

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (12): 2435.doi: 10.7503/cjcu20150397
• Organic Chemistry • Previous Articles Next Articles
WANG Gang, HAN Leiqiang, FANG Hao*( )
)
Received:2015-05-18
Online:2015-12-10
Published:2015-10-28
Contact:
FANG Hao
E-mail:haofangcn@sdu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Gang, HAN Leiqiang, FANG Hao. Syntheses and Antitumor Activities of Phenylpiperazine Derivatives†[J]. Chem. J. Chinese Universities, 2015, 36(12): 2435.
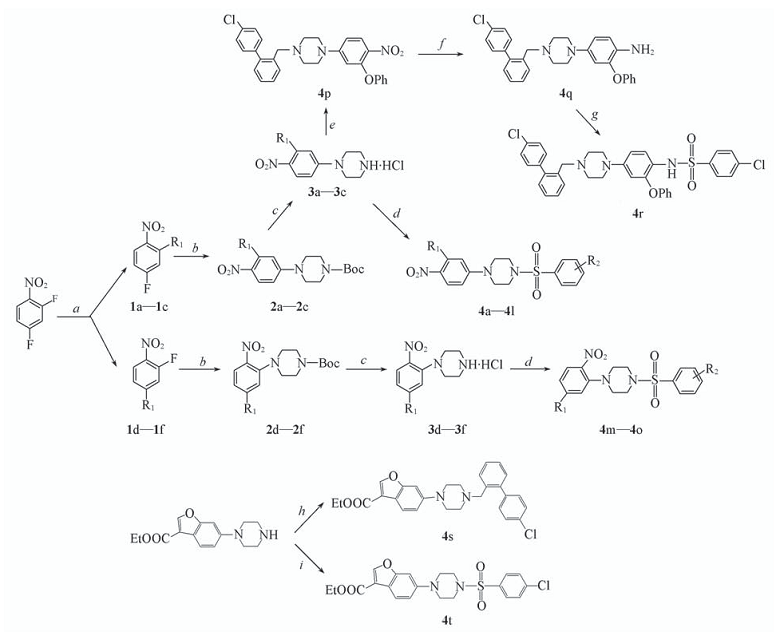
Scheme 1 Synthetic routes of target compounds 4a—4t 1a—3a, 1d—3d: R1=4-nitrophenoxyl; 1b—3b, 1e—3e: R1=phenoxyl; 1c—3c, 1f—3f: R1=8-quinolinoxyl; 4a, 4m: R1=4-nitrophenoxyl, R2=3-nitro; 4b: R1=4-nitrophenoxyl, R2=4-nitro; 4c: R1=4-nitrophenoxyl, R2=4-chloro; 4d: R1=4-nitrophenoxyl, R2=4-methoxyl; 4e, 4n: R1=phenoxyl, R2=3-nitro; 4f: R1=phenoxyl, R2=4-nitro; 4g: R1=phenoxyl, R2=4-chloro; 4h: R1=phenoxyl, R2=4-methoxyl; 4i, 4o: R1=8-quinolinoxyl, R2=3-nitro; 4j: R1=8-quinolino-xyl, R2=4-nitro; 4k: R1=8-quinolino-xyl, R2=4-chloro; 4l: R1=8-quinolinoxyl, R2=4-methoxyl a. Substituted phenol, K2CO3, DMF, r.t.; b. 1-Boc-piperazine, K2CO3, DMSO, 90 ℃; c. HCl gas, MeOH, r.t.; d. arylsulfonyl chloride, TEA, DCM, r.t.; e. R1=phenoxyl, 4'-chlorobiphenyl-2-carbaldehyde, sodium triacetoxyborohydride, DCM, r.t.; f. Zn, NH4Cl, EtOH, HOAc, reflux; g. 4-chlorobenzenesulfonyl chloride, TEA, DCM, r.t.; h. 4'-Chlorobiphenyl-2-carbaldehyde, sodium triacetoxyborohydride, DCM, r.t.; i. 4-chlorobenzenesulfonyl chloride, TEA, DCM, r.t..
