

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (6): 1128.doi: 10.7503/cjcu20150966
• Physical Chemistry • Previous Articles Next Articles
CAO Dan, LI Yuanying, SU Qingqing, WANG Bin, LIU Fengyi*( ), WANG Wenliang
), WANG Wenliang
Received:2015-12-20
Online:2016-06-10
Published:2016-05-26
Contact:
LIU Fengyi
E-mail:FengyiLiu@snnu.edu.cn
Supported by:CLC Number:
TrendMD:
CAO Dan, LI Yuanying, SU Qingqing, WANG Bin, LIU Fengyi, WANG Wenliang. CASSCF and MS-CASPT2 Studies on an Electron-tunable,1,2-Dicyanoethylene-based Optical Molecular Switch†[J]. Chem. J. Chinese Universities, 2016, 37(6): 1128.
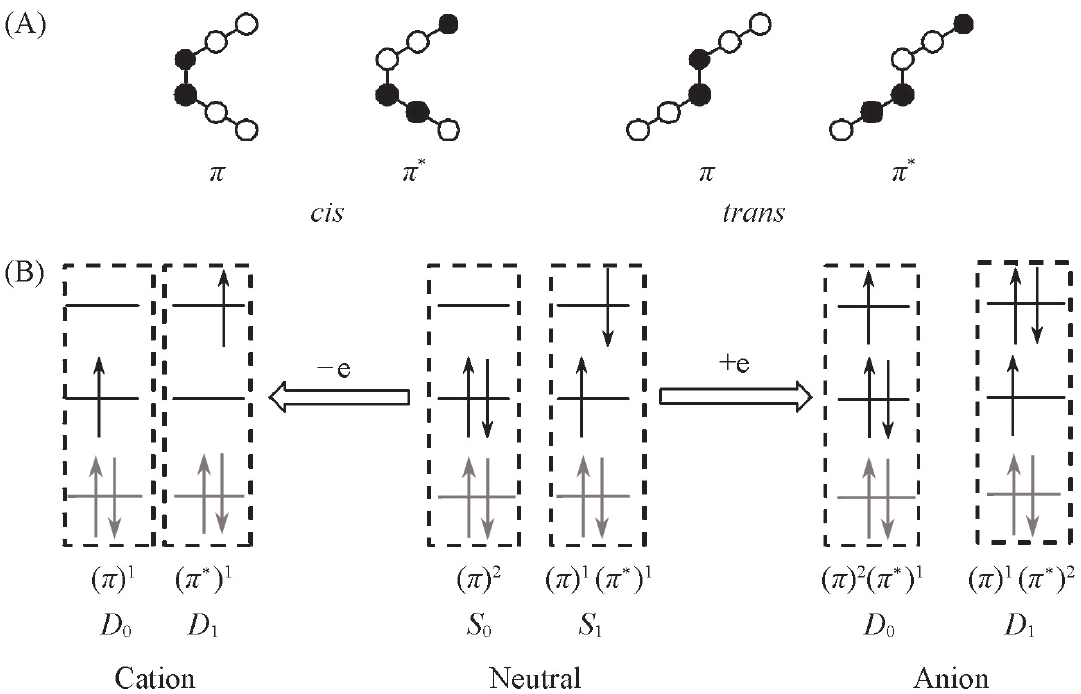
