

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (1): 79.doi: 10.7503/cjcu20150568
• Physical Chemistry • Previous Articles Next Articles
ZHU Peng, DUAN Xuemei, LIU Jingyao*( )
)
Received:2015-07-20
Online:2016-01-10
Published:2015-12-20
Contact:
LIU Jingyao
E-mail:ljy121@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
ZHU Peng, DUAN Xuemei, LIU Jingyao. Mechanism and Kinetics of the Hydrogen-abstraction Reaction of CF2ClC(O)OCH2CH3 with OH Radicals†[J]. Chem. J. Chinese Universities, 2016, 37(1): 79.
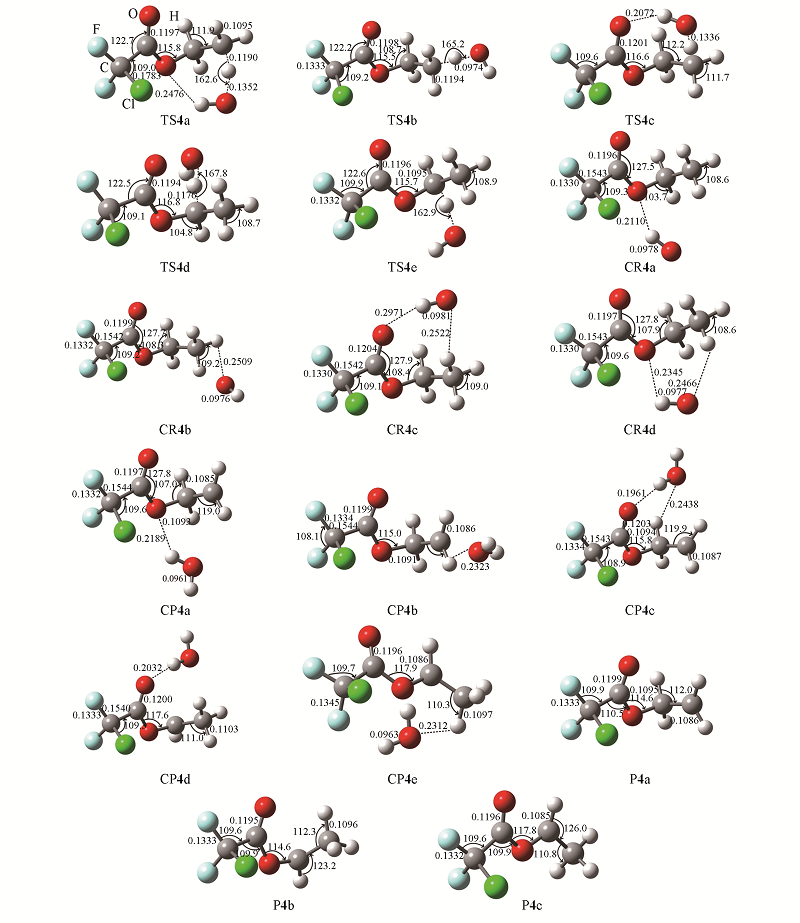
Fig.3 Optimized geometry parameters of reactants, products, transition-states, and hydrogen-bond complexes for the H-abstraction channels of R4 at the M06-2X/aug-cc-Pvdz^Bond lengths are in nm and angles are in degree.
| Species | / (kJ·mol-1) | Species | / (kJ·mol-1) | Species | / (kJ·mol-1) | Species | / (kJ·mol-1) | Species | / (kJ·mol-1) |
|---|---|---|---|---|---|---|---|---|---|
| RC1 | -838.47 | RC2 | -843.49 | RC3 | -837.22 | RC4 | -843.08 | RC5 | -842.24 |
| P1a | -625.09 | P2a | -630.53 | P3a | -626.76 | P4a | -631.78 | P5a | -631.37 |
| P1b | -628.02 | P2b | -633.04 | P3b | -649.78 | P4b | -640.99 | P5b | -654.38 |
| P1c | -649.78 | P2c | -654.13 | P3c | -636.80 | P4c | -653.54 | P5c | -640.99 |
Table 1 Standard enthalpies of formation() at 298 K calculated at the MCG3-MPWB//M06-2X/aug-cc-pVDZ level
| Species | / (kJ·mol-1) | Species | / (kJ·mol-1) | Species | / (kJ·mol-1) | Species | / (kJ·mol-1) | Species | / (kJ·mol-1) |
|---|---|---|---|---|---|---|---|---|---|
| RC1 | -838.47 | RC2 | -843.49 | RC3 | -837.22 | RC4 | -843.08 | RC5 | -842.24 |
| P1a | -625.09 | P2a | -630.53 | P3a | -626.76 | P4a | -631.78 | P5a | -631.37 |
| P1b | -628.02 | P2b | -633.04 | P3b | -649.78 | P4b | -640.99 | P5b | -654.38 |
| P1c | -649.78 | P2c | -654.13 | P3c | -636.80 | P4c | -653.54 | P5c | -640.99 |
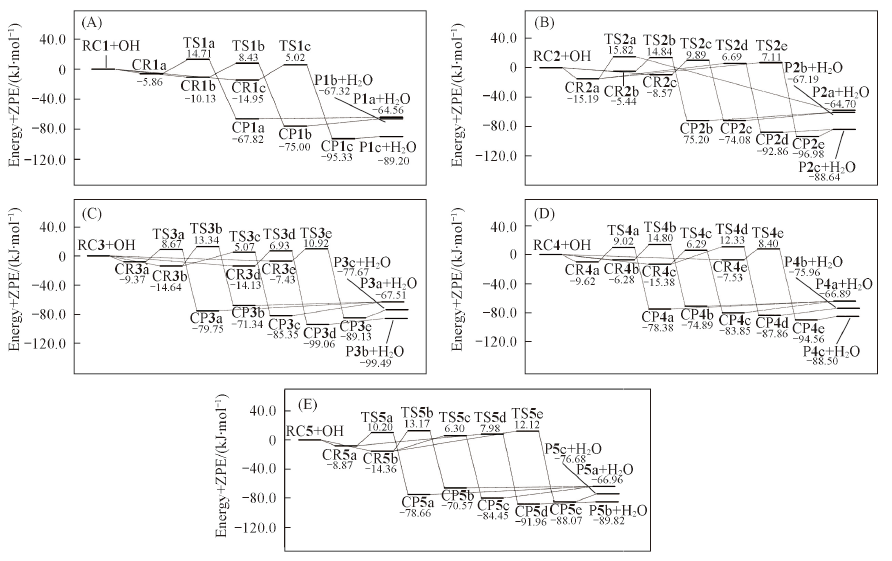
Fig.4 Schematic potential energy profiles for the RC1+OH reaction(A), the RC2+OH reaction(B), the RC3+OH reaction(C), the RC4+OH reaction(D) and the RC5 + OH reaction(E)^Relative energies with ZPE atthe MCG3-MPWB//M06-2X/aug-cc-pVDZ level are in kJ/mol.
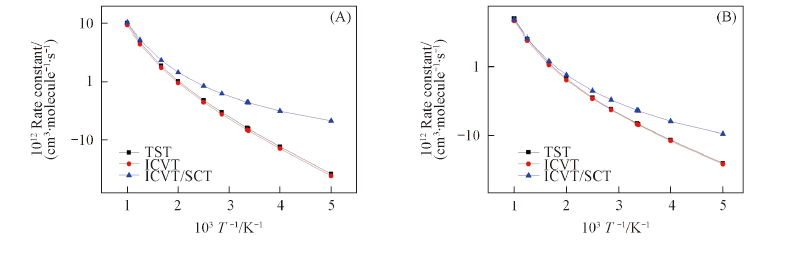
Fig.5 TST, ICVT and ICVT/SCT rate constants calculated at the MCG3-MPWB//M06-2X/aug-cc-pVDZ level versus 1000/T between 200 and 1000 K for path (R4c)(A) and for path (R4e)(B)
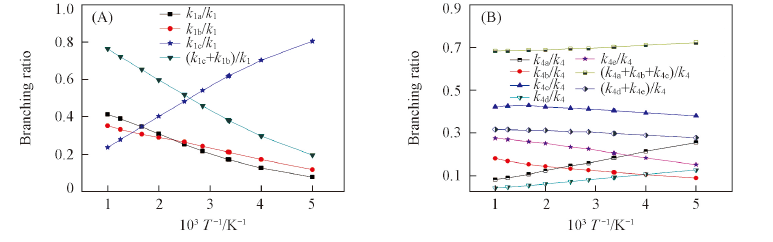
