

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (11): 2189.doi: 10.7503/cjcu20150595
• Physical Chemistry • Previous Articles Next Articles
CHI Weijie, TIAN Meng, LI Quansong*( ), LI Zesheng*(
), LI Zesheng*( )
)
Received:2015-07-30
Online:2015-11-10
Published:2015-10-10
Contact:
LI Quansong,LI Zesheng
E-mail:liquansong@bit.edu.cn;zeshengli@bit.edu.cn
CLC Number:
TrendMD:
CHI Weijie, TIAN Meng, LI Quansong, LI Zesheng. Computational Studies on Energetic Performance of Polynitro-substituted Uric Acid Derivatives[J]. Chem. J. Chinese Universities, 2015, 36(11): 2189.
| Compound | HOFg/ (kJ·mol-1) | ΔHsub/ (kJ·mol-1) | HOFs/ (kJ·mol-1) | ΔHcomb / (kJ·g-1) | Compound | HOFg/ (kJ·mol-1) | ΔHsub/ (kJ·mol-1) | HOFs/ (kJ·mol-1) | ΔHcomb / (kJ·g-1) |
|---|---|---|---|---|---|---|---|---|---|
| U1 | -513.96 | 101.97 | -615.92 | -8.36 | U24 | -436.23 | 110.54 | -546.77 | -6.61 |
| U2 | -529.67 | 109.81 | -639.48 | -8.25 | U34 | -422.47 | 106.60 | -529.07 | -6.68 |
| U3 | -550.33 | 99.51 | -649.84 | -8.20 | U123 | -336.94 | 116.09 | -453.03 | -5.47 |
| U4 | -530.90 | 101.32 | -632.22 | -8.28 | U124 | -320.26 | 119.48 | -439.74 | -5.51 |
| U12 | -420.53 | 112.64 | -533.17 | -6.67 | U134 | -307.40 | 115.58 | -422.98 | -5.57 |
| U13 | -441.04 | 106.18 | -547.21 | -6.61 | U234 | -313.71 | 113.92 | -427.63 | -5.55 |
| U14 | -418.24 | 108.65 | -526.90 | -6.69 | U1234 | -196.07 | 124.71 | -320.78 | -4.73 |
| U23 | -449.42 | 108.26 | -557.69 | -6.57 |
Table 1 Calculated heat of formation(HOFs) and the specific enthalpy(ΔHcomb) of combustion of the title compounds at B3LYP/6-31G(d,p) level
| Compound | HOFg/ (kJ·mol-1) | ΔHsub/ (kJ·mol-1) | HOFs/ (kJ·mol-1) | ΔHcomb / (kJ·g-1) | Compound | HOFg/ (kJ·mol-1) | ΔHsub/ (kJ·mol-1) | HOFs/ (kJ·mol-1) | ΔHcomb / (kJ·g-1) |
|---|---|---|---|---|---|---|---|---|---|
| U1 | -513.96 | 101.97 | -615.92 | -8.36 | U24 | -436.23 | 110.54 | -546.77 | -6.61 |
| U2 | -529.67 | 109.81 | -639.48 | -8.25 | U34 | -422.47 | 106.60 | -529.07 | -6.68 |
| U3 | -550.33 | 99.51 | -649.84 | -8.20 | U123 | -336.94 | 116.09 | -453.03 | -5.47 |
| U4 | -530.90 | 101.32 | -632.22 | -8.28 | U124 | -320.26 | 119.48 | -439.74 | -5.51 |
| U12 | -420.53 | 112.64 | -533.17 | -6.67 | U134 | -307.40 | 115.58 | -422.98 | -5.57 |
| U13 | -441.04 | 106.18 | -547.21 | -6.61 | U234 | -313.71 | 113.92 | -427.63 | -5.55 |
| U14 | -418.24 | 108.65 | -526.90 | -6.69 | U1234 | -196.07 | 124.71 | -320.78 | -4.73 |
| U23 | -449.42 | 108.26 | -557.69 | -6.57 |
| Compound | Q / (kJ·mol-1) | ρ/(g·cm-3) | D / (k·ms-1) | P/GPa | Compound | Q / (kJ·mol-1) | ρ/(g·cm-3) | D / (k·ms-1) | P/GPa |
|---|---|---|---|---|---|---|---|---|---|