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z [M+H]+ |
|---|---|---|---|---|
| 4a | Yellow solid | 72 | >250 | 530.0977(530.0976) |
| 4b | Yellow solid | 72 | 248—250 | 530.0979(530.0976) |
| 4c | Yellow solid | 88 | 220—221 | 519.0736(519.0736) |
| 4d | Slightly yellow solid | 74 | >250 | 515.1231(515.1231) |
| 4e | Yellow solid | 69 | 164—166 | 485.1125(485.1125) |
| 4f | Yellow solid | 83 | 214—216 | 485.1125(485.1125) |
| 4g | Yellow solid | 78 | 174—176 | 474.0884(474.0885) |
| 4h | Slightly yellow solid | 95 | 182—184 | 470.1384(470.1380) |
| 4i | Yellow solid | 43 | 238—240 | 536.1234(536.1234) |
| 4j | Yellow solid | 67 | 202—204 | 536.1237(536.1234) |
| 4k | Slightly yellow solid | 89 | 212—214 | 525.0994(525.0994) |
| 4l | Off-white solid | 90 | 224—226 | 521.1486(521.1489) |
| 4m | Yellow solid | 72 | 242—244 | 530.0974(530.0976) |
| 4n | Yellow solid | 98 | 164—166 | 485.1121(485.1125) |
| 4o | Slightly yellow solid | 77 | 188—189 | 536.1236(536.1234) |
| 4q | Slightly brown solid | 73 | 64—66 | 470.1992(470.1994) |
| 4r | Slightly brown solid | 44 | 84—86 | 644.1536(644.1540) |
| 4s | Slightly yellow solid | 67 | 84—86 | 475.1786(475.1783) |
| 4t | Off-white solid | 56 | 202—204 | 449.0930(449.0932) |
Table 1 Appearances, yields, melting points and HRMS data of target compounds
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z [M+H]+ |
|---|---|---|---|---|
| 4a | Yellow solid | 72 | >250 | 530.0977(530.0976) |
| 4b | Yellow solid | 72 | 248—250 | 530.0979(530.0976) |
| 4c | Yellow solid | 88 | 220—221 | 519.0736(519.0736) |
| 4d | Slightly yellow solid | 74 | >250 | 515.1231(515.1231) |
| 4e | Yellow solid | 69 | 164—166 | 485.1125(485.1125) |
| 4f | Yellow solid | 83 | 214—216 | 485.1125(485.1125) |
| 4g | Yellow solid | 78 | 174—176 | 474.0884(474.0885) |
| 4h | Slightly yellow solid | 95 | 182—184 | 470.1384(470.1380) |
| 4i | Yellow solid | 43 | 238—240 | 536.1234(536.1234) |
| 4j | Yellow solid | 67 | 202—204 | 536.1237(536.1234) |
| 4k | Slightly yellow solid | 89 | 212—214 | 525.0994(525.0994) |
| 4l | Off-white solid | 90 | 224—226 | 521.1486(521.1489) |
| 4m | Yellow solid | 72 | 242—244 | 530.0974(530.0976) |
| 4n | Yellow solid | 98 | 164—166 | 485.1121(485.1125) |
| 4o | Slightly yellow solid | 77 | 188—189 | 536.1236(536.1234) |
| 4q | Slightly brown solid | 73 | 64—66 | 470.1992(470.1994) |
| 4r | Slightly brown solid | 44 | 84—86 | 644.1536(644.1540) |
| 4s | Slightly yellow solid | 67 | 84—86 | 475.1786(475.1783) |
| 4t | Off-white solid | 56 | 202—204 | 449.0930(449.0932) |
| Compd. | 1H NMR(300 MHz), δ |
|---|---|
| 4a | 8.57(d, J=8.8 Hz, 1H, ArH), 8.39(s, 1H, ArH), 8.20—8.24(m, 3H, ArH), 8.10(d, J=9.2 Hz, 1H, ArH), 7.96(t, J=8.4 Hz, 1H, ArH), 7.07(d, J=9.2 Hz, 2H, ArH), 6.96(dd, J=2.4, 9.6 Hz, 1H, ArH), 6.87(d, J=2.4 Hz, 1H, ArH), 3.60(t, J=4.4 Hz, 4H, piperazine), 3.11(t, J=4.4 Hz, 4H, piperazine) |
| 4b | 8.44(d, J=5.6 Hz, 2H, ArH), 8.22(d, J=5.6 Hz, 2H, ArH), 8.10(d, J=6.4 Hz, 1H, ArH), 8.04(d, J=5.6 Hz, 2H, ArH), 7.07(d, J=5.6 Hz, 2H, ArH), 6.96(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.87(d, J=1.6 Hz, 1H, ArH), 3.59(d, J=2.8 Hz, 4H, piperazine), 3.10(d, J=2.8 Hz, 4H, piperazine) |
