

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (9): 1897.doi: 10.7503/cjcu20190167
• Organic Chemistry • Previous Articles Next Articles
XIAO Yanhua1,*( ),ZHANG Guangjie2,ZONG Liang1,LIU Guohong1,REN Lijun1,DONG Junxing2,*(
),ZHANG Guangjie2,ZONG Liang1,LIU Guohong1,REN Lijun1,DONG Junxing2,*( )
)
Received:2019-03-19
Online:2019-09-10
Published:2019-09-09
Contact:
XIAO Yanhua,DONG Junxing
E-mail:yanhuaxiao@163.com;djx931314@163.com
Supported by:CLC Number:
TrendMD:
XIAO Yanhua, ZHANG Guangjie, ZONG Liang, LIU Guohong, REN Lijun, DONG Junxing. Chemical Constituents and Antitumor Activity of Tupistra chinensis †[J]. Chem. J. Chinese Universities, 2019, 40(9): 1897.
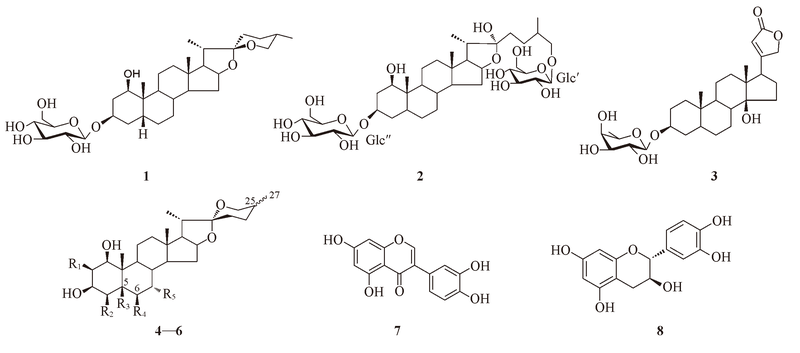
Fig.1 Structures of compounds 1-8 4: R1=OH, R2=OH, R3=OH, R4=OH, R5=OH, 25S; 5: R1=H, R2=H, R3=H, R4=H, R5=H, 25S,Δ5(6); 6: R1=H, R2=H, R3=H, R4=H, R5=H, 25R,Δ5(6)
| C atom | δH(J/Hz) | Key NOESY | δC | Key HMBC |
|---|---|---|---|---|
| 1 | 4.55(d, 6.4) | 3-H | 72.2(CH) | C3 |
| 2 | 2.39(m), 2.01(m) | 33.0(CH2) | ||
| 3 | 2.01(m) | 1-H | 74.4(CH) | |
| 4 | 4.41(d, 5.2), 2.31(m) | 30.8(CH2) | ||
| 5 | 1.97(m) | 35.3(CH) | C3 | |
| 6 | 2.02(m) | 26.3(CH2) | ||
| 7 | 1.70(m), 1.56(m) | 26.4(CH2) | ||
| 8 | 1.66(m), 1.30(m) | 31.8(CH) | ||
| 9 | 1.87(m) | 42.0(CH) | ||
| 10 | 1.16(m) | 41.0(C) | ||
| 11 | 20.9(CH2) | |||
| 12 | 2.00(m), 1.42(m) | 40.4(CH2) | ||
| 13 | 1.63(m), 1.04(m) | 40.2(C) | ||
| 14 | 16-H, 17-H | 55.3(CH) | ||
| 15 | 1.06(m) | 30.5(CH2) | ||
| 16 | 1.84(m), 1.45(m) | 14-H, 23-H | 80.6(CH) | |
| 17 | 4.36(d, 7.2), 1.54(m) | 14-H | 62.5(CH) | |
| 18 | 0.94(s) | 20-H | 19.0(CH3) | C14, C17 |
| 19 | 1.22(s) | 16.5(CH3) | C1, C5, C6, C9 | |
| 20 | 2.29(m) | 18-H | 42.0(CH) | |
| 21 | 1.00(d, 6.4) | 16.8(CH3) | C20, C22 | |
| 22 | 110.4(C) | |||
| 23 | 1.69(m), 1.49(m) | 16-H | 28.1(CH2) | |
| 24 | 1.51(m), 1.34(m) | 28.0(CH2) | ||
| 25 | 1.65(m) | 29.0(CH) | ||
| 26 | 3.69(d, 7.2), 3.63(d, 7.2) | 69.5(CH2) | ||
| 27 | 0.66(d, 6.4) | 17.2(CH3) | C24, C25, C26 | |
| 1' | 5.01(d, 8.0) | 101.1(CH) | C3 | |
| 2' | 3.98(dd, 8.0, 4.0) | 74.9(CH) | ||
| 3' | 3.95(dd, 8.0, 4.0) | 78.5(CH) | ||
| 4' | 4.26(dd, 8.4, 4.4) | 71.4(CH) | ||
| 5' | 4.24(dd, 8.4, 4.4) | 78.5(CH) | ||
| 6' | 4.57(dd, 9.2, 3.6), 4.36(dd, 9.2, 3.6) | 62.5(CH2) |
| C atom | δH(J/Hz) | Key NOESY | δC | Key HMBC |
|---|---|---|---|---|
| 1 | 4.55(d, 6.4) | 3-H | 72.2(CH) | C3 |
| 2 | 2.39(m), 2.01(m) | 33.0(CH2) | ||
| 3 | 2.01(m) | 1-H | 74.4(CH) | |
| 4 | 4.41(d, 5.2), 2.31(m) | 30.8(CH2) | ||
| 5 | 1.97(m) | 35.3(CH) | C3 | |
| 6 | 2.02(m) | 26.3(CH2) | ||
| 7 | 1.70(m), 1.56(m) | 26.4(CH2) | ||
| 8 | 1.66(m), 1.30(m) | 31.8(CH) | ||
| 9 | 1.87(m) | 42.0(CH) | ||
| 10 | 1.16(m) | 41.0(C) | ||
