

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (10): 2206.doi: 10.7503/cjcu20180182
• Organic Chemistry • Previous Articles Next Articles
LI Yang1, LI Zhiwen1, ZHU Junfei1, LIU Shihui1,*( ), HE Junlin2,*(
), HE Junlin2,*( )
)
Received:2018-03-08
Online:2018-10-10
Published:2018-09-29
Contact:
LIU Shihui,HE Junlin
E-mail:liush05@163.com;hejunlin@bmi.ac.cn
Supported by:CLC Number:
TrendMD:
LI Yang, LI Zhiwen, ZHU Junfei, LIU Shihui, HE Junlin. Construction of Pyrenyl Pairs in dsDNA: Fluorescent Properties of Multiple Pyrenyl-attached dsDNAs Through 7-Substituted 8-Aza-7-deaza-2'-deoxyadenosine Analogues†[J]. Chem. J. Chinese Universities, 2018, 39(10): 2206.
| Name | Sequence | MS(calcd.), m/z | Name | Sequence | MS(calcd.), m/z |
|---|---|---|---|---|---|
| PY01 | 5'-d(TAGGTC22TACT)-3' | 4092.2(4090.8) | PY06 | 5'-d(TAGGTC33TACT)-3' | 4100.4(4101.0) |
| PY02 | 5'-d(TAGGTC2AT2CT)-3' | 4092.7(4090.8) | PY07 | 5'-d(TAGGTC3AT3CT)-3' | 4100.1(41010) |
| PY03 | 5'-d(TAGGTC2ATACT)-3' | 3867.9(3868.7) | PY08 | 5'-d(TAGGTC3ATACT)-3' | 3871.7(3872.7) |
| PY04 | 5'-d(TAGGTCA2T2CT)-3' | 4092.0(4090.8) | PY09 | 5'-d(TAGGTCA3T3CT)-3' | 4100.2(41010) |
| PY05 | 5'-d(AGT2TTGACCTA)-3' | 3868.1(3868.7) | PY10 | 5'-d(AGT3TTGACCTA)-3' | 3872.1(3872.7) |
Table 1 MS characterization of the DNA sequences
| Name | Sequence | MS(calcd.), m/z | Name | Sequence | MS(calcd.), m/z |
|---|---|---|---|---|---|
| PY01 | 5'-d(TAGGTC22TACT)-3' | 4092.2(4090.8) | PY06 | 5'-d(TAGGTC33TACT)-3' | 4100.4(4101.0) |
| PY02 | 5'-d(TAGGTC2AT2CT)-3' | 4092.7(4090.8) | PY07 | 5'-d(TAGGTC3AT3CT)-3' | 4100.1(41010) |
| PY03 | 5'-d(TAGGTC2ATACT)-3' | 3867.9(3868.7) | PY08 | 5'-d(TAGGTC3ATACT)-3' | 3871.7(3872.7) |
| PY04 | 5'-d(TAGGTCA2T2CT)-3' | 4092.0(4090.8) | PY09 | 5'-d(TAGGTCA3T3CT)-3' | 4100.2(41010) |