Fig.2 Nature of frontier MOs for cis and trans(A) and representative electronic configurations of ground- and excited states of neutral, cationic and anionic dicyanoethylene(B)
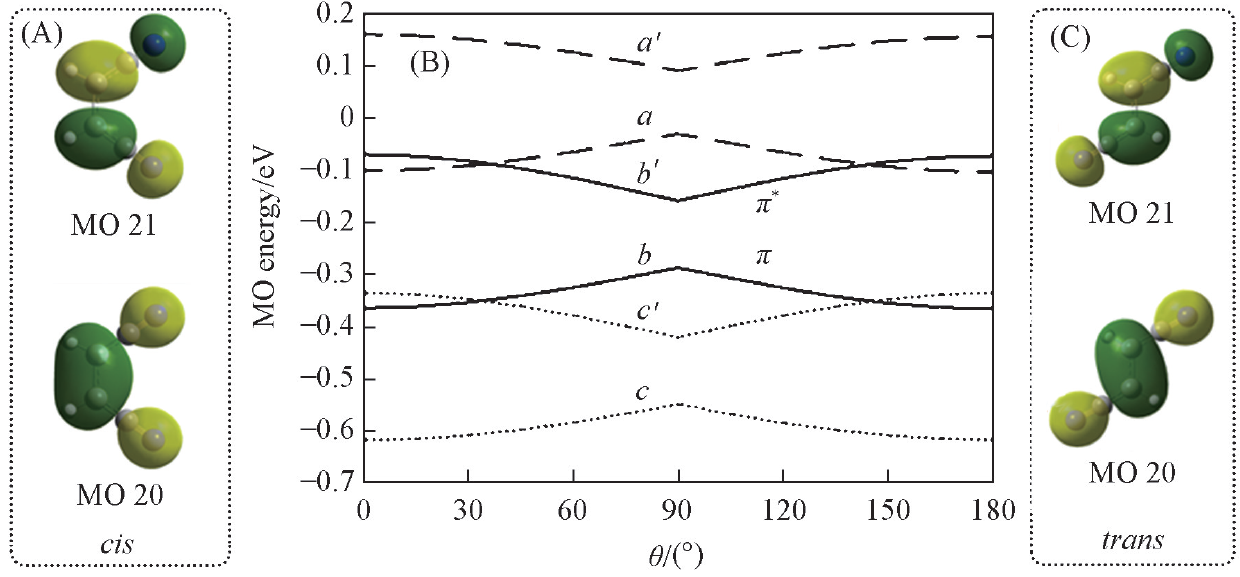
Fig.3 Nature of frontier MOs for cis(A) and trans(C) conformations and variation of MO energies along C1═C1' rotary path(B)a,a'. Anion; b,b'. neutral; c,c'. cation. a—c. MO 20; a'—c'. MO 21.
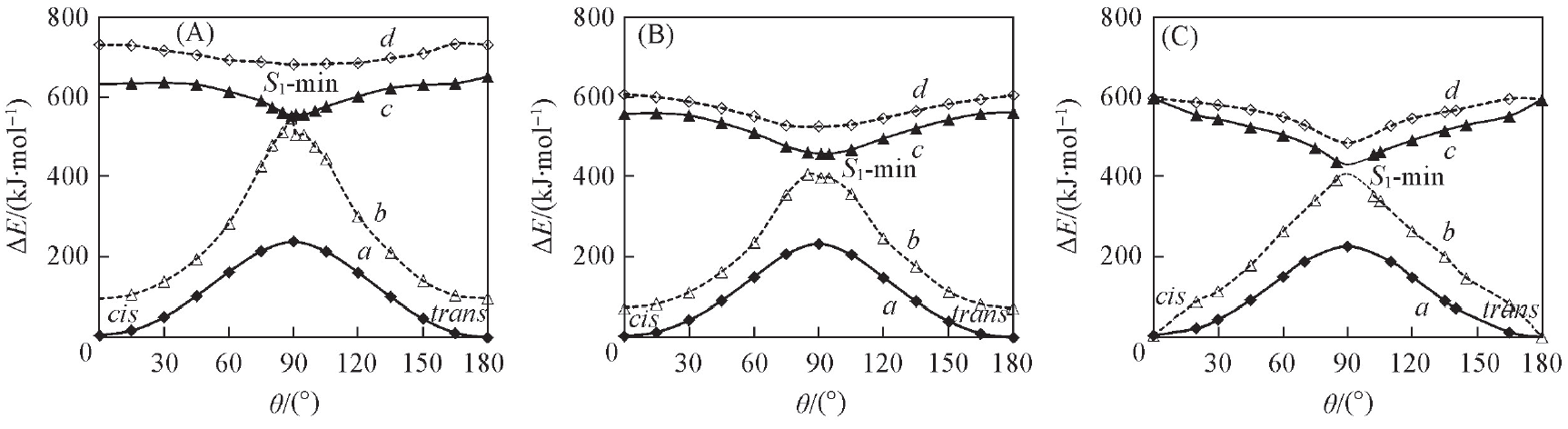
Fig.4 CASSCF(A), MS-CASPT2//CASSCF(B) and MS-CASPT2(C) computed PESs(potential energy surfaces) of 1,2-dicyanoethylene along rotary path(θ)a. S0-MEP; b. S0//S1; c. S1-MEP; d. S1//S0. The S1//S0 energy profiles indicate the S1-state energies on the basis of optimized S0-state structure(So does S0//S1 curves).