| 11 | 20.9(CH2) | |||
| 12 | 2.00(m), 1.42(m) | 40.4(CH2) | ||
| 13 | 1.63(m), 1.04(m) | 40.2(C) | ||
| 14 | 16-H, 17-H | 55.3(CH) | ||
| 15 | 1.06(m) | 30.5(CH2) | ||
| 16 | 1.84(m), 1.45(m) | 14-H, 23-H | 80.6(CH) | |
| 17 | 4.36(d, 7.2), 1.54(m) | 14-H | 62.5(CH) | |
| 18 | 0.94(s) | 20-H | 19.0(CH3) | C14, C17 |
| 19 | 1.22(s) | 16.5(CH3) | C1, C5, C6, C9 | |
| 20 | 2.29(m) | 18-H | 42.0(CH) | |
| 21 | 1.00(d, 6.4) | 16.8(CH3) | C20, C22 | |
| 22 | 110.4(C) | |||
| 23 | 1.69(m), 1.49(m) | 16-H | 28.1(CH2) | |
| 24 | 1.51(m), 1.34(m) | 28.0(CH2) | ||
| 25 | 1.65(m) | 29.0(CH) | ||
| 26 | 3.69(d, 7.2), 3.63(d, 7.2) | 69.5(CH2) | ||
| 27 | 0.66(d, 6.4) | 17.2(CH3) | C24, C25, C26 | |
| 1' | 5.01(d, 8.0) | 101.1(CH) | C3 | |
| 2' | 3.98(dd, 8.0, 4.0) | 74.9(CH) | ||
| 3' | 3.95(dd, 8.0, 4.0) | 78.5(CH) | ||
| 4' | 4.26(dd, 8.4, 4.4) | 71.4(CH) | ||
| 5' | 4.24(dd, 8.4, 4.4) | 78.5(CH) | ||
| 6' | 4.57(dd, 9.2, 3.6), 4.36(dd, 9.2, 3.6) | 62.5(CH2) |
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||
|---|---|---|---|---|---|
| LoVo | BGC-823 | LoVo | BGC-823 | ||
| 1 | >6.0 | >5.0 | 5/6 | 0.532±0.139* | 0.757±0.093* |
| 2 | >6.0 | >5.0 | 7 | 4.731±0.157 | 3.679±0.083 |
| 3 | 3.232±0.141 | 4.833±0.097 | 8 | >6.0 | 5.538±0.207 |
| 4 | >6.0 | >5.0 | Cisplatin | 5.725±0.272 | 4.623±0.742 |
| Compd. | IC50/(μmol·L-1) | Compd. | IC50/(μmol·L-1) | ||
|---|---|---|---|---|---|
| LoVo | BGC-823 | LoVo | BGC-823 | ||
| 1 | >6.0 | >5.0 | 5/6 | 0.532±0.139* | 0.757±0.093* |
| 2 | >6.0 | >5.0 | 7 | 4.731±0.157 | 3.679±0.083 |
| 3 | 3.232±0.141 | 4.833±0.097 | 8 | >6.0 | 5.538±0.207 |
| 4 | >6.0 | >5.0 | Cisplatin | 5.725±0.272 | 4.623±0.742 |
| [1] | Wu X., Fan J., Ouyang Z ., J. Pharmacol 2014, 66( 3), 453-465 |
| [2] | Zhao H., Wang H. N., Xu H., ., Journal of Shanxi University of Technology(Natural Science Edition) 2013, 29, 62-69 |
| ( 赵桦, 王慧娜, 徐皓 . 陕西理工学院学报自然科学版, 2013, 29, 62-69) | |
| [3] | Huang W., Zhang H., Zou K ., Neoplasma, 2012, 59( 613-621 |
| [4] | Xie J. Y., Zhang D. D., Li Y. Z ., Pharmacology and Clinics of Chinese Materia Medica, 2014, 30, 82-86 |
| ( 谢锦艳, 张东东, 李玉泽 . 中药药理与临床, 2014, 30, 82-86) | |
| [5] | Zou K., Wang J. Z., Guo Z. Y ., J. Magn. Reson Chem, 2009, 47( 87-91 |
| [6] | Zou K., Wang J. Z., Wu J ., J. Chin. Chem Lett, 2007, 18( 1239-1242 |
| [7] | Zou K., Wu J Du M ., J. Chin. Chem Lett, 2007, 18( 65-68 |
| [8] | Zou K., Wu J Liu C ., J. Chin. Chem Lett, 2006, 17( 1355-1338 |
| [9] | Zou K., Jun W Ming D ., J. Chin. Chem Lett, 2006, 18( 65-68 |
| [10] | Zou K., Wang J. Z., Du M ., J. Chem. Pharm Bull, 2006, 54( 1440-1442 |
| [11] | Wu H. Y., Wang Q., Liu C. X., Zou K., ., Journal of China Three Gorges University(Natural Science), 2012, 34, 85-88 |