| U1 | 488.64 | 1.83 | 6.19 | 18.66 | U24 | 811.13 | 1.97 | 7.59 | 28.19 |
| U2 | 462.21 | 1.93 | 6.33 | 20.28 | U34 | 827.53 | 1.92 | 7.49 | 26.96 |
| U3 | 450.59 | 1.87 | 6.17 | 18.76 | U123 | 1057.23 | 1.97 | 8.28 | 33.11 |
| U4 | 470.36 | 1.90 | 6.28 | 19.66 | U124 | 1067.70 | 1.98 | 8.31 | 33.42 |
| U12 | 823.73 | 1.95 | 7.57 | 27.86 | U134 | 1080.93 | 1.99 | 8.38 | 34.08 |
| U13 | 810.72 | 1.94 | 7.51 | 27.32 | U234 | 1077.26 | 1.98 | 8.33 | 33.52 |
| U14 | 829.54 | 1.89 | 7.43 | 26.35 | U1234 | 1266.12 | 2.00 | 8.86 | 37.93 |
| U23 | 801.02 | 1.94 | 7.50 | 27.26 | RDX | 1591.03 | 1.81 | 8.75 | 34.00 |
Table 2 Calculated detonation properties of the title compounds and reference compound RDX
| Compound | Q / (kJ·mol-1) | ρ/(g·cm-3) | D / (k·ms-1) | P/GPa | Compound | Q / (kJ·mol-1) | ρ/(g·cm-3) | D / (k·ms-1) | P/GPa |
|---|---|---|---|---|---|---|---|---|---|
| U1 | 488.64 | 1.83 | 6.19 | 18.66 | U24 | 811.13 | 1.97 | 7.59 | 28.19 |
| U2 | 462.21 | 1.93 | 6.33 | 20.28 | U34 | 827.53 | 1.92 | 7.49 | 26.96 |
| U3 | 450.59 | 1.87 | 6.17 | 18.76 | U123 | 1057.23 | 1.97 | 8.28 | 33.11 |
| U4 | 470.36 | 1.90 | 6.28 | 19.66 | U124 | 1067.70 | 1.98 | 8.31 | 33.42 |
| U12 | 823.73 | 1.95 | 7.57 | 27.86 | U134 | 1080.93 | 1.99 | 8.38 | 34.08 |
| U13 | 810.72 | 1.94 | 7.51 | 27.32 | U234 | 1077.26 | 1.98 | 8.33 | 33.52 |
| U14 | 829.54 | 1.89 | 7.43 | 26.35 | U1234 | 1266.12 | 2.00 | 8.86 | 37.93 |
| U23 | 801.02 | 1.94 | 7.50 | 27.26 | RDX | 1591.03 | 1.81 | 8.75 | 34.00 |
| Compound | N | Tc | Is | Compound | N | Tc | Is |
|---|---|---|---|---|---|---|---|
| U1 | 0.0270 | 9766.7 | 16.24 | U24 | 0.0271 | 7627.1 | 14.39 |
| U2 | 0.0270 | 9641.4 | 16.13 | U34 | 0.0271 | 7703.1 | 14.46 |
| U3 | 0.0270 | 9586.3 | 16.09 | U123 | 0.0272 | 6266.9 | 13.06 |
| U4 | 0.0270 | 9680.0 | 16.17 | U124 | 0.0272 | 6314.7 | 13.11 |
| U12 | 0.0271 | 7685.5 | 14.44 | U134 | 0.0272 | 6375.1 | 13.17 |
| U13 | 0.0271 | 7625.2 | 14.38 | U234 | 0.0272 | 6358.4 | 13.16 |
| U14 | 0.0271 | 7712.5 | 14.47 | U1234 | 0.0273 | 5720.7 | 12.50 |
| U23 | 0.0271 | 7580.3 | 14.34 | RDX | 14.13 |
Table 3 Calculated Is and the corresponding parameters for the title compounds and RDX
| Compound | N | Tc | Is | Compound | N | Tc | Is |
|---|---|---|---|---|---|---|---|
| U1 | 0.0270 | 9766.7 | 16.24 | U24 | 0.0271 | 7627.1 | 14.39 |
| U2 | 0.0270 | 9641.4 | 16.13 | U34 | 0.0271 | 7703.1 | 14.46 |
| U3 | 0.0270 | 9586.3 | 16.09 | U123 | 0.0272 | 6266.9 | 13.06 |
| U4 | 0.0270 | 9680.0 | 16.17 | U124 | 0.0272 | 6314.7 | 13.11 |
| U12 | 0.0271 | 7685.5 | 14.44 | U134 | 0.0272 | 6375.1 | 13.17 |
| U13 | 0.0271 | 7625.2 | 14.38 | U234 | 0.0272 | 6358.4 | 13.16 |