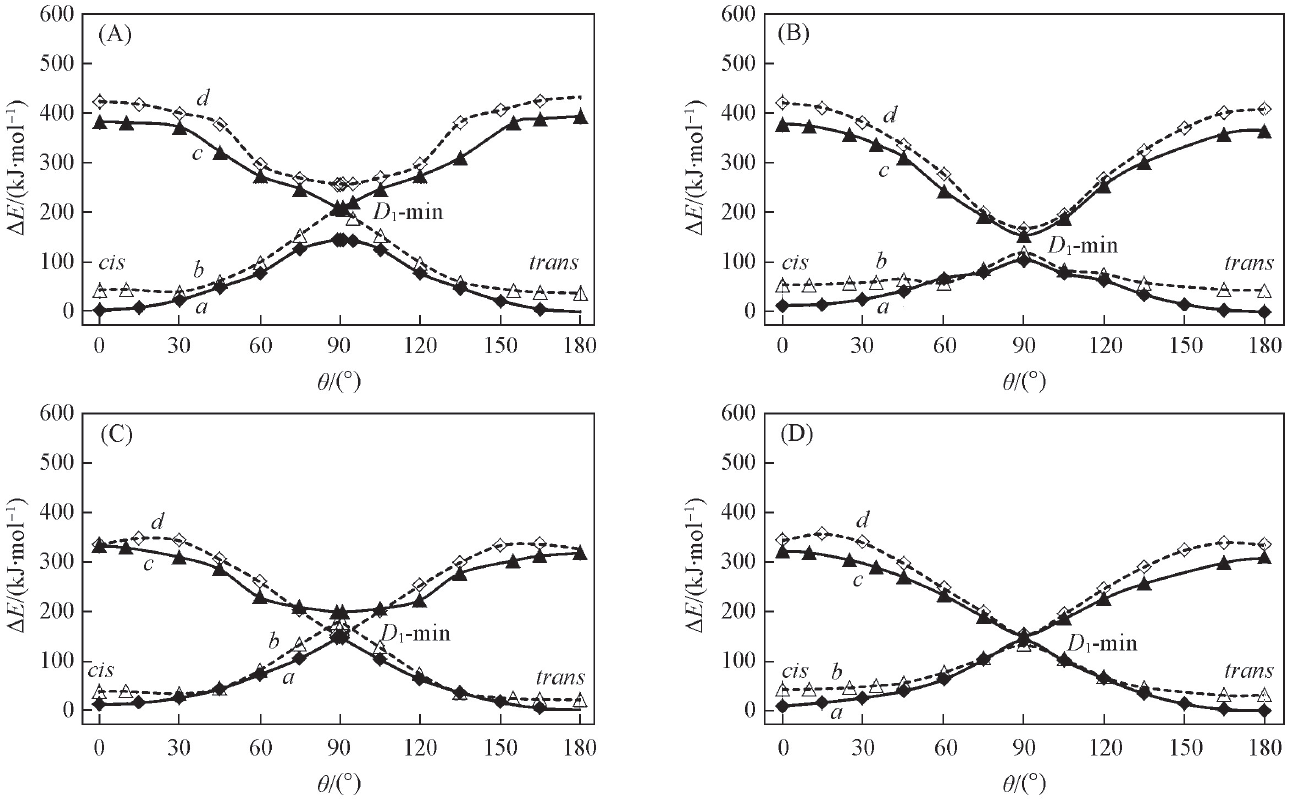
Fig.6 CASSCF computed PESs for cation(A) and anion(B) dicyanoethylene and the corresponding MS-CASPT2//CASSCF energy profiles for cationic(C) and anionic(D) dicyanoethylene along rotary path(θ)a. D0-MEP; b. D0//D1; c. D1-MEP; d. D1//D0.