| ( 邬昊洋, 王倩, 刘呈雄, 邹坤 . 三峡大学学报自然科学版, 2012, 34, 85-88) | |
| [12] | Wu G. X., Liu A. Y., Wei X. Y., Chen W. X ., Journal of Wuhan Botanical Research, 2007, 25, 89-92 |
| ( 吴光旭, 刘爱媛, 魏孝义, 陈维信 . 武汉植物学研究, 2007, 25, 89-92) | |
| [13] | Pan W. B., Wei L. M., Wei L. L., Wu Y. C ., Chem. Pharm Bull, 2006, 54( 954-958 |
| [14] | Pan W. B., Chang F. R., Wei L. M., Wu Y. C ., J. Nat Prod, 2003, 66( 161-168 |
| [15] | Pan W. B., Chang F. R ., J. Chem. Pham Bull, 2000, 48( 9), 1350-1353 |
| [16] | Pan W. B., Chang F. R., Wu Y. C ., J. Nat. Prod 2000, 63( 861-863 |
| [17] | Li Q., Zou K., Wang J. Z., ., Chinese Journal of Ethnomedicine and Ethnopharmacy, 2007, 86, 164-167 |
| ( 李青, 邹坤, 汪鋆植 . 中国民族民间医药杂志, 2007, 86, 164-167) | |
| [18] | Cai J., Zhu Z. G., Xu C. L ., Journal of Southern Medical University, 2007, 27, 188-194 |
| ( 蔡晶, 朱正光, 余传林 . 南方医科大学学报, 2007, 27, 188-194) | |
| [19] | Xiao Y. H., Yin H. L Chen L. Dong J. X., ., Fitoterapia, 2015, 102( 102-108 |
| [20] | Situ Z Q.. Wu J. Z ., Cell Culture(Revised Edition), World Publishing Corporation, Xi'an, 2004 |
| ( 司徒镇强, 吴军正 . 细胞培养修订版, 西安: 世界图书出版公司, 2004) | |
| [21] | Miyahara K., Kudo K., Kawasaki T ., Chem. Pharm Bull, 1983, 31( 348-351 |
| [22] | Tobari A., Teshima M., Koyanagi J., Kawase M ., Eur. J. Med Chem, 2000, 35( 511-527 |
| [23] | Inoue T., Mimaki Y., Sashida Y., Nikaido T., Ohmoto T ., Phytochemistry, 1995, 39( 1103-1110 |
| [24] | Patricia Y. H., Aisyah H. J., Reg L., Kerry P., William K., James J. D ., Phytochemistry, 2008, 69( 796-804 |
| [25] | Pawan K. A ., Phytochemistry, 1992, 31( 3307-3313 |
| [26] | Pan Z. H., Li Y., Liu J. L., Ning D. S., Li D. P., Wu X. D., Wen Y. X ., Fitoterapia, 2012, 83( 1489-1493 |
| [27] | Shen P., Wang S. L., Yang C. R., Cai B., Yao X. S ., Acta Bota. Sinica 2003, 45( 5), 626-629 |
| [28] | Wang Q., Miao W. J., Xiang C., Guo D. A., Ye M., ., Chinese Traditional and Herbal Drugs 2014, 45, 31-35 |
| ( 王青, 苗文娟, 向诚, 果德安, 叶敏 . 中草药, 2014, 45, 31-35) | |
| [29] | Elsohly H. N., Joshi A., Li X. C., Ross S. A ., Phytochemistry, 1999, 52( 141-145 |
| [30] | Qi L. K., Wang J., Liu H. Z ., Chinese Journal of Biochemical Pharmaceutics, 2015, 2, 45-47 |
| ( 戚利坤, 王俊, 刘洪泽 . 中国生化药物杂志, 2015, 2, 45-47) |
| [1] | LÜ Mingjun,LI Wen,YANG Xinying,FANG Hao. Synthesis and Antitumor Activity of N9 Position Aromatic Substituted Purine-8-one Derivatives† [J]. Chem. J. Chinese Universities, 2019, 40(2): 254. |
| [2] | FANG Fang,XUE Liangmin,CONG Jing,TIAN Chao,WANG Xiaowei,LIU Junyi,ZHANG Zhili. Synthesis and Anti-tumor Activity Evaluation of a Series of 2- or 4-Substituted Pyrido[3,2-d]pyrimidines as Nonclassical Antifolates † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2111. |