| U14 | 0.0271 | 7712.5 | 14.47 | U1234 | 0.0273 | 5720.7 | 12.50 |
| U23 | 0.0271 | 7580.3 | 14.34 | RDX | 14.13 |
| Compound | BO | ΔE/eV | BDE /(kJ·mol-1) | Compound | BO | ΔE/eV | BDE /(kJ·mol-1) |
|---|---|---|---|---|---|---|---|
| U1 | 0.83 | 3.80 | 188.18 | U24 | 0.92 | 3.78 | 101.16 |
| U2 | 0.89 | 4.53 | 100.76 | U34 | 0.83 | 3.80 | 73.44 |
| U3 | 0.97 | 3.60 | 117.91 | U123 | 0.84 | 3.97 | 87.32 |
| U4 | 0.93 | 3.36 | 81.46 | U124 | 0.90 | 3.77 | 91.85 |
| U12 | 0.88 | 4.42 | 101.05 | U134 | 0.79 | 3.80 | 64.50 |
| U13 | 0.96 | 3.69 | 118.49 | U234 | 0.82 | 4.05 | 88.00 |
| U14 | 0.80 | 3.34 | 70.73 | U1234 | 0.79 | 4.05 | 88.51 |
| U23 | 0.85 | 3.87 | 85.84 | RDX | 0.98 | 5.97 | 160.41 |
Table 4 Calculated bond order(BO), HOMO-LUMO energy gap(ΔE), and BDE of the title compounds and RDX at UB3LYP/6-31G(d,p) level
| Compound | BO | ΔE/eV | BDE /(kJ·mol-1) | Compound | BO | ΔE/eV | BDE /(kJ·mol-1) |
|---|---|---|---|---|---|---|---|
| U1 | 0.83 | 3.80 | 188.18 | U24 | 0.92 | 3.78 | 101.16 |
| U2 | 0.89 | 4.53 | 100.76 | U34 | 0.83 | 3.80 | 73.44 |
| U3 | 0.97 | 3.60 | 117.91 | U123 | 0.84 | 3.97 | 87.32 |
| U4 | 0.93 | 3.36 | 81.46 | U124 | 0.90 | 3.77 | 91.85 |
| U12 | 0.88 | 4.42 | 101.05 | U134 | 0.79 | 3.80 | 64.50 |
| U13 | 0.96 | 3.69 | 118.49 | U234 | 0.82 | 4.05 | 88.00 |
| U14 | 0.80 | 3.34 | 70.73 | U1234 | 0.79 | 4.05 | 88.51 |
| U23 | 0.85 | 3.87 | 85.84 | RDX | 0.98 | 5.97 | 160.41 |
| Compound | ΔV/nm3 | h50/cm | Compound | ΔV/nm3 | h50/cm |
|---|---|---|---|---|---|
| U1 | 0.028 | 21.0 | U24 | 0.028 | 38.6 |
| U2 | 0.026 | 28.2 | U34 | 0.031 | 33.4 |
| U3 | 0.024 | 48.2 | U123 | 0.034 | 21.0 |
| U4 | 0.023 | 49.3 | U124 | 0.033 | 25.7 |
| U12 | 0.029 | 23.2 | U134 | 0.033 | 22.5 |
| U13 | 0.027 | 30.3 | U234 | 0.030 | 26.5 |
| U14 | 0.032 | 35.5 | U1234 | 0.035 | 20.2 |
| U23 | 0.029 | 37.9 | RDX | 0.046 | 26.0 |
Table 5 Calculated impact sensitivity(h50) and the free space per molecule(ΔV) of the investigated molecules and RDX
| Compound | ΔV/nm3 | h50/cm | Compound | ΔV/nm3 | h50/cm |
|---|---|---|---|---|---|
| U1 | 0.028 | 21.0 | U24 | 0.028 | 38.6 |
| U2 | 0.026 | 28.2 | U34 | 0.031 | 33.4 |
| U3 | 0.024 | 48.2 | U123 | 0.034 | 21.0 |
| U4 | 0.023 | 49.3 | U124 | 0.033 | 25.7 |
| U12 | 0.029 | 23.2 | U134 | 0.033 | 22.5 |
| U13 | 0.027 | 30.3 | U234 | 0.030 | 26.5 |
| U14 | 0.032 | 35.5 | U1234 | 0.035 | 20.2 |
| U23 | 0.029 | 37.9 | RDX | 0.046 | 26.0 |
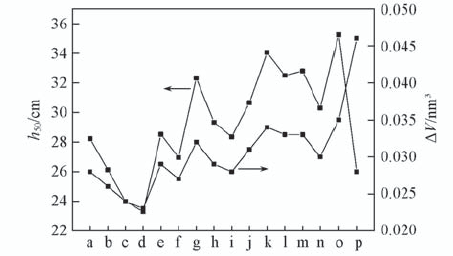
Fig.2 Calculated ΔV and h50 of the designed molecules and RDX a. U1; b. U2; c. U3; d. U4; e. U12; f. U13; g. U14; h. U23; i. U24; j. U34; k. U123; l. U124; m. U134; n. U234; o. U1234; p. RDX.