| [1] | Feringa B. L., J. Org. Chem., 2007, 72(18), 6635—6652 |
| [2] | Kazaryan A., Kistemaker J. C. M., Schafer L. V., Browne W. R., Feringa B. L., Filatov M., J. Phys. Chem. A, 2010, 114(15), 5058—5067 |
| [3] | Cnossen A., Kistemaker J. C. M., Kojima T., Feringa B. L., J. Org. Chem., 2014, 79(3), 927—935 |
| [4] | Lu X. Y., Zhu W. H., Chin. J. Org. Chem., 2007, 27(11), 1352—1357 |
| (陆欣宇, 朱为宏. 有机化学, 2007, 27(11), 1352—1357) | |
| [5] | Fang C., Oruganti B., Durbeej B., RSC Advances, 2014, 4(20), 10240—10251 |
| [6] | Filatov M., Chem. Phys. Chem., 2011, 12(17), 3348—3353 |
| [7] | Guo X., Zhou J. W., Siegler M. A., Bragg A. E., Katz H. E., Angew. Chem. Int. Ed., 2015, 54(16), 4782—4786 |
| [8] | Freund L., Klessinger M., Int. J. Quantum Chem., 1998, 70, 1023—1028 |
| 9 | [9] Barbatti M., Paier J., Lischka H., J. Chem. Phys., 2004, 121(23), 11614—11624 |
| [10] | Barbatti M., Granucci G., Persico M., Lischka H., Chem. Phys. Lett., 2005, 401(S1—S3), 276—281 |
| [11] | Barbatti M., Ruckenbauer M., Lischka H., J. Chem. Phys., 2005, 122(17), 174307 |
| [12] | Ben-Nun M., Martínez T. J., Chem. Phys. Lett., 1998, 298(1), 57—65 |
| [13] | Michael F., Massimo O., J. Org. Chem., 2014, 79(8), 3587—3600 |
| [14] | Klaiman S., Cederbaum L. S., Angew. Chem. Int. Ed., 2015, 127(36), 10616—10619 |
| [15] | Finley J., Malmqvist P. Å., Roos B. O., Serrano-Andrés L., Chem. Phys. Lett., 1998, 288(S2—4), 299—306 |
| [16] | Andersson K., Malmqvist P. Å., Roos B. O., J. Chem. Phys., 1992, 96(2), 1218—1226 |
| [17] | Malmqvist P. Å., Roos B. O., Schimmelpfennig B., Chem. Phys. Lett., 2002, 357(3), 230—240 |
| [18] | Andersson K., Theor. Chim. Acta, 1995, 91(1), 31—46 |
| [19] | Levine B. G., Ko C., Quenneville J., Martínez T. J., Mol. Phys., 2006, 104(5), 1039—1051 |
| [20] | Levine B. G., Coe J. D., Martínez T. J., J. Phys. Chem. B, 2008, 112(2), 405—413 |
| [21] | Maeda S., Ohno K., Morokuma K., J. Chem. Theory Comput., 2010, 6(5), 1538—1545 |
| [22] | Qu Z. X., Gao J. L., Chem. J. Chinese Universities, 2015, 36(11), 2236—2240 |
| (曲泽星, 高加力. 高等学校化学学报, 2015, 36(11), 2236—2240) | |
| [23] | Song Y. L., Liu Y. J., Chem. J. Chinese Universities, 2015, 36(11), 2163—2170 |
| (宋艳丽, 刘亚军. 高等学校化学学报, 2015, 36(11), 2163—2170) | |
| [24] | Aquilante F., de Vico L., Ferré N., Ghigo G., Malmqvist P. Å., Neogrády P., Pedersen T. B., Pitoňák M., Reiher M., Roos B. O., Serrano-Andrés L., Urban M., Veryazov V., Lindh R., J. Comput. Chem., 2010, 31(1), 224—247 |
| [25] | Aquilante F., Autschbach J., Carlson R. K., Chibotaru L. F., Delcey M. G., de Vico L., Galván I. F., Ferré N., Frutos L. M., Gagliardi L., Garavelli M., Giussani A., Hoyer C. E., Manni G. L., Lischka H., Ma D., Malmavist P. Å., Müller T., Nenov A., Olivucci M., Pedersen T. B., Peng D., Plasser F., Pritchard B., Reiher M., Rivalta I., Schapiro I., Segarra-Martí J., Stenrup M., Truhlar D. G., Ungur L., Valentini A., Vancoillie S., Veryazov V., Vysotskiy V. P., Weingart O., Zapata F., Lindh R., J. Comput. Chem., 2016, 37, 506—541 |
| [26] | Liu F. Y., Morokuma K., J. Am. Chem. Soc., 2012, 134(10), 4864—4876 |
| [27] | Ohmine L., J. Chem. Phys., 1985, 83(5), 2348—2362 |
| [28] | Ben-Nun M., Martínez T. J., Chem. Phys., 2000, 259(2), 237—248 |
| [29] | Krawczyk R. P., Viel A., Manthe U., Domcke W., J. Chem. Phys., 2003, 119(3), 1397—1411 |
| [30] | Yamazaki S., Kato S., J. Chem. Phys., 2005, 123(11), 114510 |