| 4c | 8.22(d, J=6.0 Hz, 2H, ArH), 8.10(d, J=6.4 Hz, 1H, ArH), 7.75(dd, J=6.0, 17.6 Hz, 4H, ArH), 7.08(d, J=6.0 Hz, 2H, ArH), 6.97(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.87(d, J=1.6 Hz, 1H, ArH), 3.58(s, 4H, piperazine), 3.07(s, 4H, piperazine) |
| 4d | 8.23(d, J=9.2 Hz, 2H, ArH), 8.10(d, J=9.6 Hz, 1H, ArH), 7.69(d, J=8.8 Hz, 2H, ArH), 7.17(d, J=8.8 Hz, 2H, ArH), 7.08(d, J=9.2 Hz, 2H, ArH), 6.96(dd, J=2.8, 9.6 Hz, 1H, ArH), 6.87(d, J=2.8 Hz, 1H, ArH), 3.85(s, 3H, OCH3), 3.58(t, J=4.8 Hz, 4H, piperazine), 2.95(t, J=4.8 Hz, 4H, piperazine) |
| 4e | 8.55(dd, J=0.8, 5.6 Hz, 1H, ArH), 8.37(s, 1H, ArH), 8.19(d, J=6.0 Hz, 1H, ArH), 8.02(d, J=6.4 Hz, 1H, ArH), 7.95(t, J=5.2 Hz, 1H, ArH), 7.34(t, J=5.2 Hz, 2H, ArH), 7.10(t, J=5.2 Hz, 1H, ArH), 6.90(d, J=5.2 Hz, 2H, ArH), 6.84(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.57(d, J=1.6 Hz, 1H, ArH), 3.50(t, J=3.2 Hz, 4H, piperazine), 3.08(t, J=3.2 Hz, 4H, piperazine) |
| 4f | 8.43(d, J=5.6 Hz, 2H, ArH), 8.02(dd, J=1.2, 6.0 Hz, 3H, ArH), 7.34(t, J=5.6 Hz, 2H, ArH), 7.10(t, J=4.8 Hz, 1H, ArH), 6.90(d, J=4.8 Hz, 2H, ArH), 6.85(dd, J=2.0, 6.4 Hz, 1H, ArH), 6.58(d, J=1.6 Hz, 1H, ArH), 3.49(t, J=3.2 Hz, 4H, piperazine), 3.08(t, J=3.2 Hz, 4H, piperazine) |
| 4g | 8.03(d, J=6.4 Hz, 1H, ArH), 7.75(dd, J=5.6, 14.8 Hz, 4H, ArH), 7.35(t, J=5.6 Hz, 2H, ArH), 7.10(t, J=5.2 Hz, 1H, ArH), 6.91(d, J=5.2 Hz, 2H, ArH), 6.85(dd, J=2.0, 6.0 Hz, 1H, ArH), 6.59(d, J=2.0 Hz, 1H, ArH), 3.48(t, J=3.2 Hz, 4H, piperazine), 3.00(t, J=3.2 Hz, 4H, piperazine) |
| 4h | 8.03(d, J=9.2 Hz, 1H, ArH), 7.68(d, J=8.8 Hz, 2H, ArH), 7.35(t, J=7.6 Hz, 2H, ArH), 7.17(d, J=8.8 Hz, 2H, ArH), 7.10(t, J=7.2 Hz, 1H, ArH), 6.91(d, J=8.4 Hz, 2H, ArH), 6.86(dd, J=2.4, 9.2 Hz, 1H, ArH), 6.59(d, J=2.4 Hz, 1H, ArH), 3.85(s, 3H, OCH3), 3.48(t, J=4.8 Hz, 4H, piperazine), 2.94(t, J=4.8 Hz, 4H, piperazine) |
| 4i | 8.85(dd, J=1.6, 4.4 Hz, 1H, ArH), 8.55(td, J=0.8, 8.4 Hz, 1H, ArH), 8.44(dd, J=1.2, 8.4 Hz, 1H, ArH), 8.34(d, J=1.2 Hz, 1H, ArH), 8.16(d, J=7.6 Hz, 1H, ArH), 8.06(d, J=9.6 Hz, 1H, ArH), 7.94(t, J=8.0 Hz, 1H, ArH), 7.79(d, J=8.0 Hz, 1H, ArH), 7.60(dd, J=4.0, 8.0 Hz, 1H, ArH), 7.54(t, J=8.0 Hz, 1H, ArH), 7.17(d, J=8.0 Hz, 1H, ArH), 6.81(dd, J=2.4, 9.2 Hz, 1H, ArH), 6.36(d, J=2.4 Hz, 1H, ArH), 3.38(t, J=4.8 Hz, 4H, piperazine), 3.02(t, J=4.8 Hz, 4H, piperazine) |
| 4j | 8.84(dd, J=0.8, 2.4 Hz, 1H, ArH), 8.41—8.44(m, 3H, ArH), 8.05(d, J=6.4 Hz, 1H, ArH), 7.98(d, J=5.6 Hz, 2H, ArH), 7.78(d, J=5.6 Hz, 1H, ArH), 7.60(dd, J=2.8, 5.6 Hz, 1H, ArH), 7.53(t, J=5.2 Hz, 1H, ArH), 7.16(d, J=4.8 Hz, 1H, ArH), 6.80(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.37(d, J=1.6 Hz, 1H, ArH), 3.37(t, J=3.2 Hz, 4H, piperazine), 3.00(t, J=3.2 Hz, 4H, piperazine) |
| 4k | 8.85(dd, J=0.8, 2.4 Hz, 1H, ArH), 8.44(dd, J=0.8, 5.2 Hz, 1H, ArH), 8.06(d, J=6.0 Hz, 1H, ArH), 7.79(d, J=5.2 Hz, 2H, ArH), 7.72(d, J=0.8 Hz, 4H, ArH), 7.60(dd, J=2.8, 5.6 Hz, 1H, ArH), 7.54(t, J=5.2 Hz, 1H, ArH), 7.16(d, J=5.2 Hz, 1H, ArH), 6.81(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.38(d, J=1.6 Hz, 1H, ArH), 2.93(t, J=3.2 Hz, 8H, piperazine) |