| PY05 | 5'-d(AGT2TTGACCTA)-3' | 3868.1(3868.7) | PY10 | 5'-d(AGT3TTGACCTA)-3' | 3872.1(3872.7) |
| Name | Sequence | Tm/℃ | ΔTm/℃ | Name | Sequence | Tm/℃ | ΔTm/℃ |
|---|---|---|---|---|---|---|---|
| D01+D02 | 5'-d(TAGGTCAATACT)-3' | 49.8 | PY06+D02 | 5'-d(TAGGTC33TACT)-3' | 45.9 | -3.9 | |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| PY03+D02 | 5'-d(TAGGTC2ATACT)-3' | 39.4 | -10.4 | PY07+D02 | 5'-d(TAGGTC3AT3CT)-3' | 61.7 | 11.9 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| PY08+D02 | 5'-d(TAGGTC3ATACT)-3' | 54.0 | 4.2 | PY09+D02 | 5'-d(TAGGTCA3T3CT)-3' | 59.5 | 9.7 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| D01+PY05 | 5'-d(TAGGTCAATACT)-3' | 41.0 | -8.8 | PY01+PY05 | 5'-d(TAGGTC22TACT)-3' | 48.8 | -1.0 |
| 3'-d(ATCCAGTT2TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| D01+PY10 | 5'-d(TAGGTCAATACT)-3' | 57.0 | 7.2 | PY02+PY05 | 5'-d(TAGGTC2AT2CT)-3' | 54.0 | 4.2 |
| 3'-d(ATCCAGTT3TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| PY03+PY05 | 5'-d(TAGGTC2ATACT)-3' | 49.8 | 0 | PY04+PY05 | 5'-d(TAGGTCA2T2CT)-3' | 60.0 | 10.2 |
| 3'-d(ATCCAGTT2TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| PY01+D02 | 5'-d(TAGGTC22TACT)-3' | 44.3 | -4.5 | PY06+PY10 | 5'-d(TAGGTC33TACT)-3' | 55.2 | 5.4 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY02+D02 | 5'-d(TAGGTC2AT2CT)-3' | 36.5 | -13.3 | PY09+PY10 | 5'-d(TAGGTCA3T3CT)-3' | 59.4 | 9.6 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY04+D02 | 5'-d(TAGGTCA2T2CT)-3' | 52.1 | 2.3 | PY07+PY10 | 5'-d(TAGGTC3AT3CT)-3' | 58.6 | 8.8 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY08+PY10 | 5'-d(TAGGTC3ATACT)-3' | 64.2 | 14.4 | ||||
| 3'-d(ATCCAGTT3TGA)-5' | |||||||
Table 2 Thermal stability(Tm) of dsDNAs containing pyrenyl group
| Name | Sequence | Tm/℃ | ΔTm/℃ | Name | Sequence | Tm/℃ | ΔTm/℃ |
|---|---|---|---|---|---|---|---|