Fig.6 Plots of the calculated branching ratios versus 1000/T between 200 and 1000 K for the reaction RC1+OH→products(A) and the reaction RC4+OH→products(B)
| T/K | 1012 k1 | 1012 k2 | 1012 k3 | 1012 k4 | 1012 k5 | 1012 koverall |
|---|---|---|---|---|---|---|
| 200 | 0.355 | 0.464 | 0.471 | 0.551 | 0.360 | 0.464 |
| 250 | 0.425 | 0.756 | 0.614 | 0.695 | 0.516 | 0.645 |
| 296 | 0.529 | 1.12 | 0.803 | 0.877 | 0.796 | 0.898 |
| 298 | 0.536 | 1.14 | 0.813 | 0.888 | 0.812 | 0.912(0.54±0.15)[ |
| 350 | 0.705 | 1.67 | 1.11 | 0.161 | 1.40 | 1.34 |
| 400 | 0.926 | 2.31 | 1.50 | 0.149 | 2.35 | 1.92 |
| 500 | 1.56 | 4.06 | 2.64 | 0.236 | 6.12 | 3.80 |
| 600 | 2.53 | 6.51 | 4.40 | 3.60 | 13.7 | 7.03 |
| 800 | 5.88 | 14.1 | 10.4 | 7.33 | 47.5 | 19.4 |
| 1000 | 11.8 | 26.1 | 20.7 | 13.2 | 121 | 43.5 |
Table 2 Rate constants for each conformer and the overall rate constant[koverall/(cm3·molecule-1·s-1)] in the temperature range 200—1000 K at the MCG3-MPWB//M06-2X level
| T/K | 1012 k1 | 1012 k2 | 1012 k3 | 1012 k4 | 1012 k5 | 1012 koverall |
|---|---|---|---|---|---|---|
| 200 | 0.355 | 0.464 | 0.471 | 0.551 | 0.360 | 0.464 |
| 250 | 0.425 | 0.756 | 0.614 | 0.695 | 0.516 | 0.645 |
| 296 | 0.529 | 1.12 | 0.803 | 0.877 | 0.796 | 0.898 |
| 298 | 0.536 | 1.14 | 0.813 | 0.888 | 0.812 | 0.912(0.54±0.15)[ |
| 350 | 0.705 | 1.67 | 1.11 | 0.161 | 1.40 | 1.34 |
| 400 | 0.926 | 2.31 | 1.50 | 0.149 | 2.35 | 1.92 |
| 500 | 1.56 | 4.06 | 2.64 | 0.236 | 6.12 | 3.80 |
| 600 | 2.53 | 6.51 | 4.40 | 3.60 | 13.7 | 7.03 |
| 800 | 5.88 | 14.1 | 10.4 | 7.33 | 47.5 | 19.4 |
| 1000 | 11.8 | 26.1 | 20.7 | 13.2 | 121 | 43.5 |
| [1] | Oyaro N., Sellevag S. R., Nielsen C. J., Environ. Sci. Technol., 2004, 38, 5567—5576 |
| [2] | Sulback Andersen M. P., Nielsen O. J., Wallington T. J., Hurley M. D., DeMoore G. W., J. Phys. Chem. A, 2005, 109, 3926—3934 |
| [3] | Blanco M. B., Teruel M. A., Chem. Phys. Lett., 2007, 441, 1—6 |
| [4] | Hu W. P., Truhlar D. G., J. Am. Chem. Soc., 1996, 118, 860—869 |
| [5] | Truhlar D. G., Garrett B. C., Klippenstein S. J., J. Phys. Chem., 1996, 100, 12771—12800 |
| [6] | Truhlar D. G., Gordon M. S., Science,1990, 249, 491—498 |
| [7] | Tucker S.C., Truhlar D. G., New Theoretical Concepts for Understanding Organic Reaction, Kluwer,Dordrecht, 1989, 291—346 |
| [8] | Truhlar D. G., Gordon M. S., Stechler R., Chem. Rev., 1987, 87, 217—236 |
| [9] | Lu D. H., Truong T. N., Melissas V. S., Lynch G. C., Liu Y. P., Grarrett B. C., Stechler R., Issacson A. D., Rai S. N., Hancock G. C., Lauderdale J. G., Joseph T., Truhlar D. G., Comput. Phys. Commum., 1992, 71, 235—262 |