| 4l | 8.84(dd, J=0.8, 2.4 Hz, 1H, ArH), 8.43(dd, J=1.2, 5.6 Hz, 1H, ArH), 8.06(d, J=6.4 Hz, 1H, ArH), 7.79(d, J=5.2 Hz, 1H, ArH), 7.64(d, J=6.0 Hz, 2H, ArH), 7.60(dd, J=2.8, 5.6 Hz, 1H, ArH), 7.53(t, J=5.2 Hz, 1H, ArH), 7.13—7.17(m, 3H, ArH), 6.81(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.37(d, J=2.0 Hz, 1H, ArH), 3.84(s, 3H, OCH3), 3.34(t, J=3.2 Hz, 4H, piperazine), 2.86(t, J=3.2 Hz, 4H, piperazine) |
| 4m | 8.58(d, J=5.6 Hz, 1H, ArH), 8.41(s, 1H, ArH), 8.29(d, J=6.0 Hz, 2H, ArH), 8.23(d, J=5.2 Hz, 1H, ArH), 7.96—8.01(m, 2H), 7.30(d, J=5.6 Hz, 2H, ArH), 7.09(d, J=0.8 Hz, 1H, ArH), 6.84(dd, J=0.8, 6.0 Hz, 1H, ArH), 3.12(s, 8H, piperazine) |
| 4n | 8.58(d, J=5.6 Hz, 1H, ArH), 8.41(s, 1H, ArH), 8.23(d, J=4.8 Hz, 1H, ArH), 8.00(t, J=5.6 Hz, 1H, ArH), 7.92(d, J=6.0 Hz, 1H, ArH), 7.47(t, J=5.6 Hz, 2H, ArH), 7.28(t, J=5.2 Hz, 1H, ArH), 7.15(d, J=5.6 Hz, 2H, ArH), 6.89(d, J=1.6 Hz, 1H, ArH), 6.54(dd, J=1.6, 6.0 Hz, 1H, ArH), 3.11(d, J=6.4 Hz, 8H, piperazine) |
| 4o | 8.84(d, J=2.4 Hz, 1H, ArH), 8.59(d, J=5.2 Hz, 1H, ArH), 8.48(d, J=5.2 Hz, 1H, ArH), 8.42(s, 1H, ArH), 8.23(d, J=5.2 Hz, 1H, ArH), 8.00(t, J=5.2 Hz, 1H, ArH), 7.97(d, J=5.6 Hz, 1H, ArH), 7.82(d, J=6.4 Hz, 1H, ArH), 7.68(t, J=5.6 Hz, 1H, ArH), 7.60—7.62(m, 2H, ArH), 6.94(d, J=1.2 Hz, 1H, ArH), 6.27(dd, J=1.6, 6.0 Hz, 1H, ArH), 3.11(d, J=2.4 Hz, 8H, piperazine) |
| 4q | 7.46—7.50(m, 5H, ArH), 7.28—7.36(m, 4H, ArH), 7.22(dd, J=0.8, 4.8 Hz, 1H, ArH), 7.01(t, J=4.8 Hz, 1H, ArH), 6.86(d, J=5.2 Hz, 2H, ArH), 6.73(d, J=5.6 Hz, 1H, ArH), 6.59(dd, J=1.6, 5.6 Hz, 1H, ArH), 6.42(d, J=1.6 Hz, 1H, ArH), 4.39(s, 2H, NH2), 3.35(s, 2H, CH2), 2.84(s, 4H, piperazine), 2.36(s, 4H, piperazine) |
| 4r | 7.57(dd, J=2.4, 8.8 Hz, 3H, ArH), 7.33—7.36(m, 6H, ArH), 7.22—7.30(m, 5H, ArH), 7.09(t, J=6.4 Hz, 1H, ArH), 6.63—6.66(m, 2H, ArH), 6.50(d, J=8.0 Hz, 2H, ArH), 6.20(d, J=2.0 Hz, 1H, ArH), 3.39(s, 2H, CH2), 3.00(s, 4H, piperazine), 2.45(s, 4H, piperazine) |
| 4s | 7.60(d, J=0.8 Hz, 1H, ArH), 7.55(d, J=6.4 Hz, 1H, ArH), 7.52(d, J=4.8 Hz, 1H, ArH), 7.49(s, 4H, ArH), 7.36—7.40(m, 2H, ArH), 7.22—7.26(m, 2H, ArH), 7.14(d, J=1.2 Hz, 1H, ArH), 4.34(q, J=4.8 Hz, 2H, OCH2), 3.40(s, 2H, ArCH2), 3.07(s, 4H, piperazine), 2.45(s, 4H, piperazine), 1.33(t, J=4.8 Hz, 3H, CH3) |
| 4t | 7.78(dd, J=5.6, 18.0 Hz, 4H, ArH), 7.62(s, 1H, ArH), 7.58(d, J=6.4 Hz, 1H, ArH), 7.22(dd, J=1.2, 6.0 Hz, 1H, ArH), 7.18(d, J=1.2 Hz, 1H, ArH), 4.34(q, J=4.8 Hz, 2H, OCH2), 3.19(s, 4H, piperazine), 3.07(s, 4H, piperazine), 1.33(t, J=4.8 Hz, 3H, CH3) |
Table 2 1H NMR data of all target compounds*
| Compd. | 1H NMR(300 MHz), δ |
|---|---|
| 4a | 8.57(d, J=8.8 Hz, 1H, ArH), 8.39(s, 1H, ArH), 8.20—8.24(m, 3H, ArH), 8.10(d, J=9.2 Hz, 1H, ArH), 7.96(t, J=8.4 Hz, 1H, ArH), 7.07(d, J=9.2 Hz, 2H, ArH), 6.96(dd, J=2.4, 9.6 Hz, 1H, ArH), 6.87(d, J=2.4 Hz, 1H, ArH), 3.60(t, J=4.4 Hz, 4H, piperazine), 3.11(t, J=4.4 Hz, 4H, piperazine) |
| 4b | 8.44(d, J=5.6 Hz, 2H, ArH), 8.22(d, J=5.6 Hz, 2H, ArH), 8.10(d, J=6.4 Hz, 1H, ArH), 8.04(d, J=5.6 Hz, 2H, ArH), 7.07(d, J=5.6 Hz, 2H, ArH), 6.96(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.87(d, J=1.6 Hz, 1H, ArH), 3.59(d, J=2.8 Hz, 4H, piperazine), 3.10(d, J=2.8 Hz, 4H, piperazine) |