| D01+D02 | 5'-d(TAGGTCAATACT)-3' | 49.8 | PY06+D02 | 5'-d(TAGGTC33TACT)-3' | 45.9 | -3.9 | |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| PY03+D02 | 5'-d(TAGGTC2ATACT)-3' | 39.4 | -10.4 | PY07+D02 | 5'-d(TAGGTC3AT3CT)-3' | 61.7 | 11.9 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| PY08+D02 | 5'-d(TAGGTC3ATACT)-3' | 54.0 | 4.2 | PY09+D02 | 5'-d(TAGGTCA3T3CT)-3' | 59.5 | 9.7 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| D01+PY05 | 5'-d(TAGGTCAATACT)-3' | 41.0 | -8.8 | PY01+PY05 | 5'-d(TAGGTC22TACT)-3' | 48.8 | -1.0 |
| 3'-d(ATCCAGTT2TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| D01+PY10 | 5'-d(TAGGTCAATACT)-3' | 57.0 | 7.2 | PY02+PY05 | 5'-d(TAGGTC2AT2CT)-3' | 54.0 | 4.2 |
| 3'-d(ATCCAGTT3TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| PY03+PY05 | 5'-d(TAGGTC2ATACT)-3' | 49.8 | 0 | PY04+PY05 | 5'-d(TAGGTCA2T2CT)-3' | 60.0 | 10.2 |
| 3'-d(ATCCAGTT2TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| PY01+D02 | 5'-d(TAGGTC22TACT)-3' | 44.3 | -4.5 | PY06+PY10 | 5'-d(TAGGTC33TACT)-3' | 55.2 | 5.4 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY02+D02 | 5'-d(TAGGTC2AT2CT)-3' | 36.5 | -13.3 | PY09+PY10 | 5'-d(TAGGTCA3T3CT)-3' | 59.4 | 9.6 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY04+D02 | 5'-d(TAGGTCA2T2CT)-3' | 52.1 | 2.3 | PY07+PY10 | 5'-d(TAGGTC3AT3CT)-3' | 58.6 | 8.8 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY08+PY10 | 5'-d(TAGGTC3ATACT)-3' | 64.2 | 14.4 | ||||
| 3'-d(ATCCAGTT3TGA)-5' | |||||||
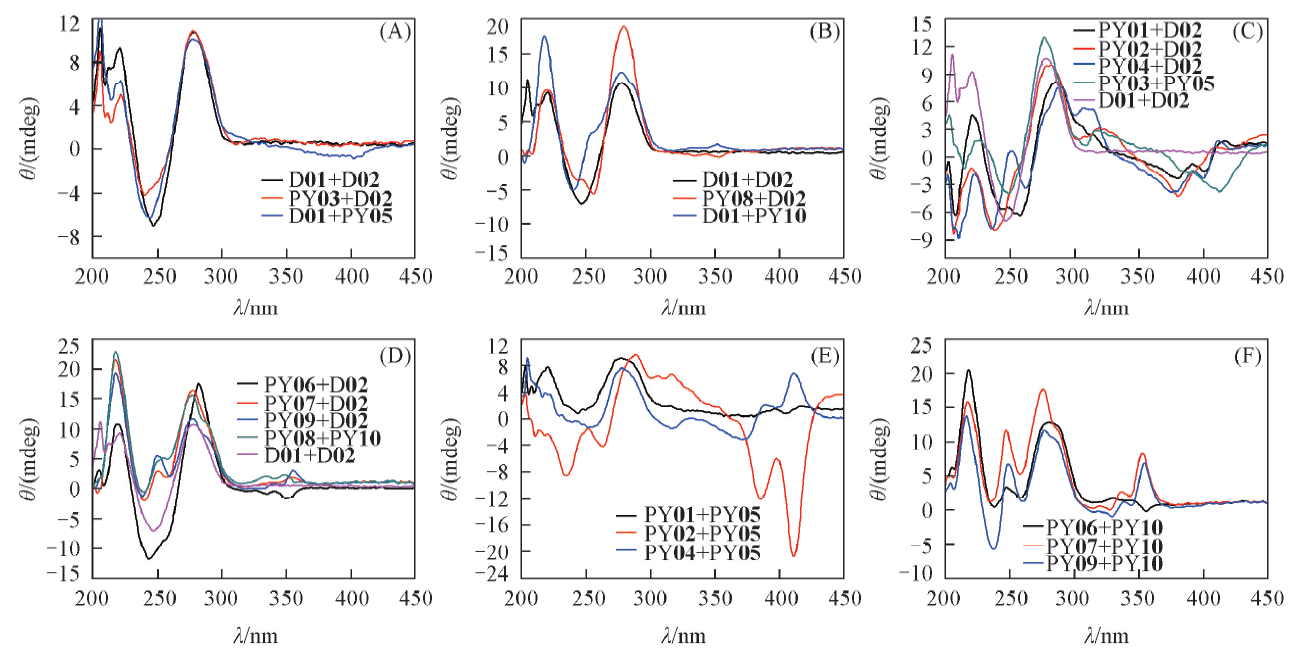
Fig.2 CD spectra of the pyrenyl-containing dsDNAsThe dsDNA in the buffer(50 mmol/L Tris-HCl, 20 mmol/L Mg2+, pH=7.5) with 1 μmol/L of pyrenyl group was measured on a MOS-450 spectropolarimeter(Biologic, France), in the quartz cuvette of 1 cm optical path length, with a speed of 100 nm/min.