| [10] | Garrett B. C., Truhlar D. G., J. Phys. Chem., 1991, 95, 10374—10379 |
| [11] | Truhlar D. G., Garrett B. C., Acc. Chem. Res., 1980, 13, 440—448 |
| [12] | Truhlar D. G., Garrett B. C., Annu. Rev. Phys. Chem., 1984, 35, 159—189 |
| [13] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A.F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A.1, Gaussian Inc.,Wallingford CT, 2009 |
| [14] | Zhao Y., Truhlar D.G., Theor. Chem. Acc., 2008, 120, 215—241 |
| [15] | Zhao Y., Truhlar D. G., J. Phys. Chem. A, 2005, 109, 4209—4212 |
| [16] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Zakrzewski V. G., Montgomery J. A. Jr., Stratmann R. E., Burant J. C., Dapprich S., Millam J. M., Daniels A. D., Kudin K. N., Strain M. C., Farkas O., Tomasi J., Barone V., Cossi M., Cammi R., Mennucci B., Pomelli C., Adamo C., Clifford S., Ochterski J., Petersson G. A., Ayala P. Y., Cui Q., Morokuma K., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Cioslowski J., Ortiz J. V., Boboul A. G., Stefnov B. B., Liu G., Liaschenko A., Piskorz P., Komaromi L., Gomperts R., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Gonzalez C., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Andres J. L., Gonzalez C., Head-Gordon M., Replogle E. S., Pople J. A, Gaussian 03, Revision A.1, Guassian Inc.,Pittsburgh PA, 2003 |
| [17] | Zhao Y., Truhlar D.G., MLGAUSS-Version 2.0, University of Minnesota, Minneapolis, 2005 |
| [18] | Chuang Y. Y., Corchado J. C., Truhlar D. G., J. Phys. Chem. A, 1999, 103, 1140—1149 |
| [19] | Garrett B. C., Truhlar D. G., Grev R. S., Magnuson A. W., J. Phys. Chem., 1980, 84, 1730—1748 |
| [20] | Truhlar D. G., J. Comput. Chem., 1991, 12, 266—270 |
| [21] | Liu Y. P., Lynch G. C., Truong T. N., Lu D. H., Truhlar D. G., Garrett B. C., J. Am. Chem. Soc., 1993, 115, 2408—2415 |
| [22] | Galano A., Alvarez-Idaboy J. R., Ruiz-Santoyo M. E., Vivier-Bunge A., Chem.Phys.Chem,2004, 5, 1379—1388 |
| [23] | Taghikhani M., Parsafar G. A., Sabzyan H., J. Phys. Chem. A, 2005, 109, 8158—8167 |
| [24] | Wang Y. X., Gao H., Wang Q., Liu J. Y., Chem. J. Chinese Universities, 2010, 31(6), 1240—1245 |
| (王永霞, 高红, 王钦, 刘靖尧. 高等学校化学学报, 2010, 31(6), 1240—1245) | |
| [25] | Ren H., Zhang L. L., Wang R. S., Pan X. M., J. Phys. Chem. A, 2012, 116, 10647—10655 |
| [26] | Jin T. Y., Wang Q., Liu J. Y., Chem. J. Chinese Universities, 2013, 34(3), 641—649 |