| 4c | 8.22(d, J=6.0 Hz, 2H, ArH), 8.10(d, J=6.4 Hz, 1H, ArH), 7.75(dd, J=6.0, 17.6 Hz, 4H, ArH), 7.08(d, J=6.0 Hz, 2H, ArH), 6.97(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.87(d, J=1.6 Hz, 1H, ArH), 3.58(s, 4H, piperazine), 3.07(s, 4H, piperazine) |
| 4d | 8.23(d, J=9.2 Hz, 2H, ArH), 8.10(d, J=9.6 Hz, 1H, ArH), 7.69(d, J=8.8 Hz, 2H, ArH), 7.17(d, J=8.8 Hz, 2H, ArH), 7.08(d, J=9.2 Hz, 2H, ArH), 6.96(dd, J=2.8, 9.6 Hz, 1H, ArH), 6.87(d, J=2.8 Hz, 1H, ArH), 3.85(s, 3H, OCH3), 3.58(t, J=4.8 Hz, 4H, piperazine), 2.95(t, J=4.8 Hz, 4H, piperazine) |
| 4e | 8.55(dd, J=0.8, 5.6 Hz, 1H, ArH), 8.37(s, 1H, ArH), 8.19(d, J=6.0 Hz, 1H, ArH), 8.02(d, J=6.4 Hz, 1H, ArH), 7.95(t, J=5.2 Hz, 1H, ArH), 7.34(t, J=5.2 Hz, 2H, ArH), 7.10(t, J=5.2 Hz, 1H, ArH), 6.90(d, J=5.2 Hz, 2H, ArH), 6.84(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.57(d, J=1.6 Hz, 1H, ArH), 3.50(t, J=3.2 Hz, 4H, piperazine), 3.08(t, J=3.2 Hz, 4H, piperazine) |
| 4f | 8.43(d, J=5.6 Hz, 2H, ArH), 8.02(dd, J=1.2, 6.0 Hz, 3H, ArH), 7.34(t, J=5.6 Hz, 2H, ArH), 7.10(t, J=4.8 Hz, 1H, ArH), 6.90(d, J=4.8 Hz, 2H, ArH), 6.85(dd, J=2.0, 6.4 Hz, 1H, ArH), 6.58(d, J=1.6 Hz, 1H, ArH), 3.49(t, J=3.2 Hz, 4H, piperazine), 3.08(t, J=3.2 Hz, 4H, piperazine) |
| 4g | 8.03(d, J=6.4 Hz, 1H, ArH), 7.75(dd, J=5.6, 14.8 Hz, 4H, ArH), 7.35(t, J=5.6 Hz, 2H, ArH), 7.10(t, J=5.2 Hz, 1H, ArH), 6.91(d, J=5.2 Hz, 2H, ArH), 6.85(dd, J=2.0, 6.0 Hz, 1H, ArH), 6.59(d, J=2.0 Hz, 1H, ArH), 3.48(t, J=3.2 Hz, 4H, piperazine), 3.00(t, J=3.2 Hz, 4H, piperazine) |
| 4h | 8.03(d, J=9.2 Hz, 1H, ArH), 7.68(d, J=8.8 Hz, 2H, ArH), 7.35(t, J=7.6 Hz, 2H, ArH), 7.17(d, J=8.8 Hz, 2H, ArH), 7.10(t, J=7.2 Hz, 1H, ArH), 6.91(d, J=8.4 Hz, 2H, ArH), 6.86(dd, J=2.4, 9.2 Hz, 1H, ArH), 6.59(d, J=2.4 Hz, 1H, ArH), 3.85(s, 3H, OCH3), 3.48(t, J=4.8 Hz, 4H, piperazine), 2.94(t, J=4.8 Hz, 4H, piperazine) |
| 4i | 8.85(dd, J=1.6, 4.4 Hz, 1H, ArH), 8.55(td, J=0.8, 8.4 Hz, 1H, ArH), 8.44(dd, J=1.2, 8.4 Hz, 1H, ArH), 8.34(d, J=1.2 Hz, 1H, ArH), 8.16(d, J=7.6 Hz, 1H, ArH), 8.06(d, J=9.6 Hz, 1H, ArH), 7.94(t, J=8.0 Hz, 1H, ArH), 7.79(d, J=8.0 Hz, 1H, ArH), 7.60(dd, J=4.0, 8.0 Hz, 1H, ArH), 7.54(t, J=8.0 Hz, 1H, ArH), 7.17(d, J=8.0 Hz, 1H, ArH), 6.81(dd, J=2.4, 9.2 Hz, 1H, ArH), 6.36(d, J=2.4 Hz, 1H, ArH), 3.38(t, J=4.8 Hz, 4H, piperazine), 3.02(t, J=4.8 Hz, 4H, piperazine) |
| 4j | 8.84(dd, J=0.8, 2.4 Hz, 1H, ArH), 8.41—8.44(m, 3H, ArH), 8.05(d, J=6.4 Hz, 1H, ArH), 7.98(d, J=5.6 Hz, 2H, ArH), 7.78(d, J=5.6 Hz, 1H, ArH), 7.60(dd, J=2.8, 5.6 Hz, 1H, ArH), 7.53(t, J=5.2 Hz, 1H, ArH), 7.16(d, J=4.8 Hz, 1H, ArH), 6.80(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.37(d, J=1.6 Hz, 1H, ArH), 3.37(t, J=3.2 Hz, 4H, piperazine), 3.00(t, J=3.2 Hz, 4H, piperazine) |
| 4k | 8.85(dd, J=0.8, 2.4 Hz, 1H, ArH), 8.44(dd, J=0.8, 5.2 Hz, 1H, ArH), 8.06(d, J=6.0 Hz, 1H, ArH), 7.79(d, J=5.2 Hz, 2H, ArH), 7.72(d, J=0.8 Hz, 4H, ArH), 7.60(dd, J=2.8, 5.6 Hz, 1H, ArH), 7.54(t, J=5.2 Hz, 1H, ArH), 7.16(d, J=5.2 Hz, 1H, ArH), 6.81(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.38(d, J=1.6 Hz, 1H, ArH), 2.93(t, J=3.2 Hz, 8H, piperazine) |