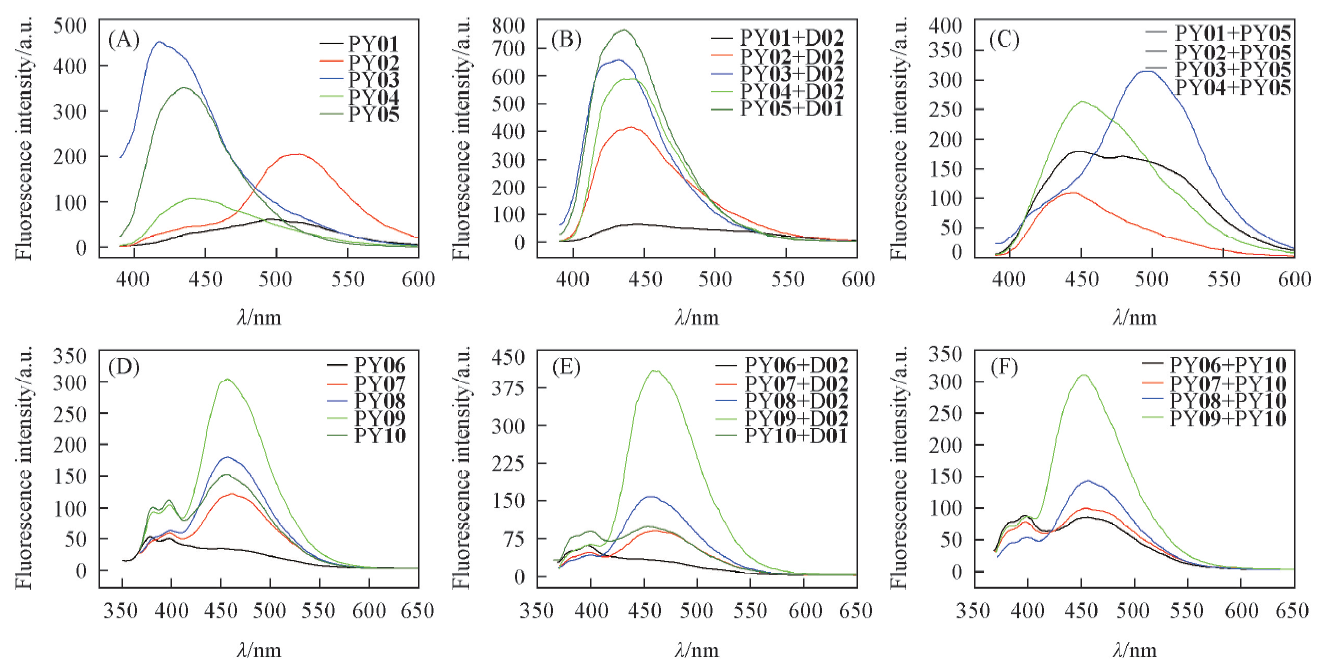
Fig.3 Fluorescence spectra of the single sequences and dsDNAs containing residues 2 and 3The single sequences and dsDNA, 1.0 μmol/L of pyrenyl group in the buffer(50 mmol/L Tris-HCl, 20 mmol/L Mg2+, pH=7.5) was measured, with λex=377 or 340 nm for the samples containing residue 2 or 3, respectively.