| [1] | Zhang J., Zhang Q., Vo T. T., Parrish D. A., Shreeve J. M., J. Am. Chem. Soc., 2015, 137(4), 1697—1704 |
| [2] | Bian C., Zhang M., Li C., Zhou Z., J. Mater. Chem. A, 2015, 3(1), 163—169 |
| [3] | Rice B. M., Larentzos J. P., Byrd E. F., Weingarten N. S., J. Chem. Theory. Comput., 2015, 11(2), 392—405 |
| [4] | Huynh M. H. V., Hiskey M. A., Chavez D. E., Naud D. L., Gilardi R. D., J. Am. Chem. Soc., 2005, 127(36), 12537—12543 |
| [5] | Yin P., Parrish D. A., Shreeve J. M., J. Am. Chem. Soc., 2015, 137(14), 4778—4786 |
| [6] | Chavez D. E., Hiskey M. A., J. Energ. Mater., 1999, 17(4), 357—377 |
| [7] | Malow M., Wehrstedt K. D., Neuenfeld S., Tetrahedron. Lett., 2007, 48(7), 1233—1235 |
| [8] | Lesnikovich A., Ivashkevich O., Levchik S., Balabanovich A., Gaponik P., Kulak A., Thermochim. Acta, 2002, 388(1), 233—251 |
| [9] | Korkin A. A., Balkova A., Bartlett R. J., Boyd R. J., von Rague Schleyer P., J. Phys. Chem., 1996, 100(14), 5702—5714 |
| [10] | Simões P., Pedroso L., Matos Beja A., Silva M. R., MacLean E., Portugal A., J. Phys. Chem. A, 2007, 111(1), 150—158 |
| [11] | Li C., Liang L., Wang K., Bian C., Zhang J., Zhou Z., J. Mater. Chem. A, 2014, 2(42), 18097—18105 |
| [12] | Sikder A., Sikder N., J. Hazard. Mater., 2004, 112(1), 1—15 |
| [13] | Liu Y., Gong X., Wang L., Wang G., Xiao H., J. Phys. Chem. A, 2011, 115(9), 1754—1762 |
| [14] | Pan Y., Zhu W., Xiao H., J. Mol. Model., 2012, 18(7), 3125—3138 |
| [15] | Chi W., Li L., Li B., Wu H., Struct. Chem., 2012, 23(6), 1837—1841 |
| [16] | Chi W., Li B., Wu H., Struct. Chem., 2013, 24(2), 375—381 |
| [17] | Chi W. J., Li Z. S., RSC Adv., 2015, 5(10), 7766—7772 |
| [18] | Chi W., Wang X., Li B., Wu H., J. Mol. Model., 2012, 18(9), 4217—4223 |
| [19] | Guo Y. Y., Chi W. J., Li Z. S., Li Q. S., RSC Adv., 2015, 5(48), 38048—38055 |
| [20] | Liu X. F., Xu W. G., Lu S. X., Chem. J. Chinese. Universities, 2009, 30(7), 1406—1409 |
| [21] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A., Vreven T. Jr., Kudin K. N., Burant J. C., Millam J. M., Iyengar S., Tomasi S. J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cui Q., Baboul A. G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Nanayakkara Y., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Gonzalez C., Pople J. A., Gaussian 09, Revision D. 01, Gaussian Inc., Wallingford CT, 2009 |
| [22] | Zhang X., Zhu W., Xiao H., J. Phys. Chem. A, 2009, 114(1), 603—612 |
| [23] | Chen Z., Xiao J., Xiao H., Chiu Y., J. Phys. Chem. A, 1999, 103(40), 8062—8066 |
| [24] | |
| [25] | Politzer P., Murray J. S., Edward Grice M., Desalvo M., Miller E., Mol. Phys., 1997, 91(5), 923—928 |
| [26] | Byrd E. F., Rice B. M., J. Phys. Chem. A, 2006, 110(3), 1005—1013 |
| [27] | Rice B. M., Pai S. V., Hare J., Combust. Flame, 1999, 118(3), 445—458 |
| [28] | Kamlet M., Jacobs S., J. Chem. Phys., 1968, 48, 23—35 |