| 4l | 8.84(dd, J=0.8, 2.4 Hz, 1H, ArH), 8.43(dd, J=1.2, 5.6 Hz, 1H, ArH), 8.06(d, J=6.4 Hz, 1H, ArH), 7.79(d, J=5.2 Hz, 1H, ArH), 7.64(d, J=6.0 Hz, 2H, ArH), 7.60(dd, J=2.8, 5.6 Hz, 1H, ArH), 7.53(t, J=5.2 Hz, 1H, ArH), 7.13—7.17(m, 3H, ArH), 6.81(dd, J=1.6, 6.4 Hz, 1H, ArH), 6.37(d, J=2.0 Hz, 1H, ArH), 3.84(s, 3H, OCH3), 3.34(t, J=3.2 Hz, 4H, piperazine), 2.86(t, J=3.2 Hz, 4H, piperazine) |
| 4m | 8.58(d, J=5.6 Hz, 1H, ArH), 8.41(s, 1H, ArH), 8.29(d, J=6.0 Hz, 2H, ArH), 8.23(d, J=5.2 Hz, 1H, ArH), 7.96—8.01(m, 2H), 7.30(d, J=5.6 Hz, 2H, ArH), 7.09(d, J=0.8 Hz, 1H, ArH), 6.84(dd, J=0.8, 6.0 Hz, 1H, ArH), 3.12(s, 8H, piperazine) |
| 4n | 8.58(d, J=5.6 Hz, 1H, ArH), 8.41(s, 1H, ArH), 8.23(d, J=4.8 Hz, 1H, ArH), 8.00(t, J=5.6 Hz, 1H, ArH), 7.92(d, J=6.0 Hz, 1H, ArH), 7.47(t, J=5.6 Hz, 2H, ArH), 7.28(t, J=5.2 Hz, 1H, ArH), 7.15(d, J=5.6 Hz, 2H, ArH), 6.89(d, J=1.6 Hz, 1H, ArH), 6.54(dd, J=1.6, 6.0 Hz, 1H, ArH), 3.11(d, J=6.4 Hz, 8H, piperazine) |
| 4o | 8.84(d, J=2.4 Hz, 1H, ArH), 8.59(d, J=5.2 Hz, 1H, ArH), 8.48(d, J=5.2 Hz, 1H, ArH), 8.42(s, 1H, ArH), 8.23(d, J=5.2 Hz, 1H, ArH), 8.00(t, J=5.2 Hz, 1H, ArH), 7.97(d, J=5.6 Hz, 1H, ArH), 7.82(d, J=6.4 Hz, 1H, ArH), 7.68(t, J=5.6 Hz, 1H, ArH), 7.60—7.62(m, 2H, ArH), 6.94(d, J=1.2 Hz, 1H, ArH), 6.27(dd, J=1.6, 6.0 Hz, 1H, ArH), 3.11(d, J=2.4 Hz, 8H, piperazine) |
| 4q | 7.46—7.50(m, 5H, ArH), 7.28—7.36(m, 4H, ArH), 7.22(dd, J=0.8, 4.8 Hz, 1H, ArH), 7.01(t, J=4.8 Hz, 1H, ArH), 6.86(d, J=5.2 Hz, 2H, ArH), 6.73(d, J=5.6 Hz, 1H, ArH), 6.59(dd, J=1.6, 5.6 Hz, 1H, ArH), 6.42(d, J=1.6 Hz, 1H, ArH), 4.39(s, 2H, NH2), 3.35(s, 2H, CH2), 2.84(s, 4H, piperazine), 2.36(s, 4H, piperazine) |
| 4r | 7.57(dd, J=2.4, 8.8 Hz, 3H, ArH), 7.33—7.36(m, 6H, ArH), 7.22—7.30(m, 5H, ArH), 7.09(t, J=6.4 Hz, 1H, ArH), 6.63—6.66(m, 2H, ArH), 6.50(d, J=8.0 Hz, 2H, ArH), 6.20(d, J=2.0 Hz, 1H, ArH), 3.39(s, 2H, CH2), 3.00(s, 4H, piperazine), 2.45(s, 4H, piperazine) |
| 4s | 7.60(d, J=0.8 Hz, 1H, ArH), 7.55(d, J=6.4 Hz, 1H, ArH), 7.52(d, J=4.8 Hz, 1H, ArH), 7.49(s, 4H, ArH), 7.36—7.40(m, 2H, ArH), 7.22—7.26(m, 2H, ArH), 7.14(d, J=1.2 Hz, 1H, ArH), 4.34(q, J=4.8 Hz, 2H, OCH2), 3.40(s, 2H, ArCH2), 3.07(s, 4H, piperazine), 2.45(s, 4H, piperazine), 1.33(t, J=4.8 Hz, 3H, CH3) |
| 4t | 7.78(dd, J=5.6, 18.0 Hz, 4H, ArH), 7.62(s, 1H, ArH), 7.58(d, J=6.4 Hz, 1H, ArH), 7.22(dd, J=1.2, 6.0 Hz, 1H, ArH), 7.18(d, J=1.2 Hz, 1H, ArH), 4.34(q, J=4.8 Hz, 2H, OCH2), 3.19(s, 4H, piperazine), 3.07(s, 4H, piperazine), 1.33(t, J=4.8 Hz, 3H, CH3) |
| Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|
| MDA-MB-231 | A549 | MCF-7 | Hela | KG1 | |
| 4t | 3.50±0.68 | 25.9±7.9 | 20.9±3.9 | > 42 | 17.3±0.4 |
| Gossypol | 1.40±0.24 | 4.02±0.41 | 3.00±0.87 | 8.81±1.22 | 2.87±0.77 |
Table 3 Cytotoxicity of compound 4t and gossypol
| Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|
| MDA-MB-231 | A549 | MCF-7 | Hela | KG1 | |
| 4t | 3.50±0.68 | 25.9±7.9 | 20.9±3.9 | > 42 | 17.3±0.4 |
| Gossypol | 1.40±0.24 | 4.02±0.41 | 3.00±0.87 | 8.81±1.22 | 2.87±0.77 |
| [1] | Yang W. S., Shimada K., Delva D., Patel M., Ode E., Skouta R., Stockwell B., ACS Med. Chem. Lett., 2012, 3(1), 35—38 |