| [1] | Guo J., Xu N., Li Z., Zhang S., Wu J., Kim D.H., Marma M. S., Meng Q., Cao H., Li X., Shi S., Yu L., Kalachikov S., Russo J. J., Turro N. J., Ju J., Proc. Natl. Acad. Sci., 2008, 105, 9145—9150 |
| [2] | Astakhova I.V., Korshun V. A., Jahn K., Kjems J., Wengel J., Bioconjugate Chem., 2008, 19, 1995—2007 |
| [3] | Manna S., Panse C.H., Sontakke V. A., Sangamesh S., Srivatsan S. G., ChemBioChem, 2017, 18, 1604—1615 |
| [4] | Sarder P., Maji D., Achilefu S., Bioconjugate Chem., 2015, 26, 963—974 |
| [5] | Iida K., Nakamura T., Yoshida W., Tera M., Nakabayashi K., Hata K., Ikebukuro K., Nagasawa K., Angew. Chem. Int. Ed., 2013, 52, 12052—12055 |
| [6] | Wang Y.F., Zhang X., Liu C. X., Zhou X., Acta Chim.Sinica, 2017, 75, 692—698 |
| (王雅芬, 张雄, 刘朝兴, 周翔. 化学学报, 2017, 75, 692—698) | |
| [7] | Ying Z.M., Wu Z., Tu B., Tan W., Jiang J. H., J. Am. Chem. Soc., 2017, 139, 9779—9782 |
| [8] | McManus S. A., Li Y., J. Am. Chem. Soc., 2013, 135, 7181—7186 |
| [9] | Lu H., Zheng Y., Zhao X., Wang L., Ma S., Han X., Xu B., Tian W., Gao H., Angew. Chem. Int. Ed., 2016, 55, 155—159 |
| [10] | Park S., Otomo H., Zheng L., Sugiyama H., Chem. Commun., 2014, 50, 1573—1575 |
| [11] | Davies M.J., Shah A., Bruce I. J., Chem. Soc. Rev., 2000, 29, 97—107 |
| [12] | Yamauchi T., Takeda T., Yanagi M., Takahashi N., Suzuki A., Saito Y., Tetrahedron Lett., 2017, 58, 117—120 |
| [13] | Wranne M.S., Füchtbauer A. F., Dumat B., Bood M., El-Sagheer A. H., Brown T., Gradén H., Grøtli M., Wilhelmsson L. M., J. Am. Chem. Soc., 2017, 139, 9271—9280 |
| [14] | Xu W., Chan K.M., Kool E. T., Nat. Chem., 2017, 9, 1043—1055 |
| [15] | Winnik F.M., Chem. Rev., 1993, 93, 587—614 |
| [16] | Nagatoishi S., Nojima T., Juskowiak B., Takenaka S., Angew. Chem. Int. Ed., 2005, 44, 5067—5070 |
| [17] | Sahoo D., Narayanaswami V., Kay C.M., Ryan R. O., Biochem., 2000, 39, 6594—6601 |
| [18] | Chen S., Wang L., Fahmi N.E., Benkovic S. J., Hecht S. M., J. Am. Chem. Soc., 2012, 134, 18883—18885 |
| [19] | Okamoto A., Kanatani K., Saito I., J. Am. Chem. Soc., 2004, 126, 4820—4827 |
| [20] | Ueda T., Kobori A., Yamayoshi A., Yoshida H., Yamaguchi M., Murakami A., Bioorg. Med. Chem., 2012, 20, 6034—6039 |
| [21] | Kostenko E., Dobrikov M., Pyshnyi D., Petyuk V., Komarova N., Vlassov V., Zenkova M., Nucleic Acids Res., 2001, 29, 3611—3620 |
| [22] | Smalley M.K., Silverman S. K., Nucleic Acids Res., 2006, 34, 152—166 |
| [23] | Martí A., Li X., Jockusch S., Li Z., Raveendra B., Kalachikov S., Russo J.J., Morozova I., Puthanveettil S. V., Ju J., Turro N. J., Nucleic Acids Res., 2006, 34, 3161—3168 |
| [24] | Yamana K., Ohtani Y., Nakanoa H., Saitob I., Bioorg. Med. Chem. Lett., 2003, 13, 3429—3431 |
| [25] | Huang P.J. J., Lin J., Cao J., Vazin M., Liu J., Anal. Chem., 2014, 86, 1816—1821 |
| [26] | Huang J., Wu Y., Chen Y., Zhu Z., Yang X., Yang C.J., Wang K., Tan W., Angew. Chem. Int. Ed., 2011, 50, 401—404 |
| [27] | Karuppannan S., Chambron J. C., Chem. Asian J., 2011, 6, 964—984 |