| [1] | GE Yicong, NIE Wanli, SUN Guofeng, CHEN Jiaxuan, TIAN Chong. Silver-catalyzed [5+1] Cyclization of 2-Vinylanilines with Benzisoxazoles [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220142. |
| [2] | WANG Mingzhi, ZHENG Yanping, WENG Weizheng. Catalytic Methane Combustion over CeO2 Supported PdO and Ce1‒x Pd x O2‒δ Species [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210816. |
| [3] | GAO Jing, HE Wentao, WANG Xinxin, XIANG Yushu, LONG Lijuan, QIN Shuhao. Preparation of DOPO Derivative Modified Carbon Nanotubes and Their Effect on Flame Retardancy of Polylactic Acid [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210670. |
| [4] | LI Xiaohui, WEI Aijia, MU Jinping, HE Rui, ZHANG Lihui, WANG Jun, LIU Zhenfa. Effects of SmPO4 Coatingon Electrochemical Performance of High-voltage LiNi0.5Mn1.5O4 Cathode Materials [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210546. |
| [5] | ZHANG Liling, LIU Liu, ZHENG Mingqiu, FANG Wenkai, LIU Da, TANG Hongwu. Dual Signal Detection of HPV16 DNA by CRISPR/Cas12a Biosensing System Based on Upconversion Luminescent Resonance Energy Transfer [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220412. |
| [6] | LI Dan, XIAO Liping, FAN Jie. Inorganic-based Surface Materials with Anti-SARS-CoV-2 Properties and Their Mechanisms of Action [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220301. |
| [7] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [8] | ZHOU Yonghui, HUANG Rujun, YAN Jianyang, LI Yajun, QIU Huanhuan, YANG Jinxuan, ZHENG Youxuan. Synthesis and Electroluminescence Properties of Two Iridium(Ⅲ) Complexes with Nitrogen Heterocycle Structures [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210415. |
| [9] | LIANG Yu, LIU Huan, GONG Lige, WANG Chunxiao, WANG Chunmei, YU Kai, ZHOU Baibin. Synthesis and Supercapacitor Properties of Biimidazole-modified {SiW12O40} Hybrid [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210556. |
| [10] | LI Haibo, XIAO Changfa, JIANG Long, HUANG Yun, DAN Yi. Copolymerization of Methyl Acrylate and 1-Octene Catalyzed by the Loaded Aluminum Chloride on MCM-41 Molecular Sieve [J]. Chem. J. Chinese Universities, 2021, 42(9): 2974. |
| [11] | LUO Qiangqiang, JIN Shaoqing, SUN Hongmin, YANG Weimin. Post-synthesis of Ti-MWW Zeolite via Titanium Incorporation in Liquid Acid Solution [J]. Chem. J. Chinese Universities, 2021, 42(9): 2742. |
| [12] | WANG Meiyin, HUANG Daofeng, CHEN Xin, ZHOU Junfu, REN Yuanhang, YE Lin, YUE Bin, HE Heyong. Liquid Phase Assembly of Mesoporous CsxH3-xPW12O40 and Characterization of Their Acidity [J]. Chem. J. Chinese Universities, 2021, 42(9): 2734. |
| [13] | HU Chuanchuan, PANG Jingxiang, HE Chuangchuang, LI Wei, SUN Shutao. Sc(OTf)3 Catalyzed 1,6-Conjugate Allylation of δ-CN p-QMs: Synthesis of Allyl Substituted Diarylacetonitrile Compounds [J]. Chem. J. Chinese Universities, 2021, 42(9): 2805. |
| [14] | MENG Fanwei, GAO Qi, YE Qing, LI Chenxi. Potassium Poisoning Mechanism of Cu-SAPO-18 Catalyst for Selective Catalytic Reduction of NOx by Ammonia [J]. Chem. J. Chinese Universities, 2021, 42(9): 2832. |
| [15] | LIU Huazheng, PAN Xiaoguang, LI Hua, WAN Renzhong, LIU Xigong. Na2CO3-catalyzed 1,6-Conjugate Addition of Trimethylsilyl Azide to δ-CF3-δ-Aryl-disubstituted Para-Quinone Methides: Efficient Construction of Diarylmethanes Bearing CF3- and N3-Substituted Quaternary Stereocenters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2772. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||