| [2] | Hamer P. C. D. W., Mir S. E., Noske D., Noorden C. J. F. V., Wudinger T., Clin. Cancer Res., 2011, 17(13), 4200—4207 |
| [3] | Tong Y. S., Torrent M., Florjancic A. S., Bromberg K. D., Buchanan F. G., Ferguson D. C., Johnson E. F., Lasko L. M., Maag D., Merta P. J., Olson A. M., Osterling D. J., Soni N., Shoemaker A. R., Penning T. D., ACS Med. Chem. Lett., 2015, 6(1), 58—62 |
| [4] | Ma Y., Lahue B. R., Gibeau C. R., Shipps G. W., Bogen S. L., Wang Y., Guo Z., Guzi T. J., ACS Med. Chem. Lett., 2014, 5(5), 572—575 |
| [5] | Deng X. M., Yang Q. K., Kwiatkowski N., Sim T., McDermott U., Settleman J. E., Lee J., Gray N. S., ACS Med. Chem. Lett., 2011, 2(3), 195—200 |
| [6] | Toogood P. L., Harvey P. J., Repine J. T., Sheehan D. J., van der Wel S. N., Zhou H., Keller P. R., McNamara D. J., Sherry D., Zhu T., Brodfuehrer J., Choi C., Barvian M. R., Fry D. W., J. Med. Chem., 2005, 48(7), 2388—2406 |
| [7] | Liu Q. S., Chang J. W., Wang J., Kang S. A., Thoreen C. C., Markhard A., Hur W., Zhang J., Sim T., Sabatini D. M., Gray N. S., J. Med. Chem., 2010, 53(19), 7146—7155 |
| [8] | Lee S., Hsu E., Chou C., Chuang H., Bai L., Kulp S. K., Chen C., J. Med. Chem., 2011, 54(18), 6364—6374 |
| [9] | Luo H. M., Yang C. L., Zhang X. Y., Zhao M. M., Jiang D., Xiao J. H., Yang X. H., Li S., Chem. Res. Chinese Universities,2013, 29(5), 906—910 |
| [10] | Chen K. X., Jiang Z. Z., Chen W. H., Xi B. M., J. South Med. Univ., 2014, 34(12), 1830—1833 |
| (陈凯旋, 江振洲, 陈文华, 习保民. 南方医科大学学报, 2014, 34(12), 1830—1833) | |
| [11] | Gong H. W., Qi H., Sun W., Jiang D., Xiao J. H., Yang X. H., Wang Y., Li S., Chem. J. Chinese Universities,2013, 34(9), 2131—2138 |
| (巩宏伟, 綦辉, 孙薇, 姜丹, 肖军海, 杨晓虹, 王应, 李松. 高等学校化学学报,2013, 34(9), 2131—2138) | |
| [12] | Shah S. S. A., Rivera G., Ashfaq M., Mini-Rev. Med. Chem., 2013, 13(1), 70—86 |
| [13] | Wang J. H., Wang Q. D., Dun Y. Y., Fang H., Chem. J. Chinese Universities,2014, 35(6), 1189—1198 |
| (王军华, 王泉德, 顿艳艳, 方浩. 高等学校化学学报,2014, 35(6), 1189—1198) | |
| [14] | Hann C. L., Daniel V. C., Sugar E. A., Dobromilskaya I., Murphy S. C., Cope L., Lin X., Hierman J. S., Wilburn D. L., Watkins D. N., Rudin C. M., Cancer Res., 2008, 68(7), 2321—2328 |
| [15] | Souers A. J., Leverson J. D., Boghaert E. R., Ackler S. L., Catron N. D., Chen J., Dayton B. D., Ding H., Enschede S. H., Fairbrother W. J., Huang D. C. S., Hymowitz S. G., Jin S., Khaw S. L., Kovar P. J., Lam L. T., Lee J., Maecker H. L., Marsh K. C., Mason K. D., Mitten M. J., Nimmer P. M., Oleksijew A., Park C. H., Park C. M., Phillips D. C., Roberts A. W., Sampath D., Seymour J. F., Smith M. L., Sullivan G. M., Tahir S. K., Tse C., Wendt M. D., Xiao Y., Xue J. C., Zhang H., Humerickhouse R. A., Rosenberg S. H., Elmore S. W., Nat. Med., 2013, 19(2), 202—208 |
| [16] | Wang X. L., Salaski E. J., Berger D. M., Powell D., Org. Lett., 2009, 11(24), 5662—5664 |
| [17] | Bourrain S., Collins I., Neduvelil J. G., Rowley M., Leeson P. D., Patel S., Patel S., Emms F., Marwood R., Chapman K. L., Fletcher A. E., Showell G. A., Bioorg. Med. Chem., 1998, 6(10), 1731—1743 |
