 张楠楠, 李红艳, 姜民, 高洁, 白钢. 药学学报,2012, 47(12), 1618—1622) | |
| [9] | Hsia T. C., Yang J. S., Chen G. W.,Chiu T. H., Lu H. F., Yang M. D., Yu F. S., Liu K. C., Lai K. C., Lin C. C., Chung J. G., Anticancer Res., 2009, 29(1), 309—318 |
| [10] | Peng L. L., Yang J. Y., Ning C., Zhang J., Xiao X. C., He D., Wang X. Y., Li Z. P., Fu S. S., Ning J. P., Biol. Pharm. Bull., 2012, 35(10), 1676—1685 |
| [11] | Liu X., Cheng J., Zheng X. C., Chen Y. G., Wu C., Li B., Fu J. F., Cao H. W., Lu Y. L., Li J., Zheng J., Zhou H., Int. Immuno-pharmacol., 2009, 9(9), 1021—1031 |
| [12] | Yu L., Xiang H., Fan J. W., Wang D. C., Yang F., Guo N., Jin Q., Deng X. M., J. Biotechnol., 2008, 135(3), 304—308 |
| [13] | Chung J. G., Tsou M. F., Wang H. H., Lo H. H., Hsieh S. E., Yen Y. S., Wu L. T., Chang S. H., Ho C. C., Hung C. F., J. Appl. Toxicol., 1998, 18(2), 117—123 |
| [14] | Hao K., Qi Q., Wan P., Zhang J. W., Hao H. P., Liang Y., Xie L., Wang G. J., Sun J. G., Basic Clin. Pharmacol. Toxicol., 2014, 114(2), 160—167 |
| [15] | Gao Q., Qin W. S., Jia Z. H., Zheng J. M., Zeng C. H., Li L. S., Liu Z. H., Planta Med., 2010, 76(1), 27—33 |
| [16] | Ye M. Y., Yao G. Y., Wei J. C., Pan Y. M., Liao Z. X., Wang H. S., Int. J. Mol. Sci., 2013, 14(5), 9424—9439 |
| [17] | Liang Y. K., Yue Z. Z., Li J. X., Tan C., Miao Z. H., Tan W. F., Yang C. H., Eur. J. Med. Chem., 2014, 84, 505—515 |
| [18] | Ji C. M., Zhen Y. Z., Fan X. Y., Wang Z. J., Jiang S. F., Zhu L. H., Zhang G. L., Basic Clin. Med., 2014, 34(2), 155—159 |
| (纪春梅, 甄永占, 范晓禹, 王志军, 蒋守芳, 朱丽华, 章广玲. 基础医学与临床,2014, 34(2), 155—159) | |
| [19] | Guo L., Cao R. H., Fan W. X., Ma Q., Chem. J. Chinese Universities, 2014, 35(3), 518—523 |
| (郭亮, 曹日晖, 范文玺, 马芹. 高等学校化学学报,2014, 35(3), 518—523) | |
| [20] | Guo L., Cao R. H., Fan W. X., Gan Z. Y., Ma Q., Chem. J. Chinese Universities, 2016, 37(6), 1093—1099 |
| (郭亮, 曹日晖, 范文玺, 甘紫云, 马芹. 高等学校化学学报,2016, 37(6), 1093—1099) | |
| [21] | Zhang S. B., Qian X., Zhang D. H., Zhu J. M., Wu Y., Guo Y., Xu L., Chem. Res. Chinese Universities, 2016, 32(1), 149—154 |
| [22] | Ma J. J., Hu G., Xie L. J., Chen L., Xu B. X., Gong P., Chem. Res. Chinese Universities, 2015, 31(6), 958—963 |
| [23] | Long E. C., Barton J. K., Acc. Chem. Res., 1990, 23(9), 271—273 |
| [24] | Sirajuddin M., Ali S., Badshah A., J. Photochem. Photobiol., B: Biol., 2013, 124, 1—19 |
| [1] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [2] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [3] | HOU Hua, WANG Baoshan. Group Additivity Theoretical Model for the Prediction of Dielectric Strengths of the Alternative Gases to SF6 [J]. Chem. J. Chinese Universities, 2021, 42(12): 3709. |
| [4] | YE Xiaodong, QI Guodong, XU Jun, DENG Feng. Glucose Oxidation on Au-supported SBA-15 Molecular Sieve † [J]. Chem. J. Chinese Universities, 2020, 41(5): 960. |
| [5] | XIAO Yanhua, ZHANG Guangjie, ZONG Liang, LIU Guohong, REN Lijun, DONG Junxing. Chemical Constituents and Antitumor Activity of Tupistra chinensis † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1897. |
| [6] | CHANG Junpeng,ZHAO Jiarui,CHEN Sijia,MENG Kai,SHI Weini,LI Ruifang. Structure-activity Relationship of Antimicrobial Peptide SAMP1 and Its Analog Peptides† [J]. Chem. J. Chinese Universities, 2019, 40(4): 705. |
| [7] | LÜ Mingjun,LI Wen,YANG Xinying,FANG Hao. Synthesis and Antitumor Activity of N9 Position Aromatic Substituted Purine-8-one Derivatives† [J]. Chem. J. Chinese Universities, 2019, 40(2): 254. |
| [8] | FANG Fang,XUE Liangmin,CONG Jing,TIAN Chao,WANG Xiaowei,LIU Junyi,ZHANG Zhili. Synthesis and Anti-tumor Activity Evaluation of a Series of 2- or 4-Substituted Pyrido[3,2-d]pyrimidines as Nonclassical Antifolates † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2111. |
| [9] | ZHANG Peiquan,YANG Qianqian,LONG Huidan,CHEN Xin. Synthesis and Antitumor Activity of Auranofin Derivatives † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2097. |
| [10] | YU Min, HUANG Jingjing, MA Min, FU Ruiyan, YAN Yan, ZHANG Fusheng, YIN Junfeng, XIE Ningning. Zinc Chelating Activity and Quantitative Structure-activity Relationship of Tripeptides† [J]. Chem. J. Chinese Universities, 2018, 39(2): 234. |
| [11] | HOU Hua, YU Xiaojuan, ZHOU Wenjun, LUO Yunbai, WANG Baoshan. Theoretical Investigations on the Structure-activity Relationship to the Dielectric Strength of the Insulation Gases† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2477. |
| [12] | WANG Lei, ZHENG Guojun, JI Qi, CHEN Bo, GONG Longlong, GAO Congmin, DU Zhenjian, ZHANG Xingmin. Synthesis and Biological Activity of Novel PI3K/mTOR Inhibitors† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1590. |
| [13] | LIU Yuming, TIAN Lijun, HU Dong, NIE Jianbing. yntheses and Anti-cholinesterase Activity of 4-N-Phenylaminoquinoline Derivatives † [J]. Chem. J. Chinese Universities, 2017, 38(3): 392. |
| [14] | BAI Xinfa, MA Xuan, XIE Xiaoxia, SHAO Mingsha, GUO Ningning, YAN Ning, YAO Lei. Synthesis and Anti-tumor Activity of Tubulysins Analogues† [J]. Chem. J. Chinese Universities, 2017, 38(1): 47. |
| [15] | LIU Benguo, LIU Jiangwei, LI Jiaqi, GENG Sheng, MO Haizhen, LIANG Guizhao. 3D-QSAR and Interaction Mechanism of Flavonoids s P-glycoprotein Inhibitors† [J]. Chem. J. Chinese Universities, 2017, 38(1): 41. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||