| [28] | Hrdlicka P.J., Karmakar S., Org. Biomol. Chem., 2017, 15, 9760—9774 |
| [29] | Zhu H., Lewis F.D., Bioconjugate Chem., 2007, 18, 1213—1217 |
| [30] | Malakhov A.D., Skorobogatyi M. V., Prokhorenko I. A., Gontarev S. V., Kozhich D. T., Stetsenko D. A., Stepanova I. A., Shenkarev Z. O., Berlin Y. A., Korshun V. A., Eur. J. Org. Chem., 2004, 2004(6), 1298—1307 |
| [31] | Ren R.X. F., Chaudhuri N. C., Paris P. L., Rumney IV S., Kool E. T., J. Am. Chem. Soc., 1996, 118, 7671—7678 |
| [32] | Hwang G.T., Seo Y. J., Kim B. H., Tetrahedron Lett., 2005, 46, 1475—1477 |
| [33] | Mayer E., Valis L., Wagner C., Rist M., Amann N., Wagenknecht H.A., ChemBioChem, 2004, 5, 865—868 |
| [34] | Wang G., Bobkov G.V., Mikhailov S. N., Schepers G., Van Aerschot A., Rozenski J., Van der Auweraer M., Herdewijn P., Feyter S. D., ChemBioChem, 2009, 10, 1175—1185 |
| [35] | Okamoto A., Ochia Y., Saito I., Chem. Commun., 2005, 1128—1130 |
| [36] | Rist M., Amann N., Wagenknecht H.A., Eur. J. Org. Chem., 2003, 2003(13), 2498—2504 |
| [37] | Hwang G.T., Seo Y. J., Kim S. J., Kim B. H., Tetrahedron Lett., 2004, 45, 3543—3546 |
| [38] | Hrdlicka P.J., Babu B. R., Sørensen M. D., Harrit N., Wengel J., J. Am. Chem. Soc., 2005, 127, 13293—13299 |
| [39] | Østergaard M.E., Cheguru P., Papasani M. R., Hill R. A., Hrdlicka P. J., J. Am. Chem. Soc., 2010, 132, 14221—14230 |
| [40] | Imincan G., Pei F., Yu L., Jin H., Zhang L., Yang X., Zhang L., Tang X., Anal. Chem., 2016, 88, 4448—4455 |
| [41] | Ingale S.A., Pujari S. S., Sirivolu V. R., Ding P., Xiong H., Mei H., Seela F., J. Org. Chem., 2012, 77, 188—199 |
| [42] | Honcharenko D., Zhou C., Chattopadhyaya J., J. Org. Chem., 2008, 73, 2829—2842 |
| [43] | Karlsen K.K., Pasternak A., Jensen T. B., Wengel J., ChemBioChem, 2012, 13, 590—601 |
| [44] | Seo Y.J., Hwang G. T., Kim B. H., Tetrahedron Lett., 2006, 47, 4037—4039 |
| [45] | Grünewald C., Kwon T., Piton N., Förster U., Wachtveitl J., Engels J.W., Bioorg. Med. Chem., 2008, 16, 19—26 |
| [46] | Dioubankova N.N., Malakhov A. D., Stetsenko D. A., Gait M. J., Volynsky P. E., Efremov R. G., Korshun V. A., Chem. Bio. Chem., 2003, 4, 841—847 |
| [47] | Li Z., Zhu J., He J., Org. Biomol. Chem., 2016, 14, 9846—9858 |
| [48] | He J., Seela F., Nucleic Acids Res., 2002, 30, 5485—5496 |
| [49] | Doluca O., Withers J.M., Loo T. S., Edwards P. J. B., González C., Filichev V. V., Org. Biomol. Chem., 2015, 13, 3742—3748 |
| [50] | Wojciechowski F., Lietard J., Leumann C.J., Org. Lett., 2012, 14, 5176—5179 |
| [51] | Fukuda M., Nakamura M., Takada T., Yamana K., Tetrahedron Lett., 2010, 51, 1732—1735 |
| [52] | Anderson B.A., Karmakar S., Hrdlicka P. J., Molecules, 2015, 20, 13780—13793 |
| [1] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [2] | ZHANG Jun, WANG Bin, PAN Li, MA Zhe, LI Yuesheng. Synthesis and Properties of Imidazolium-based Polyethylene Ionomer [J]. Chem. J. Chinese Universities, 2020, 41(9): 2070. |