| [29] | Bulat F. A., Toro-Labbé A., Brinck T., Murray J. S., Politzer P., J. Mol. Model., 2010, 16(11), 1679—1691 |
| [30] | Wei T., Zhu W., Zhang J., Xiao H., J. Hazard. Mater., 2010, 179(1), 581—590 |
| [31] | He P., Zhang J. G., Wang K., Yin X., Zhang T. L., J. Org. Chem., 2015, 80(11), 5643—5651 |
| [32] | Ghule V. D., Jadhav P. M., Patil R. S., Radhakrishnan S., Soman T., J. Phys. Chem. A, 2010, 114(1), 498—503 |
| [33] | Ghule V. D., J. Phys. Chem. A, 2012, 116(37), 9391—9397 |
| [34] | Chi W. J., Li L. L., Li B. T., Wu H. S., J. Mol. Model., 2012, 18(8), 3695—3704 |
| [35] | Ma Q., Jiang T., Zhang X., Fan G., Wang J., Huang J., J. Phys. Org. Chem., 2015, 28(1), 31—39 |
| [36] | Politzer P., Murray J. S., Cent. Eur. J. Energ. Mater., 2011, 8(3), 209—220 |
| [37] | Hoffmann R., J. Chem. Soc., 1969, 5, 240—241 |
| [38] | Liu H., Wang F., Wang G. X., Gong X. D., J. Comput. Chem., 2012, 33(22), 1790—1796 |
| [39] | Fan X. W., Ju X. H., Xiao H. M., Qiu L., J. Mol. Struct: Theochem., 2006, 801(1), 55—62 |
| [40] | Owens F., J. Mol. Struct: Theochem., 1996, 370(1), 11—16 |
| [41] | Ravi P., Mol. Phys., 2015, 113(7), 647—655 |
| [42] | Fried L. E., Manaa M. R., Pagoria P. F., Simpson R. L., Ann. Rev. Mater. Res., 2001, 31(1), 291—321 |
| [43] | Politzer P., Murray J. S., J. Mol. Struct., 1996, 376(1), 419—424 |
| [44] | Pospíšil M., Vávra P., Concha M. C., Murray J. S., Politzer P., J. Mol. Model., 2011, 17(10), 2569—2574 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [3] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [4] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [5] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [6] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [7] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [8] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [9] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [10] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [11] | HU Wei, LIU Xiaofeng, LI Zhenyu, YANG Jinlong. Surface and Size Effects of Nitrogen-vacancy Centers in Diamond Nanowires [J]. Chem. J. Chinese Universities, 2021, 42(7): 2178. |
| [12] | YANG Yiying, ZHU Rongxiu, ZHANG Dongju, LIU Chengbu. Theoretical Study on Gold-catalyzed Cyclization of Alkynyl Benzodioxin to 8-Hydroxy-isocoumarin [J]. Chem. J. Chinese Universities, 2021, 42(7): 2299. |
| [13] | YING Fuming, JI Chenru, SU Peifeng, WU Wei. λ-DFCAS: A Hybrid Density Functional Complete Active Space Self Consistent Field Method [J]. Chem. J. Chinese Universities, 2021, 42(7): 2218. |
| [14] | ZHENG Ruoxin, ZHANG Igor Ying, XU Xin. Development and Benchmark of Lower Scaling Doubly Hybrid Density Functional XYG3 [J]. Chem. J. Chinese Universities, 2021, 42(7): 2210. |
| [15] | LIU Yang, LI Qingbo, SUN Jie, ZHAO Xian. Direct Synthesis of Graphene on AlN Substrates via Ga Remote Catalyzation [J]. Chem. J. Chinese Universities, 2021, 42(7): 2271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||