| (金铜音, 王钦, 刘靖尧. 高等学校化学学报, 2013, 34(3), 641—649) | |
| [27] | Zhu P., Ai L. L., Wang H., Liu J. Y., Comput. Theor. Chem., 2014, 1029, 91—98 |
| [28] | Zhu P., Duan X. M., Liu J. Y., J. Fluorine. Chem., 2015, 176, 61—70 |
| [29] | Corchado J.C., Chuang Y. Y., Fast P. L., Hu W. P., Liu Y. P., Lynch G. C., Nguyen K. A., Jackels C. F., Ramos A. F., Ellingson B. A., Lynch B. J., Melissas V. S., Villa J., Rossi I., Coitino E. L., Pu J., Albu T. V., Steckler R., Garrett B. C., Isaacson A. D., Truhlar D. G., POLYRATE, Version 9.7, University of Minnesota,Minneapolis, 2007 |
| [30] | Jin X. H., Hu B. C., Jia H. Q., Lu C. X., Chem. J. Chinese Universities, 2013, 34(7), 1685—1690 |
| (金兴辉, 胡炳成, 贾欢庆, 吕春绪. 高等学校化学学报, 2013, 34(7), 1685) | |
| [31] | Wang H. X., Wang B. Y., Zhang J. L., Li Z. R., Li X. Y., Chem. J. Chinese Universities, 2011, 32(5), 1123—1128 |
| (王海霞, 汪必耀, 张俊玲, 李泽荣, 李象远. 高等学校化学学报, 2011, 32(5), 1123—1128) | |
| [32] | Linstrom P.JMallard W. G.NIST Chemistry Webbook, |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [3] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [4] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [5] | SUN Cuihong, LYU Liqiang, LIU Ying, WANG Yan, YANG Jing, ZHANG Shaowen. Mechanism and Kinetics on the Reaction of Isopropyl Nitrate with Cl, OH and NO3 Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210591. |
| [6] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [7] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [8] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [9] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [10] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [11] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [12] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [13] | MENG Fanwei, GAO Qi, YE Qing, LI Chenxi. Potassium Poisoning Mechanism of Cu-SAPO-18 Catalyst for Selective Catalytic Reduction of NOx by Ammonia [J]. Chem. J. Chinese Universities, 2021, 42(9): 2832. |
| [14] | LIU Changhui, LIANG Guojun, LI Yanlu, CHENG Xiufeng, ZHAO Xian. Density Functional Theory Study of NH3 Adsorption on Boron Nanotubes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2263. |
| [15] | YING Fuming, JI Chenru, SU Peifeng, WU Wei. λ-DFCAS: A Hybrid Density Functional Complete Active Space Self Consistent Field Method [J]. Chem. J. Chinese Universities, 2021, 42(7): 2218. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||