| [18] | Klioze S. S., Allen R. C., J. Med. Chem., 1980, 23(6), 677—679 |
| [19] | Ullah N., Med. Chem., 2014, 10(5), 484—496 |
| [20] | Martin E. L., Org. Synth., 1943, 2, 501—503 |
| [1] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [2] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [3] | HOU Hua, WANG Baoshan. Group Additivity Theoretical Model for the Prediction of Dielectric Strengths of the Alternative Gases to SF6 [J]. Chem. J. Chinese Universities, 2021, 42(12): 3709. |
| [4] | YE Xiaodong, QI Guodong, XU Jun, DENG Feng. Glucose Oxidation on Au-supported SBA-15 Molecular Sieve † [J]. Chem. J. Chinese Universities, 2020, 41(5): 960. |
| [5] | CHANG Junpeng,ZHAO Jiarui,CHEN Sijia,MENG Kai,SHI Weini,LI Ruifang. Structure-activity Relationship of Antimicrobial Peptide SAMP1 and Its Analog Peptides† [J]. Chem. J. Chinese Universities, 2019, 40(4): 705. |
| [6] | YU Min, HUANG Jingjing, MA Min, FU Ruiyan, YAN Yan, ZHANG Fusheng, YIN Junfeng, XIE Ningning. Zinc Chelating Activity and Quantitative Structure-activity Relationship of Tripeptides† [J]. Chem. J. Chinese Universities, 2018, 39(2): 234. |
| [7] | HOU Hua, YU Xiaojuan, ZHOU Wenjun, LUO Yunbai, WANG Baoshan. Theoretical Investigations on the Structure-activity Relationship to the Dielectric Strength of the Insulation Gases† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2477. |
| [8] | WANG Lei, ZHENG Guojun, JI Qi, CHEN Bo, GONG Longlong, GAO Congmin, DU Zhenjian, ZHANG Xingmin. Synthesis and Biological Activity of Novel PI3K/mTOR Inhibitors† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1590. |
| [9] | LIU Yuming, TIAN Lijun, HU Dong, NIE Jianbing. yntheses and Anti-cholinesterase Activity of 4-N-Phenylaminoquinoline Derivatives † [J]. Chem. J. Chinese Universities, 2017, 38(3): 392. |
| [10] | LIU Benguo, LIU Jiangwei, LI Jiaqi, GENG Sheng, MO Haizhen, LIANG Guizhao. 3D-QSAR and Interaction Mechanism of Flavonoids s P-glycoprotein Inhibitors† [J]. Chem. J. Chinese Universities, 2017, 38(1): 41. |
| [11] | GUO Liang, CAO Rihui, FAN Wenxi, GAN Ziyun, MA Qin. Design, Synthesis and in vitro Antitumor Activities of Novel Bivalent β-Carbolines† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1093. |
| [12] | ZHANG Jie, ZHOU Changjian, XIE Jianwei, DAI Bin. Synthesis and Antitumor Activities of Rhein-Valine Adducts† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2159. |
| [13] | ZHANG Jing, MU Boshuai, WU Meng, BIAN Yanqing, LI Yuan. Synthesis, Antifungal Activity and Structure-activity Relationship of -Fluorophenyl-2,3-dihydro-1,5-benzothiazepines Derivatives† [J]. Chem. J. Chinese Universities, 2015, 36(4): 687. |
| [14] | KANG Wang, BU Huijuan, LI Wenhong, LI Yuan. Preliminary Structure-activity Relationship of 2-Ethoxycarbonyl-4-aryl-1,5-benzothiazepines with Antifungal Activity† [J]. Chem. J. Chinese Universities, 2014, 35(4): 766. |
| [15] | GUO Liang, CAO Rihui, FAN Wenxi, MA Qin. Synthesis and Biological Evaluation of 1,2,7,9-Tetrasubstituted Harmine Derivatives as Potential Antitumor Agents† [J]. Chem. J. Chinese Universities, 2014, 35(3): 518. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||