| [3] | ZHANG Peiquan,YANG Qianqian,LONG Huidan,CHEN Xin. Synthesis and Antitumor Activity of Auranofin Derivatives † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2097. |
| [4] | BAI Xinfa, MA Xuan, XIE Xiaoxia, SHAO Mingsha, GUO Ningning, YAN Ning, YAO Lei. Synthesis and Anti-tumor Activity of Tubulysins Analogues† [J]. Chem. J. Chinese Universities, 2017, 38(1): 47. |
| [5] | GUO Liang, CAO Rihui, FAN Wenxi, GAN Ziyun, MA Qin. Design, Synthesis and in vitro Antitumor Activities of Novel Bivalent β-Carbolines† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1093. |
| [6] | ZHANG Jie, ZHOU Changjian, XIE Jianwei, DAI Bin. Synthesis and Antitumor Activities of Rhein-Valine Adducts† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2159. |
| [7] | ZHOU Hao, DUAN Zhigang, ZHAO Shuang, BAO Meiying, LI Zhiwei, PEI Yazhong. Design and Synthesis of Phenylpyrimidine and Their Anticancer Activity† [J]. Chem. J. Chinese Universities, 2015, 36(9): 1694. |
| [8] | WANG Gang, HAN Leiqiang, FANG Hao. Syntheses and Antitumor Activities of Phenylpiperazine Derivatives† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2435. |
| [9] | GUO Hua, YANG Chengling, WANG Wei, LAI Quanyong, YUAN Zhi. Preparation of Liver-targeted Nano-prodrug Based on Sodium Alginate Derivative and the Study on Antitumor Activity† [J]. Chem. J. Chinese Universities, 2014, 35(8): 1835. |
| [10] | SONG Xiudao, HE Jun, MA Jin, LIU Yunmei, ZHENG Xing, LEI Xiaoyong, GUO Yu. Syntheses and Anticancer Activities of Glycine Derivatives of Chrysin† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1465. |
| [11] | WANG Junhua, WANG Quande, DUN Yanyan, FANG Hao. Syntheses and Antitumor Activities of Purine-sulfonamides Derivatives† [J]. Chem. J. Chinese Universities, 2014, 35(6): 1189. |
| [12] | GUO Liang, CAO Rihui, FAN Wenxi, MA Qin. Synthesis and Biological Evaluation of 1,2,7,9-Tetrasubstituted Harmine Derivatives as Potential Antitumor Agents† [J]. Chem. J. Chinese Universities, 2014, 35(3): 518. |
| [13] | YANG Haikui, XU Wanfu, DUAN Anna, YOU Wenwei, ZHAO Peiliang. Syntheses and Biological Activities of Novel Imine and Imide Derivatives Bearing 1,2,4-Triazole Moiety† [J]. Chem. J. Chinese Universities, 2014, 35(3): 555. |
| [14] | YANG Hongliang, XU Guoxing, BAO Meiying, ZHANG Dapeng, LI Zhiwei, PEI Yazhong. Design and Synthesis of Pyridinylisoxazoles and Their Anticancer Activities† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2584. |
| [15] | DING Guobin, LI Binchun, GUO Yi, XU Li. Spectral Properties and Antitumor Activity of 10-Hydroxycamptothecin and Its Application in Cell Labeling† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2324. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||