| [3] | MA Xiangying, LIAO Yanjun, QIN Fanghong, YIN Yuanhao, HUANG Zaiyin, CHEN Qifeng. Study on the Photocatalytic Performance of Carbon Doped g-C3N4 Based on in situ Photomicrocalorimeter-fluorescence Spectrometry [J]. Chem. J. Chinese Universities, 2020, 41(11): 2526. |
| [4] | RAN Shiya,SHEN Haifeng,LI Xiaonan,WANG Zilu,GUO Zhenghong,FANG Zhengping. Effect and Mechanism of Rare Earth Trifluoromethanesulfonate on the Thermal Stability of Polypropylene† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1333. |
| [5] | FANG Xijie,LIU Ruiyun,LIN Sen,SHI Lei,WANG Runwei,LI Yi,LI Junying. Synthesis of STW-zeotype Germanosilicate via Steam-assisted Crystallization† [J]. Chem. J. Chinese Universities, 2019, 40(5): 867. |
| [6] | YIN Mengxin,LIU Dongsheng,ZHAO Dongyue,DING Tong,TIAN Ye,LI Xingang. Effect of Copper Doping on Lean NOx Trap Performance of Pt/Ba/CuxMg1-xAl2O4 Catalysts at High Temperatures † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2170. |
| [7] | BAI Lei,HUO Shuhui,CHEN Jing,LU Xiaoquan. Squaramide Fluorescence Probe for Chiral Recognition of α-Amino Acids† [J]. Chem. J. Chinese Universities, 2019, 40(1): 41. |
| [8] | LIU Yi, XU Xiaozhou, MO Song, ZHAI Lei, HE Minhui, FAN Lin. Thermal Stability of Polyimide Resins Containing Siloxane Structure and Their High Temperature Structural Evolution [J]. Chem. J. Chinese Universities, 2019, 40(1): 187. |
| [9] | MENG Jiafeng, NI Xufeng, ZHENG Hao, SHEN Zhiquan. Copolymerization of Norbornene and 1-Octene Catalyzed by Bis(phenoxy-imine) Titanium Complex† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1853. |
| [10] | DU Shanshan, LI Yang, GUO Lei, LI Pengyu, CHAI Zhilong, WANG Tao, QUAN Dongqin, HE Junlin. Modification of Aptamer TBA with Extra Functional Groups and the Biological Activities† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2445. |
| [11] | LI Caixin, LIANG Xiaorong, GU Ju. Preparation and Characterization of Bagasse Nanocellulose† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1286. |
| [12] | WAN Ting, LI Xingxing, HUANG Zaiyin, QIU Jiangyuan, ZUO Chen, TAN Xuecai. In-situ Photocatalytic Process of Rhodamine B over g-C3N4@Ag3PO4 Nanocomposites Based on Photomicrocalorimeter-fluorescence Spectrometry† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2226. |
| [13] | LIU Yaoyao, DING Tong, ZHAO Dongyue, GAO Zhongnan, GUO Lihong, TIAN Ye, LI Xingang. Effect of Potassium Loading on the NOx Storage and Reduction Performance of the CuO/K2CO3/MgAl2O4 Catalyst at High Temperature† [J]. Chem. J. Chinese Universities, 2017, 38(11): 2006. |
| [14] | LI Wei, WU Suhua, Ren Xinxin. Effect of Co-stabilizers on the Properties of Ba/Zn System [J]. Chem. J. Chinese Universities, 2017, 38(11): 2089. |
| [15] | LIU Yaohua, LIN Yu, ZHANG Dongge, CHEN Chunlei, WU Guozhang, ZHANG Yan, LUAN Weiling. Fabrication of Natural Rubber/Chemically Reduced Graphene Oxide Nanocomposites and Nuclear Radiation Resistant Behavior† [J]. Chem. J. Chinese Universities, 2016, 37(7): 1402. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||